Abstract
Liver injury is associated with inflammation, which is generally believed to accelerate the progression of liver diseases; however, clinical data show that inflammation does not always correlate with hepatocelluar damage in some patients. Investigating the cellular mechanisms underlying these events using an experimental animal model, we show that inflammation may attenuate liver necrosis induced by carbon tetrachloride (CCl4) in myeloid-specific signal transducer and activator of transcription 3 (STAT3) knockout mice. As an important anti-inflammatory signal, conditional deletion of STAT3 in myeloid cells results in markedly enhanced liver inflammation after CCl4 injection. However, these effects are also accompanied by reduced liver necrosis, correlating with elevated serum IL-6 and hepatic STAT3 activation. An additional deletion of STAT3 in hepatocytes in myeloid-specific STAT3 knockout mice restored hepatic necrosis, but decreased liver inflammation. Conclusions: Inflammation-mediated STAT3 activation attenuates hepatocellular injury induced by CCl4 in myeloid-specific STAT3 knockout mice, suggesting that inflammation associated with a predominance of hepatoprotective cytokines that activate hepatic STAT3 may reduce rather than accelerate hepatocellular damage in patients with chronic liver diseases.
Introduction
Inflammation is associated with various types of acute and chronic liver diseases, and is considered a key contributor of hepatocellular damage and progression to liver fibrosis and hepatocellular carcinoma.1–7 However, clinical data show that hepatic inflammation does not always correlate with hepatocellular damage (serum ALT elevations).8–13 In order to understand the cellular mechanism of this phenomena, a well-established experimental animal model14 to study hepatotoxin-induced liver inflammation and injury induced by CCl4 was utilized in our investigation. Once CCl4 is injected, the p450 CYP2E1 in hepatocytes metabolizes it into trichloromethyl radicals (CCl3*), which then causes lipid peroxidation and membrane damage.14 These damaged hepatocytes generate free radicals, thereby activating Kupffer cells/macrophages to produce pro-inflammatory and anti-inflammatory cytokines that control the progression of liver inflammation and injury.14 Among the cytokines produced, interleukin-6 (IL-6) has been shown to be a hepatoprotective cytokine in this model15 as well as in several other liver injury models, including ischemia/reperfusion, partial hepatectomy, alcoholic and nonalcoholic fatty liver, Con A-induced T cell hepatitis.16–20 Additionally, the anti-inflammatory cytokine, IL-10, has also been shown to play an important role in controlling inflammation in CCl4-induced chronic liver injury and fibrosis, and may therefore participate in protecting the liver from disease progression.21, 22
The hepatoprotective effects of IL-6 are mediated mainly via activation of signal transducer and activator of transcription 3 (STAT3) in hepatocytes.16 In contrast, the anti-inflammatory effects of IL-10 in the liver are likely mediated via activation of STAT3 in Kupffer cells/macrophages. In general, IL-6 binds to its corresponding gp80 receptor located on hepatocytes, and induces dimerization of the gp130 signal chain. Consequently, the Janus kinases (JAKs) associated with the gp130 chains also dimerize and autophosphorylate. Once activated, the JAKs then phosphorylate gp130 to activate STAT3 monomers. Phosphorylated STAT3 then forms dimers and translocates into the nuclei to induce expression of a wide array of genes, including anti-apoptotic and anti-oxidative genes that protect against hepatocyte damage. The protective role of hepatic STAT3 has been well documented in studies with hepatocyte-specific gp130 or STAT3 knockout mice; these mice have shown to be more susceptible to hepatocellular damage induced by various toxins.23–25 Moreover, a conditional ablation of the STAT3 gene in myeloid linage cells (eg, macrophages) have shown markedly enhanced systemic and liver inflammation,26–28 which clearly suggests the anti-inflammatory functions of STAT3 in myeloid cells. In this investigation, we found that myeloid-specific STAT3 knockout (STAT3Mye−/−) mice are more susceptible to CCl4-induced liver inflammation, but are surprisingly resistant to CCl4-induced necrosis. Further study revealed that the enhanced inflammation observed is associated with elevated hepatic STAT3 activation, which may explain the reduced necrosis observed in these mice.
Materials and Methods
Mice
Eight- to 10-week old male mice were used in all experiments. Hepatocyte-specific STAT3 knockout (KO) mice (STAT3Hep−/−) and myeloid-specific STAT3 KO mice (STAT3Mye−/−)were generated as described previously.27 The STAT3Mye−/− mice were described previously as M/N-STAT3KO mice.27 The corresponding littermates of the wild-type mice were used as controls. For deletion of STAT3 in both hepatocytes and myeloid cells, STAT3Hep−/− and STAT3Mye−/− were bred to generate 4 lines of mice, including double KO (STAT3Mye−/−Hep−/−), STAT3Hep−/−, STAT3Mye−/−, and littermate wild-type controls. All mice were fed regular chow unless specified. All animal experiments were conducted in accordance with NIH guidelines and approved by the Institutional Animal Care and Use Committee of the National Institute on Alcohol Abuse and Alcoholism.
