Abstract
Purpose
The existence of cancer stem cells (CSCs) in breast cancer has profound implications for cancer prevention. In this study, we evaluated sulforaphane, a natural compound derived from broccoli/broccoli sprouts, for its efficacy to inhibit breast CSCs and its potential mechanism.
Experimental Design
Aldefluor assay and mammosphere formation assay were used to evaluate the effect of sulforaphane on breast CSCs in vitro. A NOD/SCID xenograft model was employed to determine whether sulforaphane could target breast CSCs in vivo, as assessed by Aldefluor assay and tumor growth upon cell re-implantation in secondary mice. The potential mechanism was investigated utilizing Western blotting analysis and β-catenin reporter assay.
Results
Sulforaphane (1~5 μM) decreased aldehyde dehydrogenase (ALDH)-positive cell population by 65%~80% in human breast cancer cells (P < 0.01), and reduced the size and number of primary mammospheres by 8~125-fold and 45%~75% (P < 0.01), respectively. Daily injection with 50 mg/kg sulforaphane for two weeks reduced ALDH-positive cells by more than 50% in NOD/SCID xenograft tumors (P = 0.003). Sulforaphane eliminated breast CSCs in vivo, thereby abrogating tumor growth after re-implantation of primary tumor cells into the secondary mice (P < 0.01). Western blotting analysis and β-catenin reporter assay showed that sulforaphane down-regulated Wnt/β-catenin self-renewal pathway.
Conclusions
Sulforaphane inhibits breast CSCs and down-regulates Wnt/β-catenin self-renewal pathway. These findings support the use of sulforaphane for chemoprevention of breast cancer stem cells and warrant further clinical evaluation.
Keywords: sulforaphane, breast cancer stem cell, aldehyde dehydrogenase, mammosphere, NOD/SCID mouse
Introduction
Broccoli and broccoli sprouts contain large amounts of glucosinolates (1). Numerous studies have substantiated the chemoprevention effect of increasing cruciferous vegetable intake against cancer, which has been attributed to the activity of various isothiocyanates that are enzymatically hydrolyzed from glucosinolates (2). Sulforaphane was found to be converted from glucoraphanin, a major glucosinolate in broccoli/broccoli sprouts (3). The chemoprevention properties of sulforaphane against cancer are through both “blocking” and “suppressing” effects (2). The “blocking” function of sulforaphane is achieved through inhibiting Phase 1 metabolism enzymes that convert procarcinogens to carcinogens and inducing Phase 2 metabolism enzymes that promote excretion of carcinogens (2). Subsequent studies revealed the “suppressing” effects of sulforaphane in modulating diverse cellular activities to inhibit the growth of transformed cells (2, 4). The ability of sulforaphane to induce apoptosis and cell cycle arrest is associated with regulation of many molecules including Bcl-2 family proteins, caspases, p21, cyclins and cdks (4). Sulforaphane was also shown to suppress angiogenesis and metastasis by down-regulating VEGF, HIF-1α, MMP-2 and MMP-9 (4).
Accumulating evidence has shown that many types of cancer, including breast cancer, are initiated from and maintained by a small population of cancer stem cells (CSCs) (5, 6). This minor population produces the tumor bulk through continuous self-renewal and differentiation, which may be regulated by similar signaling pathways occurring in normal stem cells (5–8). Several pathways including Wnt/β-catenin, Hedgehog, and Notch have been identified to be critical to the self-renewal behavior of CSCs (7, 9, 10). Furthermore, CSCs have been suggested to contribute to tumor resistance/relapse because chemotherapy and radiation therapy are incapable of eradicating them (6, 11, 12). Thus, targeting these self-renewal pathways may provide an effective strategy to target CSCs and thereby overcome tumor resistance and reduce relapse (5). Several dietary compounds, such as curcumin (13, 14), quercetin and epigallocatechin-gallate (15), were found to be potentially against CSC self-renewal.
Wnt/β-catenin signaling is one of the key pathways that promotes self-renewal of breast CSCs (5). Activation of Wnt target genes are mediated by β-catenin, which translocates into nucleus and binds to the transcription factors T cell factor/lymphoid enhancer factor (TCF/LEF) (5, 16). The level of intracellular β-catenin is modulated by a multi-protein complex consisting of glycogen synthase kinase3β (GSK3β), adenomatous polyposis coli, casein kinase1α and axin (TCF/LEF) (5, 16, 17). GSK3β promotes the ubiquitin-proteasome degradation of β-catenin by phosphorylating three specific amino acids, Ser33/Ser37/Thr41, on β-catenin (17).
Sulforaphane was shown to target pancreatic tumor-initiating cells in a very recent report (18). In the present study, we examined the efficacy of sulforaphane against breast CSCs in both breast cancer cell lines and breast cancer xenografts. We demonstrated that sulforaphane eliminated breast CSCs in vivo, which was reflected by the inhibition of tumor growth in recipient mice that were inoculated with tumor cells derived from sulforaphane-treated primary xenografts. Furthermore, since sulforaphane was reported to induce down-regulation of β-catenin in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells (19), we investigated the suppressing activity of sulforaphane on Wnt/β-catenin pathway.
Materials and Methods
Cell Lines and Reagents
Human breast cancer cell lines, MCF7 and SUM159, were obtained from American Type Culture Collection and from Dr. Stephen Ethier (Karmanos Cancer Center), respectively. The source of SUM159 cell line is primary breast anaplastic carcinoma. This cell line is ER negative, PR negative, and does not have Her2 over-expression. Both cell lines were tested and authenticated in their origin sources. Authentication of these cell lines included morphology analysis, growth curve analysis, isoenzyme analysis, short tandem repeat analysis, and mycoplasma detection. Both cell lines were passaged in our laboratory for fewer than six months after receipt. To maintain the integrity of collections, stocks of the earliest-passage cells have been stored, and cell lines have been carefully maintained in culture as described below. MCF7 was maintained in RPMI1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Fisher Scientific, Pittsburgh, PA), 1% antibiotic-antimycotic (Invitrogen, Carlsbad, CA), and 5 μg/ml insulin (Sigma-Aldrich, St Louis, MO). SUM159 was maintained in Ham’s F12 medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum, 1% antibiotic-antimycotic, 5 μg/ml insulin, 1μg/ml hydrocortisone (Sigma-Aldrich, St Louis, MO), and 4 μg/ml gentamicin (Invitrogen, Carlsbad, CA).
