Abstract
Records of repeated examinations of blood pressure are increasingly available for primary care patients, but the utility of this information in predicting incident hypertension remains unclear because cohort studies with repeat blood pressure monitoring are rare. We compared the incremental value of using data on blood pressure history to a single measure as in the Framingham hypertension risk score, a validated hypertension risk prediction algorithm. Participants were 4314 London-based civil servants (1297 women) aged 35 to 68 who were free from prevalent hypertension, diabetes and coronary heart disease at baseline examination (the Whitehall II study). Standard clinical examinations of blood pressure, weight and height, current cigarette smoking and parental history of hypertension were undertaken on a 5-yearly basis. A total of 1052 incident (new-onset) cases of hypertension were observed in two 5-year baseline-follow-up data cycles. Comparison of the Framingham risk score with a score additionally incorporating 5-year blood pressure history showed, at best, modest improvements in indicators of predictive performance: C-statistics (0.796 vs 0.799), predicted-to-observed ratios (1.04, 95%CI: 0.95-1.15 vs 0.98, 95%CI: 0.89-1.08) or Hosmer-Lemeshow chi-square values (11.5 vs 6.5). The net reclassification improvement with the modified score was 9.3% (95%CI: 4.2%-14.4%) resulting from a net 17.1% increase in non-hypertensives correctly identified as being at lower risk, but a net 7.8% increase in hypertensives incorrectly identified as at lower risk. These data suggest that despite the net reclassification improvement, the clinical utility of adding repeat measures of blood pressure to the Framingham hypertension risk score may be limited.
Keywords: Hypertension, prevention, primary prevention, public health, risk assessment, risk factors
Introduction
Preventive interventions can delay the onset of hypertension (systolic/diastolic blood pressure≥140/90 mm Hg).1-4 Current risk prediction tools to target preventive interventions at individuals with the highest risk of hypertension, such as the Framingham hypertension risk score,5,6 are based on clinical data taken from a single examination. However, records of repeat blood pressure examinations are increasingly available for primary care patients. We therefore examined whether adding past blood pressure measurements to the Framingham hypertension risk algorithm actually improves its predictive power.
Methods
Population and Study Design
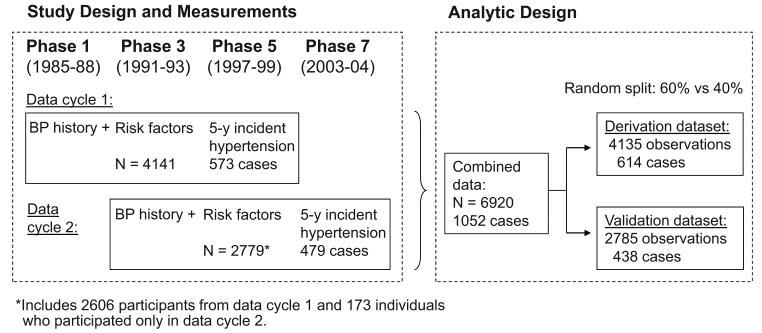
Data are taken from the Whitehall II study, a large-scale prospective cohort study of 10,308 civil servants (6895 men, 3413 women) aged 35-55 at the start of the study (Phase 1, 1985-1988).7 Since the Phase 1 medical examination, follow-up examinations have taken place approximately every 5 years: Phase 3 (1991-1993), n=8104; Phase 5 (1997-1999), n=6551; and Phase 7 (2003-2004), n=6483.
The present analysis was based on 2 history-baseline-follow-up screening cycles, each with 3 blood pressure examinations, the first for blood pressure history, the second for blood pressure at baseline and the third for follow-up blood pressure (Figure 1). Participants were eligible for inclusion if they attended three consecutive screenings between Phase 1 and Phase 7. This resulted in 6210 and 5691 eligible participants at the two baseline phases, Phase 3 and Phase 5. At the baseline for both screening cycles, we successively excluded participants who were hypertensive or had a history of hypertension (n=1642 and n=1887 at Phases 3 and 5, respectively), had cardiovascular disease (n=75 and n=137), diabetes (n=39 and n=62), or missing data on any risk factors (n=313 and n=826). After these exclusions, 4141 participants at Phase 3 and 2779 participants at Phase 5 remained and formed the sample for the analyses.