Mouse model for CCl4-induced liver injury
Liver injury was induced by intraperitoneal (i.p.) injection with 2 mL/kg body weight of 10% CCl4 (Sigma) dissolved in olive oil (Sigma).
Statistical Analysis
Data are expressed as mean±SD. To compare values obtained from 2 groups, the Student's t test was performed. To compare values obtained from three or more groups, 1-factor analysis of variance (ANOVA) was used, followed by Tukey's post hoc test. Statistical significance was taken at the P <0.05 level.
Other methods
all other methods are described in the supporting documents.
Results
STAT3Mye−/− mice are more susceptible to liver inflammation, but are resistant to liver necrosis induced by CCl4
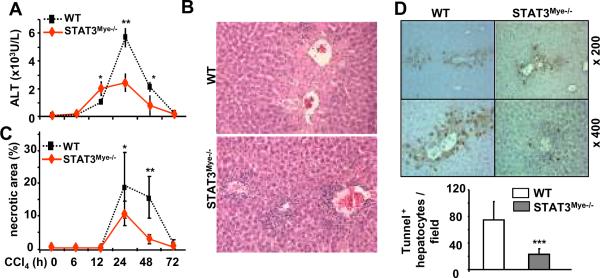
As shown in Figs. 1A–C, treatment of wild-type littermate control mice with CCl4 induced serum ALT elevation, liver necrosis, and inflammation with peak effect occurring 24 hours post injection. Compared with wild-type mice, STAT3Mye−/− mice had greater inflammatory cell infiltration around the hepatic central vein, but surprisingly, these mice had lower serum ALT levels and less liver necrosis (Figs. 1A–C). TUNEL assay results revealed that the number of apoptotic hepatocytes was significantly lower in STAT3Mye−/− mice than in wild-type mice 24 hours post CCl4 injection (Fig. 1D).
Fig. 1. STAT3Mye−/− mice are more susceptible to liver inflammation, but resistant to necrosis/apoptosis induced by CCl4.
Wild-type and STAT3Mye−/− mice were injected with CCl4, and then sacrificed at various time points. A. Serum ALT levels. B. H&E staining of liver tissues from the mice treated 24 h post CCl4 injection. C. Necrotic area was quantified. D. Liver tissues were also stained with TUNEL to determine hepatocyte apoptosis, and the number of TUNEL+ hepatocytes was quantified. Values represent means±SD (n=4–10). *P<0.05, **P<0.01, ***P<0.001 in comparison with corresponding WT groups. WT: littermate wild-type control mice (LyzMCre−STAT3flox/flox); STAT3Mye−/−(LyzMCre+STAT3flox/flox).
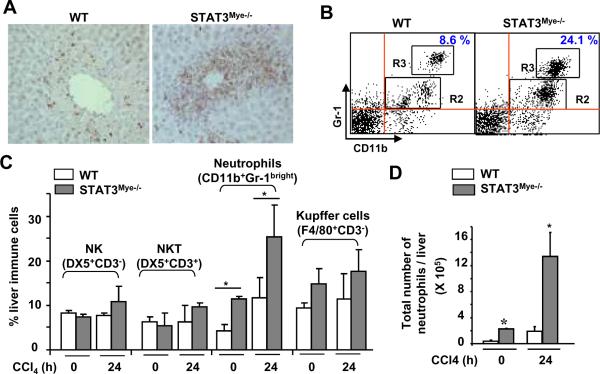
Immunohistochemical analyses with anti-MPO staining showed that the majority of cells infiltrating the liver after CCl4 injection were neutrophils (Fig. 2A). These neutrophils are either located within sinusoids or infiltrated into liver parenchyma (Fig. 2A and Fig. S1a). The number of MPO+ neutrophils was much higher in STAT3Mye−/− mice compared with wild-type mice post CCl4 injection. Flow cytometry analyses of liver inflammatory cells showed that the percentage and total number of neutrophils (CD11b+Gr-1bright cells) in the liver were significantly higher in STAT3Mye−/− mice than in wild-type mice before or 24 hours post CCl4 injection (Figs. 2B–D). Lee et al previously demonstrated that STAT3-deficient neutrophils matured normally and were functional.29 Here we also confirmed that nutrophils from wild-type and STAT3Mye−/− mice had similar respiratory burst (Fig. S1c).
Fig. 2. STAT3Mye−/− mice have more neutrophil infiltration in the liver than wild-type mice after CCl4 treatment.
Wild-type (WT) and STAT3Mye−/− mice were injected with CCl4 and sacrificed 24 h post injection. A. Liver tissues were stained with anti-MPO (myeloperoxidase) antibody for neutrophils (MPO positive brown staining). B. Liver immune cells were isolated and analyzed by flow cytometry with anti-CD11b and anti-Gr-1 antibodies. Gr-1brightCD11b+ cells in the R3 area represent neutrophils. C, D. The percentage and the total number of liver lymphocytes were quantified. Values represent means±SD (n=4–5). *P<0.05, in comparison with corresponding littermate WT groups.
