Abstract
Maternal separation has been associated with development of anxiety-like behaviour and learning impairments in adult rats. This has been linked to changes in brain morphology observed after exposure to high levels of circulating glucocorticoids during the stress-hyporesponsive period (P4 to P14). In the present study, adult rats that had been subjected to maternal separation (180 min/day for 14 days) during the stress-hyporesponsive period, received unilateral infusions of a small dose of 6-hydroxydopamine (6-OHDA, 5 μg/4 μl saline) into the medial forebrain bundle. The results showed that voluntary exercise had a neuroprotective effect in both non-stressed and maternally separated rats in that there was a decrease in forelimb akinesia (step test) and limb use asymmetry (cylinder test). Maternal separation increased forelimb akinesia and forelimb use asymmetry and reduced the beneficial effect of exercise on forelimb akinesia. It also reduced exploratory behaviour, consistent with anxiety-like behaviour normally associated with maternal separation. Exercise appeared to reduce dopamine neuron destruction in the lesioned substantia nigra when expressed as a percentage of the non-lesioned hemisphere. However, this appeared to be due to a compensatory decrease in completely stained tyrosine hydroxylase positive neurons in the contralateral, non-lesioned substantia nigra. In agreement with reports that maternal separation increases the 6-OHDA-induced loss of dopamine terminals in the striatum, there was a small increase in dopamine neuron destruction when expressed as a percentage of the non-lesioned hemisphere but there was no difference in dopamine cell number, suggesting that exposure to maternal separation did not exacerbate dopamine cell loss.
Keywords: maternal separation, exercise, dopamine, 6-OHDA, Parkinson's disease
Introduction
Exposure to early life stressors such as maternal separation may result in disruption of the brain's neurocircuitry and the development of neurological disorders such as learning deficits, attention deficit hyperactivity disorder and anxiety disorders [4,8,15,19]. Neonatal exposure to stress modifies intracellular processes that predispose the affected cells to dysfunction which may be unmasked by further exposure to trauma later on in life [3]. Immediately after birth, pups have high circulating corticosterone levels which gradually decline and remain low during the stress-hyporesponsive period [28,52]. The stress-hyporesponsive period begins on postnatal day 4 (P 4) and lasts until P 14 [15,45]. It is characterised by low circulating corticosterone levels and the inability of mild stressors to induce a corticosterone response [45]. The low levels of corticosterone are due to the adrenal glands' insensitivity to the low levels of circulating ACTH and a low glucocorticoid receptor concentration in the hippocampus [16,44,52]. The stress-hyporesponsive period is crucial in protecting the developing brain as excessively high glucocorticoid levels are associated with abnormal neural and behavioral development [41]. Maternal separation as a stressor is used as a model to study long-term neurochemical and behavioural changes in adult rats [5,26,29].
In rats, separation of pups from the dam in the first weeks after birth can permanently alter the hypothalamic-pituitary-adrenal (HPA) axis (stress response) [27] leading to increased basal CRF mRNA in the hypothalamic and extra hypothalamic nuclei in adult rats [15] and adverse effects on the behaviour of rats in adulthood [5]. Apart from its effects on the limbic system (learning, memory and anxiety), stress has been shown to affect the dopaminergic system that is responsible for the control of movement [33] and therefore might influence the pathology of movement disorders such as Parkinson's disease.
Parkinson's disease is a neurodegenerative disorder of multifactorial aetiology that is characterized by a progressive loss of dopaminergic neurons in the substantia nigra pars compacta [10,20]. Initially, the nigrostriatal pathway is able to compensate for the loss of dopamine cells and only when more than 80% of the dopamine neurons have degenerated are clinical symptoms of Parkinson's disease present [35]. Neurotoxic drugs such as 6-hydroxydopamine (6-OHDA), have made it possible to create a parkinsonian rat model [51]. 6-OHDA is specific to catecholamine neurons (dopamine and norepinephrine) and when it is transported into the cell bodies and fibres of these neurons, it causes their degeneration [48]. Injecting a small dose of 6-OHDA into the medial forebrain bundle of rats can create a preclinical parkinsonian rat model [50]. There have been a number of interventions that have been used to slow down or reverse dopamine neuron degeneration in the nigrostriatal pathway following injections of dopamine neurotoxins. Among these, physical exercise has been shown to play a significant role in improving motor function deficits associated with Parkinson's disease [12,13,49]. In both forced [47,48] and voluntary [14,23,24] exercise, it has been shown that there is a decrease in both dopamine neuron degeneration and the behavioural deficits associated with a parkinsonian rat model. It has also been suggested that physical activity early in life may protect against the development of Parkinson's disease [2]. In the present study, we developed a preclinical parkinsonian rat model and we hypothesized that early life trauma such as maternal separation leaves the striatum/substantia nigra area of the rat brain more vulnerable to the neurotoxic stress of 6-OHDA. Therefore we aimed to investigate whether maternal separation exacerbates the size of the lesion in adult rats following injection of a small dose of 6-OHDA and whether voluntary exercise pre- and post-lesion can reduce this vulnerability.
Methods
Animals
On postnatal day (P) 1, the rats were sexed and culled to eight male Sprague Dawley pups per litter. If there were less than 8 male pups per litter, the numbers were increased to eight by adding the appropriate number of females so that the same number of pups was suckling on all dams. All experiments were approved by the Research Ethics Committee of the University of Cape Town.
Maternal Separation
On P2, the dam was removed from her litter and placed in a clean cage with fresh bedding [39]. The home cage was moved to a different room (temperature range, 31-33°C) for 3 hrs before being returned to the room with the dam and the dam returned to her litter. Maternal separation occurred daily between 09h00 and 12h00 until P14. The daily light /dark cycle was 07h00 to 19h00. The home cage was cleaned once every 4 days and care was taken not to handle the pups as studies have shown that handled pups have increased hippocampal glucocorticoid receptor expression [30,31]. Increased receptor expression has been shown to reduce the response to an acute stressor [30] thus cancelling the effects of maternal separation in adulthood. Only half the bedding was changed during each cleaning session thus ensuring that the dams were familiar with the odour of the home cage when reintroduced to the litter following the 3 hr separation.