Sulforaphane was obtained from LKT Laboratories (St. Paul, MN). Propidium iodide (PI) was from Invitrogen (Carlsbad, CA). LiCl was purchased from Fisher Scientific (Pittsburgh, PA); BIO (GSK3 inhibitor IX) was from Calbiochem (EMD Biosciences, San Diego, CA); and MG132 was obtained from Assay Designs (Stressgen, Ann Arbor, MI).
Antibodies to β-catenin, phospho-β-catenin Ser33/Ser37/Thr41, phospho-GSK3β Ser9, and GSK3β were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to cyclin D1 and β-actin were acquired from Santa Cruz Biotechnology (Santa Cruz, CA).
MTS Cell Proliferation Assay
MCF7 and SUM159 were seeded in 96-well microplates at a density of 3,000~5,000 cells per well. Cells were treated with increasing concentrations of sulforaphane as indicated. After 48 hr incubation cell viability was assessed by MTS assay (Promega, Madison, WI) according to manufacturer’s instruction. The number of living cells is directly proportional to the absorbance at 490 nm of a formazan product reduced from MTS by living cells.
Caspase-3 Activity Assay
Cells were treated with different concentrations of sulforaphane and collected after 24 hrs. The caspase-3 activity assay was based on the manufacturer’s instruction of Caspase-3/CPP32 Fluorometric Assay Kit (Biovision Research Products, Mountain View, CA). Cellular protein was extracted with the supplied lysis buffer, followed by determination of protein concentration using BCA Protein Assay Reagents (Pierce, Rockford, IL). The cleavage of DEVD-AFC, a substrate of caspase-3, was quantified by using a fluorescence microtiter plate reader with a 400 nm excitation filter and a 505 nm emission filter.
Mammosphere Formation Assay
Stem/progenitor cells are enriched in mammospheres of breast cancer cells (20), based on the unique ability of stem/progenitor cells to grow and form spheres in serum-free medium (21). Mammosphere culture was performed as previously described (22, 23) in a serum-free mammary epithelium basal medium (Lonza Inc., Walkersville, MD) supplemented with B27 (Invitrogen), 1% antibiotic-antimycotic, 5 μg/ml insulin, 1μg/ml hydrocortisone, 4 μg/ml gentamicin, 20 ng/mL EGF (Sigma-Aldrich, St Louis, MO), 20 ng/mL bFGF (Sigma-Aldrich, St Louis, MO), and 1:25,000,000 β-mercaptoethanol (Sigma-Aldrich, St Louis, MO). Single cells prepared from mechanical and enzymatic dissociation were plated in six-well ultra-low attachment plates (Corning, Acton, MA) at a density of 500~1,000 cells per milliliter in primary culture and 100~500 cells per milliliter in the following passages. Different concentrations of sulforaphane were added to primary culture, while the second and third passages were grown in the absence of drug. After 7 days of culture, the number of mammospheres was counted under Nikon Eclipse TE2000-S microscope and the photos were acquired with MetaMorph 7.6.0.0.
Aldefluor Assay
A cell population with a high Aldehyde dehydrogenase (ALDH) enzyme activity was previously reported to enrich mammary stem/progenitor cells (23). Aldefluor assay was performed according to manufacturer’s guidelines (StemCell Technologies, Vancouver, BC, Canada). Single cells obtained from cell cultures or xenograft tumors were incubated in Aldefluor assay buffer containing an ALDH substrate, bodipy-aminoacetaldehyde (BAAA, 1 μmol/L per 1,000,000 cells), for 40~50 min at 37 °C. As a negative control, a fraction of cells from each sample was incubated under identical condition in the presence of ALDH inhibitor, diethylaminobenzaldehyde (DEAB). Flow cytometry was used to measure ALDH-positive cell population.
Primary NOD/SCID Mouse Model
All experimentation involving mice were conducted in accordance with standard protocol approved by the University Committee on the Use and Care of Animals (UCUCA) at University of Michigan. SUM159 cells (2,000,000) mixed with Matrigel (BD Biosciences, San Jose, CA) were injected to the mammary fat pads of 5-week-old female NOD/SCID mice (The Jackson Laboratory, Bar Harbor, ME) as previously described (24). Tumors were measured with a caliper, and the volume was calculated using V=1/2 (width2 × length). Two weeks after cell injection, the mice were randomly separated into two groups, one group intraperitoneally injected with control (0.9% NaCl solution) and the other group with 50 mg/kg sulforaphane (dissolved in 0.9% NaCl solution) daily for two weeks.
Dissociation of Tumors
At the end of drug treatment, the mice were humanely euthanized and tumors were harvested. Tumor tissues were dissociated mechanically and enzymatically to obtain single cell suspension as previously described (25). Briefly, tumors were minced by scalpel and incubated in medium 199 (Invitrogen, Carlsbad, CA) mixed with collagenase/hyaluronidase (StemCell Technologies, Vancouver, BC, Canada) at 37 °C for 15~20 min. The tissues were further dissociated by pipet trituration, and then passed through 40 μm nylon mesh to produce single cell suspension, which was used for Aldeflour assay and flow cytometry.
Secondary NOD/SCID Mouse Model
Living cells from the dissociated tumors were sorted out by Fluorescence-activated cell sorting (FACS). Two groups of mice (four in group 1 and three in group 2) were implanted with tumor cells separately. Each secondary NOD/SCID mouse was inoculated with 50,000 cells from control mouse tumors in one side of inguinal mammary fat pad and another 50,000 cells from sulforaphane-treated tumors in the contralateral mammary fat pad. The growth of tumors was monitored; and tumor volumes were measured twice a week. Mice were humanely euthanized when the larger one of the two tumors reached 300~500 mm3.
Western Blotting Analysis
Cells were treated with sulforaphane at varying concentrations for indicated time periods in figure legends. Cells were harvested, lysed in RIPA buffer (20 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 5 mM EDTA, 1 mM Na3VO4, pH 7.5) supplemented with a protease inhibitor cocktail (Pierce, Rockford, IL) and a phosphatase inhibitor (Calbiochem, EMD Biosciences, San Diego, CA), and incubated on ice for 30 min. Cell lysate was centrifuged at 14,000 rpm for 15 min, and the supernatant was recovered. Protein concentration was determined with BCA Protein Assay Reagents (Pierce, Rockford, IL). Equal amounts of protein were subject to SDS-PAGE, and transferred to PVDF membrane (BioRad, Richmond, CA). The membrane was then incubated with appropriate antibodies.