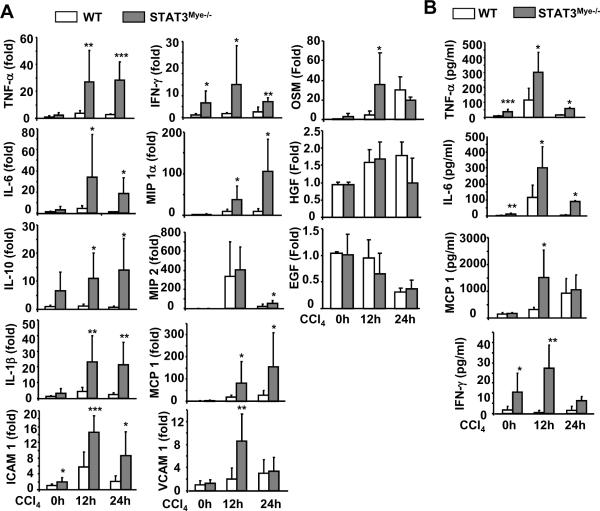
Fig. 3A shows that basal levels of various hepatic inflammatory cytokines and chemokines were higher in STAT3Mye−/− mice compared with wild-type mice. In wild-type mice, treatment with CCl4 increased the expression of these cytokines and chemokines; however, this increase was much more profound in STAT3Mye−/− mice. Serum levels of several inflammatory cytokines were also found to be elevated after CCl4 injection, and again, these serum elevations were higher in STAT3Mye−/− mice compared with wild-type mice (Fig. 3B). Serum IL-12p70 and IL-10 were below detection levels in both groups (data not shown).
Fig. 3. Upregulation of cytokines and chemokines in STAT3Mye−/− mice.
Wild-type littermate controls and STAT3Mye−/− mice were injected with CCl4 and then sacrificed 12 h and 24 h post injection. A. Liver tissues were collected for real-time PCR analyses for expression of pro-inflammatory cytokines and chemokines. B. Serum levels of cytokines and chemokines. Values represent means±SD (n=4–10) *P<0.05, **P<0.01 in comparison with corresponding WT groups.
STAT3Mye−/− mice show higher hepatic STAT3 activation and are resistant to hepatic oxidative stress after CCl4 injection compared with wild-type mice
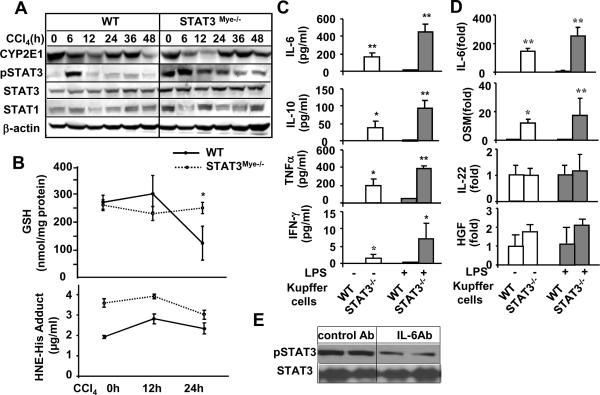
Since p450 CYP2E1-mediated CCl4 metabolism is essential for CCl4-induced liver injury,14 we examined whether alterations in CCl4 metabolism are responsible for reduced liver injury in STAT3Mye−/− mice. As shown in Fig. 4A and Fig. S1b, the basal levels of CYP2E1 expression were comparable in livers from wild-type and STAT3Mye−/− mice. After CCl4 administration, CYP2E1 expression was downregulated in both groups of STAT3Mye−/− and wild-type mice. The downregulation seemed to be more profound in the former group compared to the later group, suggesting that CCl4 metabolism is not reduced in STAT3Mye−/− mice compared to wild-type mice since the downregulation of CYP2E1 is caused by CCl4 metabolism.
Fig. 4. Elevated hepatic STAT3 activation in STAT3Mye−/− mice.
A,B: Mice were injected with CCl4 and then sacrificed at various time points. Liver tissues were collected for Western blot analyses (A) or for measurement of GSH levels and NHE-His adduct (B). C, D: Kupffer cells were isolated from wild-type and STAT3Mye−/− mice, and treated with LPS (1ng/ml) for 24 hours. The supernatants were collected for measurement of various cytokines (C), and cells were collected for real-time PCR analyses (D). Values represent means±SD (n=5). *P<0.05 in comparison with corresponding WT littermate groups. E, STAT3Mye−/− mice were treated with control Ab or anti-IL-6 antibody for 2 days. Liver tissues were then collected for Western blot analyses.
Injection of CCl4 induced increased oxidative stress, which was higher in STAT3Mye−/− mice than in wild-type mice as determined by measuring HNE-His adduct (Fig. 4B). Although STAT3Mye−/− mice had higher levels of oxidative stress, CCl4 treatment-induced GSH depletion, which was observed in wild-type mice, was not observed in STAT3Mye−/− mice (Fig. 4B). At the present, it is not clear why STAT3Mye−/− mice had higher levels of oxidative stress without GSH depletion after CCl4 treatment compared to wild-type mice. It is possible that elevated inflammation may trigger some compensatory effects to prevent GSH depletion in STAT3Mye−/− mice, which should be explored in the future studies.
