INTRODUCTORY PARAGRAPH
To identify genetic variants associated with birth weight, we meta-analyzed six genome-wide association (GWA) studies (N=10,623 Europeans from pregnancy/birth cohorts) and followed up two lead signals in thirteen replication studies (N=27,591). Rs900400 near LEKR1 and CCNL1 (P=2×10−35), and rs9883204 in ADCY5 (P=7×10−15) were robustly associated with birth weight. Correlated SNPs in ADCY5 were recently implicated in regulation of glucose levels and type 2 diabetes susceptibility,1 providing evidence that the well described association between lower birth weight and subsequent type 2 diabetes2,3 has a genetic component, distinct from the proposed role of programming by maternal nutrition. Using data from both SNPs, the 9% of Europeans with 4 birth weight-lowering alleles were, on average, 113g (95%CI 89-137g) lighter at birth than the 24% with 0 or 1 allele (Ptrend=7×10−30). The impact on birth weight is similar to that of a mother smoking 4-5 cigarettes per day in the third trimester of pregnancy.4
The extremes of birth weight are associated with high risks of perinatal morbidity and mortality.5,6 In addition, there are well-documented observational associations between lower birth weight and later life chronic disease, including type 2 diabetes, cardiovascular disease and higher blood pressure.2,3 The mechanisms underlying these associations are poorly understood. Birth weight is a complex multifactorial trait.7,8 The importance of genetic factors acting independently of the intra-uterine environment is illustrated by correlations between paternal height or weight and offspring birth weight,7,9,10 and genetic variants that are associated both with low birth weight and increased risk of type 2 diabetes may account for some of the observed correlation between these phenotypes.11-13 However, the genetic loci that influence birth weight are largely unknown.
Birth weight may be influenced directly by fetal genotype, and also indirectly by maternal genotype operating through the intra-uterine environment. This is clearly illustrated by observations of mothers and offspring with rare, heterozygous glucokinase (GCK) mutations. By reducing insulin secretion, these mutations increase offspring birth weight by 600g when inherited by the mother and reduce birth weight by 530g when inherited by the fetus.14
To search for common genetic variants associated with birth weight, we performed a meta-analysis of GWA studies. We reasoned that finding such variants, even those with modest effects, would lead to enhanced understanding of pathways important for fetal growth and those underlying the associations between fetal growth and adult disease.
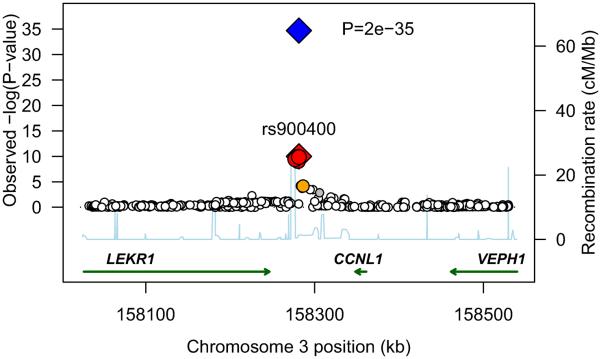
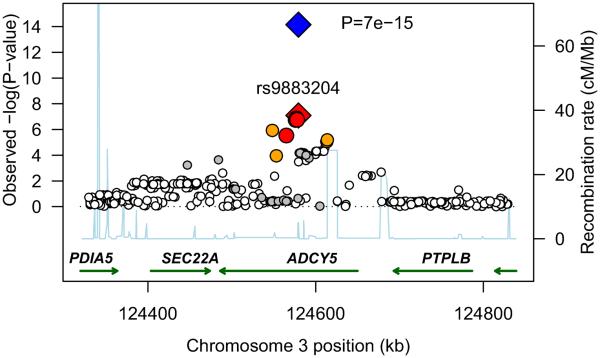
We meta-analyzed association statistics from 2,427,548 directly-genotyped and imputed SNPs in singletons of European descent from six discovery GWA studies (N=10,623; Supplementary Table 1). Birth weight (BW) was standardized to Z-scores within each study ([BW-mean]/standard deviation, SD) and adjusted for sex and gestational age. We observed SNPs at two independent loci on chromosome 3 that were associated with birth weight at, or close to, genome-wide significance (P<5×10−8; Supplementary Figure 1). The first locus was at 3q25, between CCNL1 and LEKR1; and the second, at 3q21 in ADCY5 (Figure 1). To replicate these associations, we genotyped the most strongly associated SNP from each locus (rs900400 from 3q25; rs9883204 from 3q21), or a closely-correlated proxy (HapMap r2=0.927-0.963), in thirteen further samples of European descent (N=27,591; Supplementary Table 2). Robust evidence of association was seen for both signals in these replication samples (Figure 2; P=3×10−26 and 3×10−9, respectively). Combining all discovery and replication samples, each additional C-allele of SNP rs900400 (frequency 32-47%) was associated with a 0.086 SD lower birth weight (95%CI: 0.073-0.100; P=2×10−35), while each C-allele of SNP rs9883204 (frequency 71-83%) was associated with a 0.063 SD lower birth weight (95%CI: 0.047-0.079; P=7×10−15; Table 1). These SD changes equate approximately to differences of 40g and 30g per allele, respectively (median study SD=484g). Analysis conditional on the index SNPs, rs900400 and rs9883204 did not produce any evidence for additional independent signals at either of the loci.
Figure 1.
