Abstract
A pair of recent studies has reopened debate on the subject of phase separations in model bilayer mixtures of cholesterol (Chol) and dipalmitoyl-phosphatidylcholine (DPPC). Fluorescence microscopy methods have not been able to detect phase separations in binary DPPC-Chol mixtures that have been inferred from NMR studies. However, micron-scale phase-separated liquid domains are observed by fluorescence in ternary mixtures of DPPC, Chol, and diphytanoyl-phosphatidylcholine (DiPhyPC). Here, a model of condensed complexes of Chol and DPPC is used to account for these results. In particular, it is shown that the orientation of tie-lines in ternary mixtures of DiPhyPC/DPPC/Chol is compatible with phase separation in binary DPPC/Chol mixtures.
Mixtures of cholesterol (Chol) and phospholipids have long been studied as models of the lipid bilayer region of animal cell membranes (1,2). In particular, the binary mixture of Chol and dipalmitoyl-phosphatidycholine (DPPC) has served as a prototypical model membrane and the often used ideas and terminology of liquid-ordered and liquid-disordered phases arose largely from studies of this system (3,4). This simple binary mixture continues to be a useful system to study intermolecular forces in bilayers and recent studies have led to a re-examination of its phase behavior (5,6).
Early NMR studies of mixtures of Chol and deuterated DPPC in bilayers showed a Chol-dependent increase in the deuterium quadrupole splitting due to ordering of DPPC acyl chains. In addition, line broadening was observed at intermediate Chol concentrations which was interpreted to arise from DPPC molecules moving between coexisting liquid phases (domain size ∼25 nm) and led to a much cited phase diagram for the binary DPPC/Chol mixture (4). A more recent study from the same group has reinforced this view and has additionally acknowledged the possibility of line broadening arising due to composition fluctuations close to a miscibility critical point (6).
In contrast to bilayers composed of binary mixtures of cholesterol and phospholipids, those made of ternary mixtures of DPPC, Chol, and a third lipid such as diphytanoyl-phosphatidylcholine (DiPhyPC) show micron-sized immiscible liquid domains when observed by fluorescence microscopy (5,7). The absence of micron-scale phase separation in binary mixtures combined with the orientation of tie-lines that connect compositions of coexisting phases in this ternary mixture has led to a counter-proposal that there is no liquid-liquid phase separation in the binary DPPC/Chol mixture (5).
The present study addresses these different interpretations using a thermodynamic model involving formation of a “condensed complex” between Chol and DPPC. The complex is immiscible with DPPC and DiPhyPC. The condensed complex model has been used previously to successfully describe phase diagrams, NMR relaxation rates, and other physical properties of cholesterol-containing monolayers and bilayers (8–11). Here, this model is extended to account for liquid-liquid phase separation in both binary and ternary mixtures.
As before, this study considers a liquid bilayer composed of Chol (C), DPPC (P1) and DiPhyPC (P2). The condensed complex (CP1) forms in a reversible reaction
| (1) |
Here, Keq is the equilibrium constant and 1:1 stoichiometry is chosen for simplicity. The regular solution free energy of the equilibrium mixture of C, P1, P2 and CP1 is
| (2) |
where kB is Boltzmann's constant, is the standard chemical potential of pure component i, xi is its equilibrium mole fraction and is the critical temperature of the i - j binary pair (representing a mean field repulsion). All of the standard chemical potentials are constant and are set to be zero with the exception of the complex . As has been done before, this study assumes immiscibility between the complex and DiPhyPC to model the closed miscibility loop found in this ternary mixture (9,12). The DiPhyPC/DPPC/Chol mixture is especially attractive for modeling since the closed miscibility loop can be determined in its entirety at higher temperatures without interference from the solid gel phase that forms at 41°C, the main chain melting temperature (Tm) of DPPC (13). This study also assumes immiscibility between complex and DPPC to generate phase separation in the binary DPPC/Chol mixture. The critical temperatures for the other four binary pairs are set to be zero. At a given temperature, there are only three parameters: Keq, and . Equilibrium free energies and phase boundaries are calculated as described previously (14). The experimental data that this study seeks to fit are the ternary critical temperature of DiPhyPC/DPPC/Chol which has been measured to be ∼55°C and the closed miscibility loop at lower temperatures (5). Phase separation in the binary DPPC/Chol mixture is assumed to terminate at a critical temperature of 45°C, slightly above 41°C, the Tm of DPPC. These criteria severely restrict the range of values for the parameters, best-fit values for which are , , and Keq = 150 at 298K. The temperature dependence of Keq is given by an exothermic heat of reaction of 9.6 kcal/mole of DPPC, a value consistent with previous estimates (8).
