Abstract
The medial temporal lobes (MTL) are critical for episodic memory but the functions of MTL subregions are controversial. According to memory strength theory, MTL subregions collectively support declarative memory in a graded manner. In contrast, other theories assert that MTL subregions support functionally distinct processes. For instance, one view is that perirhinal cortex (PRc) processes item information, parahippocampal cortex (PHc) processes context information, and the hippocampus binds item and context. Here, we report two experiments that tested competing predictions from these models. In these studies, subjects encoded color-word associations by imagining color either as a contextual association (context detail condition) or as a feature of the item to be encoded (item detail condition). Results showed that encoding color information as an item detail improved source recognition in amnesic patients with recollection deficits. Furthermore, event-related fMRI data from healthy subjects revealed PRc activation associated with successful retrieval of item details, whereas activation in the hippocampus and PHc was associated with recollection-based source retrieval. The qualitatively different patterns of results observed in PRc and hippocampus/PHc are inconsistent with a memory strength account and are consistent with the idea that different MTL regions process different types of episodic information.
Keywords: memory, hippocampus, parahippocampal, memory formation, medial, temporal
The medial temporal lobes (MTL) are critical for episodic memory, but the specific functions of MTL subregions, such as the hippocampus, perirhinal cortex (PRc), and parahippocampal cortex (PHc), are controversial. Some dual process theories propose a fundamental difference between these regions, such that the hippocampus is necessary for recollection of context information or relational memory, whereas the surrounding cortex is critical for familiarity-based item memory (Brown & Aggleton, 2001; N. J. Cohen & Eichenbaum, 1993; Yonelinas, 2002). Consistent with this view, functional magnetic resonance imaging (fMRI) studies have shown that hippocampal activity is correlated with successful performance on source memory tests, thought to depend on recollection, whereas PRc activity is correlated with item familiarity (e.g. Davachi, Mitchell, & Wagner, 2003; Kensinger & Schacter, 2006; Ranganath et al., 2003; Tendolkar et al., 2008; Weis et al., 2004). An alternative memory strength-based account of this dissociation is that, due to nonlinearities in the BOLD signal in fMRI studies, hippocampal activation is sensitive to gradations in high but not low strength memories, whereas PRc activation is sensitive to gradations in low but not high strength memories (Squire, Wixted, & Clark, 2007). Importantly, however, recent neuroimaging results have found that patterns of PHc activity in recognition memory experiments are more similar to patterns seen in the hippocampus than PRc, with both regions being associated with recollection/strong memory (Davachi et al., 2003; Kensinger & Schacter, 2006; Ranganath et al., 2003; Tendolkar et al., 2008). This pattern has not been accounted for by many dual process or memory strength theories.
Other theories have proposed that different MTL subregions process different kinds of information, and that their functions cannot be explained solely in terms of recollection/familiarity or memory strength (e.g. Bird & Burgess, 2008; Davachi, 2006) . For example, the Binding of Item and Context model (BIC) (Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007), proposes that PRc processes item information, PHc processes context information (defined as any information peripheral to the target of encoding), and the hippocampus binds item and context information. Recollection and familiarity processes reflect the type of information recovered during a retrieval cue. The BIC model suggests that in recognition tests that use an item cue, recollection is associated with retrieval of an item-context binding, supported by the hippocampus, and retrieval of context details, supported by PHc. Familiarity is associated with the retrieval of item representations, supported by PRc. However, the BIC model does not propose that PHc and PRc activity always reflect recollection and familiarity, respectively. For example, depending on the paradigm, recollection may reflect the retrieval of item details from a context cue. In this case subregion activation should be based on the type of information being retrieved. Thus, the functions of MTL subregions are related to recollection and familiarity but these processes are not the sole organizing principle.
The present studies test competing predictions of the different models by examining MTL involvement during a source memory test. Critically, during encoding, participants were either asked to treat the source (background color) as a context detail or an item detail.1 Our first experiment studied patients with recollection deficits, possibly due to hippocampal lesions, who are impaired on typical source memory tasks. Information-based theories predict that the item detail encoding condition enables patients to retrieve the background color based on the strength of item representations, supported by PRc, resulting in relatively spared performance in the item detail condition. However, memory strength theories would not necessarily predict different patterns of performance for the item detail and context detail source memory tasks. Our second experiment tested healthy young participants in an fMRI paradigm. Information-based theories predict that the hippocampus and PHc should support source recognition if source information is encoded as a context detail, as in standard source memory tasks, whereas PRc can also support performance if source information is encoded as an item detail. Memory strength theories predict that hippocampal, PHc, and PRc activation will vary based on memory strength, with the hippocampus BOLD response being more sensitive to differences between strong and moderate memory strength and the PRc BOLD response being more sensitive to differences between weak and moderate memory strength.
Method
Patients and Controls
Three subjects were recruited who had previously experienced mild hypoxic events, two due to cardiac arrest and one due to head trauma. These patients exhibit selective recollection deficits, based on previous testing (see Quamme, Yonelinas, & Norman, 2007; Yonelinas et al., 2002), with relatively spared familiarity-based memory. Due to pacemakers, two of these patients do not have MRI scans. Table 1 reports the demographic information and standardized test scores for the patients. For each patient participant, two age and education matched controls were recruited. In addition, twelve undergraduate students were recruited to provide control data.
Table 1.
Demographic information and standardized test results for amnesic patients. WAIS-R IQ: Wechsler Adult Intelligence scale, revised; COWA: Controlled Oral Word Association Test; WMS-R: Wechsler memory scale, revised
| WMS-R | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age | Education | WAIS-R IQ |
Trail making |
COWA category |
Verbal | Visual | General | Delayed | Attention/ Concentration |
| F | 50 | 16 | 96 | 0.51 | 0.16 | −2.53 | 2.00 | −1.4 | −1.13 | −0.2 |
| M | 28 | 16 | 111 | −1.36 | −1.12 | −1.33 | 0.33 | −0.87 | −2.13 | 0.2 |
| M | 51 | 13 | 108 | −0.56 | −0.24 | −0.4 | −1.53 | −0.87 | −1.33 | −0.27 |
fMRI Subjects
15 people (10 female) from the University of California, Davis community served as participants. Data from three subjects were excluded from analyses because of excessive head movement during at least one functional imaging block.