TOP-dGFP Lentiviral β-Catenin Reporter Assay
TCF/LEF-1 (TOP-dGFP, FOP-dGFP) lentiviral reporter system was kindly gifted by Dr. Wiessman at Ludwig Center, Stanford University School of Medicine (26). Cells were infected with TOP-dGFP or control reporter FOP-dGFP with mutated TCF/LEF-1 binding sites. TOP-dGFP MCF7 and FOP-dGFP MCF7 cells were maintained in the same RPMI1640 medium as MCF7 cells. MCF7, TOP-dGFP MCF7 and FOP-dGFP MCF7 cells were cultured in the same serum-free mammary epithelium basal medium as mammospheres in six-well ultra-low attachment plates at a density of 1,000~1,500 cells per milliliter for 5 days. Single cells prepared from the primary spheres were incubated in medium containing 5 μM sulforaphane or/and 0.5 μM BIO for 48 hrs. After dissociation, single cell suspension was subject to flow cytometry analysis for dGFP-positive cell population. Parental MCF7 cells served as a control for autofluorescence. The photos of mammospheres were taken with Nikon Eclipse TE2000-S microscope and acquired with MetaMorph 7.6.0.0.
Statistical Analysis
Statistical differences were determined using two-tailed Student t-test. Data are presented as mean ± SD (n ≥3).
Results
Sulforaphane Inhibits Proliferation and Induces Apoptosis of Breast Cancer Cells
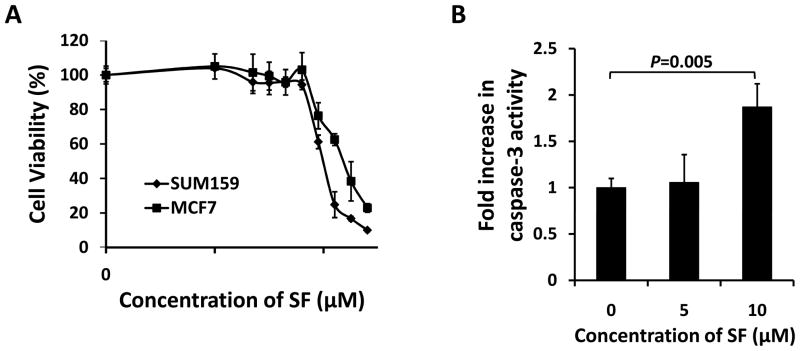
Sulforaphane was previously shown to inhibit proliferation (27) and induce apoptosis (28) in breast cancer cells. We first evaluated the anti-proliferative effects of sulforaphane in two human breast cancer cell lines, SUM159 and MCF7, by MTS assay. Cells were treated with increasing concentrations of sulforaphane for 48 hrs; and the ratio of viable cells of treatment relative to control is plotted in Figure 1A. Cell survival decreased as the concentration of sulforaphane increased, with the IC50 around 10 μM for SUM159 and 16 μM for MCF7. Caspase-3 fluorometric assay showed that sulforaphane (10 μM) significantly (P = 0.005) induced activation of caspase-3 (Figure 1B).
Figure 1. Sulforaphane inhibited proliferation and induced apoptosis in breast cancer cells.
(A) SUM159 and MCF7 cells growing in log phase were treated with increasing concentrations of sulforaphane for 48 hrs. The anti-proliferation effect of sulforaphane was measured by MTS assay. (B) Sulforaphane enhanced caspase-3 activity in SUM159 cells. Data are presented as mean ± SD(n≥ 3). SF = sulforaphane.
Sulforaphane Inhibits Breast Cancer Stem/Progenitor Cells in Vitro
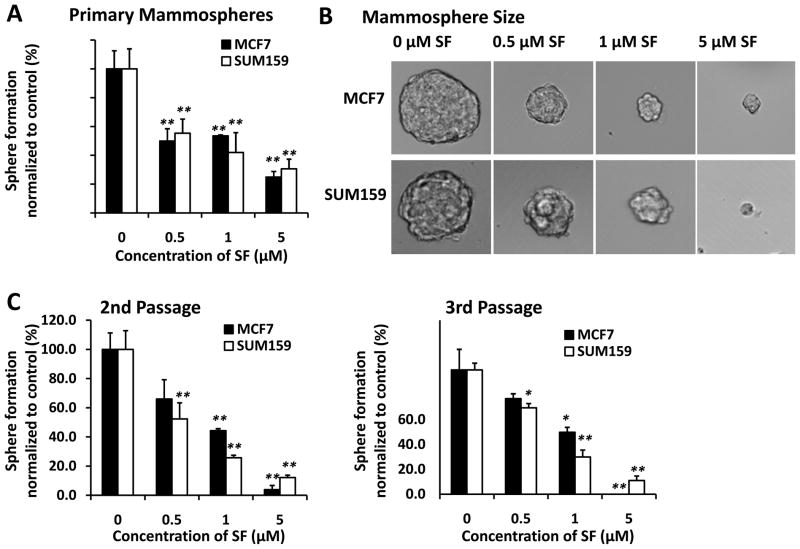
It has been demonstrated that mammary stem/progenitor cells are enriched in non-adherent spherical clusters of cells, termed mammospheres (22). These cells are capable of yielding secondary spheres and differentiating along multiple lineages (22). In order to evaluate whether sulforaphane could suppress the formation of mammospheres in vitro, we exposed primary MCF7 and SUM159 spheres to varying concentrations of sulforaphane and then cultured them two additional passages in the absence of drug. As shown in Figure 2A and 2B, sulforaphane inhibited the formation of primary spheres. Not only the number of spheres declined by 45%~75% (P < 0.01) (Figure 2A), but also the size of spheres was reduced by 8~125-fold (Figure 2B). Furthermore, a significant decrease in the number of sphere-forming cells in subsequent passages indicated a reduced self-renewal capacity of these stem/progenitor cells (Figure 2C) (22). MCF7 Cells initially propagated in the presence of 5 μM sulforaphane barely produced secondary spheres, with no cells passaged to third generation (Figure 2C). It is worth noting that the concentrations of sulforaphane that were capable of suppressing mammosphere formation (IC50 around 0.5~1 μM for both SUM159 and MCF7 spheres) were approximately 10-fold lower than those exhibiting anti-proliferative effects in MTS assay (IC50 around 10 μM for SUM159 and 16 μM for MCF7).
Figure 2. Inhibitory effect of sulforaphane on mammosphere formation.