To understand the mechanism by which STAT3Mye−/− mice are resistant to CCl4-induced liver injury, we measured activation of hepatic STAT3, a signaling molecule that has been shown to promote hepatocyte survival in the liver.23–25 Shown in Fig. 4A, basal STAT3 activation (pSTAT3) was higher in STAT3Mye−/− mice than in wild-type mice. Injection with CCl4 induced much higher and prolonged STAT3 activation in STAT3Mye−/− mice compared with wild-type mice. Expression of STAT3 protein was also slightly higher in STAT3Mye−/− mice than in wild-type mice, while expression of STAT1 protein was comparable between these groups. Fig. 4A shows that the basal levels (0 h time point) of hepatic pSTAT3 are higher in STAT3Mye−/− mice than wild-type mice. Our previous study showed that STAT3Mye−/− mice had similar basal levels of hepatic pSTAT3 compared to wild-type mice (Fig. 2C in Reference 28). The discrepancy between our current and previous studies was likely due to the mice fed regular chow in the current study and a medicated diet in our previous study. Fig. S2a confirmed that feeding with a medicated diet abolished the basal levels of hepatic pSTAT3 in STAT3Mye−/− mice. Despite the diminished basal levels of hepatic pSTAT3 activation after feeding with a medicated diet, STAT3Mye−/− mice were still resistant to CCl4-induced liver injury (elevation of serum ALT/AST) (Fig. S2b). In addition, the basal levels of p38 MAPK were higher in the liver of STAT3Mye−/− mice compared to wild-type mice, while activation of ERK in the liver was lower in STAT3Mye−/− mice than in wild-type mice (Fig. S3). Activation of phospho-NF-κB p65 was higher in the liver of STAT3Mye−/− mice compared to wild-type mice after CCl4 injection (Fig. S3).
To understand the mechanisms underlying elevated hepatic STAT3 activation in STAT3Mye−/− mice, the production and expression of several cytokines (IL-6, IL-22, and oncostatin M [OSM]) and growth factors (HGF, EGF), which stimulate STAT3 activation in hepatocytes, were examined in Kupffer cells. As shown in Figs. 4C and D, the production and expression of IL-6 were markedly higher in Kupffer cells from STAT3Mye−/− mice than from wild-type mice with or without LPS stimulation. Expression of OSM was also higher, to a lesser extent, in STAT3Mye−/− Kupffer cells than in wild-type cells. Expressions of IL-22 and HGF were comparable between these two types of cells (Fig. 4D) while expression of EGF was undetectable in Kupffer cells (data not shown). In addition, STAT3Mye−/− Kupffer cells produced higher levels of IL-10, TNF-α, and IFN-γ compared to wild-type Kupffer cells with or without LPS stimulation. Finally, IL-6 neutralizing antibody significantly diminished pSTAT3 levels in the liver of STAT3Mye−/− mice (Fig. 4E).
STAT3Hep−/− mice are more susceptible to liver necrosis/apoptosis, but less sensitive to inflammation induced by CCl4 injection
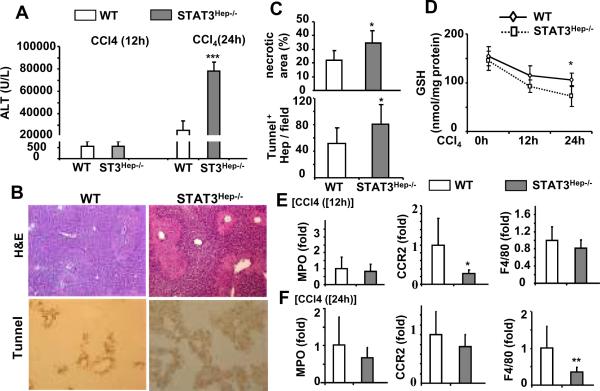
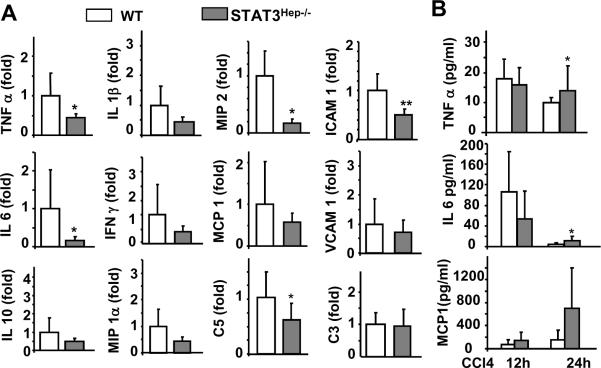
Since STAT3 has been shown to play an important role in hepatoprotection in several murine models of liver injury,23–25 we hypothesized that increased STAT3 activation in the liver of STAT3Mye−/− mice may contribute to the reduced necrosis found in these mice after CCl4 injection. To test this hypothesis, we used hepatocyte-specific STAT3 knockout (STAT3Hep−/−) mice to first examine whether STAT3 in hepatocytes protects against CCl4-induced liver injury. As illustrated in Fig. 5A–C, CCl4 injection induced greater liver damage in STAT3Hep−/− mice than in wild-type mice, as evidenced by increased serum ALT levels, and more severe necrosis and apoptosis. Additionally, injection of CCl4 induced more profound GSH depletion in STAT3Hep−/− mice compared with wild-type mice (Fig. 5D). Although STAT3Hep−/− mice had more liver necrosis, the expression of inflammatory cell markers (eg, CCR2 and F4/80) and pro-inflammatory cytokines (eg, TNF-α, IL-6, MIP-2, and ICAM-1) were lower in these mice compared with wild-type mice (Fig. 5E–F, Fig. 6A). Serum TNF-α and IL-6 levels were slightly higher in STAT3Hep−/− mice than in wild-type mice 24 hours post CCl4 injection (Fig. 6B).