Regional plots of two novel associations with birth weight. For each of the two regions, 3q25 [A] and 3q21 [B], directly genotyped and imputed SNPs are plotted using filled circles with their meta-analysis P values (as −log10 values) as a function of genomic position (NCBI Build 35). In each plot, the discovery stage SNP taken forward to replication stage is represented by a blue diamond (defining a global meta-analysis P value), with its discovery meta-analysis P value denoted by a red diamond. Local LD structure is reflected by the plotted estimated recombination rates (taken from HapMap) in the region around the associated SNPs and their correlated proxies. Each analyzed SNP is represented by circle. The colour scheme of the circles respects LD patterns (HapMap CEU pair-wise r2 correlation coefficients) between top discovery SNP and surrounding variants: white r2<0.2, grey 0.5> r2 >= 0.2, orange 0.8> r2 >= 0.5, red r2 >= 0.8. Gene annotations were taken from the University of California Santa Cruz genome browser.
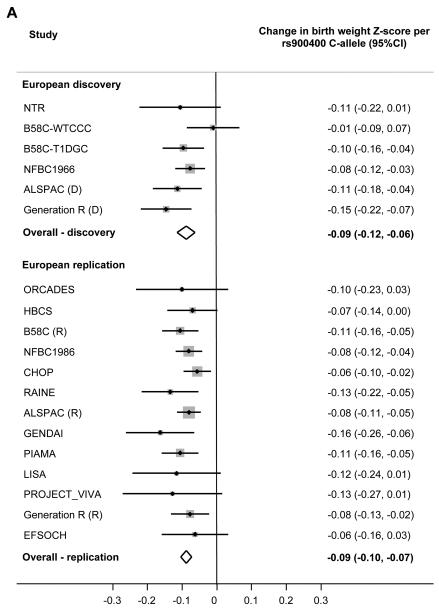
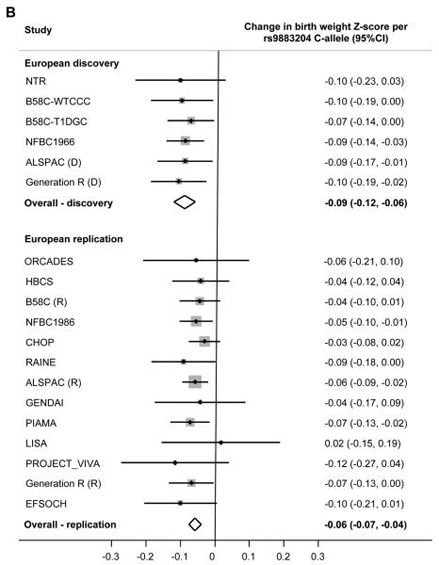
Figure 2.
Forest plots of the association between birth weight and genotype at each locus.
[A] Index SNP rs900400 at 3q25.
[B] Index SNP rs9883204 at 3q21.
If the index SNP was unavailable, a closely-correlated proxy (HapMap r2>0.9) was used.
Table 1.
Associations between novel birth weight loci and anthropometric traits at birth
| Phenotype | Locus 3q25, nearest genes: CCNL1, LEKR1a | Locus 3q21, ADCY5b | ||||||
|---|---|---|---|---|---|---|---|---|
| N | Effect | 95%CI | P valuec | N | Effect | 95%CI | P valuec | |
| Birth weight Z-scored | 37745 | −0.086 SD |
−0.100, −0.073 | 2×10−35 | 38214 | −0.063 SD |
−0.079, −0.047 | 7×10−15 |
| Birth length Z-score | 21512 | −0.028 SD |
−0.046, −0.010 | 0.002 | 21782 | −0.044 SD |
−0.066, −0.022 | 4×10−5 |
| Birth head circumference Z-score | 17349 | −0.024 SD |
−0.044, −0.004 | 0.017 | 17693 | −0.025 SD |
−0.048, −0.004 | 0.030 |
| Ponderal indexe Z-score | 21515 | −0.094 SD |
−0.113, −0.074 | 5×10−21 | 21785 | −0.032 SD |
−0.055, −0.009 | 0.006 |
|
| ||||||||
| Odds ratio for SGA <10th percentilef |
30370 | 1.16 | 1.10, 1.23 | 1×10−7 | 30778 | 1.09 | 1.02, 1.16 | 0.009 |
Results are from inverse variance, fixed effects meta-analysis of all 19 study samples of European ancestry. The effect allele for each SNP is labelled on the positive strand according to HapMap. The effect is the beta coefficient (or odds ratio) for genotype, assuming an additive genetic model. If the index SNP was unavailable, this was substituted with a closely-correlated (HapMap r2>0.9) proxy (rs1482853 or rs900399 for rs900400; rs2877716 or rs6798189 for rs9883204). There was no evidence of between-study heterogeneity of effect size estimates (all P>0.18; I2<26%).
Index SNP rs900400, effect allele C (40% frequency in HapMap CEU; range 32-47% in our European study samples).
Index SNP rs9883204, effect allele C (73% frequency in HapMap CEU; range 71-83% in our European study samples).
The P value for the birth weight meta-analysis includes the double-GC correction of the discovery meta-analysis.
Excluding the three studies that were unable to adjust for gestational age, the beta (s.e.m.), N and P values in the birth weight analysis were: −0.089(0.008), N=31510, P=7×10−32 (3q25); −0.068(0.009), N=31901, P=8×10−15 (3q21).
Ponderal index = birth weight/length3.
SGA <5th percentile, OR (P value): rs900400=1.11 (0.004); rs9883204=1.04 (0.41).