Materials
The patient study used 200 concrete English nouns. The words were randomly assigned to study condition (item detail or context detail) and background color (red or green) for each participant and separated into 2 study lists of 100 words each. The fMRI study used an additional 160 words for a total of 360 concrete nouns. These stimuli were divided into 4 study lists of 90 words each. The 360 study words from the two encoding conditions were intermixed and randomly assigned to one of 4 test lists. The test lists were ordered according to the optseq algorithm (Dale, 1999), which was used to maximize the ability to discriminate task-related hemodynamic responses, based on the study condition of each test word.
Procedure (patient study)
All subjects were given identical instructions based on previous behavioral investigations (Diana, Yonelinas, & Ranganath, 2008). They were told that they would be studying words and background colors for a memory test. In the item detail condition, based on the encoding task used by Staresina and Davachi (2006), they were asked to imagine the study item as though it were the same color as the background, read the provided explanation for why the item is that color, and then indicate whether imagining that explanation was easy or difficult. In the context detail condition, they were asked to imagine the study item interacting with a stop sign (red background) or dollar bill (green background), read the provided explanation for why the item is associated with the stop sign or dollar bill, and then indicate whether the imagining that explanation was easy or difficult. One list was studied in each encoding condition with list order counterbalanced between subjects (list order was also held constant such that each patient received the same list order and trial order as the two matched controls). On each study trial, subjects viewed the study word, background color, and study sentence until a response was given. The sentences that explained the item-source association were piloted with a separate group of undergraduate participants and selected to match performance between the two encoding conditions.
Upon completion of each study list, subjects were told that they would be tested on all of the study words but no new words. They judged whether each study item was seen on a red or green background, using a 1 to 6 confidence scale to make responses, with “1” and “6” representing high confidence red and green responses respectively, “2” and “5” responses representing moderate confidence red and green responses, and “3” and “5” responses representing low confidence red and green responses. On each test trial, participants were shown a studied word and the confidence scale and asked to judge whether the item was presented on a red or green background during study. Trials were self-paced. In order to more closely approximate a between-subjects procedure, which had shown matched performance for healthy undergraduates in previous studies (Diana et al., 2008), patients and matched controls were given source memory tests immediately following each encoding condition. A delay of 30 minutes was included between completion of the first study/test procedure and the second study/test procedure in order to reduce contamination between the two encoding tasks. The undergraduate participants were run in a between-subjects version of the experiment.
Procedure (fMRI study)
The procedure was similar to the patient study with the following exceptions. In the fMRI experiment, the number of study trials was increased from 200 to 360 in order to support a full ROC analysis and to provide sufficient numbers of trials for the fMRI analysis. In addition, the study duration was fixed at 5 seconds per item and subjects were required to covertly generate their own explanations during the encoding tasks, rather than receiving sentences providing explanations. Thus, in the item detail condition, participants imagined a scenario in which the item would be red or green while in the context detail condition participants imagined a scenario in which the item was associated with a stop sign or dollar bill. Note that sentence frames were provided in the patient study because older participants would likely have had difficulty rapidly generating their own scenarios. At test, the confidence instructions were modified such that the 1 and 6 responses were to be used when subjects recollected that the background color was red or green, respectively. Recollection was defined as recall of specific details about the study phase including memory for the story imagined on the study trial or any other details about the study phase that indicated background color. The “2” and “5” responses indicated high confidence that the item was shown against a red or green background, respectively, and the “3” and “5” responses indicated low confidence. In this way, the neural responses related to the 1 and 6 responses could be used to index recollection, which would not be possible using standard confidence instructions in which high confidence responses could reflect recollection or familiarity. During the scanning session, the participant completed four experimental runs including 90 test words each. Each run was preceded by 18 seconds of scanning (9 TRs) to allow the baseline magnetization to reach a steady state. Within each run, each trial consisted of a 2 second stimulus presentation, including the response scale, followed by a fixation screen lasting between 4 and 8 seconds. Thus, the mean inter-trial interval was 6s. Subjects were asked to make their response while the test item was on the screen if possible and were able to respond within the allotted 2 seconds for most trials.
Imaging Acquisition and Preprocessing
MRI data were acquired at the University of California Davis Imaging Research Center using a 3T Siemens Trio scanner equipped with an 8-channel phased array head coil. Earplugs were provided to attenuate acoustic noise from the scanner. Padding and adjustable head restraints were used to minimize head motion.
Functional data were obtained with a gradient echoplanar imaging (EPI) sequence (repetition time, 2000 ms; echo time, 25 ms; field of view, 220; 64 × 64 matrix); each volume consisted of 34 axial slices (interleaved acquisition), each with a slice thickness of 3.4 mm with no interslice gap, resulting in a voxel size of 3.4375×3.4375×3.4 mm. Additionally, T1-weighted images coplanar with the EPIs were acquired using an MP-RAGE sequence (matrix size = 256 × 256, voxel size = 1×1×1mm, number of slices = 192). A simple motor-response task (Aguirre, Zarahn, & D'Esposito, 1998) was performed to estimate subject-specific hemodynamic response functions (HRF).
Preprocessing was performed using Statistical Parametric Mapping (SPM5) software. EPI data were slice-timing corrected using sinc interpolation to account for timing differences in acquisition of adjacent slices, realigned using a six-parameter, rigid-body transformation, spatially normalized to the Montreal Neurological Institute (MNI) EPI template, resliced into 3mm isotropic voxels, and spatially smoothed with an isotropic 8 mm full-width at half-maximum Gaussian filter.
fMRI data analysis
Event-related blood oxygen level-dependent (BOLD) responses associated with each experimental trial were deconvolved with linear regression (Postle, Zarahn, & D'Esposito, 2000) under the assumption that these responses represent patterns of neural activity convolved with a hemodynamic response function (HRF). Regression analyses were conducted to analyze both familiarity responses (low confidence and high confidence, including responses of 2 and 3 for red backgrounds and 4 and 5 for green backgrounds) and recollection responses (1 responses for red backgrounds and 6 responses for green backgrounds). Within these analyses, covariates of interest modeled encoding condition (item detail vs. context detail) and response type (incorrect judgments vs. correct familiar judgments and incorrect judgments vs. correct recollection judgments). These covariates were convolved with a subject-specific estimated HRF (estimated from BOLD responses in the central sulcus during the performance of a motor response task). In all regression analyses, covariates of no interest modeled spikes in the time series, global signal changes that could not be attributed to variables in the design matrix (Desjardins, Kiehl, & Liddle, 2001), scan-specific baseline shifts, and an intercept. Regression analyses were performed on single-subject data using the general linear model with filters applied to remove frequencies above 0.25 Hz and below 0.005 Hz. These analyses yielded a set of parameter estimates for each participant, the magnitude of which can be interpreted as an estimate of the BOLD response amplitude associated with each test trial.