Figure 1. Flow Chart of Study Design and Analytic Design.
Assessment of Risk Factors and Prevalent Disease
Assessment of risk factors has been described previously.6 Briefly, we measured systolic and diastolic blood pressure twice in the sitting position after 5 minutes rest with the Hawksley random-zero sphygmomanometer (Phases 1 to 5) and OMRON HEM 907 (Phase 7) (hypertension risk prediction was not sensitive to the measure of blood pressure used).6 The average of each of the systolic and diastolic blood pressure readings was used. Current smoking and parental hypertension were self-reported. Weight was measured in underwear to the nearest 0.1 kg on Soehnle electronic scales. Height was measured in bare feet to the nearest 1 mm using a stadiometer with the participant standing erect with head in the Frankfort plane. Body mass index (BMI) was calculated as weight (kilograms)/height (meters) squared.
Prevalent coronary heart disease was defined using MONICA criteria,8 or positive responses to questions about chest pain9 and physician diagnoses, or evidence from medical records, or positive ECG findings. Diabetes was defined as a fasting glucose ≥7.0 mmol/L, a 2-hr postload glucose ≥11.1 mmol/L (75g oral glucose tolerance test), reported doctor-diagnosed diabetes, or use of diabetes medication.10
Assessment of Incident Hypertension
Hypertension was defined according to the 7th report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (systolic/diastolic≥140/90 mmHg or use of antihypertensive medication).1 At both screening cycles, we determined incident (new cases) hypertension by presence of hypertension at follow-up among participants free of this condition at baseline.
Statistical Analysis
Participants were followed across the two screening cycles until incident hypertension or last study phase, whichever came first, contributing to a total of 6920 person-examinations (each participant contributed observations to one or two person-examinations) (Figure 1). As in previous analyses,6 we selected at random 60% of these observations (0, 1 or 2 per participant) for a ‘derivation’ dataset and allocated the remaining 40% of the observations to a ‘validation’ dataset. We developed a risk prediction score based on the derivation data, using the same variables as those used for the Framingham hypertension risk score and, additionally, records of systolic and diastolic blood pressure from the phase preceding the baseline. We identified significant predictors and interaction terms for incident hypertension in multivariable adjusted Weibull regression models for interval censored data. To examine the robustness of this model, we repeated the analysis in a subcohort limited to the participants of the first data cycle only (ie individuals with data on blood pressure history obtained from phase 1, other risk prediction components including the Framingham risk score at phase 3, and incident hypertension status at phase 5).
We calculated a risk prediction score (‘the repeat measure risk score’) for the validation dataset from the β-coefficients obtained from the derivation dataset and calculated the Framingham risk score, using the β-coefficients derived in the Framingham study5 as described previously.6 We tested the performance of the repeat measure risk score and the Framingham risk score in the validation dataset using three methods: first, discrimination based on C-statistics (1 indicates perfect discrimination and 0.5 indicates no discrimination); second, the predicted-to-observed risk ratio calculations and calibration indicated by the Hosmer-Lemeshow chi-square statistics (<20 indicates good calibration); and, third, net reclassification index (NRI) to examine whether prediction based on the Framingham risk score was significantly improved following reclassification based on the repeat measure risk score.11
We then developed two alternative repeat measure risk prediction scores in the derivation dataset: the average blood pressure risk score and the ‘usual’ blood pressure risk score. For the first algorithm, we calculated the average of the current and previous blood pressure measurements from different time points and entered this, instead of current and previous blood pressure measurements, in the risk prediction score. To obtain the latter score, we calculated ‘usual’ systolic and diastolic blood pressures at the previous time point according to the formula: UBPi = BPbm + (RDR × (BPbi - BPbm) where UBPi refers to each participant's usual blood pressure, BPbm to the average blood pressure in the population, RDR to the regression dilution ratio, and BPbi to the participant's blood pressure.12 We derived the regression dilution ratio for a non-hypertensive population by using the mean values of the previous and current blood pressures which were computed within quartiles of the previous blood pressure. The difference in mean blood pressure between the lowest and highest quartiles for the previous blood pressure (Δ1) and the current blood pressures (Δ3) were calculated and their ratio (Δ3/Δ1) used to estimate the regression dilution ratio. We then entered ‘usual’ blood pressure as a component of the risk prediction algorithm in addition the Framingham score variables. We tested the performance of using the average blood pressure and ‘usual’ plus current blood pressure risk scores in the validation dataset in a similar manner to that used for the repeat measure risk score.