We found no evidence of heterogeneity between the studies examined (P>0.5; I2=0%),15 despite differences in mean birth weight (reflecting secular and population differences in birth weight distribution; Table 2), and the associations with birth weight were similar in males and females (P>0.05 for difference in effect sizes). Gestational age was not available as a covariate in three of our replication studies (combined N=6235; Supplementary Table 2), but these studies did not introduce detectable heterogeneity, and their removal from the meta-analysis changed the results very little (Figure 2 and Table 1 footnote). We also assessed the effects of the two SNPs on birth weight in a limited number of non-European or admixed samples from 2 studies (N=1415 Filipino subjects from the Cebu Longitudinal Health and Nutrition Survey, and N=298-448 African descended, Moroccan and Turkish subjects from Generation R; Supplementary Tables 2 and 3). There was no difference in the effect sizes observed relative to the European studies (P>0.5), but power to detect association was limited. Further well-powered studies will be needed to investigate these associations in non-Europeans.
Table 2.
Mean birth weight (SD) by genotype and individual association results by study
| Study type | Study | Year(s) of birth |
Total Na |
% mal e |
Locus 3q25, nearest genes: CCNL1, LEKR1b | Locus 3q21, ADCY5b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| TT | CT | CC |
P valuec |
TT | CT | CC |
P valuec |
|||||
|
|
|
|||||||||||
| Mean BW in g (SD) |
Mean BW in g (SD) |
Mean BW in g (SD) |
Mean BW in g (SD) |
Mean BW in g (SD) |
Mean BW in g (SD) |
|||||||
| Discovery | NTR | 1923-86 | 414 | 37.9 | 3470 (652) | 3401 (615) | 3329 (646) | 0.08 | 3500 (720) | 3402 (604) | 3359 (633) | 0.09 |
| B58C-WTCCC | 1958 | 1227 | 50.4 | 3367 (444) | 3337 (455) | 3364 (454) | 0.77 | 3459 (457) | 3357 (456) | 3336 (455) | 0.05 | |
| B58C-T1DGC | 1958 | 2037 | 49.2 | 3399 (468) | 3339 (464) | 3308 (461) | 1×10−3 | 3396 (463) | 3375 (484) | 3341 (463) | 0.07 | |
| NFBC1966 | 1966 | 4333 | 48.1 | 3567 (458) | 3519 (458) | 3503 (458) | 5×10−4 | 3630 (459) | 3559 (459) | 3529 (459) | 4×10−3 | |
| ALSPAC (D) | 1991-2 | 1418 | 48.8 | 3486 (481) | 3419 (482) | 3374 (467) | 2×10−3 | 3451 (458) | 3462 (465) | 3405 (514) | 0.03 | |
| Generation R (D) |
2002-6 | 1194 | 53.1 | 3633 (435) | 3562 (447) | 3492 (448) | 1×10−4 | 3655 (449) | 3593 (444) | 3549 (456) | 0.01 | |
|
| ||||||||||||
| Replication | ORCADES | 1920-88 | 328 | 43.3 | 3635 (599) | 3615 (594) | 3487 (602) | 0.12 | 3542 (612) | 3670 (605) | 3566 (595) | 0.74 |
| HBCS | 1934-44 | 1566 | 42.7 | 3462 (436) | 3434 (438) | 3403 (430) | 0.06 | 3391 (426) | 3479 (434) | 3431 (418) | 0.33 | |
| B58C (R) | 1958 | 2550 | 51.6 | 3407 (454) | 3341 (451) | 3308 (456) | 7×10−5 | 3338 (457) | 3387 (448) | 3340 (477) | 0.14 | |
| NFBC1986 | 1985-6 | 5008 | 49.1 | 3656 (440) | 3607 (440) | 3591 (440) | 4×10−5 | 3674 (441) | 3646 (441) | 3620 (441) | 0.03 | |
| CHOP | 1987- 2009 |
5149 | 53.3 | 3384 (634) | 3333 (646) | 3318 (628) | 5×10−3 | 3389 (641) | 3357 (647) | 3341 (609) | 0.19 | |
| RAINE | 1989-92 | 988 | 52.4 | 3507 (428) | 3432 (417) | 3384 (429) | 1×10−3 | 3472 (426) | 3489 (431) | 3427 (425) | 0.06 | |
| ALSPAC (R) | 1991-2 | 5695 | 54.6 | 3303 (547) | 3259 (568) | 3229 (493) | 3×10−6 | 3305 (464) | 3288 (580) | 3257 (626) | 3×10−3 | |
| GENDAI | 1994-6 | 758 | 45.5 | 3401 (530) | 3215 (528) | 3235 (529) | 1×10−3 | 3291 (539) | 3286 (539) | 3260 (539) | 0.53 | |
| PIAMA | 1996-7 | 1789 | 51.3 | 3629 (438) | 3575 (443) | 3512 (427) | 9×10−5 | 3619 (441) | 3607 (425) | 3554 (430) | 0.01 | |
| LISA | 1998-9 | 387 | 56.9 | 3476 (366) | 3454 (363) | 3368 (363) | 0.07 | 3532 (365) | 3429 (366) | 3443 (367) | 0.84 | |
| PROJECT VIVA | 1999- 2003 |
300 | 50.0 | 3711 (406) | 3646 (411) | 3594 (407) | 0.08 | 3698 (412) | 3703 (402) | 3625 (408) | 0.15 | |
| Generation R (R) | 2002-6 | 1885 | 50.3 | 3558 (435) | 3527 (423) | 3481 (413) | 6×10−3 | 3615 (433) | 3534 (435) | 3518 (430) | 0.04 | |
| EFSOCH | 2003-4 | 719 | 53.1 | 3556 (427) | 3509 (432) | 3504 (431) | 0.20 | 3660 (433) | 3513 (435) | 3503 (432) | 0.07 | |
BW, birth weight. All birth weight values are adjusted for sex and, where available, gestational age. Gestational age was not available for the ORCADES, CHOP and GENDAI studies.