Contrast images were created for each subject and entered into a second-level one-sample t test. Significant regions of activation were identified using an uncorrected threshold of p < 0.005 and a minimum cluster size of 13 contiguous voxels. With these probability and extent thresholds, the mapwise false positive rate for MTL (i.e., hippocampus, PHc, and PRc), estimated using a Monte Carlo procedure (implemented in the AlphaSim program in AFNI), was p < 0.05. Suprathreshold clusters of voxels in the hippocampus and adjacent MTL cortical structures were used to define regions of interest (ROIs) from which mean parameter estimates were extracted for further analysis. Below, we focus on results observed in MTL, but significant findings from a whole-brain analysis are presented in Table 2.
Table 2.
Whole-brain findings with corrected threshold p < .001, cluster > 21. No significant clusters outside MTL were found for either the item detail or context detail familiarity contrasts.
| Brain Region | X | Y | Z | t-value |
|---|---|---|---|---|
| Context detail, recollection/strong memory contrast | ||||
| L.supramarginal gyrus | −57 | −48 | 48 | 7.97 |
| R. supramarginal gyrus | 66 | −57 | 36 | 6.87 |
| L. posterior cingulate gyrus | −9 | −45 | 36 | 5.12 |
| Item detail, recollection/strong memory contrast | ||||
| L. angular gyrus | −30 | −57 | 27 | 10.55 |
| R. putamen | 30 | −6 | 3 | 8.98 |
| L. perirhinal cortex | −33 | 6 | −45 | 8.73 |
| R. superior occipital gyrus | 12 | −90 | 21 | 8.00 |
| L. middle temporal gyrus | −51 | −24 | −18 | 6.88 |
| R. central sulcus | 36 | −24 | 57 | 6.15 |
| L. amygdala | −30 | −6 | −15 | 6.08 |
| L. cingulate | −15 | −48 | −24 | 5.86 |
| L. anterior cingulate | −9 | 51 | 9 | 5.78 |
| R. central sulcus | 30 | −48 | 69 | 5.73 |
| L. precentral gyrus | −18 | −51 | 66 | 5.58 |
| L. superior frontal gyrus | −15 | 24 | 60 | 5.14 |
| R. lateral fissure | 66 | −33 | 27 | 4.56 |
Results
Patient Study
In our first experiment, we examined source recognition in patients with mild hypoxia that have previously been shown to exhibit selective recollection deficits (Yonelinas et al., 2002; Quamme et al., 2007). On the basis of previous studies of mild hypoxia (e.g. Di Paola et al., 2008; Rempel-Clower, Zola, Squire, & Amaral, 1996), we expected that the patients’ memory impairments were due to hippocampal atrophy. However, structural MRI scans were not available thus we could not verify that the lesions were limited to the hippocampus. Although patients with recollection deficits are typically impaired on source memory tasks, we predicted that the item detail encoding condition would enable patients to retrieve the source information based on the strength of item representations supported by PRc. We therefore predicted better memory performance for patients in the item detail condition than in the context detail condition. However, if performance is based on an overall assessment of memory strength, the patients should show similar patterns of impairment in both encoding conditions. Because our hypotheses for the patient studies were directional, our t-tests are one-tailed, unless otherwise noted.
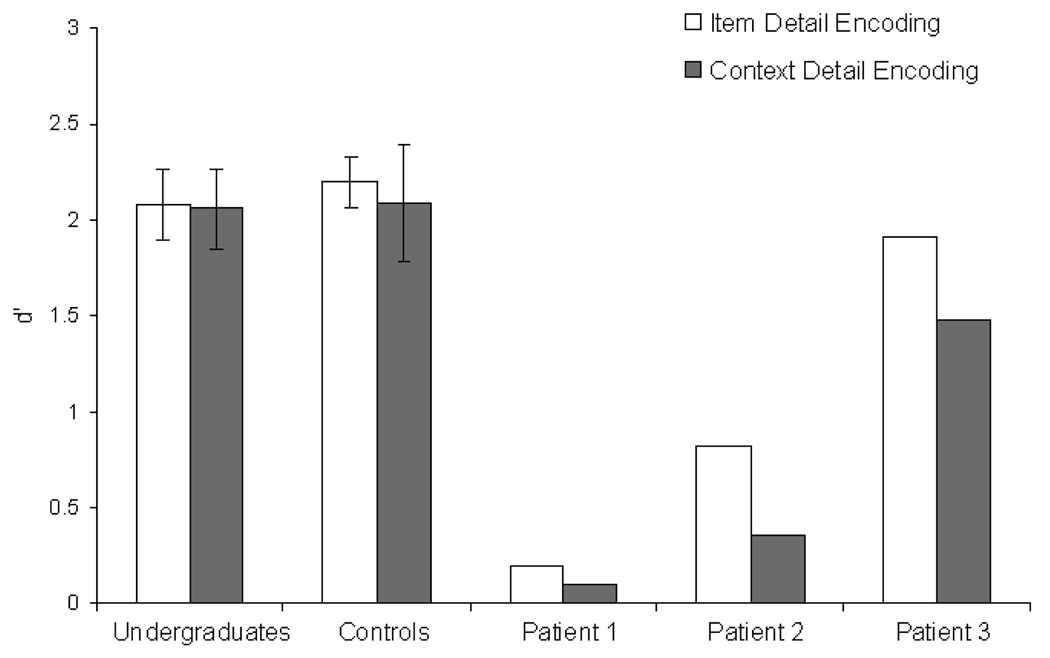
Figure 1 shows that, for both undergraduate pilot subjects and age/education matched control subjects, source recognition performance was comparable across the item detail and context detail encoding conditions (both t < 0.5). This finding indicates that any difference in performance between the two conditions for the patient participants could not be attributed to differences in overall effectiveness of either encoding task. As shown in Figure 1, patients were generally impaired relative to the matched controls (t(7)=3.14, p<.05). However, most importantly, patients’ source recognition was more accurate in the item detail condition than the context detail condition (t(2)=2.85, p=0.05, eta2=0.80), and this pattern was seen in each of the patients.
