Abstract
Objectives To investigate the distribution of month of birth in people with multiple sclerosis in Australia. To use the large regional and seasonal variation in ambient ultraviolet radiation in Australia to explore the association between exposure to ultraviolet radiation during pregnancy and subsequent risk of multiple sclerosis in offspring.
Design Data were gathered on birth month and year (1920-1950), sex, and state of birth for all patients surveyed in 1981 in Queensland, Western Australia, New South Wales (including Australian Capital Territory), South Australia, and Hobart (Tasmania). Population denominators were derived from the 1981 census and supplementary birth registration data. A variable for exposure to ambient ultraviolet radiation “at birth” was generated from monthly averages of daily total ambient ultraviolet radiation for each region. Negative binomial regression models were used to investigate exposure to ambient ultraviolet radiation at birth and at various intervals before birth.
Setting Patient data from multiple sclerosis prevalence surveys carried out in 1981; 1981 Australian census (giving the total number of people born in Australia and still alive and living in Australia in 1981 by year of birth 1920-50); supplementary Australian birth registration data covering the same birth years by month and state.
Participants 1524 patients with multiple sclerosis born in Australia 1920-50 from total population of 2 468 779.
Main outcome measure Cumulative incidence rate of multiple sclerosis.
Results There was a pattern of risk of multiple sclerosis with month of birth (adjusted incidence rate ratio 1.32, 95% confidence interval 1.10 to 1.58, P<0.01, for those born in November-December compared with those born in May-June). This pattern mirrored that previously reported in the northern hemisphere. Region of birth was related to risk. After adjustment for region of birth and other factors, there was an inverse association between ambient ultraviolet radiation in the first trimester and risk of multiple sclerosis (with ≥25 erythemal (skin reddening) dose units as reference (that is, adjusted incidence rate ratio=1.00), the rates were 1.54 (1.10 to 2.16) for 20-<25 units; 1.58 (1.12 to 2.22) for 15-<20 units; 1.65 (1.17 to 2.33) for 10-<15 units; 1.65 (1.18 to 2.29) for 5-<10 units; and 1.67 (1.18 to 2.37) for <5 units). After adjustment for this exposure during early pregnancy, there was no residual association between month of birth and multiple sclerosis.
Conclusion Region of birth and low maternal exposure to ultraviolet radiation in the first trimester are independently associated with subsequent risk of multiple sclerosis in offspring in Australia.
Introduction
Multiple sclerosis is a chronic demyelinating disorder that most commonly presents in the second to fourth decade of life. Studies of migrants indicate that risk is strongly associated with place of residence in early life.1 In Australia2 and elsewhere,3 4 there is a latitudinal gradient with increasing prevalence of multiple sclerosis, or incidence of first demyelinating event, as one moves away from the equator. This latitudinal gradient seems environmentally related because the risk associated with latitude alters if people move after birth.4 5 A strong environmental candidate is the level of ambient regional ultraviolet radiation, acting either directly or through the generation of vitamin D.6 Higher exposure to ultraviolet radiation,7 higher vitamin D intake,8 and also higher serum vitamin D concentrations9 seem to be associated with a reduced risk of onset of multiple sclerosis. This evidence comes from both case-control and cohort studies and indicates that age of operation for such a protective effect might include both childhood and early adulthood.6 7 8 9 10 11
Exposure to ultraviolet radiation in early life has not yet been formally examined but might be linked to excess risk of multiple sclerosis at birth through seasonal deficiency in maternal vitamin D concentrations.12 A study of half siblings with multiple sclerosis has also shown that risk can be maternally mediated.13 Pregnancy is a vulnerable time for vitamin D deficiency because of increased physiological needs and reduced maternal outdoor activity.14 Experimental data on animal fetal development indicate that cerebral white matter is responsive to vitamin D and that oligodendrocytes in the brain and spinal cord have vitamin D receptors.15 16 Furthermore, maternal vitamin D depletion alters neurogenesis in the developing rat brain,17 with subsequent altered gene expression in adult life.18 A recent genetic study in humans has further implicated vitamin D as a strong environmental candidate by showing direct functional interaction with the major locus that determines susceptibility to multiple sclerosis.19 Although human evidence pertaining to fetal development has been difficult to obtain, the body of related evidence to date has led some to recommend antenatal supplementation with vitamin D to prevent multiple sclerosis.14
As an indicator of possible perinatal environmental exposures, such as ultraviolet radiation, individual studies in the northern hemisphere have examined month of birth and risk of multiple sclerosis with varied results.20 21 22 23 24 A large recent pooled analysis of births in the northern hemisphere, however, showed an excess of multiple sclerosis among people born in May and a relative deficit among those born in November; these results were stronger in familial cases and suggested interactions between genes and environment that are related to climate and that might act during gestation or shortly after birth.12 We examined month of birth and risk of multiple sclerosis in Australia. We used the large regional and seasonal variation in ambient ultraviolet radiation across the continent to investigate the association between exposure to ambient ultraviolet radiation during pregnancy and risk of multiple sclerosis among the offspring.