Study N in the birth weight association analysis for rs900400 genotype. Total numbers of European discovery and replication samples, respectively, were N=10623 and N=27122 for rs900400; N=10623 and N=27591 for rs9883204.
If the index SNP was unavailable, this was substituted with a closely-correlated (HapMap r2>0.9) proxy (rs1482853 or rs900399 for rs900400 at 3q25; rs2877716 or rs6798189 for rs9883204 at 3q21).
P value is from linear regression of birth weight Z score against SNP (additive model), with sex and gestational age, where available, as covariates. All study samples were of European descent.
Key to study names: NTR, Netherlands Twin Register; B58C-WTCCC, British 1958 Birth Cohort – Wellcome Trust Case Control Consortium subset; B58C-T1DGC, British 1958 Birth Cohort – Type 1 Diabetes Genetics Consortium subset; NFBC1966, Northern Finland Birth Cohort 1966; ALSPAC (D), Avon Longitudinal Study of Parents and Children Discovery subset; Generation R (D), Generation R Discovery subset; ORCADES, Orkney Complex Disease Study; HBCS, Helsinki Birth Cohort Study; B58C (R), British 1958 Birth Cohort Replication subset; NFBC1986, Northern Finland Birth Cohort 1986; CHOP, Children's Hospital Of Philadelphia; RAINE, The Raine Study; ALSPAC (R), Avon Longitudinal Study of Parents and Children Replication subset; GENDAI, GENe and Diet Attica Investigation; PIAMA, Prevention and Incidence of Asthma and Mite Allergy; LISA, Lifestyle – Immune System – Allergy; Project Viva, The Project Viva Cohort; Generation R (R), Generation R Replication subset; EFSOCH, Exeter Family Study Of Childhood Health.
Maternal and fetal genotypes are correlated due to segregation. In a previous study, an observed association between fetal TCF7L2 genotype and birth weight was driven by the effects of maternal TCF7L2 variation on the intra-uterine environment, rather than a direct effect on fetal growth.16 To distinguish between these two mechanisms, we tested whether the novel birth weight associations were independent of maternal genotype. We genotyped both SNPs in all available maternal DNAs (N=9127; 5 study samples). Meta-analysis of associations between birth weight and fetal genotype, conditional on maternal genotype, yielded results which were very similar to the original associations at both loci (Supplementary Table 4), showing that these are direct fetal effects. As expected, there was no association between fetal genotype and various covariates of birth weight that were not included in our main analysis (maternal smoking, BMI, parity, education, age at delivery; all P>0.05; data not shown).
Birth weight may be influenced by skeletal growth or fat mass. In available samples, we analyzed the association between each locus and birth length, birth head circumference and ponderal index (Table 1 and Supplementary Figures 2-4). The association with ponderal index, relative to the birth length and head circumference associations, was particularly strong for the rs900400 SNP (0.094 SD [95%CI: 0.074-0.113] per C-allele; P=5×10−21), suggesting a greater association with fat mass than skeletal growth. For the rs9883204 SNP, the measures showed more proportionate changes (Table 1). We investigated associations with adult height and BMI using published GWA meta-analyses from the GIANT consortium.17,18 Only the rs900400 signal was captured in the published height data at r2>0.8 (since that study only included direct genotypes from the Affymetrix Genechip 500k), and there was no association (P=0.64; N=9818). There was no association with adult BMI for either locus (N≈32500, P>0.1). This is consistent with the weak observational association between birth weight and adult BMI,19 indicating that they are largely governed by different processes.
Although birth weight is a continuous trait, standard clinical cut-offs are used to identify neonates who are small for gestational age and who may require further observation. We therefore assessed whether each SNP increased the odds of gestational age-adjusted birth weight <10th percentile. Both loci were associated with smallness for gestational age: odds ratios (OR) 1.16 [95%CI: 1.10-1.23] (P=1×10−7) and 1.09 [1.02-1.16] (P=0.009) per C-allele of rs900400 and rs9883204, respectively (Table 1; Supplementary Figure 5).
The birth weight signal marked by rs900400 maps approximately 35kb 3-prime to the leucine, glutamate and lysine rich 1 (LEKR1) locus and 67kb 3-prime to cyclin L1 (CCNL1). Neither gene has previously been implicated in fetal growth. The CCNL1 protein may be involved in pre-mRNA splicing and RNA processing, and associates with cyclin-dependent kinases.20 A non-coding RNA of unknown function, 682bp from rs900400 (AK311218, Human March 2006 Assembly 18), overlaps with the signal. We found no evidence for association at a genome-wide level (P>5×10−8) between our 3q25 birth weight signal and mRNA expression in lymphocytes, using the publicly available ‘mRNA by SNP Browser 1.0’,21 and there was no association between rs900400 or rs900399 and type 2 diabetes or related adult glycemic traits in the recent GWA meta-analysis from the MAGIC consortium (P>0.1).1 A range of approaches (including resequencing and functional studies) will be required to establish which gene (CCNL1, LEKR1 or another gene) is mediating the effect on fetal growth.