Figure 1.
Source recognition performance for the patient study. Discriminability (d') values are separately plotted for the item detail (open bars) and context detail (filled bars) conditions for undergraduate subjects, age and education-matched controls, and 3 hypoxic patients.
Given that amnesic patients’ overall performance was worse than that seen in controls, we ran further analyses to assess whether poor-performing control participants would also show reduced performance in the context detail condition. A median split analysis revealed that d' values were comparable across encoding conditions for both low performing control participants (item detail M=1.8; context detail M=1.7) and high performing control participants (M=2.5 for both groups). The difference in performance across encoding condition was not statistically significant for either group (both groups: p=0.24, eta2=0.26). Thus, the lack of an effect of encoding condition in the control participants cannot be attributed to high levels of performance.
These results indicate that amnesic patients’ deficit in source recognition was less pronounced if source information (i.e., color) had been encoded as an item detail rather than as a context detail. In addition, to the extent that the damage suffered in these patients was restricted to the hippocampus, the results are consistent with the claim that source memory for item details was supported by PRc. Although the location of the patients’ MTL damage cannot be determined with certainty, if the damage was more extensive and encroached on the surrounding MTL cortex, this would make it more difficult to observe our expected pattern of results. To more directly test the specific functional roles of separate MTL subregions, we next examined brain activity in young, healthy subjects using fMRI.
fMRI study
Our second experiment used event-related fMRI in healthy undergraduates to assess the extent to which activity in the hippocampus, PRc, and PHc is related to the way source information was encoded. The context detail condition was designed to be similar to encoding tasks used in typical source memory experiments, and thus we predicted that successful source memory would be associated with increased hippocampal and PHc activation (Cansino, Maquet, Dolan, & Rugg, 2002; Davachi et al., 2003; Kensinger & Schacter, 2006; Ranganath et al., 2003; Tendolkar et al., 2008; Vilberg & Rugg, 2007). However for stimuli that were encoded in the item detail condition, we predicted that the strength of item representations processed in PRc could also support source recognition.
Behavioral Analyses
We used confidence ratings from the source memory test to calculate receiver operating characteristics (ROCs) for each participant (Macmillan & Creelman, 2005), allowing us to assess the extent to which source memory was supported by familiarity and recollection (Yonelinas, 1994). We predicted that source memory performance in the context detail encoding condition would be based primarily on hippocampally-dependent recovery of contextual information associated with each item (e.g., remembering that an elephant walked near a red stop sign). For stimuli encoded on item detail trials, however, we predicted that source recognition could also be based on gradations in item memory strength (e.g., after studying a red elephant, the idea of a red elephant might seem more familiar than a green elephant). Accordingly, we expected that source ROCs for the item detail condition would be more curvilinear than those for the context detail condition. This effect would be evident as an increase in familiarity estimates according to a dual process model or memory strength estimates in a signal detection model.
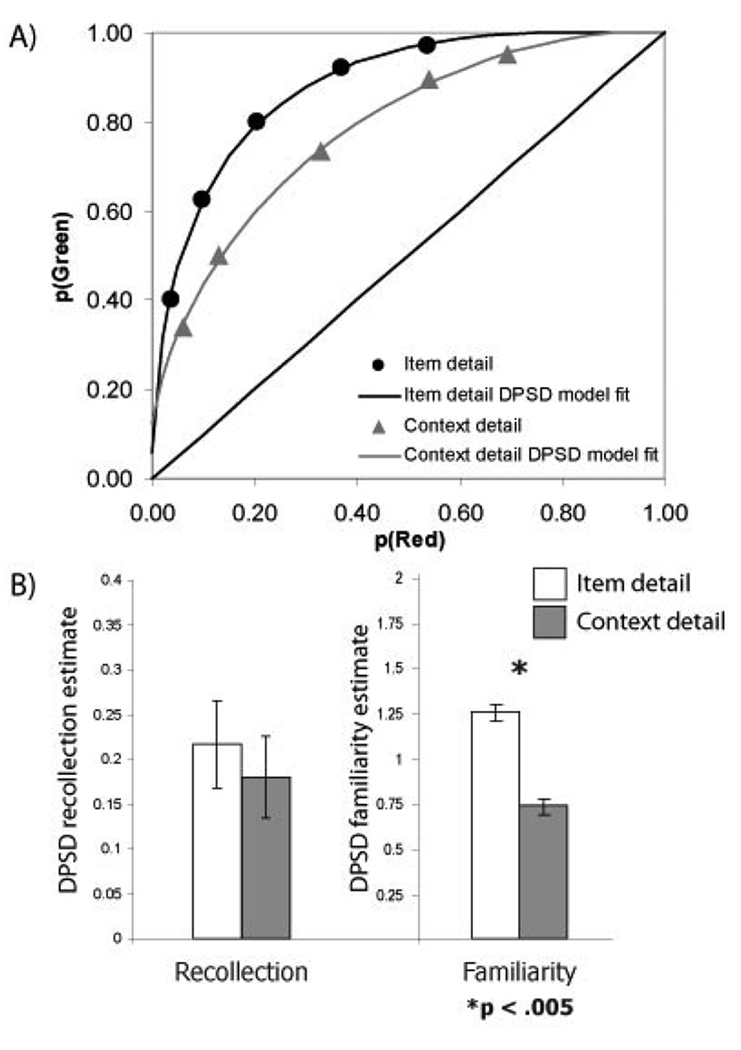
Inspection of the ROCs indicated that overall source recognition performance was better for the item detail condition than for the context detail condition (see Figure 2).2 The dual process signal detection (DPSD) model (Yonelinas, 1994), was fit to these data in order to estimate the relative contributions of recollection and familiarity to source recognition in each condition. A factorial ANOVA on these data revealed an interaction, indicating that the encoding conditions had differential effects on recollection and familiarity estimates (F(1,11) = 9.03, p = .01). Follow-up analyses indicated that familiarity estimates were significantly higher in the item detail condition than in the context detail condition, t(11) = 4.19, p < .005, but there was no significant difference in recollection between the two encoding conditions, t(11) = 0.83, p = .42. A formal analysis of the same results using an unequal variance signal detection model supported similar conclusions, showing that memory strength was greater in the item detail than context detail condition, t(11) = 3.85, p < .005, but that the old/new variance ratio did not differ across conditions, t(11) = −0.12, p = 0.9. Thus, the behavioral results indicate that encoding source information as an item detail affected estimates of familiarity (or mean memory strength), but did not affect recollection (or the variance ratio).