The pups not involved in the maternal separation protocol were kept with their dams under normal animal house conditions (07h00-19h00 light/dark cycle) and a temperature range between 21 and 24°C. All rats were weaned on P21 and then kept 4 per cage until P46.
Exercise
On P46, the rats were moved to a room with a 23h00-11h00 light/dark cycle. On P53, maternally separated rats (n = 20) and non-stressed rats (n = 19) were weighed and divided into two groups each. Ten maternally separated rats and nine non-stressed rats were placed individually into cages that had running wheels attached. The remaining rats were placed individually into plexiglass cages (no access to running wheels). The rats received food and water ad libitum. The running wheels were fitted with counters which measured the revolutions made by the rats. One complete revolution was one meter in distance. Running in the wheels was recorded daily between 10h00 and 11h00 which was 1 hr before the dark cycle began. On P60, the rats in the four groups were weighed and taken to the lab where they were to undergo stereotaxic surgery. The rats were taken to the surgical laboratory at least 1 hr before surgery so as to acclimatize to the new environment.
Stereotaxic surgery
The rats were anaesthetised using a mixture of oxygen and halothane administered via a calibrated Blease Vaporiser (DATUM). The rats also received an injection of desipramine HCl (15 mg/kg i.p., Sigma St. Louis, MO, U.S.A), a norepinephrine reuptake blocker 30 min before 6-OHDA infusion. All 39 animals received 6-OHDA HCl (5 μg/4 μl saline; Sigma, St. Louis, MO, U.S.A) infusion unilaterally (0.5 μl/min) using a 32G dental needle into the left medial forebrain bundle (4.7 mm anterior to lambda, 1.6 mm lateral to midline and 8.4 mm ventral to dura [11,37]. The infusion needle was left in the medial forebrain bundle for 1 min prior to the infusion and for a further 5 min post infusion in order to allow time for the 6-OHDA to diffuse into the tissue. Post infusion, the burr-hole was closed with bone wax and the scalp incision was sutured. The rats were allowed to recover in plexiglass cages (one per cage) for 2 hrs in the surgical laboratory before they were returned to their respective cages. Daily revolutions were recorded for a further two weeks for cages with attached running wheels.
Behavioural tests
Two weeks post lesion (P74), each rat was assessed for motor function deficit using three behavioural tests in a behavioural testing room with light intensity of 48 lux. Care was taken to clean the testing apparatus with 70% alcohol before each test.
Forelimb akinesia (Step test)
Step length provides a robust measure of impairment of initiation of movement, a striking characteristic of Parkinson's disease, and thus measures the severity of the effect of the 6-OHDA lesion on limb function [42]. The rat's hindlimbs were suspended in mid air while the animal supported its entire weight on one forelimb [48]. To minimize head turning, the head and the forelimb not being tested were gently oriented forward by using the thumb and index finger [43]. The length of the step taken by each forelimb was measured and recorded. Each forelimb was tested three times and the mean was recorded as the step taken by each forelimb.
Limb use asymmetry (Cylinder test)
This test assesses forelimb use during exploratory activity in a plexiglass cylinder [48]. Activity was analyzed by videotaping rats in a transparent cylinder (20 cm diameter and 30 cm height, with the bottom and top ends open) for 5 min [42]. Limb use asymmetry was scored as the percentage of left, right or both limb wall placement (touch), wall movement and floor landing [43,48]. To measure the percentage preference of the rats to use the unimpaired limb we used the formula: [(ipsi+½both) divided by (ipsi+contra+both)] × 100.
Ipsi stands for the limb ipsilateral to the lesioned hemisphere which is the unimpaired limb and contra is the limb contralateral to the lesioned hemisphere and therefore the impaired limb.
Open field apparatus
To allow time for recovery, there was a 2 hr interval between the limb use asymmetry test and the test in the open field apparatus. The open field apparatus measures activity of the rats in a novel environment [4,7,53]. Locomotor activity in the open field apparatus was recorded with the aid of a video camera for a period of 5 min. Activity measured included distance travelled, rearing and entry into the inner zone.
Transcardial perfusion
One day after completion of the behavioural tests (P75), rats were deeply anaesthesized with halothane and then transcardially perfused with 0.15 M phosphate buffered saline (PBS) followed by 4% parafomaldehyde (MERCK, Germany). The rat brains were further post fixed in 4% parafomaldehyde for 24 hrs after which they were cryoprotected in 20% sucrose for 48 hrs. The brains were frozen in liquid nitrogen vapour before being stored at -80°C in preparation for immunohistochemistry.
Tyrosine hydroxylase immunohistochemistry
The brain was sliced coronally (60 μm sections) and striatal and substantia nigra tissue was collected in the -20°C environment of the cryostat machine. Brain slices were washed in PBS (0.15 M, pH 7.6) and then incubated for 15 min in 3% hydrogen peroxide. Following incubation, the slices were washed several times in PBS and then incubated for 1 hr in blocking solution containing PBS, normal horse serum (Vector Laboratories) and Triton-X (MERCK, Germany). After exposure to the blocking solution, the slices were incubated in primary monoclonal anti-Tyrosine hydroxylase mouse antibody (Sigma) at 1:16000 dilution for 48 hrs at 4°C. The slices were washed in PBS and then incubated in biotinylated Mouse IgG secondary antibody (Vectastain) for 90 min. The slices were washed and then treated in diaminobenzidine tetrahydrochloride tablets (DAB) in Tris buffer (pH 7.2, Sigma) at room temperature for 10 min or until staining appeared. Following the DAB reaction, the slices were dried on gelatinous slides (10 g commercial gelatine in 500 ml distilled water) and then washed 6 times with distilled water. They were dehydrated in ethanol (96-100%) and then mounted with Entellan (MERCK, Germany). The tyrosine hydroxylase positive dopamine neurons in the substantia nigra of both hemispheres were counted using a Nikon Microphot-fx microscope (10× magnification). Only complete dopamine neurons with stained cell bodies, dendrites and axons were counted.
