Abstract
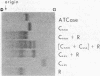
Succinylation of the catalytic subunits of ATCase yielded a relatively homogeneous, inactive electrophoretic variant which upon mixing with native regulatory subunits formed a complex the size of the native enzyme. Hybridization experiments with mixtures of this variant and the native catalytic subunits in the presence of excess regulatory subunits yielded three different molecular complexes which were separated and individually characterized. The number and properties of the various components indicated that each ATCase molecule contains two catalytic subunits. Hybridization was also effected at the intrasubunit level by dissociation and reconstitution of mixtures of the native and modified catalytic subunits. These experiments produced four components showing thereby that each catalytic subunit is composed of three polypeptide chains. The potential use of the various hybrids is discussed in relation to the unique properties manifested by regulatory enzymes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Changeux J. P., Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry. 1968 Feb;7(2):531–538. doi: 10.1021/bi00842a007. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Holoubek H. The purification of aspartate transcarbamylase of Escherichia coli and separation of its protein subunits. J Biol Chem. 1967 Jun 25;242(12):2886–2892. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Hervé G. L., Stark G. R. Aspartate transcarbamylase. Amino-terminal analyses and peptide maps of the subunits. Biochemistry. 1967 Dec;6(12):3743–3747. doi: 10.1021/bi00864a017. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem. 1956 Aug;221(2):757–770. [PubMed] [Google Scholar]
- Porter R. W., Modebe M. O., Stark G. R. Aspartate transcarbamylase. Kinetic studies of the catalytic subunit. J Biol Chem. 1969 Apr 10;244(7):1846–1859. [PubMed] [Google Scholar]
- Steitz T. A., Wiley D. C., Lipscomb W. N. The structure of aspartate transcarbamylase, I. A molecular twofold axis in the complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1859–1861. doi: 10.1073/pnas.58.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K. Aspartate transcarbamylase from Escherichia coli. Characterization of the polypeptide chains by molecular weight, amino acid composition, and amino-terminal residues. J Biol Chem. 1968 Feb 10;243(3):543–546. [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]