Methods
Case ascertainment
We obtained data on the number of patients with multiple sclerosis born in Australia for each birth month of every (birth) year, 1920-50, by sex and state of birth for Queensland, Western Australia, New South Wales (including Australian Capital Territory), South Australia, and Hobart (Tasmania) from prevalence surveys carried out in 1981.2 25 26 In these surveys, cases were ascertained from hospital records, treating doctors, multiple sclerosis societies, records from the Department of Veterans’ Affairs, and the Australian Bureau of Statistics.2 In the Hobart region the State Chronic Care Hospital Register and Commonwealth Department of Health notifications were also used.26 All patients were interviewed and examined for verification of multiple sclerosis, except in New South Wales, where only 57% of the patients were interviewed and examined because of the large number of patients notified. Almost all of the remaining patients had been examined previously by a neurologist.2 All patients in whom the diagnosis of multiple sclerosis was considered to be correct were classified clinically according to the diagnostic criteria of Rose et al.27
Analysis dataset
We constructed a longitudinal dataset in frequency (or count) format, spanning every (birth) month of every year over the chosen study period, 1920-50, from the original unit record cross sectional 1981 survey data. The constructed dataset comprised numerator data taken directly from the surveyed cases of multiple sclerosis in 1981,2 together with reference population denominators (n=2 468 779) derived from the 1981 Australian census (giving the number of people still alive and living in Australia in 1981 by year of birth and the proportion who were born in Australia) and supplementary data on 1920-50 births registered in Australia by month, year, and state provided by the Australian Bureau of Statistics. We adjusted denominators to account for the main sampling losses from surveyed to unsurveyed state regions between the time of birth and time of survey (1981). A restricted dataset of those people with multiple sclerosis born in Australia between 1920 and 1950 (inclusive) was chosen to minimise problems arising from differential survival (for those born before 1920) or age of onset (for those born after 1950) from use of the whole year of birth range (1897-1969) in the original cross sectional dataset. The resulting dataset of adjusted rates of multiple sclerosis comprised numerator and denominator data by month and by year for 1524 people with multiple sclerosis born in any of the five surveyed states during this 1920-50 period.
We generated a variable for exposure to ambient ultraviolet radiation at birth from monthly averages of daily total ambient effective ultraviolet radiation for each region (table 1).
Table 1.
Monthly averages of daily ambient ultraviolet radiation in minimum erythemal dose units*, 1996-2000, for capital cities of Australian states included in 1981 multiple sclerosis survey (data provided by H P Gies, personal communication) (capital city latitudes shown in decimal degrees in parentheses)
| Brisbane (Qld) (27.5º S) | Perth (WA) (31.9º S) | Sydney (NSW) (33.9º S) | Adelaide (SA) (34.9º S) | Hobart† (Tas) (42.9º S) | |
|---|---|---|---|---|---|
| January | 24.7 | 30.4 | 22.2 | 28.1 | 20.4 |
| February | 22.3 | 27.9 | 21.7 | 24.5 | 18.6 |
| March | 19.0 | 18.9 | 16.2 | 13.7 | 12.4 |
| April | 12.5 | 11.8 | 9.8 | 9.4 | 6.5 |
| May | 8.6 | 7.1 | 5.6 | 5.2 | 3.7 |
| June | 6.6 | 4.9 | 3.9 | 3.6 | 1.7 |
| July | 7.5 | 5.4 | 4.1 | 3.7 | 1.6 |
| August | 10.4 | 7.9 | 6.9 | 6.3 | 3.9 |
| September | 14.6 | 12.1 | 10.4 | 10.0 | 7.0 |
| October | 18.9 | 18.8 | 15.3 | 15.5 | 12.0 |
| November | 22.7 | 23.8 | 19.1 | 21.5 | 15.7 |
| December | 24.4 | 29.2 | 21.9 | 26.1 | 18.1 |
| Seasonal variation‡ | |||||
| Max:min | 3.7 | 6.2 | 5.7 | 7.8 | 12.8 |
*Minimum erythemal dose is measure of ultraviolet radiation exposure required to induce erythema or sunburn.28
†1991 data.28
‡Seasonal variation as given by ratio of summer maximum to winter minimum ultraviolet radiation.
Statistical analysis
Our main outcome measure was the number of people with multiple sclerosis born in each month relative to the general population, expressed as a cumulative incidence rate of multiple sclerosis.
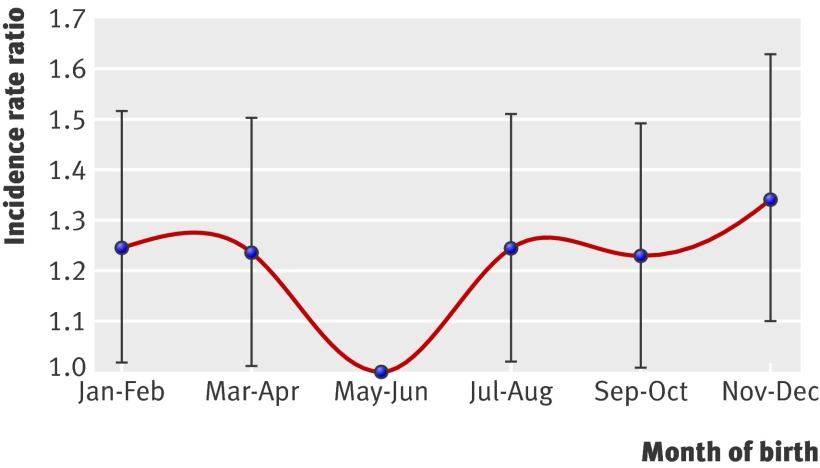
We used negative binomial regression models to provide an estimate of the incidence rate for each month of birth, expressed as an incidence rate ratio for each time period relative to a single reference period. Because of the small case numbers for the month of birth analysis, we collapsed months into two monthly periods (fig 1). May-June was the reference period because the average ambient ultraviolet radiation was generally lowest then.