All analyses were run with SAS version 9.2.
Results
Table 1 presents clinical features for the baseline participants (those 4141 with Phase 3 as the baseline and additionally those 173 whose first baseline was phase 5) and the derivation and validation subcohorts. As expected, the cohorts were very similar. During the 2 examination cycles (median length from baseline to follow-up 5.8 years), we recorded a total of 1052 incident hypertension cases.
Table 1. Characteristics of the Participants.
| Person-examinations across follow-up | |||
|---|---|---|---|
| Characteristic | Baseline population | Derivation dataset | Validation dataset |
| Number of participants/observations | 4314* | 4135 | 2785 |
| Mean age (SD), y | 48.9 (6.0) | 51.0 (6.4) | 51.0 (6.5) |
| Women, n (%) | 1297 (30) | 1201 (29) | 869 (31) |
| White, n (%) | 4002 (93) | 3854 (93) | 2599 (93) |
| Mean blood pressure (SD), mm Hg | |||
| Systolic | 115.9 (10.3) | 115.7 (10.8) | 115.8 (10.6) |
| Diastolic | 76.5 (7.4) | 75.2 (7.9) | 75.4 (7.6) |
| Prehypertensive, n (%) | 2168 (50.3) | 1968 (47.6) | 1330 (47.8) |
| Current smoker, n (%) | 524 (12) | 471 (11) | 290 (10) |
| Parental hypertension, n (%) | 1489 (35) | 1421 (34) | 917 (33) |
| Mean body mass index (SD), kg/m2 | 24.7 (3.4) | 24.9 (3.4) | 25.0 (3.4) |
| Mean of previous record of blood pressure (SD), mm Hg | |||
| Systolic | 117.7 (10.3) | 116.6 (10.3) | 116.2 (10.1) |
| Diastolic | 73.6 (7.8) | 74.4 (7.6) | 74.3 (7.7) |
| Median (IQR) follow-up for incident hypertension, y | 5.8 (5.5-6.0) | 5.7 (5.4-5.9) | 5.7 (5.3-5.9) |
Includes all participants with phase 3 as the baseline (n = 4141) and additionally those whose first baseline was phase 5 (n = 173).
Repeat Measure Risk Prediction Score
The multivariable-adjusted Weibull β-coefficients for incident hypertension, based on the derivation dataset, showed a significant effect of blood pressure history on hypertension independently of the Framingham score components (please see http://hyper.ahajournals.org, Table S1) and this finding was replicated in a sensitivity analysis of participants from the first data cycle only (Table S2). The coefficients from the derivation dataset were used to calculate the repeat measure risk score for the validation dataset.
The observed 5-year risk of incident hypertension was 13.1 per 100 (438 incident hypertension cases). The C-statistic was 0.796 for the Framingham risk score and 0.799 for the repeat measure risk score, indicating good discrimination for both. The agreement between the predicted and observed incidence of hypertension was also equally good for the Framingham risk score [predicted risk 13.5 per 100, predicted-to-observed ratio 1.04 (95% CI 0.95 to 1.15)] and the repeat measure risk score [12.8 per 100, 0.98 (0.89 to 1.08)]. Hosmer-Lemeshow chi-square values of 11.5 for the Framingham score and 6.5 for the repeat measure risk score were both lower than 20, indicating good calibration.
Table 2 shows the reclassification of individuals between risk categories after replacing the Framingham risk score with the repeat measure risk score. The net reclassification improvement was 9.3% (95% CI 4.2 to 14.4), suggesting that replacing the Framingham risk score with the repeat measure risk score results in a statistically significant improvement in the prediction of incident hypertension. Repeating this analysis with other categorizations of risk led to similar results [for risk categories: <5%, 5%-20%, >20%: NRI 6.5% (2.2 to 10.8); for risk categories: <5%, 5%-10%, >10%: NRI 10.2% (6.7 to 13.8)].