Fig. 5. STAT3Hep−/− mice are more susceptible to liver necrosis/apoptosis, but resistant to inflammation induced by CCl4.
Mice were injected with CCl4 and then sacrificed 12 h and 24 h post injection. A. Serum ALT levels. B. Liver tissues collected for H&E staining and Tunnel staining to determine hepatocyte apoptosis (magnification X200). C. Necrotic area and Tunnel+ hepatocytes in panel B were quantified. D. Hepatic GSH levels. E, F. Liver tissues were collected for real-time PCR analyses for MPO, CCR2, and F4/80 expression. Values represent means±SD (n=5–6 in each group). *P<0.05, **P<0.01, ***P<0.001 in comparison with corresponding WT littermate groups.
Fig. 6. Reduced expression of hepatic cytokines and chemokines in STAT3Hep−/− mice post CCl4 injection.
Mice were injected with CCl4 and then sacrificed 12 h and 24 h. A. Liver tissues were collected for real-time PCR analyses for cytokine and chemokine expression at 12 h post injection. B. Serum cytokines and chemokines. Values represent means±SD (n=5–6 in each group) *P<0.05, **P<0.01, ***P<0.001 in comparison with corresponding WT littermate groups.
Disruption of STAT3 in hepatocytes restores the sensitivity of STAT3Mye−/−mice to CCl4-induced necrosis but reduces CCl4-induced inflammation
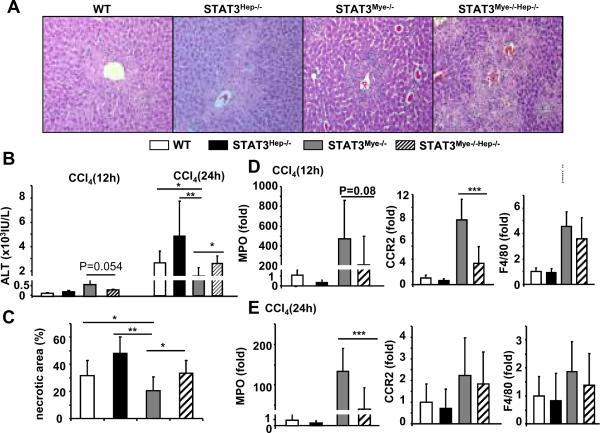
To test the hypothesis that the resistance of STAT3Mye−/− mice to CCl4-induced liver injury is caused by enhanced STAT3 activation in hepatocytes, we deleted STAT3 from the hepatocytes of STAT3Mye−/− mice by generating hepatocyte and macrophage/neutrophil specific double KO (STAT3Mye−/−Hep−/− double KO). Double KO mice showed greater ALT elevation and more necrosis at 24 hours than STAT3Mye−/− mice (Figs. 7A–C). Regarding liver inflammation, double KO mice showed significantly lower expression of MPO and CCR2 at, respectively, 24 and 12 hours post CCl4 injection, compared with STAT3Mye−/− mice, (Figs. 7D and 7E). Expression of F4/80 was comparable between STAT3Mye−/− mice and double KO mice.
Fig. 7. Disruption of STAT3 in hepatocytes restores the sensitivity of STAT3Mye−/− mice to CCl4-induced liver injury.
Four different lines of mice were injected with CCl4 and then sacrificed 12 h and 24 h post injection. A. H&E staining of liver tissues 24 h post CCl4 treatment. B. Serum ALT levels. C. Necrotic area in panel A was quantified. D, E. Real-time PCR analyses for MPO, CCR2, and F4/80 expression 12 h and 24 h after CCl4 injection. Values represent mean±SD (n=7–8 in each group). *P<0.05, **P<0.01, ***P<0.001 in comparison with groups as indicated.
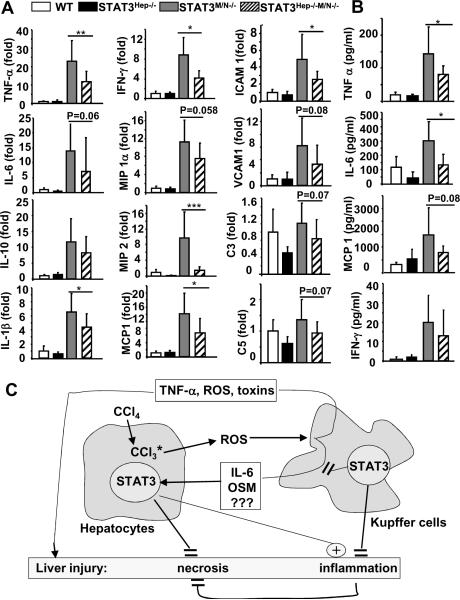
Hepatic expression of several cytokines (TNF-α, IL-1β, IFN-γ) and chemokines (MIP2, MCP-1, ICAM1) and serum levels of pro-inflammatory cytokines (TNF-α and IL-6) were lower in double KO than in STAT3Mye−/− mice 12 hours post CCl4 injection (Fig. 8).