Fig 1 Risk of multiple sclerosis for each two month period of birth. May-June is reference period. July and January represent southern hemisphere mid-winter and mid-summer, respectively
To examine the associations between levels of ambient ultraviolet radiation and multiple sclerosis, we modelled ambient ultraviolet radiation as a continuous variable against incidence of multiple sclerosis. Monthly averages were lagged a number of months so that they then expressed the ambient ultraviolet radiation pertaining to a particular length of time before birth for each individual and for each region of birth. Nine such (continuous) variables, representing one to nine months before the birth month of an individual, were generated and included in separate negative binomial regression models (see table 2), as previously done for the categorical variable for period of birth. We thus assessed different gestational periods, in terms of the associated month and region specific levels of ultraviolet radiation, in relation to risk of multiple sclerosis. P values assessing effect modification were derived from likelihood ratio tests of nested regression models with and without the relevant interaction term. Analyses were conducted with Stata 8.0.
Results
There was a large variation in average total daily ambient ultraviolet radiation, from 1.6 minimum erythemal dose units/day in Hobart, Tasmania in July to 30.4 units/day in Perth, Western Australia in January. Overall, the seasonal variation in total daily ambient ultraviolet radiation increased with increasing latitude from Brisbane, Queensland at lowest south latitude to Hobart, Tasmania at highest latitude (table 1).
For year of birth from 1920 to 1950, we identified 1524 cases in the prevalence study from a denominator population of 2 468 779. As expected from the previous Australian surveys,2 the incidence rate of multiple sclerosis was higher among women than men (incidence rate ratio 2.28, 95% confidence interval 2.03 to 2.55). There was also a latitudinal gradient in cumulative incidence rate by region of birth. Compared with the reference birth region, New South Wales/Australian Capital Territory, the risk was lower for those born in Queensland (0.59, 0.51 to 0.69) and higher for those born in Tasmania (2.70, 2.06 to 3.51). The capital cities of these northernmost and southernmost states are located at 27.5º south and 42.9º south, respectively (table 1).
Figure 1 shows the pattern of risk for multiple sclerosis for each two month period of birth, expressed as an incidence rate ratio for each period of birth relative to the reference incidence rate ratio (1.0) for May-June. The risk was 1.23-fold to 1.34-fold higher (P<0.05) for people born in all periods other than May-June; the highest magnitude of risk was for those born in the early summer months of November-December compared with the early winter months of May-June (1.34, 1.10 to 1.63; P<0.01). This pattern of month of birth persisted after adjustment for sex, age, and region of birth in Australia (1.32, 1.10 to 1.58; P<0.01, for November-December compared with May-June). We also examined whether the November-December to May-June risk ratio differed by region of birth; this ratio of around 1.3 was the same over the Queensland-Tasmania range, with no effect modification by region of birth (P=0.25).
We then examined the role of prenatal exposure to ambient ultraviolet radiation. We found no association between daily ambient ultraviolet radiation at the time of birth and subsequent risk of multiple sclerosis (table 2). Similarly, lags of one to four months before birth (late second to third trimesters) were not informative. For lags of five to nine months (first to early second trimesters), however, there were inverse associations between prenatal ambient ultraviolet radiation levels and multiple sclerosis (unadjusted incidence rate ratio ranging from 0.74 (0.63 to 0.85) to 0.81 (0.70 to 0.94); P<0.01). Table 2 summarises these results and shows a strong inverse association for the first trimester (0.72, 0.62 to 0.84), P<0.001).
Table 2.
Risk of multiple sclerosis as unadjusted and adjusted incidence rate ratio for ambient ultraviolet radiation at and before time of birth
| Ambient ultraviolet radiation* | Unadjusted | Adjusted† | |||
|---|---|---|---|---|---|
| Ratio (95% CI) | P value | Ratio (95% CI) | P value | ||
| At birth | 1.01 (0.87 to 1.17) | 0.906 | 1.01 (0.88 to 1.16) | 0.851 | |
| Time before birth (months): | |||||
| 1 | 1.03 (0.89 to 1.19) | 0.706 | 1.03 (0.90 to 1.19) | 0.630 | |
| 2 | 1.00 (0.86 to 1.16) | 0.991 | 1.01 (0.88 to 1.16) | 0.894 | |
| 3 | 0.94 (0.81 to 1.10) | 0.448 | 0.96 (0.83 to 1.10) | 0.525 | |
| 4 | 0.88 (0.76 to 1.02) | 0.100 | 0.89 (0.78 to 1.03) | 0.117 | |
| 5 | 0.81 (0.70 to 0.94) | 0.007 | 0.83 (0.72 to 0.95) | 0.007 | |
| 6 | 0.77 (0.67 to 0.90) | 0.001 | 0.78 (0.68 to 0.90) | 0.001 | |
| 7 | 0.74 (0.63 to 0.85) | <0.001 | 0.75 (0.65 to 0.86) | <0.001 | |
| 8 | 0.74 (0.63 to 0.85) | <0.001 | 0.75 (0.65 to 0.86) | <0.001 | |
| 9 | 0.79 (0.68 to 0.92) | 0.002 | 0.80 (0.70 to 0.92) | 0.002 | |
| First trimester | 0.72 (0.62 to 0.84) | <0.001 | 0.73 (0.63 to 0.84) | <0.001 | |
*Based on composite month and region specific values for each individual (see table 1) and expressed in units of 20 minimum erythemal dose units/day to gain meaningful incidence rate ratio in terms of difference in minimum erythemal dose between summer and winter (20 minimum erythemal dose units/day is approximate average difference between summer and winter ultraviolet radiation levels for Australian state regions, table 1). First trimester ultraviolet radiation variable obtained by averaging monthly values for seven and eight months before birth, for each region.