Statistical analysis
Repeated measures analysis of variance (ANOVA) was used to compare daily distance travelled in the running wheels by maternally separated and non-stressed rats. A paired t-test was used to compare left and right forelimb step length. Two-way factorial ANOVA was performed on the behavioural and tyrosine hydroxylase data with ‘stress’ and ‘exercise’ as between rat factors. Significant main effects or interaction were followed by Tukey HSD test for multiple comparisons of mean values. Results are reported as mean ± standard error of the mean (SEM).
Results
Locomotor activity
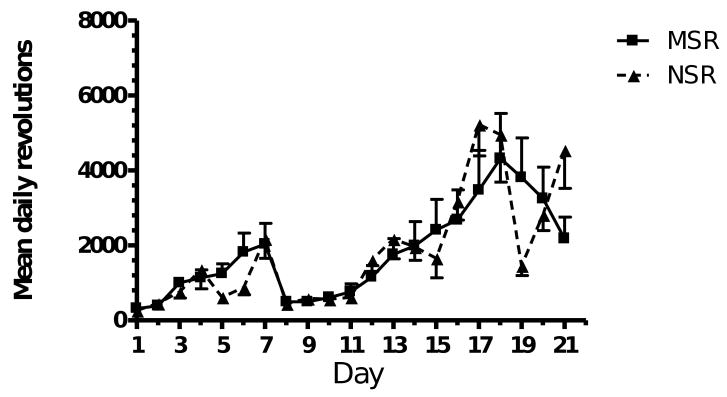
Stress did not have an effect on the mean distance covered by the maternally separated rats (MSR) and the non-stressed rats (NSR) in the cages with running wheels (Fig.1). The mean number of revolutions of the running wheels increased steadily from day 1 until day 7. Following stereotaxic surgery (day 7), there was a dramatic decrease in the mean number of revolutions traveled by the rats (day 8) and it took the rats 5 days to achieve pre-lesion levels of activity in the running wheels.
Fig. 1.
Mean daily distance run by maternally separated (MSR) rats (n = 10) and non-stressed (NSR) rats (n = 9). Repeated measures ANOVA revealed no significant difference between maternally separated and non-stressed rats.
Rat Weights
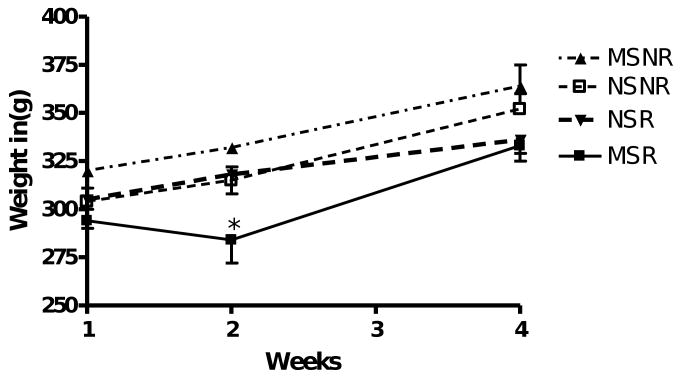
There was no significant difference between the weights of maternally separated and non-stressed rats (Fig. 2). However, at the beginning of week 2 (P60), one week after access to the running wheels, the MSR rats weighed significantly less than the maternally separated rats that did not have access to a running wheel (MSNR rats, p < 0.01).
Fig. 2.
Weight of maternally separated rats with (MSR, n = 10) or without (MSNR, n-10) access to running wheels and weight of the non-stressed rats with (NSR, n = 9) or without (NSNR, n = 10) running wheels. Repeated measures ANOVA revealed no significant difference between the weights of maternally separated and non-stressed rats. However, at the beginning of week 2 (P60), the MSR rats weighed significantly less than the maternally separated rats that did not have access to a running wheel. *(MSR vs MSNR, p < 0.01, week 2).
Forelimb akinesia (Step test)
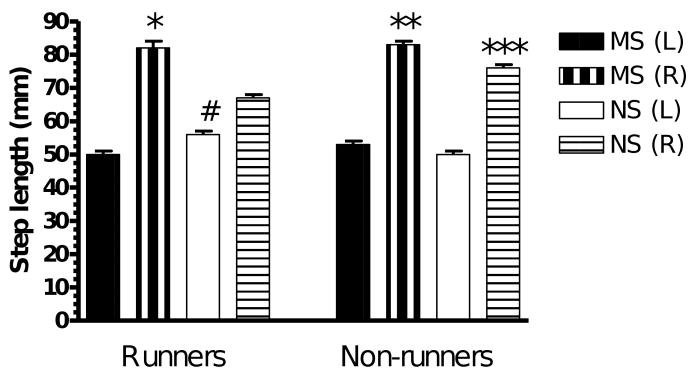
The step taken by the unimpaired limb (L) was significantly shorter than that of the impaired limb (R) in all four groups (L vs R, p < 0.001, paired t-test; Fig. 3). A two-way ANOVA revealed significant main effects of stress (F(1,35) = 68, p < 0.0001) and exercise (F(1,35) = 13.2, p < 0.001) and a significant interaction between stress and exercise (F(1,35) = 9.15, p < 0.01). Exercise significantly decreased step length while maternal separation increased step length. Access to running wheels resulted in the length of the step taken by the impaired limb of the non-stressed rats (NSR (R)) being significantly shorter than the step taken by non-stressed rats that did not have access to a running wheel (NSNR(R), p < 0.001) and the impaired limbs (R) of the maternally separated exercised rats (MSR(R), p < 0.001). In the rats without access to running wheels, the length of the step taken by the impaired limb of the non-stressed rats (NSNR (R)) was significantly shorter that the step taken by the impaired limb of the maternally separated rats (MSNR (R), p < 0.01).
Fig. 3.