Table 2. Reclassification of the Predicted Risk of Incident Hypertension between Phases of Follow-up, Based on the Framingham vs. Repeated Measures Risk Prediction Score in the Validation Dataset (2785 Observations).
| Predicted 5-year risk (Repeat data) | Reclassified | |||||||
|---|---|---|---|---|---|---|---|---|
| Status at follow-up examination | Predicted 5-year risk (Framingham) | <5% (low) | 5%-10% | 11%-20% | >20% (high) | Increased risk | Decreased risk | Net correctly reclassified (%) |
| Hypertensive | ||||||||
| (N=438) | <5% | 13 | 2 | 0 | 0 | 34 | 68 | -7.8% |
| 5-10% | 11 | 21 | 7 | 2 | ||||
| 11-20% | 2 | 20 | 42 | 23 | ||||
| >20% | 0 | 0 | 35 | 260 | ||||
| Non-hypertensive | ||||||||
| (N=2347) | <5% | 608 | 38 | 1 | 0 | 183 | 584 | 17.1% |
| 5-10% | 227 | 275 | 60 | 0 | ||||
| 11-20% | 27 | 196 | 306 | 84 | ||||
| >20% | 1 | 6 | 127 | 391 | ||||
| Net Reclassification Improvement (95% CI) | 9.3% (4.2 to 14.4) | |||||||
If the >20% predicted 5-year risk of developing hypertension category is used as the criterion to initiate preventive intervention, the risk prediction with repeat measure score would lead to 20.2% (475/2347, Table 2) of the subjects unnecessarily targeted for preventive treatment compared to 22.4% (525/2347) using the Framingham score. Use of the repeat measure score would correctly predicted 65.1% (285/438) of the incident hypertension cases while the corresponding figure for the Framingham score is slightly greater (67.4% (295/438). With a 10%-predicted-risk threshold for the intervention, the corresponding figures for the repeat measure score and the Framingham score would be 41.3% vs. 48.5% (969 vs. 1138 unnecessary treatments) and 84.2% vs. 87.2% (369 vs. 382 correctly targeted treatment).
Risk Prediction Score Based on Average and Usual Blood Pressures
The multivariable-adjusted Weibull β-coefficients for incident hypertension, based on the derivation dataset, showed the effect of average blood pressure on hypertension (please see http://hyper.ahajournals.org, Table S3) to be stronger than those of blood pressure history and baseline blood pressure as separate terms (Table S1). However, the C-statistic of 0.794 and the predicted-to-observed ratio of 0.96 (95% CI 0.88 to 1.06) did not indicate superior predictive performance for the risk score based on average blood pressure compared to the Framingham risk score or the repeat measure risk score. The reclassification improvement of individuals between risk categories after replacing the Framingham risk score with the average blood pressure risk score was 5.8% (95% CI 0.1 to 11.4)(Table S5), suggesting that replacing the Framingham risk score with the average blood pressure risk score results in a modest improvement in the prediction of incident hypertension. However, comparing this risk score which incorporates average blood pressure with the risk score which incorporates current and previous blood pressure as separate terms resulted in a reclassification improvement of -3.4% (95% CI -7.0 to 0.1) (Table S5). This suggests that the explicit use of separate terms for current and previous blood pressure is more beneficial than the use of average blood pressure in the prediction of future hypertension risk. When using ‘usual’ blood pressure, these terms in the risk score gave larger hazard ratios for incident hypertension (Table S4) than those based on observed previous blood pressure (Table S1). However, the predictive performance of using ‘usual’ blood pressure together with the current blood pressure in the risk score gave identical results, in terms of prediction, to those using observed previous and current blood pressure. (Tables 2 and S5).
Discussion
In this study of a large non-hypertensive British population, repeat measures of blood pressure independently predicted the risk of developing hypertension. However, information from repeat measures of blood pressure and use of average or usual blood pressures in the risk algorithm improved indices of calibration and the ability of the Framingham hypertension risk score to discriminate future hypertension events only marginally.