†Adjusted for age (year of birth) and sex.
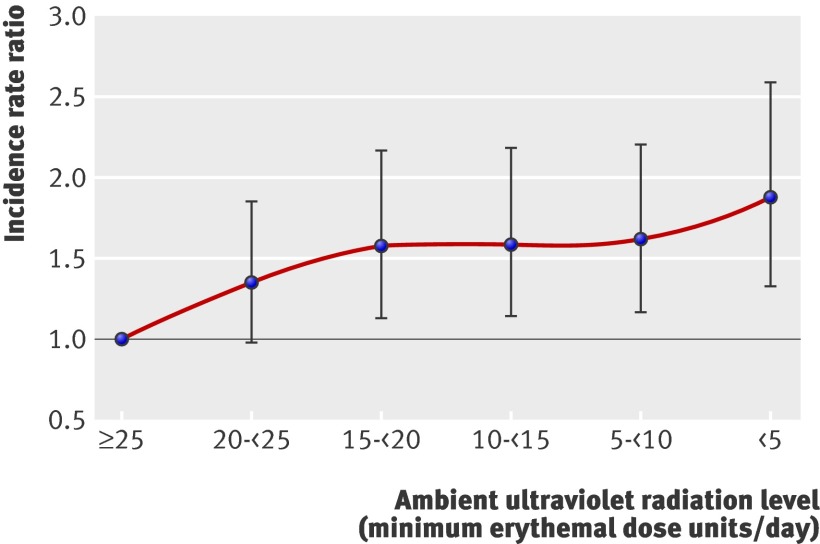
We examined the shape of the association between inversed first trimester exposure and risk of multiple sclerosis in greater detail by using six categories of ultraviolet radiation (in minimum erythemal dose units/day, see table 1) and comparing each of the five lowest levels with the highest (table 3). The association was non-linear with a particular increase in risk for levels below a monthly average of 20 minimum erythemal dose units/day (fig 2). This “threshold” level is reached only in January in Hobart, Tasmania, but from November to February in Brisbane, Queensland.
Table 3.
Risk of multiple sclerosis as incidence rate ratio for six levels of ambient ultraviolet radiation during first trimester, with and without other factors
| Factor | Unadjusted | Adjusted* | |||
|---|---|---|---|---|---|
| Ratio (95% CI) | P value | Ratio (95% CI) | P value | ||
| Ambient ultraviolet radiation exposure in first trimester† (minimum erythemal doses/day): | |||||
| ≥25 | 1.00 (reference) | — | 1.00 (reference) | — | |
| 20-<25 | 1.35 (0.97 to 1.87) | 0.071 | 1.54 (1.10 to 2.16) | 0.013 | |
| 15-<20 | 1.58 (1.14 to 2.20) | 0.007 | 1.58 (1.12 to 2.22) | 0.009 | |
| 10-<15 | 1.58 (1.13 to 2.20) | 0.008 | 1.65 (1.17 to 2.33) | 0.004 | |
| 5-<10 | 1.62 (1.17 to 2.23) | 0.004 | 1.65 (1.18 to 2.29) | 0.003 | |
| <5 | 1.90 (1.35 to 2.67) | <0.001 | 1.67 (1.18 to 2.37) | 0.004 | |
| Sex: | |||||
| Men | 1.00 (reference) | — | 1.00 (reference) | — | |
| Women | 2.28 (2.03 to 2.55) | <0.001 | 2.27 (2.03 to 2.53) | <0.001 | |
| Region of birth (latitude of capital city, degrees south): | |||||
| Queensland (27.5º) | 0.59 (0.51 to 0.69) | <0.001 | 0.60 (0.52 to 0.70) | <0.001 | |
| Western Australia (31.9º) | 0.76 (0.62 to 0.92) | 0.005 | 0.85 (0.70 to 1.04) | 0.113 | |
| New South Wales/Australian Capital Territory (33.9º) | 1.00 (reference) | — | 1.00 (reference) | — | |
| South Australia (34.9º) | 1.03 (0.88 to 1.21) | 0.723 | 1.10 (0.94 to 1.29) | 0.237 | |
| Tasmania (42.9º) | 2.70 (2.06 to 3.51) | <0.001 | 2.71 (2.08 to 3.52) | <0.001 | |
| Decade of birth: | |||||
| 1920-29 | 1.09 (0.95 to 1.24) | 0.222 | 1.06 (0.94 to 1.20) | 0.346 | |
| 1930-40 | 1.00 (reference) | — | 1.00 (reference) | — | |
| 1941-50 | 0.73 (0.64 to 0.83) | <0.001 | 0.73 (0.64 to 0.82) | <0.001 | |
*Adjusted for all other factors in multivariate model—for example, ultraviolet radiation in first trimester adjusted for sex, region of birth, and decade of birth.
†Ambient ultraviolet radiation in first trimester based on composite month and region specific values for each individual (see table 1).
Fig 2 Risk of multiple sclerosis by average level of daily ambient ultraviolet radiation during first trimester. Incidence rate ratio adjusted for age (year of birth) and sex
Table 3 also shows the increase in risk for women and people born at higher latitudes; the result for decade of birth, showing a deficit in more recently born affected people, probably reflects ascertainment because for some in this group onset of multiple sclerosis might not have occurred by the time of the 1981 survey. The association between first trimester ultraviolet radiation and multiple sclerosis persisted, however, after adjustment for region of birth and the other factors listed in table 3.