MCF7 and SUM159 cells were cultured in mammosphere forming conditions. (A) Primary mammospheres were incubated with sulforaphane (0.5, 1, and 5 μM) or DMSO for 7 days. Sulforaphane treatment reduced the number of primary mammospheres. (B) Sulforaphane reduced the size of primary mammospheres(magnification × 100). The size of mammospheres was estimated using V = (4/3)π R3. (C) In the absence of drug, the 2nd and 3rd passages that were derived from sulforaphane-treated primary mammospheres yielded smaller numbers of spheres in comparison with control. Data are presented as mean ± SD(n = 3). *, P < 0.05; **, P < 0.01. SF = sulforaphane.
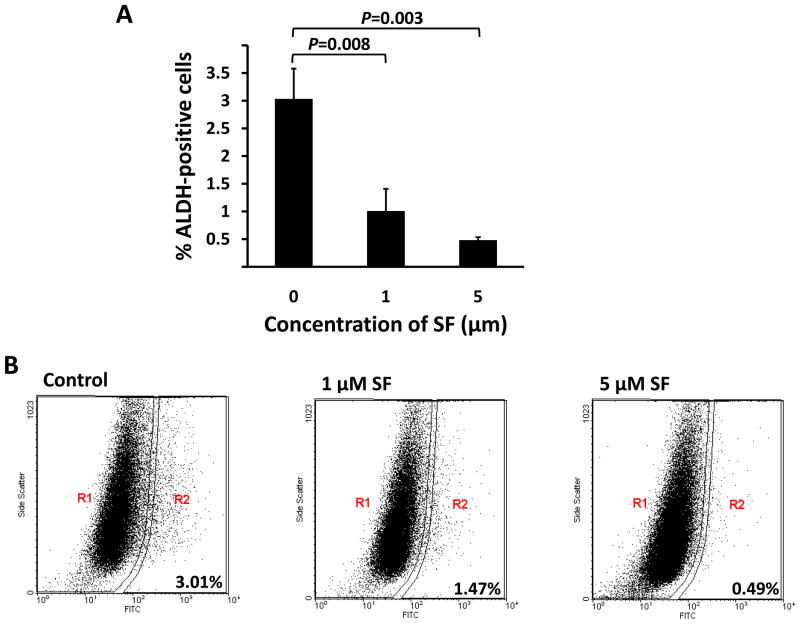
In breast carcinomas, a cell population with high aldehyde dehydrogenase (ALDH) activity as assessed by the Aldefluor assay has been demonstrated to enrich tumorigenic stem/progenitor cells (23). This cell population is capable of self-renewal and generating tumors resembling the parental tumor (23). Since SUM159 has a relatively high percentage of ALDH-positive cells, we selected SUM159 to examine whether sulforaphane inhibits the tumor-initiating ALDH-positive cells in vitro. As shown in Figure 3A, 1 μM sulforaphane significantly decreased the ALDH-positive population of SUM159 cells by over 65% (P = 0.008), while 5 μM produced greater than an 80% reduction of ALDH-positive population (P < 0.008). Representative flow cytometry dot plots are presented in Figure 3B. These data showed that sulforaphane inhibited the ALDH-positive cells at similar concentrations to those inhibited mammosphere formation and at 10-fold lower concentrations than those inhibited cancer cells as determined by MTS assay.
Figure 3. Inhibitory effect of sulforaphane on ALDH-positive cell population.
SUM159 cells were treated with sulforaphane (1 and 5 μM) or DMSO for 4 days, and subject to Aldefluor assay and flow cytometry analysis. (A) Sulforaphane decreased the percentage of ALDH-positive cells. Data are presented as mean ± SD(n =3). (B) A set of representative flow cytometry dot plots. R2 covers the region of ALDH-positive cells. SF = sulforaphane; ALDH = aldehyde dehydrogenase.
Therefore, these findings demonstrate sulforaphane in reducing the breast cancer stem/progenitor cell population in vitro. An interesting observation is that sulforaphane was able to inhibit stem/progenitor cells at the concentrations (0.5~ 5 μM) that hardly affected the bulk population of cancer cells, implying that sulforaphane is likely to preferentially target stem/progenitor cells compared to the differentiated cancer cells.
Sulforaphane Eliminates Breast Cancer Stem Cells in Vivo
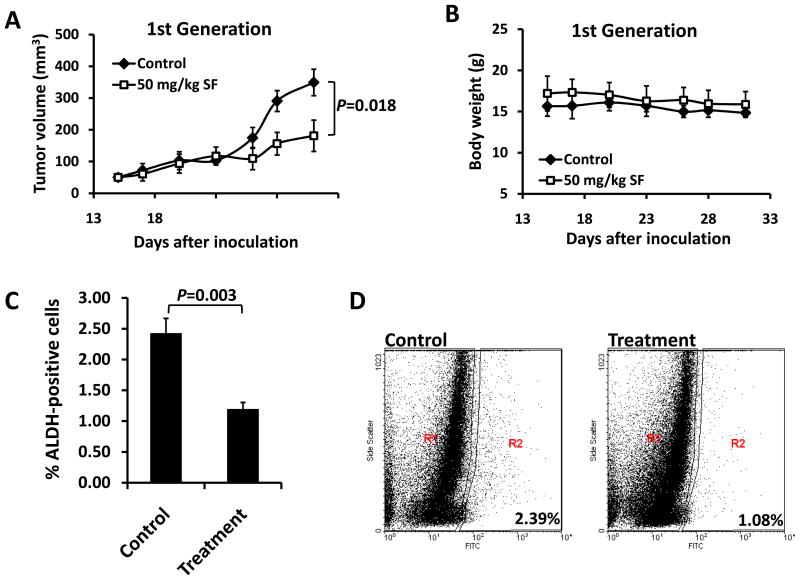
In order to determine whether sulforaphane could target breast CSCs in vivo, we utilized a xenograft model of SUM159 cells in NOD/SCID mice. Two weeks after cell inoculation, animals were daily injected with 50 mg/kg sulforaphane. After two weeks of treatment, tumors in sulforaphane-treated mice were 50% of the size of 0.9% NaCl solution control animals (P = 0.018) (Figure 4A), while sulforaphane had no apparent toxicity as determined by body weight (Figure 4B). Tumors were isolated from animals and the tumor cells were analyzed by Aldefluor assay. As shown in Figure 4C and 4D, sulforaphane reduced ALDH-positive population by more than 50% compared to that from control mice (P = 0.003).
Figure 4. Sulforaphane decreased tumor size and ALDH-positive cell population in primary breast cancer xenografts.