Fig. 8. Disruption of hepatic STAT3 suppresses cytokine and chemokine production in STAT3Mye−/− mice.
Mice were injected with CCl4 and then sacrificed 12 h post injection. A. Real-time PCR analyses of cytokine and chemokine expression in the liver. B. Serum levels of cytokines and chemokines. Values represent means±SD (n=7–8) *P<0.05, **P<0.01, ***P<0.001 denotes significant differences between STAT3Mye−/− and STAT3Mye−/−Hep−/− mice. C. Schematic of the interaction of hepatic and myeloid STAT3 in controlling liver necrosis and inflammation. Activation of Kupffer cells not only produces toxins that cause liver injury and inflammation, but also produces hepatoprotective cytokines (such as IL-6, OSM, and maybe other factors) that activate STAT3 in hepatocytes and ameliorate hepatocellular damage.
Discussion
In this paper, we reported a model of STAT3Mye−/− mice in which inflammation does not correlate with hepatocellular injury induced by CCl4 and whereby inflammation-associated hepatic STAT3 activation reduces liver necrosis (see summarized Fig. 8C). This may help explain a lack of correlation between inflammation and hepatocellular damage (serum ALT levels) observed in some patients with chronic liver diseases.8–13
In our study, injection with CCl4 induced much higher levels of systemic and hepatic inflammation in STAT3Mye−/− mice than in wild-type mice, suggesting that STAT3 in myeloid cells plays an important role in inhibiting inflammation in a model of CCl4-induced liver injury. This is consistent with previously well-documented studies showing that the anti-inflammatory effects of STAT3 in myeloid cells using various models of organ injury.26–28 Surprisingly, despite higher levels of inflammation in STAT3Mye−/− mice, they had much lower serum ALT levels and less liver necrosis than wild-type mice after CCl4 administration. The resistance of STAT3Mye−/− mice to CCl4-induced liver necrosis may be due to either the impaired ability of STAT3-deficient neutrophils/macrophages to induce hepatocellular damage or to the resistance of hepatocytes to CCl4-induced hepatocellular damage in STAT3Mye−/− mice. Several lines of evidence suggest that it is the second mechanism that contributes to reducing liver necrosis in STAT3Mye−/− mice due to enhanced STAT3 activation in the liver (see discussion below).
The hepatotoxicity of CCl4 is mediated by the direct induction of hepatocyte damage and indirect activation of Kupffer cells/macrophages and neutrophils.14 Activated Kupffer cells/macrophages produce free radicals and pro-inflammatory cytokines such as TNF-α that further trigger hepatocellular damage and induce neutrophil accumulation and activation.2, 5 Activated neutrophils can cause hepatocyte damage via releasing oxygen species and proteases.2, 5 We have previously shown that Kupffer cells from STAT3Mye−/− mice produce much higher levels of reactive oxygen species and TNF-α compared with those from wild-type mice.27 By using four different assays, Lee et al previously demonstrated that STAT3-deficient neutrophils mature normally and are functional.29 In the present study we also confirmed that STAT3-deficient neutrophils from STAT3Mye−/− mice are functional in vitro because they produced similar respiratory burst after PMA stimulation compared to PMA-stimulated wild-type mouse neutrophils (Fig. S1c). Moreover, an additional deletion of STAT3 in hepatocytes restored liver necrosis in STAT3Mye−/− mice after CCl4 administration, suggesting that neutrophils from STAT3Mye−/− mice are functional in vivo. Collectively, these findings suggest that STAT3-deficient macrophages and neutrophils in STAT3Mye−/− mice have normal ability to induce inflammatory responses and hepatocellular damage; and that the reduced liver necrosis observed in STAT3Mye−/− mice is not due to a defect in STAT3-deficient macrophages and neutrophils to induce hepatocellular damage.