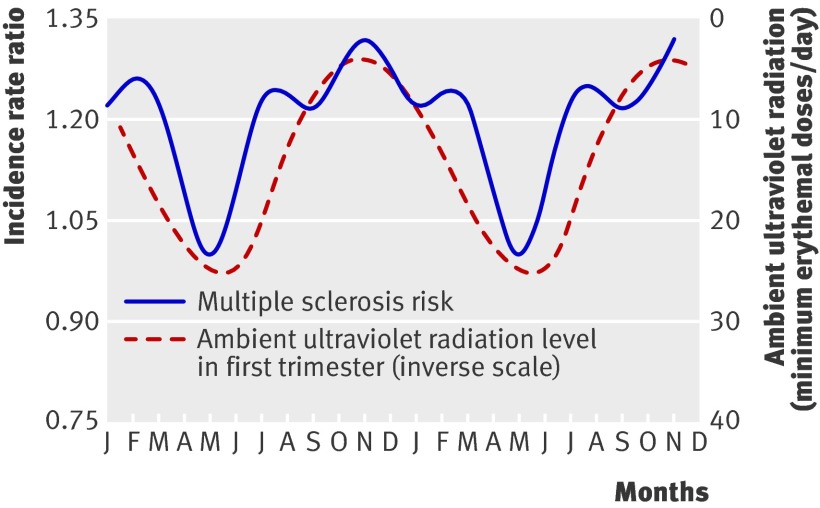
We examined the association between prenatal exposure to ultraviolet radiation and risk of multiple sclerosis related to month of birth. Figure 3 shows the inverse relation between first trimester ultraviolet radiation and risk related to months of birth. That is, when we lagged the ultraviolet radiation values by seven to eight months, there was overall similarity between the two curves, such that infants experiencing low levels of ultraviolet radiation in the first trimester are associated with a higher risk of multiple sclerosis by birth month.
Fig 3 Multiple sclerosis risk by two month periods of birth with monthly averages of daily ambient ultraviolet radiation in first trimester of pregnancy on inverse scale. Time interval is two annual cycles
After adjustment for ultraviolet radiation in the first trimester, we found no residual association between period of birth and risk of multiple sclerosis. That is, once we accounted for first trimester ultraviolet radiation (as in table 3) there was no improvement in model fit by also including variation in period of birth (likelihood ratio χ2 (5 df) =3.79; P=0.58). In contrast, region of birth remained significantly associated with risk of multiple sclerosis even after adjustment for ultraviolet radiation in the first trimester (table 3).
There was no effect modification of the association between ultraviolet radiation in the first trimester and multiple sclerosis by sex (P=0.71) or region of birth (P=0.80).
Discussion
In the southern hemisphere there is a relative excess of multiple sclerosis in people born in November-December compared with the May-June reference minimum. This pattern is consistent with the pattern reported by Willer et al in their larger study in the northern hemisphere,12 given that the seasons are reversed. Our results show a trough in multiple sclerosis in people born in May-June, when a protective effect is evident, compared with a peak in those born in November-December, thus mirroring the northern hemisphere pattern of a peak associated with May births and a deficit associated with November births. We found no interaction between this pattern and region of birth within Australia, nor with sex or decade of birth; this was also consistent with findings of Willer et al for region of ascertainment, sex, and decade of birth in Canada.12
The higher risk of multiple sclerosis for people born in November-December is consistent with these infants having experienced lower levels of ultraviolet radiation during the first trimester. In fact, the pattern of month of birth in the southern hemisphere was accounted for by the month and region specific ambient ultraviolet radiation during the first trimester—that is, the effect of month of birth did not persist after adjustment for first trimester ultraviolet radiation. Lower average daily levels of ambient ultraviolet radiation during the first trimester predicted a higher subsequent risk of multiple sclerosis independently of month of birth. This association was non-linear, with a particular increase in risk under the (monthly average) level of 20 units of minimum erythemal dose of daily ambient ultraviolet radiation. We have thus shown an inverse association between low ultraviolet radiation in the first trimester and increased risk of multiple sclerosis in the offspring.
Strengths and weaknesses
In this longitudinal study we used a prospective exposure—prenatal ambient ultraviolet radiation—the levels of which were heterogeneously distributed among the study participants because of the large variation in ultraviolet radiation linked to month and region of birth across Australia. A further strength is the standardised case ascertainment of multiple sclerosis from the national study in 1981,2 even though this study was smaller than the extensive northern hemisphere analysis by Willer et al12 because of the much smaller population in Australia.
We could not examine the vitamin D status of the fetus directly but instead chose the ambient ultraviolet radiation level experienced by the mother during gestation. This proxy has some limitations in that it does not take into account individual personal behaviour, concurrent dietary vitamin D intake, or skin pigmentation. These omissions, however, would probably obscure rather than create the patterns observed. Recent work by Sayers et al also indicates that ambient erythemal ultraviolet radiation levels during pregnancy, even in a single location, can be used to indicate vitamin D status.29 Further work could confirm the timing of the observed effect of prenatal ultraviolet radiation.
Other considerations
Prenatal exposure to ambient ultraviolet radiation during the first trimester was probably not just a marker for postnatal exposure because the association had temporal specificity and was not evident for exposure at the time of birth. Furthermore, the association was independent of region of birth, a probable marker for postnatal sun exposure correlated with long term residence.