Average length of step taken by MSR rats (n = 10), NSR rats (n = 9), MSNR rats (n = 10) and NSNR rats (n = 10). L represents the left forelimb and R is the right forelimb (L vs R, p < 0.001 in all groups, paired t-test). A two-way ANOVA of results obtained for the right (R) impaired forelimb revealed significant main effects of stress (F(1,35) = 68, p < 0.0001) and exercise (F(1,35) = 13.2, p < 0.001) and a significant interaction between stress and exercise (F(1,35) = 9.15, p < 0.01) *(NSR (R) vs MSR (R), p < 0.001, post-hoc Tukey HSD test), **(NSNR (R) vs MSNR (R), p < 0.01, post-hoc Tukey HSD test), ***(NSR (R) vs NSNR (R), p < 0.001, post-hoc Tukey HSD test). A two-way ANOVA of results obtained for the left (L) unimpaired forelimb revealed a significant interaction between stress and exercise (F(1,35) = 25.89, p < 0.0001). #(NSR (L) vs MSR (L) and NSNR (L), p < 0.001, post-hoc Tukey HSD test).
Interestingly, a two-way ANOVA revealed a significant interaction between stress and exercise (F(1.35) = 25.8, p < 0.0001) in the ipsilateral forelimb step length. Post-hoc Tukey's test revealed that the step length of the unimpaired limb of NSR rats was greater than that of NSNR and MSR rats (p < 0.001) and the step taken by the MSNR rats was longer than that of the NSNR rats (p < 0.05). In contrast to non-stressed rats, exercise failed to reduce the asymmetry between ipsi- and contralateral forelimb of maternally separated rats in the step test of initiation of movement.
Limb use asymmetry (Cylinder test)
Landing
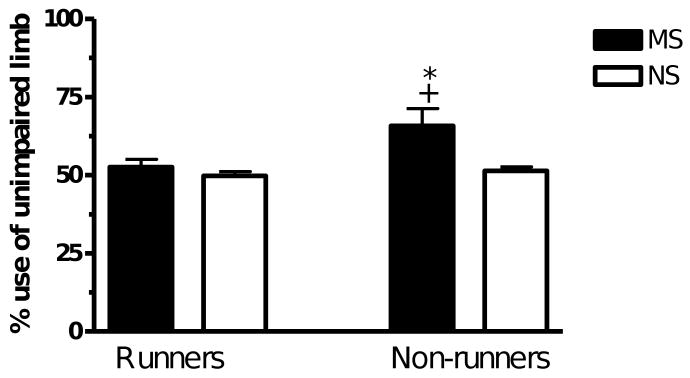
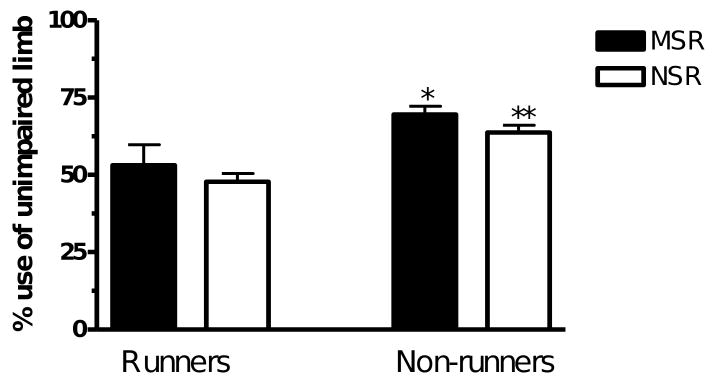
A two-way ANOVA revealed significant main effects of stress and exercise as well as a significant interaction between stress and exercise (F(1.35) > 5, p < 0.05). The rats that were maternally separated displayed an effect of exercise (MSNR v MSR, p < 0.05) and the non-runners displayed a significant stress effect (MSNR v NSNR, p < 0.05) on preference for use of the unimpaired limb when landing (Fig. 4).
Fig. 4.
The number of times the rat used the unimpaired forelimb to land on the floor expressed as a percentage of the total number of times it landed on the floor of the cylinder. MSR rats (n = 10), NSR rats (n = 9), MSNR rats (n = 10) and NSNR rats (n = 10). A two-way ANOVA revealed significant main effects of stress and exercise as well as a significant interaction between stress and exercise (F(1.35) > 5, p < 0.05). *(MSR vs MSNR, p < 0.05) and +(NSNR vs MSNR, p < 0.05).
Wall touch & wall movement
A two-way ANOVA revealed that exercise significantly reduced the use of the unimpaired limb when touching the wall of the cylinder (F(1.35) = 9.6, p < 0.01) and also reduced limb use asymmetry during wall movement (F(1.35) = 15.6, p < 0.001; MSR v MSNR, p < 0.05; NSR v NSNR, p < 0.05; Fig. 5). Maternal separation did not affect percentage limb use preference.
Fig. 5.
The number of times the rat preferred to use the unimpaired limb when moving across the wall of the cylinder while the rat was standing on its hindlimbs expressed as a percentage of the total number of times it used its forelimbs to move across the wall of the cylinder. A two-way ANOVA revealed that exercise significantly reduced forelimb use asymmetry during wall movement (F(1.35) = 15.6, p < 0.001). MSR rats (n = 10), NSR rats (n = 9), MSNR rats (n = 10) and NSNR rats (n = 10). *(MSR vs MSNR, p < 0.05) and **(NSR vs NSNR, p < 0.05).
Open field apparatus
Distance traveled
Exploration in the open field showed a stress effect (F(1.35) = 21.5, p = 0.0001). MSNR covered a significantly shorter distance than the NSNR rats (p < 0.001) in the 5 min interval (Fig. 6). However, there was no significant difference in the distances covered by the rats that had access to running wheels.
Fig. 6.