Figure 2.
Source recognition performance for the fMRI study. A) ROC curves for the item detail and context detail conditions, fit using DPSD model. B) Recollection and familiarity estimates for unitized and nonunitized source retrieval, based on DPSD model fits.
fMRI Analyses
The fMRI analyses assessed memory for the background color (i.e. source memory) in the item detail and context detail conditions based on the confidence ratings given for each item. Different models of source recognition lead to different interpretations of confidence ratings. For instance, the DPSD model suggests that recollection primarily supports the highest confidence ratings (correct “1” and “6” responses, which were explicitly labeled as recollection in this experiment), whereas familiarity supports the entire range of confidence judgments. In contrast, pure signal detection models argue that the most confident responses are based on the strongest memories, whereas low confidence responses are based on relatively weaker memories. In our analyses of the fMRI data, we sought to examine the relationship between brain activation and source recognition in a manner that did not strictly depend on the assumptions of either model. Accordingly, two analyses were performed for each encoding condition. In one analysis, trials with high confidence source hits (i.e., on which a 1 or a 6 response was correctly given) were contrasted against incorrect source recognition trials (i.e., a 1, 2, or 3 response for green sources or a 4, 5, or 6 response for red sources). This “recollection/strong memory” contrast was designed to identify brain activity correlated with accurate source retrieval based on recollection (dual process model) or based on strong memory (memory strength model). In a second contrast, trials with low confidence source hits (i.e., a 2 or 3 response for red sources or a 4 or 5 response for green sources) were contrasted against trials with incorrect source recognition responses. This “familiarity/weak memory” contrast was designed to identify neural correlates of source retrieval based on familiarity (dual process model) or weak memory (strength model).
As noted earlier, the context detail condition was designed to elicit patterns of MTL activation typical of most previous fMRI studies, in which successful source memory performance was correlated with PHc and hippocampal activation. According to the BIC and DPSD, this is because source retrieval is typically based on recollection of contextual information associated with an item cue. However, according to a memory strength account, such results reflect the fact that studies typically contrast trials with high confidence responses against trials with medium confidence responses. Specifically, it has been argued that, due to across-region differences in the relationship between memory strength and BOLD signal, hippocampal activity measured by fMRI is only sensitive to differences at the higher end of the memory strength scale, whereas PRc activity measured by fMRI is only sensitive to changes at lower points in the memory strength scale (Kirwan, Wixted, & Squire, 2008; Wais, Squire, & Wixted, 2009). In the present study, the recollection/strong memory analysis contrasted the most confident responses against responses at the weakest end of the memory strength scale. Accordingly, an account based solely on memory strength should predict activation in the hippocampus, PHc, and PRc in this contrast.
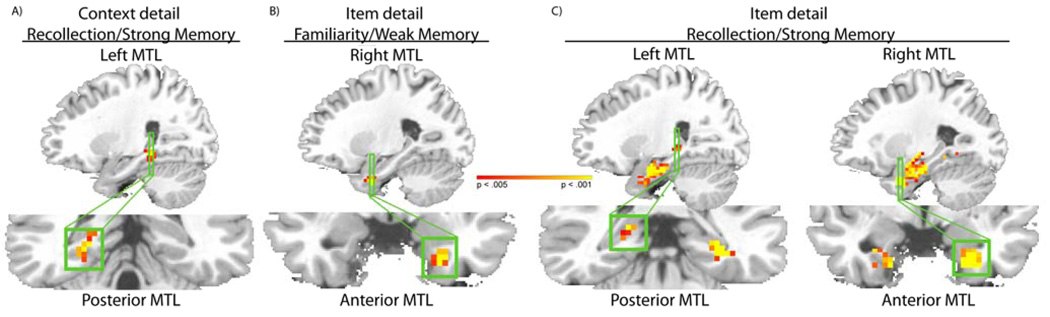
Consistent with results from previous studies (Cansino et al., 2002; Davachi et al., 2003; Kensinger & Schacter, 2006; Ranganath et al., 2003; Tendolkar et al., 2008; Vilberg & Rugg, 2007; Weis et al., 2004), the recollection/strong memory contrast for context detail trials identified a region that included left PHc and the hippocampus (peak voxel x=−27, y=−39, z=−9, t(11) = 4.56, p < .001; see Figure 3), but no suprathreshold voxels in PRc. The familiarity/weak memory contrast for context detail trials did not reveal any suprathreshold voxels in the MTL. These results are not consistent with a simple memory strength account, which would have predicted PRc activation associated with source retrieval in the recollection/strong memory contrast.
Figure 3.
Different MTL subregions show dissociable patterns of activation during source recognition. Suprathreshold voxels in the MTL ROI are shown for the (A) context detail recollection/strong memory contrast, (B) item detail familiarity/weak memory contrast, and C) item detail recollection/strong memory contrast. Whereas activation in the hippocampal and PHc was specifically evident in the recollection/strong memory contrasts, PRc activation was specifically evident in the item detail contrasts.
Our next analyses focused on activity elicited by stimuli encoded in the item-detail condition. We predicted that accurate source recognition on these trials could be supported by recollection of contextual details (e.g., recollection of the story generated at study), and that the recollection/strong memory contrast would reveal activation in PHc and the hippocampus. In addition, based on the hypothesis that PRc is important for processing item information (Diana et al., 2007; Eichenbaum et al., 2007; Staresina & Davachi, 2006, 2008), we predicted that PRc activation on item detail trials should correlate with familiarity- and recollection-based source recognition. In contrast, according to a memory strength account, MTL activation would be expected to reflect sensitivity of MTL regions to strong and weak memories. Specifically, this account would suggest that the weak memory contrast should reveal activation in PRc, whereas the strong memory contrast should reveal activation in the hippocampus and PRc.