Here, and in the larger northern hemisphere analysis,12 the pattern of month of birth was not smoothly sinusoidal but had a few months of particularly altered risk of multiple sclerosis. This would support an underlying seasonal factor that was not altering smoothly throughout the year but was more evident in particular months or was a threshold biological effect of a continuous variable. Consistent with this, when we examined different levels of prenatal exposure to ultraviolet radiation in the first trimester, we clearly observed that risk was specifically increased in the lower levels of exposure, below a monthly average of 20 minimum erythemal dose units a day. We could not, however, control for other factors such as nutrition or physical activity that could be associated with prenatal exposure to ultraviolet radiation in the first trimester and that could also determine risk of multiple sclerosis. In this setting, there is strong a priori evidence that sun exposure or vitamin D, or both, is likely to be the central exposure6 and little a priori evidence for any other strong determinant of multiple sclerosis likely to be linked to maternal ambient ultraviolet radiation exposure during the first trimester.
Maternal exposure and health of offspring
This report adds to other work showing that maternal exposure to ambient ultraviolet radiation during pregnancy might influence subsequent health in the offspring.30 31 32 33 In a New Zealand birth cohort, infants whose mothers were exposed to peak sunshine during the first trimester were significantly heavier at birth than infants whose mothers experienced trough levels of sunshine during the same trimester.30 In the United Kingdom, maternal ambient ultraviolet B exposure in the third trimester has been positively related to bone mineral density and content at age 9 in offspring.34 There is now growing interest in the role of maternal vitamin D deficiency in pregnancy and the development of central nervous system and immune disorders, particularly schizophrenia,33 type 1 diabetes, and other disorders.35 Although active vitamin D3 concentrations in the mother increase substantially during pregnancy, early fetal supplies are lower36 and directly dependent on the mother. By the time of delivery, maternal and infant cord serum 25-hydroxyvitamin D3 concentrations are highly correlated.37 Unfortunately, much remains unknown, leading to large international variations in maternal vitamin D monitoring and supplementation during pregnancy.35 38 39
A maternal effect operating antenatally would also be consistent with the stronger maternal than paternal “parent of origin” effect in familial multiple sclerosis.13 40 It has been previously proposed14 that maternal vitamin D deficiency, a problem for some dark skinned women migrating to regions with low ambient ultraviolet radiation, such as the UK, might explain the increase in incidence of disease seen among second generation migrants in such locations.41 Our findings are consistent with this explanation. Because season of birth has previously also been related to the clinical course of multiple sclerosis,42 43 44 it is possible that early life exposures determine not only onset of disease but also resistance to the demyelinating process of multiple sclerosis. The mechanisms involved could include neurological or immunological factors. Ultraviolet radiation exposure during the first trimester would be expected to specifically influence vitamin D concentrations up to early in the second trimester, given that vitamin D has a half life of one to two months.45 In recent Australian work, higher levels of ambient ultraviolet radiation were associated with higher vitamin D concentrations at the population level with a lag of one and a half months.46
Possible mechanisms
First trimester vitamin D concentrations might be particularly important in the development of the central nervous system because during early embryonic development, vitamin D receptors are expressed in the neuroepithelium and later in the subventricular zone.15 Myelination occurs later; even in mid-gestation (19-24 weeks) cortical axonal tracts are not yet myelinated,47 with major myelination of several areas occurring as late as 29-39 weeks.48 In addition, the in utero development of immune central tolerance occurs in the first trimester and vitamin D has immunomodulating properties. The first trimester is also a sensitive period with regard to prenatal thymocyte differentiation, with animal studies showing that chemicals such as dioxin can alter this process, disrupt the development of central tolerance, and lead to increased auto-reactive peripheral T cells.49 Furthermore, indirect effects of vitamin D should be considered. For example, vitamin D can down-regulate interleukin 6, an important mediator of the adverse effect of maternal infection during pregnancy on neural development in the fetus.50 51 52
Prenatal and postnatal timing
Our results do not indicate that the possible beneficial effect of ultraviolet radiation exposure is confined only to the prenatal period. The finding that both first trimester ultraviolet radiation and region of birth were independent predictors of risk of multiple sclerosis is consistent with birth region also acting as an indicator of postnatal exposure to ultraviolet radiation. Overall, epidemiological studies support a protective role for vitamin D in autoimmune disease, particularly in childhood and adolescence, and vitamin D supplementation in early adulthood effectively reduces the risk of multiple sclerosis; therefore, supplementation of adolescents and young adults could be effectively used for prevention.6 The findings here provide the first population based evidence beyond month of birth patterns to indicate that vitamin D supplementation for the prevention of multiple sclerosis might also need to be considered during in utero development.14 They are consistent with a multi-hit causal cascade for multiple sclerosis, with putative adverse environmental factors, such as low vitamin D concentrations, acting at more than one stage of life.53
What is already known on this topic
In the northern hemisphere, there are more cases of multiple sclerosis in people born in May and fewer in those born in November
Maternal exposure to ambient ultraviolet radiation in pregnancy can be used as an instrumental variable for vitamin D status during pregnancy
Low vitamin D concentrations have been associated with a higher risk of multiple sclerosis
What this study adds
In Australia there is a reciprocal pattern of month of birth and risk of multiple sclerosis, with a higher risk for those born in November-December compared with May-June
This pattern was accounted for by maternal exposure to ambient ultraviolet radiation in the first trimester
Low maternal exposure to ultraviolet radiation in the first trimester was inversely related to risk of multiple sclerosis in the offspring after adjustment for either month of birth or place of birth
We gratefully acknowledge the assistance of J G McLeod, S R Hammond, and P Macaskill in providing case data and advice on the conduct of the original 1981 national multiple sclerosis survey. H P Gies, Australian Radiation Protection and Nuclear Safety Agency (ARPANSA), kindly provided the regional ultraviolet radiation data for the Australian State capital cities for 1996-2000. We also thank A J McMichael and J G McLeod for their comments on the manuscript and H Raschella and A Pezic for literature review and manuscript preparation.