NOD/SCID mice bearing SUM159 cells in fat pads as xenografts were treated with daily i.p. injection of control or 50 mg/kg sulforaphane for 2 weeks. Tumor volumes (A) and mouse body weights (B) were determined as described in “Materials and Methods”. Tumors in sulforaphane-treated mice were 50% the size of control animals at the end of drug treatment. (C) Sulforaphane decreased the percentage of ALDH-positive cells in xenograft breast tumors. (D) A set of representative flow cytometry plots. Data are presented as mean ± SD(n = 6). ALDH = aldehyde dehydrogenase.
Although the decreased ALDH-positive cell population in sulforaphane-treated tumors suggests that sulforaphane may target breast cancer stem/progenitor cells, the ability of residual cancer cells to initiate tumors upon re-implantation in secondary mice is a more definitive assay (6). Therefore, we examined the growth of secondary tumors in NOD/SCID mice inoculated with primary tumor cells obtained from primary xenografts. In order to avoid potential variations due to mouse heterogeneity, each recipient mouse was injected with 50,000 cells obtained from sulforaphane-treated tumors in one side of inguinal mammary fat pad and another 50,000 cells obtained from control tumors in the contralateral fat pad. The results showed that cancer cells from control animals exhibited rapid tumor re-growth, reaching a final tumor size ranging from 300 to 500 mm3 in secondary NOD/SCID mice. However, the cancer cells obtained from sulforaphane-treated mice largely failed to produce any tumors in recipient mice up to 33 days after implantation (Figure 5A). Figure 5A & 5B showed that tumor cells derived from sulforaphane-treated mice only gave rise to one small tumor (6 mm3) out of 7 inoculations at day 19, while control tumor cells yielded tumors as early as day 7 (P < 0.01). All control inoculations produced tumors by day 15 (Figure 5B). These results suggest that sulforaphane was able to eliminate breast CSCs in primary xenografts, thereby abrogating the re-growth of tumors in secondary mice. Taken together with the in vivo Aldefluor assay results, these findings suggest that sulforaphane targets breast CSCs with high potency.
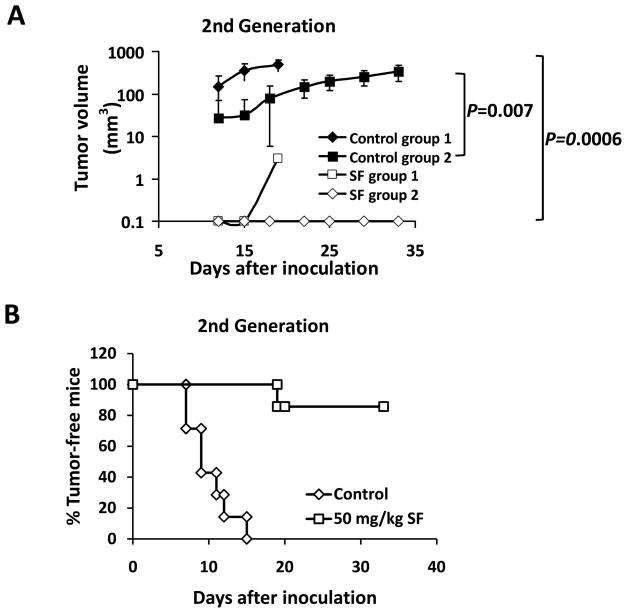
Figure 5. Sulforaphane eradicated breast cancer stem cells in vivo asassessed by re-implantation in secondary mice.
Each secondary NOD/SCID mouse received 50,000 cells from control tumorsin one side of mammary fat pad and another 50,000 cells from sulforaphane-treated tumorsin the contralateral fat pad. (A) Tumor growth curves of the recipient NOD/SCID mice. Data for group 1 are presented as mean ± SD(n = 4), and for group 2 are presented as mean ± SD(n = 3). Sulforaphane abrogated the tumorigenicity of breast cancer stem cells. (B) Percentage of tumor-free mice by the day of euthanization for each group. Four mice were euthanized at day 20 and three at day 33 due to the mass tumor burden on control side.
Sulforaphane Down-regulates Wnt/β-Catenin Pathway in Breast Cancer Cells
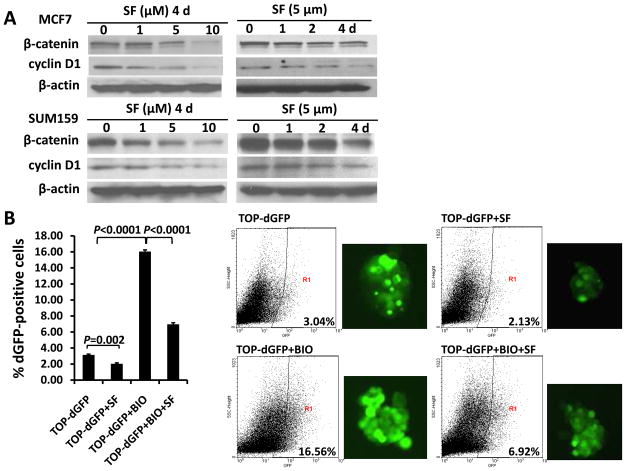
Next, we investigated the mechanisms that may contribute to the effects of sulforaphane on breast CSCs. Wnt/β-catenin pathway is known to be an important regulator of stem cell self-renewal (8). Since sulforaphane was reported to down-regulate β-catenin in human cervical carcinoma and hepatocarcinoma cell lines (19), we examined whether β-catenin and Wnt/β-catenin downstream targets are down-regulated by sulforaphane in human breast cancer cells. As shown in Figure 6A, sulforaphane decreased the protein level of β-catenin by up to 85% in MCF7 and SUM159 cells; and the expression of cyclin D1, one of the Wnt/β-catenin target genes, declined by up to 77% as well. To further confirm that the down-regulation of β-catenin protein level decreased its transcriptional activity, we utilized a TCF/LEF TOP-dGFP lentiviral reporter system. The β-catenin activates TCF/LEF in nucleus, driving the transcription of destabilized GFP (dGFP) gene; and the dGFP expression was analyzed by fluorescence microscopy and quantified by flow cytometry. As determined by flow cytometry, approximately 3% of transfected cells are dGFP-positive, and 5 μM sulforaphane reduced this population by 30% ~40% (P = 0.002) (Figure 6B).
Figure 6. Sulforaphane down-regulated Wnt/β-catenin self-renewal pathway.