We observed a 9.3% improvement in reclassification of hypertension risk by using past blood pressure measurements in addition to the Framingham risk score variables. This improvement was a result of a 17.1% increase in non-hypertensives correctly identified as being at lower risk but also a 7.8% increase in hypertensives incorrectly identified as at lower risk. Thus, the adoption of the repeated measures risk prediction model would reduce any harm related to unnecessary preventive treatments (e.g., waste of health care resources, side-effects related to the treatment), but increases missed prevention opportunities. The reduction in the number of unnecessary treatments was meaningful only when applying a low (10% rather than 20%) risk threshold for treatment, but it came with the cost of missing 2-3% of patients who actually develop hypertension.
Perspective
This appears to be the first report estimating the clinical utility of adding past blood pressure data to the Framingham hypertension risk score. Despite the statistically significant net reclassification improvement, our findings suggest that incorporating previous blood pressure records or estimates of average or usual blood pressure in the risk score provides relatively limited incremental value to the prediction of the development of hypertension.
Supplementary Material
Acknowledgments
Sources of Funding: Medical Research Council; British Heart Foundation; Wellcome Trust; Health and Safety Executive; Department of Health; Agency for Health Care Policy Research, UK; John D and Catherine T MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health; National Heart, Lung, and Blood Institute (R01HL036310) and National Institute on Aging (R01AG013196 and R01AG034454), NIH, US; Academy of Finland, Finland; BUPA Foundation, UK; and European Science Foundation. GDB is a Wellcome Trust Fellow. AS-M is supported by a “European Young Investigator Award” from the European Science Foundation. MM is supported by an MRC Research Professorship. MJS is supported by the British Heart Foundation.
Footnotes
Conflict(s) of Interest/Disclosure(s) Statement: No author has anything to disclose.
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure. The Trials of Hypertension Prevention, phase II. The Trials of Hypertension Prevention Collaborative Research Group. Arch Intern Med. 1997;157:657–667. [PubMed] [Google Scholar]
- 3.Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, Kaciroti N, Black HR, Grimm RH, Jr, Messerli FH, Oparil S, Schork MA. Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med. 2006;354:1685–1697. doi: 10.1056/NEJMoa060838. [DOI] [PubMed] [Google Scholar]
- 4.He J, Whelton PK, Appel LJ, Charleston J, Klag MJ. Long-term effects of weight loss and dietary sodium reduction on incidence of hypertension. Hypertension. 2000;35:544–549. doi: 10.1161/01.hyp.35.2.544. [DOI] [PubMed] [Google Scholar]
- 5.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D'Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: the Framingham Heart Study. Ann Intern Med. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Kivimäki M, Batty GD, Singh-Manoux A, Ferrie JE, Tabak AG, Jokela M, Marmot MG, Davey Smith G, Shipley MJ. Validating the Framingham Hypertension Risk Score. Results From the Whitehall II Study. Hypertension. 2009;54:496–501. doi: 10.1161/HYPERTENSIONAHA.109.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmot MG, Davey Smith G, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A. Health inequalities among British civil servants: the Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 8.Tunstall-Pedoe H, Kuulasmaa K, Amouyel P, Arveiler D, Rajakangas AM, Pajak A. Myocardial infarction and coronary deaths in the World Health Organization MONICA Project. Registration procedures, event rates, and case-fatality rates in 38 populations from 21 countries in four continents. Circulation. 1994;90:583–612. doi: 10.1161/01.cir.90.1.583. [DOI] [PubMed] [Google Scholar]
- 9.Rose GA, Blackburn H, Gillum RF, Prineas RJ. Cardiovascular Survey Methods. 2nd. Geneva: World Health Organization; 1982. [Google Scholar]
- 10.WHO. Definition, diagnosis and classification of diabetes mellitus and its complications. Geneva: World Health Organization; 1997. [Google Scholar]
- 11.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 12.Gasowski J, Li Y, Kuznetsova T, Richart T, Thijs L, Grodzicki T, Clarke R, Staessen JA. Is “usual” blood pressure a proxy for 24-h ambulatory blood pressure in predicting cardiovascular outcomes. Am J Hypertens. 2008;21:994–1000. doi: 10.1038/ajh.2008.231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.