Mean total distance covered in the open field by rats that had access to running wheels (MSR, n = 10), (NSR, n = 9) and rats that were in plexiglass cages (MSNR, n = 10), (NSNR, n = 10). A two-way ANOVA revealed a significant effect of maternal separation (F(1,35) = 21.5. p < 0.0001). * (NSNR vs MSNR, p < 0.001, post-hoc Tukey HSD test).
Rearing
There was no significant effect of maternal separation or exercise on the number of times the rats reared during the 5 min test in the open field.
Entries into the inner zone
There was no significant effect of maternal separation or exercise on the number of times the rats entered the inner zone of the open field apparatus during the 5 min test.
Tyrosine hydroxylase immunohistochemistry
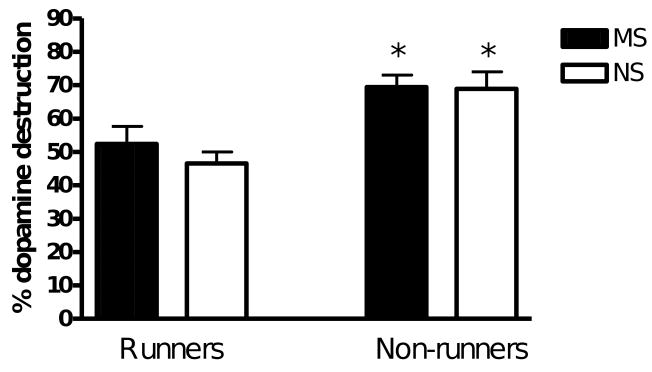
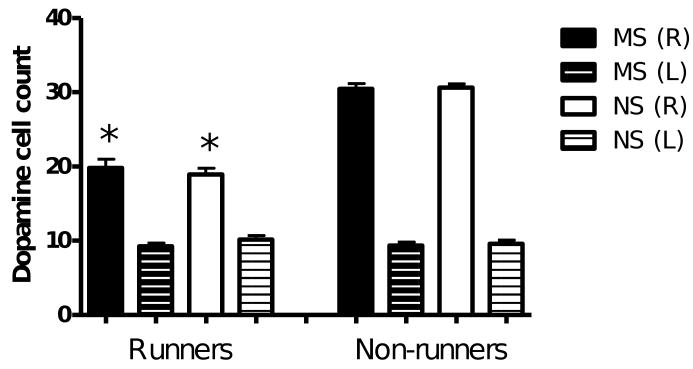
Dopamine neuron destruction in the lesioned hemisphere was calculated as a percentage of the total number of completely stained tyrosine hydroxylase positive cells in the non-lesioned hemisphere. A two-way ANOVA revealed significant main effects of stress (F(1,35) = 12.1, p < 0.01) and exercise (F(1,35) = 347.8, p < 0.0001) and a significant interaction between stress and exercise (F(1,35) = 8.8, p < 0.01). Exercise significantly reduced the percentage dopamine neuron destruction in maternally separated and non-stressed rats (MSR vs MSNR, p < 0.05 and NSR vs NSNR, p < 0.01; Fig. 7). This was mainly due to a decrease in completely stained neurons (with stained axons and dendrites) in the contralateral, non-lesioned substantia nigra of exercised rats (F(1.35) = 188, p < 0.0001; MSR vs MSNR, p < 0.001; NSR vs NSNR, p < 0.001; Fig. 8). The effect of exercise on percentage dopamine neuron destruction was smaller in maternally separated rats, suggesting that maternal separation decreased the beneficial effect of exercise.
Fig. 7.
The percentage of dopamine neuron destruction in lesioned hemispheres of the MSR (n = 10), NSR (n = 9), MSNR (n = 10) and NSNR (n = 10) rats. A two-way ANOVA revealed main effects of stress (F(1,35) = 12.1, p < 0.01) and exercise (F(1,35) = 347.8, p < 0.0001) and a significant interaction between stress and exercise (F(1,35) = 8.8, p < 0.01).*(MSR vs MSNR, p < 0.001, and NSR vs NSNR, p < 0.001, post-hoc Tukey HSD test).
Fig. 8.
Complete dopamine neuron count in substantia nigra sections of lesioned and non-lesioned hemispheres of the MSR (n = 10), NSR (n = 9), MSNR (n = 10) and NSNR (n = 10) rats. A two-way ANOVA revealed a significant effect of exercise in the non-lesioned (R) hemisphere (F(1.35) = 188, p < 0.0001). *(MSR vs MSNR, p < 0.001 and NSR vs NSNR, p < 0.001, post-hoc Tukey HSD test)
Discussion
In agreement with previous findings, exercise significantly reduced forelimb akinesia and forelimb use asymmetry [14,23,24]. In addition, the present study showed that exercise decreased the percentage dopamine cell loss in the lesioned relative to the non-lesioned substantia nigra. However, this was not due to a decrease in cell loss in the lesioned substantia nigra but a decrease in complete tyrosine hydroxylase staining of dopamine cell bodies, dendrites and axons in the contralateral, non-lesioned substantia nigra. Downregulation of tyrosine hydroxylase expression in the contralateral hemisphere may be part of a compensatory mechanism to correct the behavioural asymmetry. Maternal separation exacerbated forelimb akinesia and forelimb use asymmetry and caused a slight increase in dopamine cell loss when expressed as a percentage of the non-lesioned substantia nigra, however, it did not alter dopamine cell number.
The results of this study indicate that in a parkinsonian rat model, adult rats that were maternally separated and had no access to running wheels displayed increased forelimb akinesia, increased asymmetrical bias towards use of the unimpaired forelimb in the cylinder test and greater relative dopamine neuron destruction in the lesioned substantia nigra (expressed as percentage of the non-lesioned substantia nigra) than rats that had access to running wheels. Exercise did not reduce forelimb akinesia in maternally separated rats but it did cancel the bias towards use of the unimpaired forelimb and also reduced the relative dopamine neuron destruction in the lesioned hemisphere suggesting that exercise produced a similar adaptive change to dopamine neurons in the substantia nigra of maternally separated rats following 6-OHDA lesion as observed in non-stressed rats.