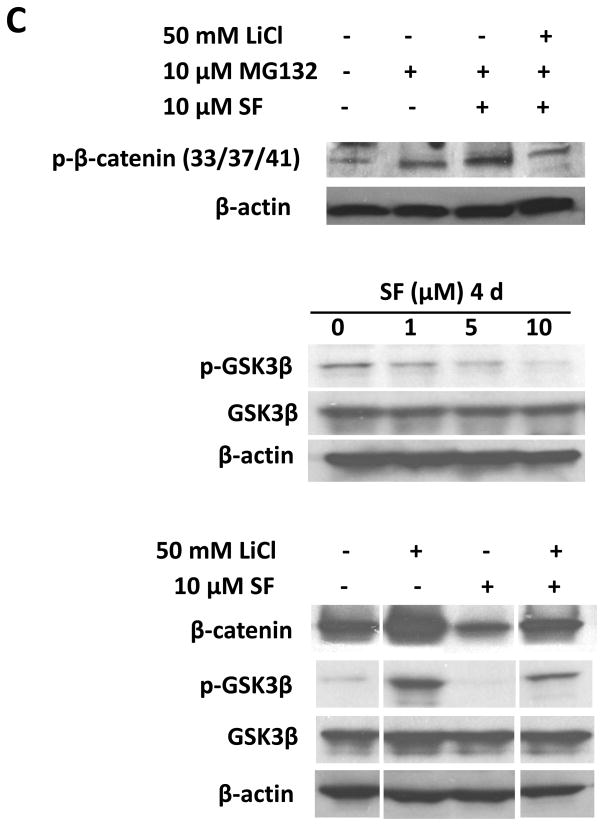
(A) Sulforaphane decreased protein levels of β-catenin and cyclin D1 in both SUM159 and MCF7 cell lines. (B) TOP-dGFP reporter lentivirus-infected MCF7 mammospheres were treated with indicated compounds (0.5 μM BIO and 5 μM sulforaphane) either alone or in combination for 2 days. Sulforaphane decreased the percentage of dGFP-positive cells by 30% ~40%. BIO increased this population, while sulforaphane decreased it by over 60% in the presence of BIO. Representative flow cytometry results of TOP-dGFP mammospheres and their pictures under fluorescence microscope(magnification × 100)are shown on the right. (C) Sulforaphane promoted β-catenin phosphorylation at Ser33/37/Thr41, while LiCl suppressed the phosphorylation by inactivating GSK3β (upper panel). Sulforaphane decreased phospho-GSK3β (Ser9)level, whereas total GSK3β remained unchanged(middle panel). LiCl increased protein level of β-catenin by phosphorylating/inactivating GSK3β at Ser9, while sulforaphane attenuated LiCl-induced GSK3β phosphorylation and β-catenin accumulation(bottom panel). SF = sulforaphane; dGFP = destabilized green fluorescent protein.
The intracellular level of β-catenin is regulated by its phosphoryaltion status and subsequent proteasomal degradation. When β-catenin is phosphorylated at Ser33/Ser37/Thr41 by GSK3β, it is immediately subject to ubiquitin-proteasome degradation (17). Phospharylation of GSK3β at Ser9 may decrease the activity of GSK3β, thereby stabilizing β-catenin (29, 30). Thus, we used a proteasome inhibitor, MG132, to block proteasome function and observed an accumulation of phospho-β-catenin (Ser33/Ser37/Thr41) in response to sulforaphane (Figure 6C, upper panel). The sulforaphane-induced β-catenin phosphorylation was reversed when LiCl, a GSK3β inhibitor, was present (Figure 6C, upper panel) (31). As shown in Figure 6B, 0.5 μM BIO, another specific GSK3β inhibitor (31, 32), enhanced the dGFP-positive cell population by more than 5-fold (P < 0.0001), and sulforaphane (5 μM) decreased this population by over 60% in the presence of BIO (P < 0.0001). Furthermore, our result demonstrated a decreased level of phospho-GSK3β (Ser9) by up to 74% in cells with increasing concentrations of sulforaphane (Figure 6C, middle panel). LiCl was demonstrated to inactivate GSK3β through Ser9 phosphorylation, which in turn reduce phosphorylation of β-catenin at Ser33/Ser37/Thr41 and its degradation (31, 32). As shown in the bottom panel of Figure 6C, sulforaphane was able to attenuate LiCl-induced GSK3β phosphorylation and β-catenin accumulation.
Taken together, these data suggest that the down-regulation of Wnt/β-catenin self-renewal pathway might contribute to the inhibitory effects of sulforaphane on breast CSCs. This warrants further studies to establish the conclusive role of this down-regulation in inhibition of breast CSCs by sulforaphane.
Discussion
The anti-cancer efficacy of sulforaphane, a natural compound derived from broccoli/broccoli sprouts, has been evaluated in various cancers. For instance, oral or intraperitoneal administration of sulforaphane inhibited the tumor growth in prostate PC-3 and pancreatic Panc-1 xenografts (33, 34). The risk of premenopausal breast cancer was shown to be inversely associated with broccoli consumption (35). The orally administered sulforaphane reached mammary gland and increased the detoxification enzyme activity (36). Additionally, it has been suggested that sulforaphane may have the potential to act against tumor resistance and relapse/recurrence (37). A very recent study demonstrated the effectiveness of sulforaphane in abrogating pancreatic tumor resistance to TRAIL by interfering with NF-κB induced anti-apoptotic signaling (18). Another study indicated that sulforaphane could overcome doxorubicin resistance and restore apoptosis induction in cells (38). These findings provide a strong rationale for investigating the chemoprevention property of sulforaphane or broccoli/broccoli sprouts in clinical trials.
Increasing evidence supports the cancer stem cell theory, which states that a variety of cancers are driven and sustained by a small proportion of CSCs (8). The concept of CSCs has profound clinical implications for cancer therapeutics and prevention (8, 39). Recent studies indicate that CSCs have the capacity to drive tumor resistance and relapse/recurrence (40, 41). Lack of efficacy of current chemotherapies in advance and metastatic disease requires novel approaches to specifically target CSC population (8, 42, 43). Thus, therapies that are directed against both differentiated cancer cells and CSCs may provide advantages to treat these diseases. Researchers have found that several dietary compounds are promising chemoprevention agents against CSCs, such as curcumin (13, 14). Therefore, based on the chemopreventive activity of sulforaphane and the implications of CSC theory, we have utilized both in vitro and in vivo systems to determine whether sulforaphane acts against breast CSCs.