Consistent with the predictions of the BIC model, the recollection/strong memory contrast for item details was associated with activation across MTL, including bilateral PRc (peak voxels: left PRc, x=−33, y=6, z=−45, t(11)=8.73, p < .001 and right PRc, x=18, y=0, z=−39, t(11)=6.64, p < .001), left hippocampus (x=−28, y=−23, z=−13, p < .005) and right PHc (x=30, y=−39, z=−9, p < .001) extending into the hippocampus. Additionally, the familiarity/weak memory contrast revealed activation in right PRc (peak voxel x=15, y=−12, z=−30, t(11)=5.45, p < .001), and no suprathreshold voxels were seen in other MTL regions. In order to assess whether PRc activation was differentially associated with the item detail condition as compared to the context detail condition, we conducted a subsequent voxel-wise analysis contrasting the magnitude of source memory success effects between the item detail and context detail conditions. For recollection/strong memory and familiarity/weak memory, regions in PRc were identified as showing greater activation on item detail trials than on context detail trials. This interaction was significant in right PRc (p < .01, cluster size > 4, resulting in a mapwise false positive rate of p < .05) for both recollection/strong memory (peak voxel: x=−24, y=6, z=−42) and familiarity/weak memory (x=−27, y=3, z=−42).
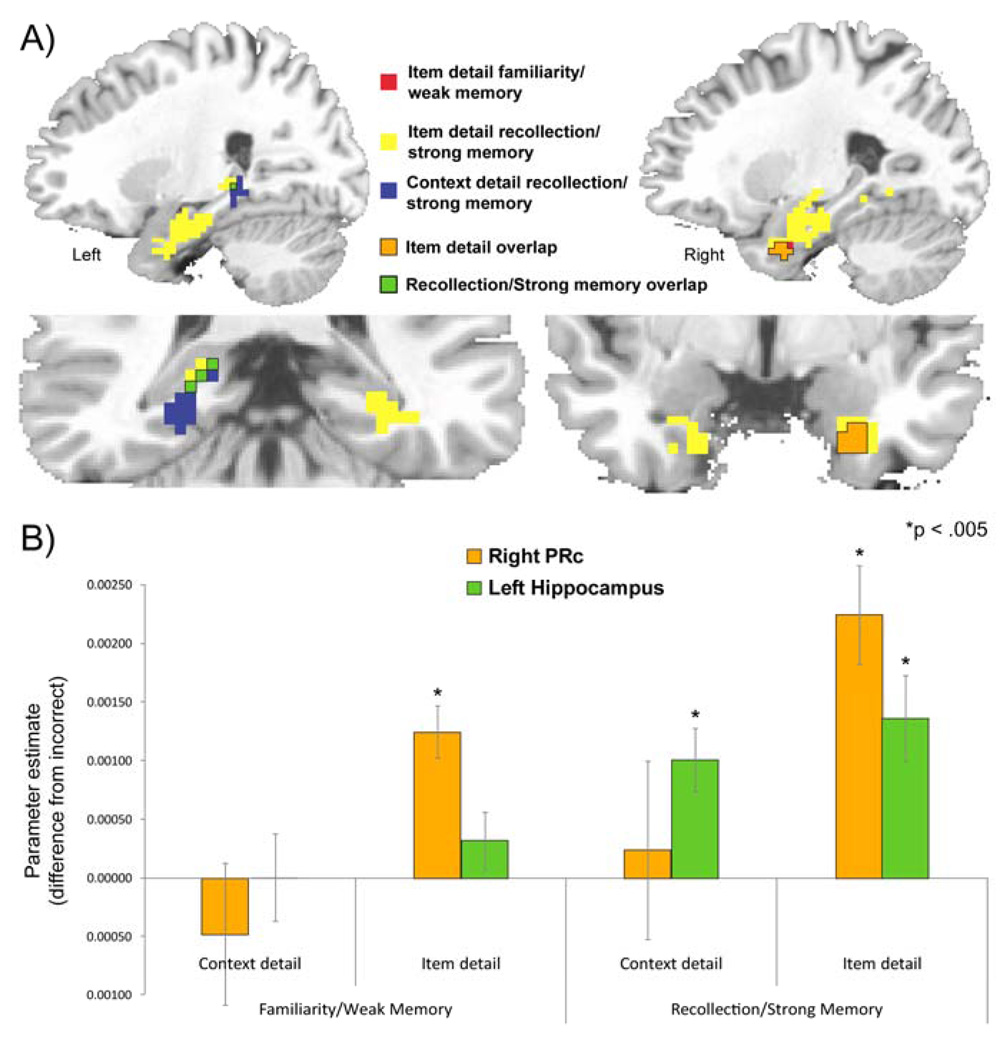
To more clearly visualize the topography of MTL activation during source recognition, we overlaid the thresholded statistical maps from the three conditions that showed significant MTL activation (familiarity and recollection in the item detail condition and recollection in the context detail condition). As shown in Figure 4, overlapping voxels in PRc were identified for recollection and familiarity in the item detail condition and in the hippocampus for recollection-based retrieval in both the item detail and context detail conditions. No overlapping voxels were seen in PHc, but separate regions in right and left PHc were identified in the recollection contrasts for the item and context detail conditions, respectively. These results suggest that PHc and hippocampal activation was generally associated with recollection, whereas PRc activation was specifically associated with source recognition in the item detail condition.
Figure 4.
Overlap of source memory success effects in the MTL. (A) Regions showing MTL activation in each contrast are plotted simultaneously to reveal areas of overlap across: familiarity/weak memory in the item detail condition (red), recollection/strong memory in the item detail condition (yellow), and recollection/strong memory in the context detail condition (blue). Common areas of activation for item detail familiarity/weak memory and recollection/strong memory occurred in PRc (plotted in orange). Common areas of activation for recollection/strong memory in both the item detail and context detail conditions occurred in the hippocampus (plotted in green). (B) Parameter estimates extracted from the common areas of activation in A (orange and green areas) reveal significant PRc activation for the item detail conditions only and significant hippocampal activation for recollection responses only.