Contributors: All authors were involved in the study design and analysis, participated in the preparation and writing of the manuscript, and approved the final version. JS is guarantor.
Funding: The research was supported by an Australian National University (ANU) Graduate School scholarship and a supplementary scholarship from the National Centre for Epidemiology and Population Health, ANU, awarded to JS.
Competing interests: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) JS, ALP, LL have no relationships with companies that might have an interest in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) JS, ALP, LL have no non-financial interests that may be relevant to the submitted work.
Ethical approval: This study was approved by the human research ethics committee, Australian National University.
Data sharing: No additional data available.
Cite this as: BMJ 2010;340:c1640
References
- 1.Dean G. Annual incidence, prevalence, and mortality of multiple sclerosis in white South-African-born and in white immigrants to South Africa. BMJ 1967;ii:724-30. [DOI] [PMC free article] [PubMed]
- 2.McLeod JG, Hammond SR, Hallpike JF. Epidemiology of multiple sclerosis in Australia. With NSW and SA survey results. Med J Aust 1994;160:117-22. [PubMed] [Google Scholar]
- 3.Alter M. Epidemiology of multiple sclerosis in Israel. In: Alter M, Kurtzke JF, eds. The epidemiology of multiple sclerosis. Charles C Thomas, 1968:83-109.
- 4.Kurtzke JF, Beebe GW, Norman JE Jr. Epidemiology of multiple sclerosis in US veterans: III. Migration and the risk of MS. Neurology 1985;35:672-8. [DOI] [PubMed] [Google Scholar]
- 5.Hammond SR, English DR, McLeod JG. The age-range of risk of developing multiple sclerosis: evidence from a migrant population in Australia. Brain 2000;123:968-74. [DOI] [PubMed] [Google Scholar]
- 6.Ascherio A, Munger K. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol 2008;28:17-28. [DOI] [PubMed] [Google Scholar]
- 7.Van der Mei IA, Ponsonby AL, Dwyer T, Blizzard L, Simmons R, Taylor BV, et al. Past exposure to sun, skin phenotype, and risk of multiple sclerosis: case-control study. BMJ 2003;327:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology 2004;62:60-5. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832-8. [DOI] [PubMed] [Google Scholar]
- 10.Islam T, Gauderman WJ, Cozen W, Mack TM. Childhood sun exposure influences risk of multiple sclerosis in monozygotic twins. Neurology 2007;69:381-8. [DOI] [PubMed] [Google Scholar]
- 11.Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol 2007;254:471-7. [DOI] [PubMed] [Google Scholar]
- 12.Willer CJ, Dyment DA, Sadovnick AD, Rothwell PM, Murray TJ, Ebers GC. Timing of birth and risk of multiple sclerosis: population based study. BMJ 2005;330:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebers GC, Sadovnick AD, Dyment DA, Yee IML, Willer CJ, Risch N. Parent of origin effect in multiple sclerosis: observations in half siblings. Lancet 2004;363:1773-4. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhuri A. Why we should offer routine vitamin D supplementation in pregnancy and childhood to prevent multiple sclerosis. Med Hypotheses 2005;64:608-18. [DOI] [PubMed] [Google Scholar]
- 15.Wion D, MacGrogan D, Neveu I, Jehan F, Houlgatte R, Brachet P. 1,25-Dihydroxyvitamin D3 is a potent inducer of nerve growth factor synthesis. J Neurosci Res 1991;28:110-4. [DOI] [PubMed] [Google Scholar]
- 16.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100-5. [DOI] [PubMed] [Google Scholar]
- 17.Cui X, McGrath JJ, Burne TH, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci 2007;25:227-32. [DOI] [PubMed] [Google Scholar]
- 18.Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007;103:538-45. [DOI] [PubMed] [Google Scholar]
- 19.Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton S-M, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PLoS Genet 2009;5:e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Templer DI, Trent NH, Spencer DA, Trent A, Corgiat MD, Mortensen PB, et al. Season of birth in multiple sclerosis. Acta Neurol Scand 1992;85:107-9. [DOI] [PubMed] [Google Scholar]
- 21.Wiberg M. Season of birth in multiple sclerosis in Sweden: replication of Denmark findings. J Orthomol Med 1994;9:71-4. [Google Scholar]
- 22.Sadovnick AD, Yee IM. Season of birth in multiple sclerosis. Acta Neurol Scand 1994;89:190-1. [DOI] [PubMed] [Google Scholar]
- 23.Salemi G, Ragonese P, Aridon P, Reggio A, Nicoletti A, Buffa D, et al. Is season of birth associated with multiple sclerosis? Acta Neurol Scand 2000;101:381-3. [DOI] [PubMed] [Google Scholar]
- 24.Torrey EF, Miller J, Rawlings R, Yolken RH. Seasonal birth patterns of neurological disorders. Neuroepidemiology 2000;19:177-85. [DOI] [PubMed] [Google Scholar]
- 25.Hammond SR, de Wytt C, Maxwell IC, Landy PJ, English D, McLeod JG, et al. The epidemiology of multiple sclerosis in Queensland, Australia. J Neurol Sci 1987;80:185-204. [DOI] [PubMed] [Google Scholar]