In the present study, rats that had access to running wheels (both non-stressed and maternally separated rats) steadily increased their mean daily running revolutions from day 1 until the day of the lesion. The decrease in the mean daily running distance between days 8 and 13 was due to the effect of surgery. Maternally separated and non-lesioned rats were similarly affected. In the second week of running, there was a significant decrease in the weight of the maternally separated rats housed in cages with attached running wheels compared to the maternally separated rats housed in cages without running wheels. This weight loss could have been the result of a physiological response to exercise. However, the absence of a weight decrease in non-stressed rats with access to running wheels suggests that the physiological stress of running may have been significantly greater in the maternally separated rats. Studies have shown that exposure to early life stressors such as maternal separation changes the HPA axis response to stress at a later stage of life, resulting in increased glucocorticoid secretion [32]. Exercise is a stressor. Ploughman et al. [40] showed that voluntary exercise results in an increase in basal plasma corticosterone concentration in rats. Glucocorticoids and catecholamines stimulate gluconeogenesis and mobilization of amino acids and fatty acids for energy production during stress [32,46] and therefore the decline in weight of the maternally separated rats that had access to running wheels might be a result of depletion of the body's energy stores. It is also possible that weight loss may have contributed to the beneficial effects of exercise. Dietary restriction has been shown to increase the resistance of dopamine neurons to the neurotoxic effects of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP) in mice [6] and gherlin, a gut hormone that is elevated during negative energy balance, has been shown to protect dopamine neurons by uncoupling mitochondrial respiration [1]. However, the rats that lost weight in the present study were the maternally separated rats that appeared to have less neuroprotection than the non-stressed rats that did not lose weight, so this was not a contributory factor.
Non-stressed rats that had access to running wheels had less difficulty in initiating stepping movements (shorter step length) with the impaired limb than the other groups. One of the symptoms of Parkinson's disease is paucity of movement and difficulty in initiating movement [34]. The decrease in the length of the step taken by the impaired forelimb during the step test in non-stressed rats with access to running wheels suggests that the lesion size in these rats may have been relatively small therefore allowing sufficient dopamine release from the nigrostriatal terminals in the striatum to initiate stepping and maintain normal control of movement. It appears that exercise did not provide the same amount of protection in maternally separated rats as there was no difference between exercised and non-exercised maternally separated rats and the mean step length taken by the impaired limb of the exercised, maternally separated rats was significantly longer than that taken by the non-stressed rats that had access to running wheels. This may indicate that there is a greater loss of dopamine terminals in the striatum, as suggested by Pienaar et al [38] or the neural circuitry involved in movement initiation or dragging is altered in maternally separated rats.
In the cylinder test, the non-stressed and maternally separated rats with access to running wheels used the impaired limb significantly more than the non-stressed and maternally separated rats without running wheels when moving across the wall of the cylinder and when landing on the floor of the cylinder. This is in agreement with Tillerson et al. [47,48] who showed that exercise abolishes forelimb use asymmetries associated with unilateral 6-OHDA lesions, in the cylinder test. As the cylinder test is designed to analyze forelimb use for postural support [42], the absence of a significant difference between the maternally separated rats with and without running wheels in touching the wall of the cylinder might suggest that this test is not as robust as movement initiation in the wall movement test or landing. Wall movement and floor landing require a significant weight transfer to the forelimbs whereas in the wall touch, the hindlimbs bear most of the weight as the rats can rear without touching the wall. The impaired forelimb of the maternally separated rats with no access to running wheels was not flaccid as shown by the ability to brace without the snout touching the table top during movement initiation (step test). This suggests that movements that require weight bearing are more impaired in rats with excessive dopamine neuron loss. In all of these tests, the rat appears to prefer to use the unimpaired limb in the presence of excessive dopamine neuron depletion.
In the open field test, there was less inclination for the maternally separated rats to explore the novel environment than the non-stressed rats. Studies have shown that maternally separated rodents develop anxiety-like behaviour which manifests itself as lack of exploration in the open field [9,36]. This might explain why maternally separated rats showed a tendency to explore the open field apparatus less than the non-stressed rats. The reduction in locomotor activity in the maternally separated rats that did not have access to running wheels is consistent with a greater lesion of nigrostriatal dopamine neurons and therefore reduced initiation of movement. However, the absence of a significant difference between the number of completely stained, tyrosine hydroxylase positive cells in the lesioned substantia nigra of maternally separated and non-stressed rats suggests that dopamine neuron loss was not the reason for reduced locomotor activity but that dopamine terminal density in the striatum was reduced, as suggested by the findings of Pienaar et al. [38]. The absence of a significant difference between exercised maternally separated and non-separated rats suggests that exercise reduced the anxiety-like behaviour of the maternally separated rats. Maternal separation studies using the maternal separation protocol used in this study have shown that glucocorticoid levels are significantly increased following exposure to an acute stressor [30,39]. Lee et al. [21] suggested that glucocorticoids may increase the expression of glial cell-like derived neurotrophic factor (GDNF) which plays a role in dopamine neuroprotection. As mentioned earlier, exercise exacerbates glucocorticoid secretion. This might explain the lack of significant difference between the percent dopamine neuron destruction in the substantia nigra of maternally separated and non-stressed rats that had access to running wheels.
The absence of significant effect of maternal separation on dopamine neuron survival contrasts with previous findings that prenatal stress increased dopamine cell loss in non-exercised rats [24]. These are both critical stages of development. The difference is possibly due to the more advanced stage of maturation of the dopaminergic system in the postnatal period. Male rats exposed to elevated dexamethasone (synthetic glucocorticoid) levels during the prenatal period displayed increased tyrosine hydroxylase-positive cell numbers in substantia nigra whereas postnatal dexamethasone treatment had no effect on tyrosine hydroxylase expression [25]. Rats exposed to stress during the prenatal period, specifically the last week of gestation, have been shown to produce offspring with altered dopamine metabolism and increased levels of the transcription factor, Nurr1, which is essential for early differentiation of midbrain dopamine neurons as well as the maintenance of dopamine neurons in adulthood [17,18]. Ablation of the Nurr1 gene in adult mice produced a slowly progressing loss of dopamine neurons [17]. Overexpression of Nurr1 in prenatally stressed rats signals a compensatory mechanism that persists into adulthood [18] that may confer increased vulnerability of dopamine neurons to toxic insult.