Several techniques have been developed to isolate and characterize breast CSCs in vitro. Mammosphere culture was first used to isolate and expand mammary stem/progenitor cells by Dontu et al. (22), based on the ability of stem/progenitor cells to grow in serum-free suspension, while differentiated cells fail to survive under the same condition (21). By employing this technique, we have demonstrated that sulforaphane (0.5~5 μM) significantly suppressed the mammospheres formation of both SUM159 and MCF7 cells (Figure 2). Another technique is to utilize cell makers, e.g., CD44+CD24−/lowlin− and ALDH-positive (21, 23, 25), to distinguish mammary stem/progenitor cells from differentiated cancer cells. It has been reported that as few as 500 ALDH-positive cells were able to generate a breast tumor within 40 days, while 50,000 ALDH-negative cells failed to form tumor (23). ALDH-positive and CD44+CD24−/lowlin− were identified a small overlap that has the highest tumorigenic capacity, generating tumors from as few as 20 cells (23). In contrast, ALDH-positive cells without the CD44+CD24−/lowlin− marker were able to produce tumors from 1,500 cells, whereas 50,000 CD44+CD24−/lowlin− ALDH-negative cells did not (23). Thus, we utilized Aldefluor assay to evaluate the ability of sulforaphane to target breast cancer stem/progenitor cells. We have demonstrated that sulforaphane (1~5 μM) could inhibit the tumor-initiating ALDH-positive cells in vitro by 65% to 80% (Figure 3). Of special note, concentrations of sulforaphane which inhibit stem/progenitor cells in both mammosphere formation assay and Aldefluor assay had only minimal effects on the bulk population of breast cancer cell lines, which implies the preferential targeting of stem/progenitor cells by sulforaphane.
The injection of human breast cancer cells into the mammary fat pad of immune-deficient NOD/SCID mice provides a reliable and sensitive in vivo system for studying human breast cancer (25, 44). We demonstrated that sulforaphane was able to target breast CSCs in vivo by using this xenograft model. Daily injection of sulforaphane for two weeks suppressed tumor growth in primary NOD/SCID mice and reduced ALDH-positive cell population of the tumors by ~50% (Figure 4). More importantly, we found that the tumor cells derived from sulforaphane-treated mice were not able to form secondary tumors in recipient mice up to 33 days (Figure 5). There are two possible reasons that may explain the difference between the 50% reduction of ALDH-positive population and the failure of tumor growth in secondary mice. One is that although ALDH-positive cells are enriched with stem/progenitor cells, not all ALDH-positive cells have tumor-initiating capacity. Another possible reason is the experimental setting we used for the primary NOD/SCID mice. We inoculated 2,000,000 SUM159 cells into the primary NOD/SCID mice, and treated them with the drug after two weeks of cell inoculation, both of which could lead to an under-estimation of the effect of sulforaphane on ALDH-positive cell population. However, the ability of CSCs to self-renew and differentiate as determined by reimplantation of primary tumor cells in secondary animals is a more definitive functional assay (6). These are consistent with the in vitro observation that sulforaphane preferentially targeted cancer stem/progenitor cells instead of bulk cell population. The preference of sulforaphane in killing CSCs may be significant for chemoprevention.
The well-known curcumin was shown to interfere with self-renewal pathways, Wnt and Notch, in colon and pancreatic cancer cells respectively (13, 14). Apple-derived quercetin and green tea epigallocatechin-gallate were reported to regulate key elements of Wnt and Notch pathways in human colon cancer cells (15). Park et al. previously reported that β-catenin was down-regulated in HeLa and HepG2 cells (19). In consistent with this study, we demonstrated that sulforaphane was able to down-regulate Wnt/β-catenin self-renewal pathway in breast cancer cells, and sulforaphane-induced β-catenin phosphorylation (Ser33/Ser37/Thr41) and proteasome degradation was possibly through activation of GSK3β (Figure 6). Myzak et al. reported that sulforaphane increased β-catenin activity without altering its protein level in HDAC1-transfected HEK293 cells (45). The differences among the studies could arise from distinct cell lines and treatment conditions.
As a chemoprevention agent, sulforaphane possesses many advantages, such as high bioavailability and low toxicity (4). Sulforaphane from broccoli extracts is efficiently and rapidly absorbed in human small intestine, and distributed throughout the body (2, 46). Plasma concentrations of sulforaphane equivalents peaked 0.94~2.27 μM in humans 1 hr after a single dose of 200 μmol broccoli sprout isothiocyanates (mainly sulforaphane) (47). A recent pilot study detected an accumulation of sulforaphane in human breast tissue, with 1.45 ± 1.12 pmol/mg for the right breast and 2.00 ± 1.95 pmol/mg for the left, in eight women who consumed broccoli sprout preparation containing 200 μmol sulforaphane about 1 hr before the surgery (36). These concentrations of sulforaphane are expected to be effective against breast CSCs, based on our in vitro results. Although sulforaphane itself has not been evaluated in humans, broccoli sprouts were tested for toxicity in clinical trials (4). A Phase I trial showed that broccoli sprouts caused no significant toxicity when administered orally at 8-hr intervals for 7 days as 25 μmol isothiocyanates (mainly sulforaphane) (48). In another study, it was well tolerated in 200 adults who consumed broccoli sprout solution containing 400 μmol glucoraphanin (precursor of sulforaphane) nightly for 2 weeks (49). Additionally, sulforaphane at concentrations below 10 μM did not show significant effect on cell cycle arrest and apoptosis induction of human non-transformed T-lymphocytes (50).
In conclusion, we have demonstrated that sulforaphane was able to target breast CSCs as determined by the mammosphere formation assay, Aldefluor assay, and tumor growth upon reimplantation in secondary mice. Furthermore, our study identified the down-regulation of Wnt/β-catenin self-renewal pathway by sulforaphane as one of the possible mechanisms for its efficacy. These studies support the use of sulforaphane for breast cancer chemoprevention. These findings provide a strong rationale for preclinical and clinical evaluation of sulforaphane or broccoli/broccoli sprouts for breast cancer therapies.
Translational Relevance
Sulforaphane, the natural compound derived from broccoli/broccoli sprouts, has been proved to possess anti-cancer activity. This study demonstrates that sulforaphane inhibits breast cancer stem cells in vitro and in vivo, which provides a strong rationale for future clinical evaluation of sulforaphane or extract of broccoli/broccoli sprouts for breast cancer chemoprevention. Breast cancer is initiated from and maintained by a small population of breast cancer stem cells. Currently available chemotherapy and radiation therapy are incapable of suppressing cancer stem cell population. Aldefluor assay and mammosphere formation assay showed that sulforaphane inhibited breast cancer stem cells in vitro. NOD/SCID mouse model exhibited that sulforaphane eliminated breast cancer stem cells in vivo.