- 26.Hammond SR, McLeod JG, Millingen KS, Stewart-Wynne EG, English D, Holland JT, et al. The epidemiology of multiple sclerosis in three Australian cities: Perth, Newcastle and Hobart. Brain 1988;111:1-25. [DOI] [PubMed] [Google Scholar]
- 27.Rose AS, Ellison GW, Myers LW, Tourtellotte WW. Criteria for the clinical diagnosis of multiple sclerosis. Neurology 1976;26:20-2. [DOI] [PubMed] [Google Scholar]
- 28.Gies HP, Roy CR, Toomey S, Tomlinson D. The ARL solar UVR measurement network: calibration and results. SPIE 1994;2282:274-284. [Google Scholar]
- 29.Sayers A, Tilling K, Boucher BJ, Noonan K, Tobias JH. Predicting ambient ultraviolet from routine meteorological data; its potential use as an instrumental variable for vitamin D status in pregnancy in a longitudinal birth cohort in the UK. Int J Epidemiol 2009;38:1681-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tustin K, Gross J, Hayne H. Maternal exposure to first-trimester sunshine is associated with increased birth weight in human infants. Dev Psychobiol 2004;45:221-30. [DOI] [PubMed] [Google Scholar]
- 31.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat 2005;29:21-30. [DOI] [PubMed] [Google Scholar]
- 32.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641-53. [DOI] [PubMed] [Google Scholar]
- 33.McGrath JJ, Feron FP, Burne TH, Mackay-Sim A, Eyles DW. Vitamin D3-implications for brain development. J Steroid Biochem Mol Biol 2004;89-90:557-60. [DOI] [PubMed]
- 34.Javaid MK, Crozier SR, Harvey NC, Gale CR, Dennison EM, Boucher BJ, et al. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 2006;367:36-43. [DOI] [PubMed] [Google Scholar]
- 35.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev 2008;66:710-20. [DOI] [PubMed] [Google Scholar]
- 36.Delvin EE, Glorieux FH, Salle BL, David L, Varenne JP. Control of vitamin D metabolism in preterm infants: feto-maternal relationships. Arch Dis Child 1982;57:754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maghbooli Z, Hossein-Nezhad A, Shafaei AR, Karimi F, Madani FS, Larijani B. Vitamin D status in mothers and their newborns in Iran. BMC Pregnancy Childbirth 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Munns C, Zacharin MR, Rodda CP, Batch JA, Morley R, Cranswick NE, et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: a consensus statement. Med J Aust 2006;185:268-72. [DOI] [PubMed] [Google Scholar]
- 39.Vitamin D supplementation: recommendations for Canadian mothers and infants. Paediatr Child Health 2007;12:583-98. [PMC free article] [PubMed] [Google Scholar]
- 40.Herrera BM, Ramagopalan SV, Lincoln MR, Orton SM, Chao MJ, Sadovnick AD, et al. Parent-of-origin effects in multiple sclerosis: observations from avuncular pairs. Neurology 2008;71:799-803. [DOI] [PubMed] [Google Scholar]
- 41.Datta S, Alfaham M, Davies DP, Dunstan F, Woodhead S, Evans J, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population—an interventional study. BJOG 2002;109:905-8. [DOI] [PubMed] [Google Scholar]
- 42.Tremlett HL, Devonshire VA. Does the season or month of birth influence disease progression in multiple sclerosis? Neuroepidemiology 2006;26:195-8. [DOI] [PubMed] [Google Scholar]
- 43.Koch M, De Keyser J, Tremlett H. Timing of birth and disease progression in multiple sclerosis. Mult Scler 2008;14:793-8. [DOI] [PubMed] [Google Scholar]
- 44.Sadovnick AD, Duquette P, Herrera B, Yee IM, Ebers GC. A timing-of-birth effect on multiple sclerosis clinical phenotype. Neurology 2007;69:60-2. [DOI] [PubMed] [Google Scholar]
- 45.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999;69:842-56. [DOI] [PubMed] [Google Scholar]
- 46.Tremlett H, van der Mei IA, Pittas F, Blizzard L, Paley G, Mesaros D, et al. Monthly ambient sunlight, infections and relapse rates in multiple sclerosis. Neuroepidemiology 2008;31:271-9. [DOI] [PubMed] [Google Scholar]
- 47.Jakovcevski I, Mo Z, Zecevic N. Down-regulation of the axonal polysialic acid-neural cell adhesion molecule expression coincides with the onset of myelination in the human fetal forebrain. Neuroscience 2007;149:328-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abe S, Takagi K, Yamamoto T, Okuhata Y, Kato T. Semiquantitative assessment of myelination using magnetic resonance imaging in normal fetal brains. Prenat Diagn 2004;24:352-7. [DOI] [PubMed] [Google Scholar]
- 49.Gogal RM Jr, Holladay SD. Perinatal TCDD exposure and the adult onset of autoimmune disease. J Immunotoxicol 2008;5:413-8. [DOI] [PubMed] [Google Scholar]
- 50.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist 2007;13:241-56. [DOI] [PubMed] [Google Scholar]
- 51.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci 2007;27:10695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 2009;132:1146-60. [DOI] [PubMed] [Google Scholar]
- 53.Goodin DS. The causal cascade to multiple sclerosis: a model for multiple sclerosis pathogenesis. PLoS One 2009;4:e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]