The second birth weight locus at 3q21 (index SNP rs9883204) maps within the adenylyl cyclase 5 gene (ADCY5). ADCY5 belongs to the family of enzymes responsible for the synthesis of cyclic adenosine monophosphate (cAMP).22-24 Allele A of rs11708067, in linkage disequilibrium (LD) with the birth weight-lowering C-allele of rs9883204 (r2=0.75), is associated with a higher risk of type 2 diabetes (OR: 1.12 [95%CI: 1.04-1.15]; P=9.9×10−21; 40,655 cases/87,022 controls), higher fasting glucose (0.027 mmol/l [95%CI: 0.021-0.033]; P=7.1×10−22; N=118,475), and reduced values of the Homeostatic Model Assessment (HOMA-) derived index of beta-cell function (HOMA-B; P=7.1×10−12; N=94,212),1 suggesting that it may impact on insulin secretion. Fetal insulin is a key fetal growth factor, and these metabolic associations suggest that one mechanism explaining the ADCY5 association with birth weight might be a direct effect of the fetal risk allele on fetal growth via reduced insulin secretion, consistent with the fetal insulin hypothesis.11
However, our previous studies suggest that an association between fetal genotype and birth weight is not characteristic of all type 2 diabetes loci. For example, susceptibility variants at CDKN2A/B, IGF2BP2 and SLC30A8 and TCF7L2 were not associated with birth weight in previous studies of N>15000, after adjusting for maternal genotype.12,16 To test this more comprehensively, we examined the associations between birth weight and all published type 2 diabetes (N=24) and fasting glucose (N=16) loci in our discovery GWA meta-analysis (N=10,623).1,25,26 Only ADCY5 and the previously observed birth weight association at CDKAL112,13 showed evidence of association at P<0.01 (Supplementary Table 5). One explanation for the variable effects of different type 2 diabetes susceptibility loci on birth weight is that they may influence beta-cell function at different points of the life course, with ADCY5 having a more marked effect in utero than the other loci. However, other mechanisms could be partially or wholly responsible for the ADCY5 association with birth weight including the regulation of placental glucose transporter expression,27 vitamin B2 uptake in the placenta28 and the architecture and permeability of the materno-fetal placental barrier.29
The associations at 3q25 and 3q21 explained 0.3% and 0.1% of the variance in birth weight, respectively. Given that estimates of the fetal genetic contribution to birth weight from twin and family studies are generally between 10 and 40%,30,31 the proportion of heritability explained may be up to ten times greater. The variance explained by the first locus is comparable to that of maternal age (0.5%). We used a weighted risk allele score to estimate the differences in birth weight attributable to combinations of birth weight-lowering alleles at both loci. The 9% of Europeans with 4 birth weight-lowering alleles were, on average, 113g (95%CI 89-137g) lighter at birth than the 24% with 0 or 1 allele (P for trend =7×10−30). For comparison, this effect on birth weight is similar to the impact of a mother smoking 4-5 cigarettes per day,4 and is approximately one-third of the impact of the severe malnutrition of the Dutch Famine of 1944-45, during which pregnant women consumed, on average, <1000 calories/day.32
To conclude, we have identified novel, robust associations between fetal genotype and birth weight at loci near CCNL1 and at ADCY5. The causal mechanisms are not yet known, but the ADCY5 locus has pleiotropic effects on glucose regulation and type 2 diabetes in adulthood. This is robust evidence that the widely described association between lower birth weight and subsequent type 2 diabetes has a genetic component, distinct from the proposed role of programming by maternal nutrition. Further understanding of these associations will illuminate the biological pathways important for fetal growth and its relationship with adult diseases.
METHODS
Stage 1: GWA meta-analysis of birth weight
Discovery samples, genotyping and imputation
We selected six population-based European studies with birth weight, gestational age and GWA data available by the beginning of May 2009 (combined N=10,623): the Northern Finland 1966 Birth Cohort (NFBC1966; N=4333); Netherlands Twin Register (NTR; N=414; singletons only); and sub-samples from the 1958 British Birth Cohort (B58C-WTCCC, N=1227; B58C-T1DGC, N=2037), Generation R (N=1194) and Avon Longitudinal Study of Parents And Children (ALSPAC; N=1418). The B58C-WTCCC and B58C-T1DGC were analyzed separately because they were genotyped on different platforms at different times. However, there is no systematic phenotypic difference between these sub-samples. Genotypes were obtained using high-density SNP arrays, and then imputed for ~2.4 million HapMap SNPs (Phase II, release 21/22, http://hapmap.ncbi.nlm.nih.gov/). The basic characteristics, exclusions (e.g. samples of non-European ancestry), genotyping, quality control and imputation methods for each discovery sample are presented in Supplementary Table 1.
Statistical analysis within discovery samples
Multiple and preterm births (gestational age <37 weeks) were excluded from all analyses. Birth weight was transformed into a Z-score (= [value-mean]/SD) to allow comparison of the data across studies. The overall (as opposed to sex-stratified) mean and SD from each study were used to create Z-scores. The association between each SNP and birth weight was assessed in each study sample using linear regression of birth weight Z-score against genotype (additive model), with sex and gestational age as covariates. Imputed genotypes were used only where directly-assayed genotypes were unavailable. In addition to this “UNIFORM” analysis, a second analysis (“BEST”) was performed, in which the analysis details were decided within each study. Details of the BEST analysis, GWA analysis software, and any additional corrections for study-specific population structure in the UNIFORM analysis are given in Supplementary Table 1.