A ternary phase diagram calculated using the above parameters at 50°C is shown in Fig. 1 A. The closed miscibility loop bears strong resemblance to that observed experimentally for this mixture (5). The two-phase region shrinks with increasing temperature due to dissociation of complexes and increased entropy of mixing, and disappears at the ternary critical temperature of 55°C. The pair of critical points at 50°C (open circles, Fig. 1 A) eventually merges into a single ternary critical point at 55°C (large solid circle, Fig. 1 A). At lower temperatures, liquid-liquid immiscibility is seen in the binary DPPC/Chol mixture as shown in Fig. 1 B. This two-phase region terminates in an upper critical point at 45°C (solid circle, Fig. 1 B) and is bounded at lower temperatures by the eutectic at 41°C, where DPPC undergoes a liquid-to-solid phase transition. At temperatures below 41°C (gray region, Fig. 1 B), the liquid-liquid two phase region would be obscured by the formation of solid phase DPPC. This study focuses only on liquid-liquid immiscibility, and in particular on details of the tie-lines.
Figure 1.
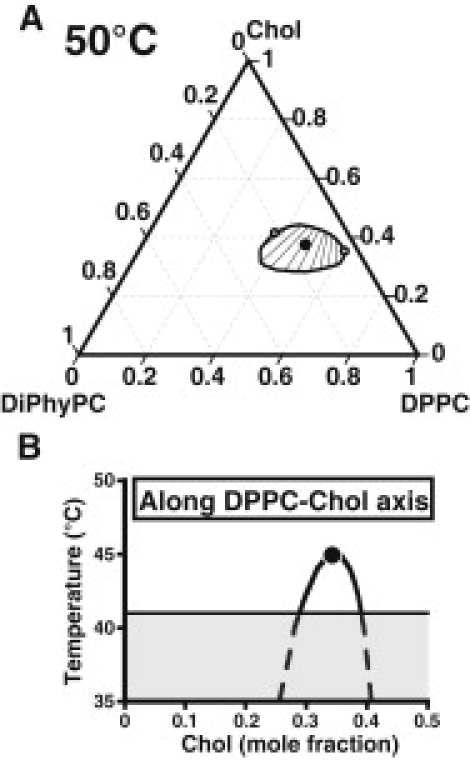
Calculated phase diagrams for mixtures of DiPhyPC, DPPC and Chol. (A) Closed miscibility loop for ternary mixtures at 50°C showing tie-lines in the two-phase region, a pair of critical points at 50°C (open circles) and the ternary critical point at 55°C (solid circle). (B) Temperature-composition phase diagram for the binary DPPC/Chol mixture. An upper critical point at 45°C (solid circle) and the solid line at 41°C (Tm of DPPC) bound the two-phase region of coexisting liquids. The area below 41°C (gray shaded region) is made complicated by solid phase formation, not treated here.
As can be seen in Fig. 1 A, the orientation of tie-lines within the closed miscibility loop is a strong function of the ratio of DPPC:DiPhyPC. When this ratio is low (close to the left critical point in Fig. 1 A), the tie-lines lie at an angle almost perpendicular to the binary DPPC/Chol axis. As the DPPC:DiPhyPC ratio increases (approaching the right critical point in Fig. 1 A), the tie-lines rotate counterclockwise approaching an angle almost parallel to the DPPC/Chol axis. A hypothetical extrapolation of tie-lines beyond the two-phase region shows that they originate from a common point on the DPPC/Chol axis where xChol = xDPPC = 0.5 and the concentration of complex is highest. The competing immiscibilities between condensed complex and phospholipid (DiPhyPC or free DPPC) drive the tie-lines into a fan-like arrangement. Fig. 2 shows a phase diagram calculated at 42°C where the tie-line splaying is more easily visible. The closed miscibility loop has fused with the DPPC/Chol binary axis at this lower temperature, eliminating one of the critical points and yielding two co-existing liquid phases in both the ternary and binary mixtures. Proximity of the upper critical temperature in the DPPC/Chol mixture to the eutectic at 41°C may complicate experimental detection of a two-phase region. Fig. 2 B shows binodal curves at 50°C and 42°C along with a line of critical points. The large solid circles represent the ternary critical point at 55°C and the binary critical point at 45°C.