The interval and timing of prenatal stress is critical. The last week of gestation is usually chosen since the basic structures of the brain are in place and the HPA axis and modulatory neurotransmitter systems (such as dopamine) are in the early stages of development [22]. The timing of running wheel exercise in relation to the lesion and the age of the rats at the time of lesion was the same in both studies and so could not have contributed to the difference in results between the two studies.
Adverse effects of stress are not limited to the perinatal period, adult rats subjected to mild stressors in the period immediately following 6-OHDA infusion into the medial forebrain bundle did not benefit from the beneficial effects of exercise and suffered a greater loss of dopamine neurons in the substantia nigra [14].
Conclusion
A number of neurological disorders including those affecting cognition and the limbic system have been found to be linked to early life stressors that alter the developing neuronal circuitry such as maternal separation [22]. A number of interventions aimed at reversing or attenuating progression of neurological disorders have been used. Parkinson's disease is a progressive neurological disease that results from the destruction of dopamine neurons and their terminals in the nigrostriatal pathway and is largely of unknown aetiology. Exercise has been shown to have a beneficial effect in attenuating the progressive nature of the disease [12-14,47,48]. In the present study, we have shown that exercise attenuated dopamine neuron destruction in the rats that had access to running wheels. This indicates that exercise plays a role in providing neuroprotection to rats that are exposed to 6-OHDA infusion into the medial forebrain bundle. Although maternal separation exaggerated limb use asymmetry and reduced the beneficial effects of exercise on forelimb akinesia, it did not exacerbate dopamine neuron destruction following injection of a mild dose of 6-OHDA. Therefore exposure to maternal separation does not predispose rats to greater dopamine neurodegeneration suggesting that individuals who are exposed to emotional trauma in early childhood may not have a greater predisposition to Parkinson's disease than less exposed individuals.
Acknowledgments
This work was supported by the University of Cape Town and the National Institutes of Health (NIH) Fogarty International Center grant R01TW008040 to Michael J. Zigmond, principal investigator. The authors wish to express their thanks to Dr Michael Zigmond, Dr Willie Daniels, Dr Amanda Smith and Ms Sandy Castro for their invaluable advice and training provided for Dr Musa Mabandla. This work forms part of the PhD thesis of Dr Musa Mabandla.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andrews ZB, Erion D, Beiler R, Liu ZW, Abizaid A, Zigman J, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci. 2009;29:14057–14065. doi: 10.1523/JNEUROSCI.3890-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasted PJ, Watts C, Torres EM, Robbins TW, Dunnett SB. Behavioural recovery following striatal transplantation: effects of postoperative training and P-zone volume. Exp Brain Res. 1999;128:535–538. doi: 10.1007/s002210050877. [DOI] [PubMed] [Google Scholar]
- 3.Ceccatelli S, Tamm C, Zhang Q, Chen M. Mechanisms and modulation of neural cell damage induced by oxidative stress. Physiol Behav. 2007;92:87–92. doi: 10.1016/j.physbeh.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Colorado RA, Shumake J, Conejo NM, Gonzalez-Pardo H, Gonzalez-Lima F. Effects of maternal separation, early handling, and standard facility rearing on orienting and impulsive behavior of adolescent rats. Behav Processes. 2006;71:51–58. doi: 10.1016/j.beproc.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Daniels WM, Pietersen CY, Carstens ME, Stein DJ. Maternal separation in rats leads to anxiety-like behavior and a blunted ACTH response and altered neurotransmitter levels in response to a subsequent stressor. Metab Brain Dis. 2004;19:3–14. doi: 10.1023/b:mebr.0000027412.19664.b3. [DOI] [PubMed] [Google Scholar]
- 6.Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 7.Furchtgott E, Wechkin S, Dees JW. Open-field exploration as a function of age. J Comp Physiol Psychol. 1961;54:386–388. doi: 10.1037/h0044087. [DOI] [PubMed] [Google Scholar]
- 8.Furukawa TA, Ogura A, Hirai T, Fujihara S, Kitamura T, Takahashi K. Early parental separation experiences among patients with bipolar disorder and major depression: a case-control study. J Affect Disord. 1999;52:85–91. doi: 10.1016/s0165-0327(98)00054-8. [DOI] [PubMed] [Google Scholar]
- 9.Gilmer WS, McKinney WT. Early experience and depressive disorders: human and non-human primate studies. J Affect Disord. 2003;75:97–113. doi: 10.1016/s0165-0327(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 10.Godoy MC, Tarelli R, Ferrari CC, Sarchi MI, Pitossi FJ. Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson's disease. Brain. 2008;131:1880–1894. doi: 10.1093/brain/awn101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan J, Krishnamurthi R, Waldvogel HJ, Faull RL, Clark R, Gluckman P. N-terminal tripeptide of IGF-1 (GPE) prevents the loss of TH positive neurons after 6-OHDA induced nigral lesion in rats. Brain Res. 2000;859:286–292. doi: 10.1016/s0006-8993(00)01988-0. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch MA, Farley BG. Exercise and neuroplasticity in persons living with Parkinson's disease. Eur J Phys Rehabil Med. 2009;45:215–229. [PubMed] [Google Scholar]
- 13.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 14.Howells FM, Russell VA, Mabandla MV, Kellaway LA. Stress reduces the neuroprotective effect of exercise in a rat model for Parkinson's disease. Behav Brain Res. 2005;165:210–220. doi: 10.1016/j.bbr.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res. 2002;950:52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- 16.Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 17.Kadkhodaei B, Ito T, Joodmardi E, Mattsson B, Rouillard C, Carta M, et al. Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci. 2009;29:15923–15932. doi: 10.1523/JNEUROSCI.3910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katunar MR, Saez T, Brusco A, Antonelli MC. Ontogenetic Expression of Dopamine-Related Transcription Factors and Tyrosine Hydroxylase in Prenatally Stressed Rats. Neurotox Res. 2009 doi: 10.1007/s12640-009-9132-z. [DOI] [PubMed] [Google Scholar]