Discussion
The current experiments tested the hypothesis that the hippocampus and PHc support source memory based on recollection of context details, but that PRc can support source recognition if source information is encoded as an item detail. Critically, stimuli from the item detail and context detail conditions were equivalent, with the only difference being the way color information was processed during encoding. Nonetheless, we found that encoding color as an item or context detail had a significant impact on the relative roles of different MTL regions in source recognition. In the patient study, even when performance was matched in controls, patients with likely recollection-specific deficits and likely hippocampal damage were more impaired for the context detail condition than for the item detail condition. In the fMRI study, activity in the hippocampus and PHc was specifically correlated with recollection-based source memory as found in previous experiments. However, PRc activation during retrieval was associated with source memory accuracy specifically if color was encoded as an item detail. Our findings are consistent with information-based theories of MTL subregion function (N. J. Cohen & Eichenbaum, 1993; Davachi, 2006), such as the BIC model (Diana et al., 2007; Eichenbaum et al., 2007).
The results from the study of mild hypoxia patients provide insight into the retrieval processes involved in the item detail and context detail encoding conditions, indicating that encoding source information as an item detail facilitates the use of item familiarity in source recognition. We cannot conclusively determine whether these patients have hippocampal damage and spared PRc function, but if the damage was extensive and encroached on the surrounding MTL cortex, this would make it more difficult to observe the expected pattern of results. Furthermore, our conclusions do not depend on assumptions about lesion site in the patients, as the roles of specific MTL regions were more directly tested in the fMRI study.
The fMRI results provide evidence against the proposal that dissociations between activation in different MTL subregions are based purely on differences in sensitivity to weak vs. strong memories (Squire et al., 2007). The methods used to analyze fMRI results from the present study were chosen so that the conclusions would not depend on the assumptions of a specific recognition memory model. However, even under these conditions, the pattern of results was inconsistent with the predictions of a memory strength theory because such theories must predict that activity within a given MTL subregion will have a monotonic relationship with memory strength. In our results, PRc activity was related to recollection of item details (i.e., a condition of high memory strength), and to familiarity of item details (i.e., a condition of low memory strength), whereas it was not related to recollection of contextual details (i.e., a condition of high memory strength). Thus the relationship between PRc activation and memory strength is nonmonotonic. The present results therefore suggest that MTL regions do not merely differ in the relationship between memory strength and BOLD signal but rather in a more fundamental manner that is related to the kind of information that is processed by these regions.
The results are consistent with dual process models that propose that PRc is particularly critical for familiarity-based retrieval (Brown & Aggleton, 2001) and less consistent with dual process models that do not distinguish between the functions of PRc and PHc (N. J. Cohen & Eichenbaum, 1993; Yonelinas, Hopfinger, Buonocore, Kroll, & Baynes, 2001). To fully account for the present results, a dual process model would need to explain the functional differences we observed between PRc and PHc, and the differences in MTL activity for source information encoded as an item or a context feature.
Although the item detail and context detail conditions used identical stimuli, one might imagine that the context detail condition could evoke spatial imagery to a greater degree than the item detail condition because it involves associating multiple objects. This is relevant because some theories claim that the hippocampus and PHc are important for perception and memory of scene and object images (e.g. Barense, Gaffan, & Graham, 2007; Taylor, Henson, & Graham, 2007). If one assumes that the context detail condition involved more spatial processing, then a purely spatial account would predict that activity in the hippocampus and PHc would be correlated with accurate familiarity- and recollection-based responses only for the context detail condition. In contrast, hippocampal and PHc activation was specifically increased during recollection-based responses across both the item detail and context detail memory conditions. Parameter estimates in these regions did not significantly differ between encoding conditions (see Figure 4), and there was not even a trend for increased hippocampal activation in the context detail condition. In addition, such an account would not explain why PRc was preferentially involved in the item context condition compared to the context condition. Accordingly, it is unlikely that the present results reflect a confound between the encoding tasks and spatial processing. We note, however, that our encoding tasks were not intended to systematically vary spatial vs. object processing, and that the results do not refute the idea that such differences could modulate MTL activation. Indeed, differential responses during spatial and object memory retrieval could be compatible with models such as BIC.
Our results are consistent with a growing body of findings suggesting that PRc can play an important role in memory for certain kinds of associative information. For instance, studies by Staresina and Davachi have found that PRc activation during encoding predicted successful memory for item-color associations, labeled as intra-item associations, (Staresina & Davachi, 2006) but not memory for other, more typical, source details such as the orienting question (Staresina & Davachi, 2008). Critically, in the studies by Staresina and Davachi, participants imagined each item in the designated color, which is consistent with the current findings showing that PRc activity during source retrieval was increased if participants encoded color as an item feature. Other studies have found that pairs of items (e.g. "motor" and "bear") can be unitized into a single new item representation ("motorbear") using tasks that encourage processing of the word pair as a single compound word (e.g. Haskins, Yonelinas, Quamme, & Ranganath, 2008; Quamme et al., 2007). Consistent with findings in the current patient study, unitization has been shown to increase associative recognition in patients with hippocampal damage (Quamme et al., 2007; Giovanello, Keane, & Verfaellie, 2006; Yonelinas, Kroll, Dobbins, & Soltani, 1999). PRc activation during encoding also increases during unitization and is correlated with subsequent associative recognition (Haskins et al., 2008). Critically, the present results also add to previous findings by demonstrating a dissociation between the role of PRc in retrieval of item details and that of PHc and the hippocampus in retrieval of context details even when stimulus materials and test demands were held constant.
Another implication of the current findings is that source memory tests do not provide pure measures of recollection as some have argued, but rather that both recollection and familiarity can contribute to source discriminations. The current data and related findings (Quamme et al., 2007; Yonelinas et al., 1999; Haskins et al., 2008; Diana et al., 2008) indicate that the way information is encoded, along with other factors, may enable the use of familiarity to support associative memory judgments.