Acknowledgments
Grant support: This work was supported by the National Institutes of Health (RO1 CA120023); University of Michigan Cancer Center Research Grant (Munn); and University of Michigan Cancer Center Core Grant to Duxin Sun.
We would like to thank Dr. Stephen P. Ethier at Karmanos Cancer Center for the SUM159 cell line. We also would like to thank Dr. Irving L. Weissman at Ludwig Center, Stanford University School of Medicine for kindly providing the TCF/LEF-1 (TOP-dGFP, FOP-dGFP) lentiviral reporter system.
References
- 1.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–72. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–54. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Dontu G, Wicha MS. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast CancerRes. 2005;7:86–95. doi: 10.1186/bcr1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Korkaya H, Paulson A, Charafe-Jauffret E, et al. Regulation of mammary stem/progenitor cells by PTEN/Akt/beta-catenin signaling. PLoS Biol. 2009;7:e1000121. doi: 10.1371/journal.pbio.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–71. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 9.Dontu G, Jackson KW, McNicholas E, Kawamura MJ, Abdallah WM, Wicha MS. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 2004;6:R605–15. doi: 10.1186/bcr920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smalley MJ, Dale TC. Wnt signalling in mammalian development and cancer. Cancer Metastasis Rev. 1999;18:215–30. doi: 10.1023/a:1006369223282. [DOI] [PubMed] [Google Scholar]
- 11.Shafee N, Smith CR, Wei S, et al. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–50. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambardzumyan D, Squatrito M, Holland EC. Radiation resistance and stem-like cells in brain tumors. Cancer Cell. 2006;10:454–6. doi: 10.1016/j.ccr.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106:2503–13. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21:8414–27. doi: 10.1038/sj.onc.1205947. [DOI] [PubMed] [Google Scholar]
- 15.Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54:7075–82. doi: 10.1021/jf0612530. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Li Y, Semenov M, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108:837–47. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 18.Kallifatidis G, Rausch V, Baumann B, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–63. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 19.Park SY, Kim GY, Bae SJ, Yoo YH, Choi YH. Induction of apoptosis by isothiocyanate sulforaphane in human cervical carcinoma HeLa and hepatocarcinoma HepG2 cells through activation of caspase-3. Oncol Rep. 2007;18:181–7. [PubMed] [Google Scholar]
- 20.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–11. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 21.Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: current opinion and future challenges. Pathobiology. 2008;75:75–84. doi: 10.1159/000123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dontu G, Abdallah WM, Foley JM, et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–70. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo M, Fan H, Nagy T, et al. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009;69:466–74. doi: 10.1158/0008-5472.CAN-08-3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–14. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 27.Azarenko O, Okouneva T, Singletary KW, Jordan MA, Wilson L. Suppression of microtubule dynamic instability and turnover in MCF7 breast cancer cells by sulforaphane. Carcinogenesis. 2008;29:2360–8. doi: 10.1093/carcin/bgn241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pledgie-Tracy A, Sobolewski MD, Davidson NE. Sulforaphane induces cell type-specific apoptosis in human breast cancer cell lines. Mol Cancer Ther. 2007;6:1013–21. doi: 10.1158/1535-7163.MCT-06-0494. [DOI] [PubMed] [Google Scholar]
- 29.Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–32. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 30.Cohen P, Frame S. The renaissance of GSK3. Nat Rev Mol Cell Biol. 2001;2:769–76. doi: 10.1038/35096075. [DOI] [PubMed] [Google Scholar]
- 31.Hedgepeth CM, Conrad LJ, Zhang J, Huang HC, Lee VM, Klein PS. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. [DOI] [PubMed] [Google Scholar]
- 32.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–9. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanatesulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–48. [PubMed] [Google Scholar]
- 34.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 35.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–8. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- 36.Cornblatt BS, Ye L, Dinkova-Kostova AT, et al. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–90. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 37.Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: keep one eye beyond Keap1. Cancer Lett. 2006;233:208–18. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fimognari C, Nusse M, Lenzi M, Sciuscio D, Cantelli-Forti G, Hrelia P. Sulforaphane increases the efficacy of doxorubicin in mouse fibroblasts characterized by p53 mutations. Mutat Res. 2006;601:92–101. doi: 10.1016/j.mrfmmm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Kakarala M, Wicha MS. Implications of the cancer stem-cell hypothesis for breast cancer prevention and therapy. J Clin Oncol. 2008;26:2813–20. doi: 10.1200/JCO.2008.16.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakariassen PO, Immervoll H, Chekenya M. Cancer stem cells as mediators of treatment resistance in brain tumors: status and controversies. Neoplasia. 2007;9:882–92. doi: 10.1593/neo.07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang C, Chua CL, Ang BT. Insights into the cancer stem cell model of glioma tumorigenesis. Ann Acad Med Singapore. 2007;36:352–7. [PubMed] [Google Scholar]
- 42.Lippman ME. High-dose chemotherapy plus autologous bone marrow transplantation for metastatic breast cancer. N Engl J Med. 2000;342:1119–20. doi: 10.1056/NEJM200004133421508. [DOI] [PubMed] [Google Scholar]
- 43.Williams SD, Birch R, Einhorn LH, Irwin L, Greco FA, Loehrer PJ. Treatment of disseminated germ-cell tumors with cisplatin, bleomycin, and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–40. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 44.Dick JE. Breast cancer stem cells revealed. Proc Natl Acad Sci U S A. 2003;100:3547–9. doi: 10.1073/pnas.0830967100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 46.Petri N, Tannergren C, Holst B, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–13. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 47.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 48.Shapiro TA, Fahey JW, Dinkova-Kostova AT, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 49.Kensler TW, Chen JG, Egner PA, et al. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in arandomized clinical trial in He Zuo township, Qidong, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–13. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]
- 50.Fimognari C, Nusse M, Berti F, Iori R, Cantelli-Forti G, Hrelia P. Isothiocyanates as novel cytotoxic and cytostatic agents: molecular pathway on human transformed and non-transformed cells. Biochem Pharmacol. 2004;68:1133–8. doi: 10.1016/j.bcp.2004.03.044. [DOI] [PubMed] [Google Scholar]