Figure 2.
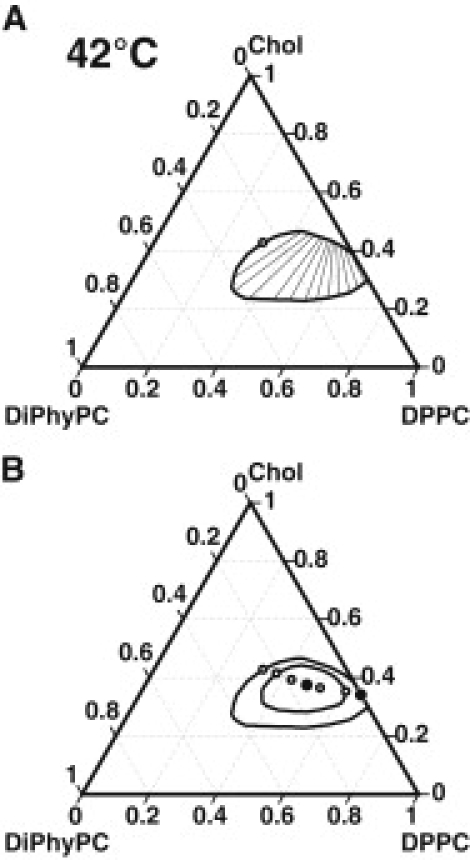
Calculated phase diagrams for mixtures of DiPhyPC, DPPC, and Chol. (A) Phase diagram at 42°C where the two-phase region encompasses ternary and binary compositions. The tie-lines adopt a fan-like arrangement starting parallel to the DPPC/Chol axis and ending at the critical point (open circle). (B) Critical points at various temperatures ranging from 55°C (ternary critical point, solid circle) to 41°C (binary critical point on DPPC/Chol axis, solid circle). Open circles denote critical points at intermediate temperatures. The binodal curves bounding two-phase regions at 42°C and 50°C are also shown.
In contrast to studies of bilayer mixtures to date, monolayers at the air-water interface composed of binary DPPC/Chol mixtures do show coexisting liquid phases when observed by fluorescence microscopy (15). The phase diagrams of monolayer mixtures have been modeled by regular solution theory involving condensed complexes (8), much like the calculations presented here. A repulsion between complex and DPPC (similar to the parameter used here) was used in the earlier monolayer studies. Unfortunately, the monolayer critical temperatures are strongly pressure-dependent which complicates quantitative comparisons with the bilayer systems in this study.
Closed miscibility loops arise in ternary mixtures when the ternary critical temperature is higher than the critical temperatures of the three possible binary pairs and many examples have been reported (see Radhakrishnan and McConnell (9) for a partial list). The study by Raub and Engel (16) on the ternary alloy mixture of Au/Ni/Cu is especially informative as it shows a closed miscibility loop at higher temperatures which fuses with the Au/Ni binary axis as the temperature is lowered. Both the shape of the two-phase regions and fan-like arrangement of tie-lines in this ternary alloy mixture are in general agreement with the calculations in Fig. 1 A and Fig. 2 A. The model used for Chol-phospholipid mixtures here could be relevant to the alloy mixture as well, assuming a complex AuCu3 which is immiscible with Ni and Cu.