The present results revealed remarkably similar findings in PHc and the hippocampus, which is consistent with observations from studies of source memory encoding (Davachi et al., 2003; Kensinger & Schacter, 2006; Ranganath et al., 2003). However, we expect that these regions have qualitatively different functions, with PHc involved in processing context information and the hippocampus involved in binding item and context information. In the current paradigm, we predicted that both of these regions should be involved in recollection of context. This is because item cues were provided at retrieval and thus recovery of context information (e.g., memory for the story that was generated during encoding) would first require retrieval of the item-context binding. It should be possible to dissociate the functions of PHc and the hippocampus in a paradigm in which retrieval of the episodic binding is not necessary in order to retrieve context information.
In conclusion, results from this study support the hypothesis that the involvement of PRc, PHc, and the hippocampus in episodic memory relates to the type of information processed by these regions (Diana et al., 2007; Davachi, 2006; Eichenbaum et al., 2007). Models that assume MTL subregion involvement is determined solely by overall memory strength or retrieval process (without taking encoding processing into account) cannot explain these results. Instead, the results indicate that the types of memorial information involved at all stages of processing should be considered when assessing the roles of MTL subregions in episodic memory.
Acknowledgements
This work was supported by National Institute of Mental Health grants MH068721 and MH059352 and a Kirschstein NRSA fellowship MH079621 to RD. We thank Colleen Parks and Linda Murray for assistance with ROC analyses and the patient study.
Footnotes
We use the term “source memory” to refer to a memory task in which the participant is asked to retrieve a particular detail that was associated with an item, and “source” to refer to the detail itself. Our use of the terms “item” and “context” refer to the kinds of representations that can be used to make source attributions and which we manipulated with our encoding conditions.
This overall accuracy difference did not occur in the patient study because patients and controls were provided with performance-matched sentences on each trial, whereas in the fMRI study, participants were asked to generate their own scenario for each item.
References
- Aguirre GK, Zarahn E, D'Esposito M. The variability of human, BOLD hemodynamic responses. NeuroImage. 1998:360–369. doi: 10.1006/nimg.1998.0369. [DOI] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45(13):2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. doi: S0028-3932(07)00216-3. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nature Reviews. Neuroscience. 2008;9(3):182–194. doi: 10.1038/nrn2335. doi: nrn2355. [DOI] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus. Nature Reviews Neuroscience. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cerebral Cortex. 2002;12:1047–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT; 1993. [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current opinion in neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner A. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins AE, Kiehl KA, Liddle PF. Removal of confounding effects of global signal in functional MRI analyses. NeuroImage. 2001;13(4):751–758. doi: 10.1006/nimg.2000.0719. doi: 11305902. [DOI] [PubMed] [Google Scholar]
- Di Paola M, Caltagirone C, Fadda L, Sabatini U, Serra L, Carlesimo GA. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus. 2008;18(7):719–728. doi: 10.1002/hipo.20432. doi: 10.1002/hipo.20432. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in cognitive sciences. 2007;11(9):379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. The effects of unitization on familiarity-based source memory: testing a behavioral prediction derived from neuroimaging data. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2008;34(4):730–740. doi: 10.1037/0278-7393.34.4.730. doi: 2008-08549-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Keane MM, Verfaellie M. The contribution of familiarity to associative memory in amnesia. Neuropsychologia. 2006;44:1859–1865. doi: 10.1016/j.neuropsychologia.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59(4):554–560. doi: 10.1016/j.neuron.2008.07.035. doi: S0896-6273(08)00634-X. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Schacter DL. Amygdala activity is associated with successful encoding of item, but not source, information for positive and negative stimuli. The Journal of Neuroscience. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Wixted JT, Squire LR. Activity in the medial temporal lobe predicts memory strength, whereas activity in the prefrontal cortex predicts recollection. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2008;28(42):10541–10548. doi: 10.1523/JNEUROSCI.3456-08.2008. doi: 10.1523/JNEUROSCI.3456-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: A user's guide. 2nd ed. New York: Cambridge University Press; 2005. [Google Scholar]
- Mayes A, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Postle BR, Zarahn E, D'Esposito M. Using event-related fMRI to assess delay-period activity during performance of spatial and nonspatial working memory tasks. Brain Research. 2000;5:57–66. doi: 10.1016/s1385-299x(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Quamme JR, Yonelinas AP, Norman KA. Effect of unitization on associative recognition in amnesia. Hippocampus. 2007;17(3):192–200. doi: 10.1002/hipo.20257. doi: 10.1002/hipo.20257. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rempel-Clower NL, Zola SM, Squire LR, Amaral DG. Three cases of enduring memory impairment after bilateral damage limited to the hippocampal formation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 1996;16(16):5233–5255. doi: 10.1523/JNEUROSCI.16-16-05233.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews. Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. doi: nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and Shared Contributions of the Hippocampus and Perirhinal Cortex to Episodic Item and Associative Encoding. Journal of cognitive neuroscience. 2008 doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KJ, Henson RNA, Graham KS. Recognition memory for faces and scenes in amnesia: dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45(11):2428–2438. doi: 10.1016/j.neuropsychologia.2007.04.004. doi: S0028-3932(07)00145-5. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus-Dumke A, van Eijndhoven P, et al. Contributions of the medial temporal lobe to declarative memory retrieval: manipulating the amount of contextual retrieval. Learning & Memory (Cold Spring Harbor, N.Y.) 2008;15(9):611–617. doi: 10.1101/lm.916708. doi: 10.1101/lm.916708. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45(10):2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Squire LR, Wixted JT. In Search of Recollection and Familiarity Signals in the Hippocampus. Journal of Cognitive Neuroscience. 2009 doi: 10.1162/jocn.2009.21190. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis S, Specht K, Klaver P, Tendolkar I, Willmes K, Ruhlmann J, et al. Process dissociation between contextual retrieval and item recognition. Neuroreport. 2004;15(18):2729–2733. [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1994;206(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. Neuroreport. 2001;12(2):359–363. doi: 10.1097/00001756-200102120-00035. doi: 11209950. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins IG, Soltani M. Recognition memory for faces: When familiarity supports associative recognition. Psychonomic Bulletin & Review. 1999;6:418–661. doi: 10.3758/bf03212975. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Quamme JR, Lazzara MM, Sauvé M, Widaman KF, et al. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5(11):1236–1241. doi: 10.1038/nn961. doi: 12379865. [DOI] [PubMed] [Google Scholar]