Meta-analysis of discovery samples
Data exchange was facilitated by the SIMBioMS platform (simbioms.org).33 Prior to meta-analysis, SNPs with a minor allele frequency <1% and poorly-imputed SNPs (proper_info ≤0.4 [SNPTEST]; r2 ≤0.3 [MACH2QTL]) were filtered. Fixed effects meta-analyses of the UNIFORM and BEST analyses were each run in parallel in two different study centers. Each was performed using different software packages: METAL (http://www.sph.umich.edu/csg/abecasis/metal/index.html); and MetaMapper (developed in-house at Imperial College London, UK). Genomic control34 was applied twice at the meta-analysis stage: first, to adjust the statistics generated within each cohort (see Supplementary Table 1 for individual study λ-values); and second, to adjust the overall meta-analysis statistics (λ=1.032). The results from the UNIFORM analysis were meta-analyzed using the inverse-variance method, whereas for the BEST analysis a Z-score weighted method that allows for differences in the units of beta coefficients and standard errors was applied.35 SNPs available for less than half of the total expected sample were excluded. Final meta-analysis results were obtained for 2,427,548 SNPs. Those SNPs that reached a p-value threshold of <10−7 in the UNIFORM analysis (N=10 SNPs, representing 2 distinct genomic regions on chromosome 3 were considered for further follow-up. The UNIFORM (reported here) and BEST analyses (data not shown) gave very similar results.
Checking for independent associations at the two loci
To test for the presence of additional association signals around the most strongly associated SNP in each region (rs900400 and rs9883204), we re-ran the UNIFORM association analysis on chromosome 3 in each discovery sample, including rs900400 and rs9883204 genotypes as additional covariates. Where these SNPs were imputed, genotype dosage was calculated from the genotype probabilities and used in the model. We meta-analyzed results using the inverse-variance method.
Stage 2: Follow-up of two lead signals in additional samples
Follow-up samples, genotyping and analysis
We used 17 study samples (combined N=30,098) to follow up the two lead signals from the GWA meta-analysis (represented by index SNPs rs900400 and rs9883204). Details of these samples are presented in Supplementary Table 2. Thirteen of the samples (combined N=27,591) were of European ancestry and were used for replication of the birth weight associations. We also examined associations in four further non-European or admixed study samples (combined N=2507). Informed consent was obtained from all discovery and follow-up study participants (or parental consent, as appropriate), and study protocols were approved by the local ethics committees. If the index SNP was unavailable, a closely correlated proxy was substituted (rs1482853 or rs900399 for rs900400 [HapMap r2=1 and 0.96, respectively]; rs2877716 or rs6798189 for rs9883204 [HapMap r2=0.95 and 0.93, respectively]). In four of the replication studies, the index SNPs were imputed from genome-wide genotype data (see Supplementary Table 2). The UNIFORM birth weight analysis (described above) was performed within each study sample. To investigate whether the associations were similar in the sexes, we repeated the analysis in males and females separately.
Meta-analyses
We performed fixed effects inverse variance meta-analyses of the UNIFORM results as follows: (i) including all 13 European replication samples; (ii) including all 19 discovery and replication samples of European descent, (iii) a sensitivity analysis, excluding the three studies without gestational age; and (iv) including all 23 study samples, regardless of ethnic background. We meta-analyzed the sex-stratified results from all European studies. All meta-analyses were performed in parallel at two different study centers, using different software packages (the METAN module, developed for Stata v.10,36 MetaAnalyst [Beta 3.13],37 RMeta in R [v.2.7.0]). We used the Cochran Q test and the I2 statistic15 to assess evidence of between-study heterogeneity of effect sizes.
Analysis of additional phenotypes
Birth length, birth head circumference, ponderal index and small for gestational age
Where available, we created Z-scores (value-mean/SD) within each study for birth length, head circumference and ponderal index (birth weight/length3). We used linear regression to assess the association between each outcome and each SNP (rs900400 or rs9883204, or proxies), with sex and gestational age as covariates. To examine the odds of small-for-gestational age (SGA), we created sex- and gestational age-adjusted birth weight Z-scores (SDS) within 15 of the available European studies using Growth Analyser 3.0 (http://www.growthanalyser.org; Dutch Growth Research Foundation, Rotterdam, the Netherlands). The reference was a cohort of 475,588 children born between 1977 and 1981 in Sweden.38 Subsequently, each study defined SGA as below the 10th percentile of birth weight SDS within their study population. We analysed the associations between the two top hits and SGA using logistic regression. Analyses were repeated with a 5th percentile cut-off. We combined the results across studies using fixed effects inverse variance meta-analysis.
Combined allele score
To estimate the birth weight effect sizes attributable to the two loci in combination, we created an allele score using information from both SNPs, which was weighted by effect size. This allowed us to estimate the differences in birth weight between individuals with different numbers of birth weight-lowering alleles at the two loci. We used nine European replication samples in which gestational age was available (N=20,190). After verifying that the two SNPs were statistically independent, we generated the score using the formula
where sj is score for individual j, gij is number of birth weight-lowering alleles (0, 1, 2) for SNP i carried by individual j and wi is effect size for SNP i from the UNIFORM analysis within the cohort. We performed linear regression of birth weight (grams) against the allele score (additive model), with sex and gestation as covariates. We combined the coefficients from the nine studies using fixed effects inverse variance meta-analysis. We then rounded the weighted score to the nearest integer, grouping scores “0” and “1” together, and performed linear regression of birth weight against the rounded score as indicator variables, with sex and gestation as covariates. The beta coefficients from the comparison of score 4 versus 0/1 in all nine studies were meta-analyzed (inverse variance, fixed effects).
Variance explained
To estimate the percentage of variation in birth weight explained by each of the associated loci, we obtained the adjusted-R2 from univariate linear regression of birth weight against genotype. We then calculated a mean value from all European studies, weighted by sample size. For comparison, we also calculated the variance explained by variables such as gestational age, maternal age and smoking.