The measured tie-lines in DiPhyPC/DPPC/Chol ternary mixtures are roughly perpendicular to the DPPC/Chol axis (5), whereas any phase separation in binary DPPC/Chol mixtures would be along tie-lines parallel to that binary axis. An argument has been made that this orthogonal arrangement of tie-lines would be implausible on thermodynamic grounds, thereby implying that there is no phase separation in the binary mixture (5). However, the calculations here show a dramatic rotation of tie-lines from parallel to roughly perpendicular with respect to the DPPC/Chol axis (Fig. 1 A and 2 A). The calculations use a model of condensed complexes of DPPC and Chol which has already been successful in accounting for several properties of membranes containing cholesterol (8–11). Other models that are consistent with the known phase diagrams may also result in rotation of tie-lines. Based on the calculations presented here, it is proposed that the direction of tie-lines in ternary mixtures does not preclude possible phase separation in binary DPPC/Chol mixtures. A putative two-phase region such as that shown in Fig. 1 B may have escaped detection by microscopy methods due to proximity of the binary critical temperature to the Tm of DPPC. It is also possible that NMR line broadening in mixtures of DPPC/Chol that has been interpreted in terms of coexisting liquid phases (4,6) may instead arise due to composition fluctuations in the vicinity of a miscibility critical point or the kinetics of complex formation and dissociation (10).
Acknowledgments
The author thanks Harden McConnell for many useful discussions.
References and Footnotes
- 1.Leathes J.B. Role of fats in vital phenomena. Lancet. 1925;208:853–856. [Google Scholar]
- 2.Finegold L. CRC Press; Ann Arbor, MI: 1993. Cholesterol in Membrane Models. [Google Scholar]
- 3.Ipsen J.H., Karlström G., Zuckermann M.J. Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim. Biophys. Acta. 1987;905:162–172. doi: 10.1016/0005-2736(87)90020-4. [DOI] [PubMed] [Google Scholar]
- 4.Vist M.R., Davis J.H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990;29:451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- 5.Veatch S.L., Gawrisch K., Keller S.L. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys. J. 2006;90:4428–4436. doi: 10.1529/biophysj.105.080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis J.H., Clair J.J., Juhasz J. Phase equilibria in DOPC/DPPC-d62/cholesterol mixtures. Biophys. J. 2009;96:521–539. doi: 10.1016/j.bpj.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veatch S.L., Polozov I.V., Keller S.L. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J. 2004;86:2910–2922. doi: 10.1016/S0006-3495(04)74342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McConnell H.M., Radhakrishnan A. Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta. 2003;1610:159–173. doi: 10.1016/s0005-2736(03)00015-4. [DOI] [PubMed] [Google Scholar]
- 9.Radhakrishnan A., McConnell H. Condensed complexes in vesicles containing cholesterol and phospholipids. Proc. Natl. Acad. Sci. USA. 2005;102:12662–12666. doi: 10.1073/pnas.0506043102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConnell H., Radhakrishnan A. Theory of the deuterium NMR of sterol-phospholipid membranes. Proc. Natl. Acad. Sci. USA. 2006;103:1184–1189. doi: 10.1073/pnas.0510514103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radhakrishnan A., McConnell H. Composition fluctuations, chemical exchange, and nuclear relaxation in membranes containing cholesterol. J. Chem. Phys. 2007;126:185101. doi: 10.1063/1.2730805. [DOI] [PubMed] [Google Scholar]
- 12.McConnell H. Complexes in ternary cholesterol-phospholipid mixtures. Biophys. J. 2005;88:L23–L25. doi: 10.1529/biophysj.104.058834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silvius J.R. Thermotropic phase transitions of pure lipids in model membranes and their modification by membrane proteins. In: Jost P.C., Griffith O.H., editors. Lipid-Protein Interactions. John Wiley and Sons; New York: 1982. [Google Scholar]
- 14.Radhakrishnan A., McConnell H.M. Condensed complexes of cholesterol and phospholipids. Biophys. J. 1999;77:1507–1517. doi: 10.1016/S0006-3495(99)76998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller S.L., Radhakrishnan A., McConnell H.M. Saturated phospholipids with high melting temperatures form complexes with cholesterol in monolayers. J. Phys. Chem. B. 2000;104:7522–7527. [Google Scholar]
- 16.Raub E., Engel A. Ternary system of gold, nickel and copper. Metallforschung. 1947;2:11–16. [Google Scholar]