The hepatoprotective effect of hepatic STAT3 has been well documented in various models of liver injury,23–25 which is further confirmed in our study using an experimental model of CCl4-induced liver damage (Fig. 5). This suggests that elevated liver STAT3 activation in STAT3Mye−/− mice likely contributes to the resistance of these mice to CCl4-induced liver necrosis and oxidative stress. This concept is further supported by conclusive evidence showing that deletion of STAT3 in hepatocytes restores liver necrosis in STAT3Mye−/− mice after CCl4administration (Fig. 7). Interestingly, STAT3Mye−/− mice are resistant to CCl4-induced liver necrosis as demonstrated in the current study but more susceptible to Con A-induced T cell hepatitis despite of enhanced STAT3 activation in the liver (Fig. 2 in Reference 28). This discrepancy could be due to the deletion of STAT3 in myeloid cells preferentially enhancing the Th1 cytokine (IFN-γ) response during Con A-induced T cell hepatitis, dominating over the hepatoprotective effect of STAT3 and resulting in accelerated liver injury in this model.28 Such preferential induction of IFN-γ was not observed in STAT3Mye−/−mice in the CCl4-induced liver injury model (2500 pg/ml serum IFN-γ in Con A model28 vs. 25 pg/ml IFN-γ in CCl4 model in STAT3Mye−/− mice in the current study). In addition, STAT3Mye−/− mice are also more susceptible to chronic ethanol feeding-induced liver inflammation and injury.27 It has been well documented that chronic ethanol consumption inhibits STAT3 activation in the liver.30 Therefore, it is probable that chronic ethanol feeding diminishes hepatic STAT3 activation and abolishes the hepatoprotective effect of STAT3 in STAT3Mye−/− mice, resulting in enhanced liver injury in this alcohol-induced liver injury model.27
We have previously shown that STAT3 activation in hepatocytes promotes liver inflammation in alcohol-induced liver injury.27 Our findings in present studies showed that STAT3 activation in hepatocytes also play a pro-inflammatory role in CCl4-induced liver injury because deletion of STAT3 reduced systemic and hepatic inflammation although enhanced CCl4-induced liver damage (Fig. 5). In addition, an additional deletion of STAT3 in hepatocytes enhanced CCl4-induced liver necrosis but partially counteracted the strong inflammation in STAT3Mye−/− mice after CCl4 administration (Fig. 8). This is probably because deletion of STAT3 in hepatocytes reduced the hepatic expression of several STAT3-controlled inflammatory mediators (such as complement 3/5, IL-1, MIP-2, MCP-1, and ICAM-1) in STAT3Mye−/− mice (Fig. 7). Taken together, these findings suggest that enhanced hepatocellular STAT3 activation in STAT3Mye−/− mice may contribute to not only the reduced liver necrosis but also the enhanced inflammation after CCl4 administration in these mice. In addition, elevated NF-κB activation (Fig. S3) may also contribute to the reduced necrosis in STAT3Mye−/− mice because of the hepatoprotective functions of NFκB.
With respect to what factors are responsible for enhanced STAT3 activation in the liver of STAT3Mye−/− mice (Fig. 4A), many cytokines and growth factors including IL-6, IL-6 family cytokines (such as OSM, IL-11, cardiotrophin-1, CNTF-1, LIF), IL-22, EGF, and HGF have been shown to stimulate STAT3 in the liver.16, 31–33 Here, we provided several lines of evidence suggesting that IL-6 is an important factor responsible for the higher levels of pSTAT3 in the liver of STAT3Mye−/− mice compared to wild-type mice. First, the basal levels of serum and hepatic IL-6 were higher in STAT3Mye−/− mice than in wild-type mice, which is consistent with previous reports.27 Second, Kupffer cells from STAT3Mye−/− mice produced much higher levels of IL-6 than wild-type Kupffer cells (200–500 pg/ml from STAT3Mye−/− vs.10 pg/ml from wild-type mice) (Fig. 4C). Finally, blockage of IL-6 with a neutralizing antibody diminished the basal levels of pSTAT3 in the liver of STAT3Mye−/− mice (Fig. 4E). In addition, OSM may also contribute, to a lesser extent, to the enhanced pSTAT3 in the liver of STAT3Mye−/− mice because Kupffer cells from these mice expressed higher levels of OSM compared to wild-type cells (Fig. 4D).
Clinical implication of this study
In general, it is believed that inflammation plays a key role in contributing to the progression of liver diseases;1–6 however, many studies have reported that inflammation does not always correlate with hepatocellular damage in patients with chronic liver diseases.8–13 Based on the findings from this and other previous studies, we speculate that inflammation associated with a predominance of hepatoprotective cytokines such as IL-6, IL-6-related cytokines, and IL-22 may not correlate with hepatocellular damage, while inflammation with a predominant expression of Th1 cytokines (such as IFN-γ) may be closely associated with liver injury. Indeed, the downstream targets of IFN-γ, such as STAT1 and IP-10, have been shown to correlate with hepatocellular damage in patients with viral hepatitis C infection.34 Thus, understanding the effects of different types of liver inflammation on hepatocellular damage may help us design better strategies to treat patients with chronic liver diseases.
Supplementary Material
Acknowledgement
This work was supported by the intramural program of National Institute on Alcohol Abuse and Alcoholism, NIH. Dr. Norio Horiguchi was supported partially by the Japan Society for the Promotion of Science (JSPS) award at NIH.