Analyses of potential confounders
To assess whether the associations were independent of maternal genotype, we used mother-offspring pairs from the 5 studies with both maternal and fetal genotype available (see Supplementary Table 4; total N=8880 for rs900400; N=9127 for rs9883204). Within each study, we performed the UNIFORM analysis, with maternal genotype as an additional covariate. For direct comparison, we repeated this without maternal genotype, using only subjects for whom maternal genotype was available. We performed two inverse variance meta-analyses (fixed effects) for each SNP, combining regression coefficients for (i) fetal genotype, and (ii) fetal genotype adjusted for maternal genotype.
To verify that the SNPs were not associated with maternal covariates of birth weight which could theoretically confound the observed associations with birth weight (including maternal age, BMI, parity, smoking, pre-eclampsia, education), we used linear or logistic regression to model the association between each covariate and genotype, using nine European replication cohorts with gestational age available. Where possible, we meta-analyzed results to assess overall evidence of association.
Supplementary Material
ACKNOWLEDGMENTS
See also Supplementary Note for detailed acknowledgments by study.
Major funding for the research in this paper is as follows: Academy of Finland (project grants 104781, 120315, 209072, 129255 and Center of Excellence in Complex Disease Genetics); Biocentrum Helsinki; Biocenter, University of Oulu, Finland; British Heart Foundation; Canadian Institutes of Health Research (grant MOP 82893); Center for Medical Systems Biology; Centre for Neurogenomics and Cognitive Research (CNCR-VU) (grant EU/QLRT-2001-01254); The Chief Scientist Office of the Scottish Government; The Children's Hospital of Philadelphia (Institute Development Award); Coca-Cola Hellas; Cotswold Foundation (Research Development Award); Darlington Trust; Department of Health's National Institute of Health Research UK; Diabetes UK (grant RD08/0003704); Dutch Asthma Foundation; Dutch Ministry of the Environment; Erasmus Medical Center Rotterdam; Erasmus University Rotterdam; European Commission (EURO-BLCS, Framework 5 award QLG1-CT-2000-01643); The European Community's Seventh Framework Programme (FP7/2007-2013), ENGAGE project, grant agreement HEALTH-F4-2007- 201413; The European Union Framework Program 6 EUROSPAN Project (LSHG-CT-2006-018947); Exeter NHS Research and Development; Friedrich-Schiller-University Jena; Genetic Association Information Network; Healthway Western Australia; Helmholtz Zentrum Muenchen - German Research Center for Environment and Health; Institute of Epidemiology Neuherberg; IUF-Institut für Umweltmedizinische Forschung Düsseldorf; Juvenile Diabetes Research Foundation International; Kompetenznetz Adipositas (Competence Network Obesity) funded by the Federal Ministry of Education and Research (FKZ: 01GI0826); Marien-Hospital Wesel; Medical Research Council UK (grants G0601261, G0600705, studentship grant G0500539, G0000934, G0601653); Munich Center of Health Sciences (MCHEALTH); Municipal Health Service Rotterdam; National Health and Medical Research Council of Australia (grant 572613); National Human Genome Research Institute; National Institute of Allergy and Infectious Diseases; National Institute of Child Health and Human Development; National Institute of Diabetes and Digestive and Kidney Diseases; National Public Health Institute, Helsinki, Finland; NHLBI (grant 5R01HL087679-02 through the STAMPEED program (1RL1MH083268-01)); NIH (grants 5R01MH63706:02, 1R01HD056465-01A1, 1R01DK075787, DK078150, TW05596, HL085144, HL068041, HD034568, HD05450, RR20649, ES10126, and DK56350); NWO/ZonMW (grants SPI 56-464-14192, 904-61-090, 904-61-193, 480-04-004, 400-05-717); Office of Population Studies Foundation; Peninsula NIHR Clinical Research Facility; Raine Medical Research Foundation; Rotterdam Homecare Foundation; South West NHS Research and Development; Spinoza; St.Georg Hospital Leipzig; Stichting Astmabestrijding; Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR) Rotterdam; Technical University Munich; Telethon Institute for Child Health Research; Type 1 Diabetes Genetics Consortium; UFZ-Centre for Environmental Research Leipzig-Halle; University Hospital Oulu Biocenter, University of Oulu Finland; University of Bristol; University of Leipzig; Wellcome Trust (grants 085301, 068545/Z/02, 076113/B/04/Z); Western Australian DNA Bank; Western Australian Genetic Epidemiology Resource; Wind Over Water Foundation; and ZonMw.
Data exchange for the meta-analyses was facilitated by the SIMBioMS platform (simbioms.org).
Personal funding is as follows: R.M.F. by a Sir Henry Wellcome Postdoctoral Fellowship (Wellcome Trust grant 085541/Z/08/Z); E.W. by the Academy of Finland (grant 120315 and 129287); H.N.L. by NIH grant 1R01DK075787; E.H. by the Career Scientist Award, Department of Health, UK; C.M.L. is a Wellcome Trust Research Career Development Fellow; A.R. by the UK Department of Health Policy Research Programme; B.M.S, B.A.K and A.T.H are employed as core members of the Peninsula NIHR Clinical Research Facility; J.F.W. by The Royal Society; L.P. by Wellcome Trust (grant 89061/Z/09/Z); and V.W.V.J by the Netherlands Organization for Health Research (ZonMw 90700303).
Footnotes
COMPETING INTERESTS STATEMENT
The authors declare no competing financial interests.