- 19.Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. Childhood parental loss and adult psychopathology in women. A twin study perspective. Arch Gen Psychiatry. 1992;49:109–116. doi: 10.1001/archpsyc.1992.01820020029004. [DOI] [PubMed] [Google Scholar]
- 20.Kibel A, Drenjancevic-Peric I. Impact of glucocorticoids and chronic stress on progression of Parkinson's disease. Med Hypotheses. 2008;71:952–956. doi: 10.1016/j.mehy.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Jang MK, Kim OS, Lee OH, Kim NY, Yoo KH, et al. Activation of the GDNF-inducible transcription factor (GIF) gene promoter by glucocorticoid and progesterone. J Steroid Biochem Mol Biol. 2009;115:30–35. doi: 10.1016/j.jsbmb.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 23.Mabandla M, Kellaway L, St Clair GA, Russell VA. Voluntary running provides neuroprotection in rats after 6-hydroxydopamine injection into the medial forebrain bundle. Metab Brain Dis. 2004;19:43–50. doi: 10.1023/b:mebr.0000027416.13070.c3. [DOI] [PubMed] [Google Scholar]
- 24.Mabandla MV, Kellaway LA, Daniels WM, Russell VA. Effect of exercise on dopamine neuron survival in prenatally stressed rats. Metab Brain Dis. 2009 doi: 10.1007/s11011-009-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol. 2005;17:475–482. doi: 10.1111/j.1365-2826.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- 26.McCormick CM, Kehoe P, Mallinson K, Cecchi L, Frye CA. Neonatal isolation alters stress hormone and mesolimbic dopamine release in juvenile rats. Pharmacol Biochem Behav. 2002;73:77–85. doi: 10.1016/s0091-3057(02)00758-x. [DOI] [PubMed] [Google Scholar]
- 27.Meaney MJ. The sexual differentiation of social play. Psychiatr Dev. 1989;7:247–261. [PubMed] [Google Scholar]
- 28.Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- 29.Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behav Neurosci. 1985;99:765–770. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- 30.Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Neonatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 31.Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, et al. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meaney MJ, Diorio J, Francis D, Widdowson J, Laplante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 33.Metz GA. Stress as a modulator of motor system function and pathology. Rev Neurosci. 2007;18:209–222. doi: 10.1515/revneuro.2007.18.3-4.209. [DOI] [PubMed] [Google Scholar]
- 34.Mink JW. The basal ganglia. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental Neuroscience. San Diego: Academic Press; 2003. pp. 815–839. [Google Scholar]
- 35.Offen D, Shtaif B, Hadad D, Weizman A, Melamed E, Gil-Ad I. Protective effect of insulin-like-growth-factor-1 against dopamine-induced neurotoxicity in human and rodent neuronal cultures: possible implications for Parkinson's disease. Neurosci Lett. 2001;316:129–132. doi: 10.1016/s0304-3940(01)02344-8. [DOI] [PubMed] [Google Scholar]
- 36.Pan Y, Liu Y, Young KA, Zhang Z, Wang Z. Post-weaning social isolation alters anxiety-related behavior and neurochemical gene expression in the brain of male prairie voles. Neurosci Lett. 2009;454:67–71. doi: 10.1016/j.neulet.2009.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. Academic Press Inc; 1986. [DOI] [PubMed] [Google Scholar]
- 38.Pienaar IS, Kellaway LA, Russell VA, Smith AD, Stein DJ, Zigmond MJ, et al. Maternal separation exaggerates the toxic effects of 6-hydroxydopamine in rats: implications for neurodegenerative disorders. Stress. 2008;11:448–456. doi: 10.1080/10253890801890721. [DOI] [PubMed] [Google Scholar]
- 39.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 40.Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136:991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 41.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 42.Schallert T, Tillerson JL. Intervention strategies for degeneration of dopamine neurons in parkinsonism: Optimizing behavioral assessment of outcome. In: Emerich DF, Dean RLI, Sandberg PI, editors. Central nervous system disease: Innovative models of CNS diseases from molecule to therapy. Totowa, NJ: Humana Press; 2000. pp. 131–151. [Google Scholar]
- 43.Schallert T, Woodlee MT. Motor systems: Orienting and placing. In: Whishaw IQ, Kolb B, editors. The behaviour of the laboratory rat: A handbook with tests. Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- 44.Schmidt M, Enthoven L, van Woezik JH, Levine S, de Kloet ER, Oitzl MS. The dynamics of the hypothalamic-pituitary-adrenal axis during maternal deprivation. J Neuroendocrinol. 2004;16:52–57. doi: 10.1111/j.1365-2826.2004.01123.x. [DOI] [PubMed] [Google Scholar]
- 45.Schmidt MV, Oitzl MS, Levine S, de Kloet ER. The HPA system during the postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- 46.Tharp GD. The role of glucocorticoids in exercise. Med Sci Sports. 1975;7:6–11. [PubMed] [Google Scholar]
- 47.Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Toole T, Hirsch MA, Forkink A, Lehman DA, Maitland CG. The effects of a balance and strength training program on equilibrium in Parkinsonism: A preliminary study. NeuroRehabilitation. 2000;14:165–174. [PubMed] [Google Scholar]
- 50.Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model of Parkinson's disease: a study of behaviour in rats with graded 6-OHDA lesions. Behav Brain Res. 2006;169:1–9. doi: 10.1016/j.bbr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 51.Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 52.Walker CD, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 53.Whitmoyer DI, Masco D, Carrer HF. An electronic open field. Physiol Behav. 1983;30:635–637. doi: 10.1016/0031-9384(83)90233-0. [DOI] [PubMed] [Google Scholar]