Abbreviations
- ALT
alanine transaminase
- CCl4
Carbon tetrachloride
- CCR2
CC chemokine receptor 2
- CNTF
ciliary neurotrophic factor
- GSH
glutathione
- ICAM1
intracellular adhesion molecule 1
- LIF
leukaemia inhibitory factor
- MPO
myeloperoxydase
- OSM
oncostatin M
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- STAT3Hep−/− mice
hepatocyte-specific STAT3 knock out mice
- STAT3Mye−/− mice
myeloid cell (macrophage/neutrophil)-specific STAT3 knockout mice
- STAT3Hep−/−Mye−/− mice
hepatocyte and myeloid cell-specific STAT3 double knockout mice
- WT
wild-type mice
References
- 1.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–350. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 2.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 3.Eksteen B, Afford SC, Wigmore SJ, Holt AP, Adams DH. Immune-mediated liver injury. Semin Liver Dis. 2007;27:351–366. doi: 10.1055/s-2007-991512. [DOI] [PubMed] [Google Scholar]
- 4.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke H, Hasegawa T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006;26:912–919. doi: 10.1111/j.1478-3231.2006.01327.x. [DOI] [PubMed] [Google Scholar]
- 6.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heydtmann M, Adams DH. Chemokines in the immunopathogenesis of hepatitis C infection. Hepatology. 2009;49:676–688. doi: 10.1002/hep.22763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed A, Keeffe EB. Chronic hepatitis C with normal aminotransferase levels. Gastroenterology. 2004;126:1409–1415. doi: 10.1053/j.gastro.2004.02.073. [DOI] [PubMed] [Google Scholar]
- 9.Sanai FM, Benmousa A, Al-Hussaini H, Ashraf S, Alhafi O, Abdo AA, et al. Is serum alanine transaminase level a reliable marker of histological disease in chronic hepatitis C infection? Liver Int. 2008;28:1011–1018. doi: 10.1111/j.1478-3231.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- 10.Luo JC, Hwang SJ, Lai CR, Lu CL, Li CP, Tsay SH, et al. Relationships between serum aminotransferase levels, liver histologies and virological status in patients with chronic hepatitis C in Taiwan. J Gastroenterol Hepatol. 1998;13:685–690. doi: 10.1111/j.1440-1746.1998.tb00714.x. [DOI] [PubMed] [Google Scholar]
- 11.Naito M, Hayashi N, Hagiwara H, Hiramatsu N, Kasahara A, Fusamoto H, et al. Serum hepatitis C virus RNA quantity and histological features of hepatitis C virus carriers with persistently normal ALT levels. Hepatology. 1994;19:871–875. [PubMed] [Google Scholar]
- 12.Mofrad P, Contos MJ, Haque M, Sargeant C, Fisher RA, Luketic VA, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- 13.Fracanzani AL, Valenti L, Bugianesi E, Andreoletti M, Colli A, Vanni E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 14.Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003;189:113–127. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 15.Kovalovich K, DeAngelis RA, Li W, Furth EE, Ciliberto G, Taub R. Increased toxin-induced liver injury and fibrosis in interleukin-6- deficient mice. Hepatology. 2000;31:149–159. doi: 10.1002/hep.510310123. [DOI] [PubMed] [Google Scholar]
- 16.Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2:92–100. [PubMed] [Google Scholar]
- 17.Jin X, Zhang Z, Beer-Stolz D, Zimmers TA, Koniaris LG. Interleukin-6 inhibits oxidative injury and necrosis after extreme liver resection. Hepatology. 2007;46:802–812. doi: 10.1002/hep.21728. [DOI] [PubMed] [Google Scholar]
- 18.Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 19.Camargo CA, Jr., Madden JF, Gao W, Selvan RS, Clavien PA. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology. 1997;26:1513–1520. doi: 10.1002/hep.510260619. [DOI] [PubMed] [Google Scholar]
- 20.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, et al. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607–1615. doi: 10.1002/hep.510280621. [DOI] [PubMed] [Google Scholar]
- 22.Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597–1606. doi: 10.1002/hep.510280620. [DOI] [PubMed] [Google Scholar]
- 23.Klein C, Wustefeld T, Assmus U, Roskams T, Rose-John S, Muller M, et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115:860–869. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, et al. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology. 2007;46:1564–1573. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 27.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lafdil FWH, Park O, Zhang W, Moritoki Y, Yin S, Fu XY, Gershwin ME, Lian ZX, Gao B. Myeloid STAT3 Inhibits T Cell-Mediated Hepatitis by Regulating T Helper 1 Cytokine and Interleukin-17 Production. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.08.004. doi:10.1053/j.gastro.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, et al. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Bao H, Sawyer S, Kunos G, Gao B. Effects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liver. Biochem Biophys Res Commun. 1997;239:666–669. doi: 10.1006/bbrc.1997.7531. [DOI] [PubMed] [Google Scholar]
- 31.Marques JM, Belza I, Holtmann B, Pennica D, Prieto J, Bustos M. Cardiotrophin-1 is an essential factor in the natural defense of the liver against apoptosis. Hepatology. 2007;45:639–648. doi: 10.1002/hep.21508. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635–644. doi: 10.1002/hep.20086. [DOI] [PubMed] [Google Scholar]
- 33.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 34.Reiberger T, Aberle JH, Kundi M, Kohrgruber N, Rieger A, Gangl A, et al. IP-10 correlates with hepatitis C viral load, hepatic inflammation and fibrosis and predicts hepatitis C virus relapse or non-response in HIV-HCV coinfection. Antivir Ther. 2008;13:969–976. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.