REFERENCES
- 1.Dupuis J, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, et al. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–7. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- 3.Jarvelin MR, et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–46. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein IM, et al. Maternal smoking and its association with birth weight. Obstet Gynecol. 2005;106:986–91. doi: 10.1097/01.AOG.0000182580.78402.d2. [DOI] [PubMed] [Google Scholar]
- 5.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–63. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 6.Acker DB, Sachs BP, Friedman EA. Risk factors for shoulder dystocia. Obstet Gynecol. 1985;66:762–8. [PubMed] [Google Scholar]
- 7.Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- 8.Jarvelin MR, et al. Ecological and individual predictors of birthweight in a northern Finland birth cohort 1986. Paediatr Perinat Epidemiol. 1997;11:298–312. doi: 10.1111/j.1365-3016.1997.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 9.Knight B, et al. Evidence of genetic regulation of fetal longitudinal growth. Early Hum Dev. 2005;81:823–31. doi: 10.1016/j.earlhumdev.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Klebanoff MA, Mednick BR, Schulsinger C, Secher NJ, Shiono PH. Father's effect on infant birth weight. Am J Obstet Gynecol. 1998;178(5):1022–6. doi: 10.1016/s0002-9378(98)70542-3. [DOI] [PubMed] [Google Scholar]
- 11.Hattersley AT, Tooke JE. The fetal insulin hypothesis: an alternative explanation of the association of low birthweight with diabetes and vascular disease. Lancet. 1999;353:1789–92. doi: 10.1016/S0140-6736(98)07546-1. [DOI] [PubMed] [Google Scholar]
- 12.Freathy RM, et al. Type 2 Diabetes Risk Alleles are Associated with Reduced Size at Birth. Diabetes. 2009 doi: 10.2337/db08-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, et al. Examination of type 2 diabetes loci implicates CDKAL1 as a birth weight gene. Diabetes. 2009;58:2414–8. doi: 10.2337/db09-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattersley AT, et al. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19:268–70. doi: 10.1038/953. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freathy RM, et al. Type 2 diabetes TCF7L2 risk genotypes alter birth weight: a study of 24,053 individuals. Am J Hum Genet. 2007;80:1150–61. doi: 10.1086/518517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weedon MN, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–83. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willer CJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons TJ, Power C, Manor O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: longitudinal study. Bmj. 2001;323:1331–5. doi: 10.1136/bmj.323.7325.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loyer P, et al. Characterization of cyclin L1 and L2 interactions with CDK11 and splicing factors: influence of cyclin L isoforms on splice site selection. J Biol Chem. 2008;283:7721–32. doi: 10.1074/jbc.M708188200. [DOI] [PubMed] [Google Scholar]
- 21.Dixon AL, et al. A genome-wide association study of global gene expression. Nat Genet. 2007;39:1202–7. doi: 10.1038/ng2109. [DOI] [PubMed] [Google Scholar]
- 22.Tesmer JJ, Sprang SR. The structure, catalytic mechanism and regulation of adenylyl cyclase. Curr Opin Struct Biol. 1998;8:713–9. doi: 10.1016/s0959-440x(98)80090-0. [DOI] [PubMed] [Google Scholar]
- 23.Hanoune J, et al. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Mol Cell Endocrinol. 1997;128:179–94. doi: 10.1016/s0303-7207(97)04013-6. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig MG, Seuwen K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J Recept Signal Transduct Res. 2002;22:79–110. doi: 10.1081/rrs-120014589. [DOI] [PubMed] [Google Scholar]
- 25.Prokopenko I, McCarthy MI, Lindgren CM. Type 2 diabetes: new genes, new understanding. Trends Genet. 2008;24:613–21. doi: 10.1016/j.tig.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prokopenko I, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2008 doi: 10.1038/ng.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogura K, et al. 8-bromo-cyclicAMP stimulates glucose transporter-1 expression in a human choriocarcinoma cell line. J Endocrinol. 2000;164:171–8. doi: 10.1677/joe.0.1640171. [DOI] [PubMed] [Google Scholar]
- 28.D'Souza VM, et al. cAMP-Coupled riboflavin trafficking in placental trophoblasts: a dynamic and ordered process. Biochemistry. 2006;45:6095–104. doi: 10.1021/bi060138f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leach L. The phenotype of the human materno-fetal endothelial barrier: molecular occupancy of paracellular junctions dictate permeability and angiogenic plasticity. J Anat. 2002;200:599–606. doi: 10.1046/j.1469-7580.2002.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Baal CG, Boomsma DI. Etiology of individual differences in birth weight of twins as a function of maternal smoking during pregnancy. Twin Res. 1998;1:123–30. doi: 10.1375/136905298320566258. [DOI] [PubMed] [Google Scholar]
- 31.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165:734–41. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 32.Stein AD, Zybert PA, van de Bor M, Lumey LH. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J Epidemiol. 2004;33:831–6. doi: 10.1093/ije/dyh083. [DOI] [PubMed] [Google Scholar]
- 33.Krestyaninova M, et al. A System for Information Management in BioMedical Studies--SIMBioMS. Bioinformatics. 2009;25:2768–2769. doi: 10.1093/bioinformatics/btp420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 35.de Bakker PI, et al. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet. 2008;17:R122–8. doi: 10.1093/hmg/ddn288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris R, et al. Statistical Software Components S456798. Boston College Department of Economics; 2006. METAN: Stata module for fixed and random effects meta-analysis. revised 19 Feb 2007. < http://ideas.repec.org/c/boc/bocode/s456798.html>. [Google Scholar]
- 37.Wallace BC, Schmid CH, Lau J, Trikalinos TA. Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data. BMC Med Res Methodol. 2009;9:80. doi: 10.1186/1471-2288-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niklasson A, et al. An update of the Swedish reference standards for weight, length and head circumference at birth for given gestational age (1977-1981) Acta Paediatr Scand. 1991;80:756–62. doi: 10.1111/j.1651-2227.1991.tb11945.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.