Abstract
Lysozyme from lambda bacteriophage (λ lysozyme) is an 18 kDa globular protein displaying some of the structural features common to all lysozymes; in particular, λ lysozyme consists of two structural domains connected by a helix, and has its catalytic residues located at the interface between these two domains. An interesting feature of λ lysozyme, when compared to the well-characterised hen egg-white lysozyme, is its lack of disulfide bridges; this makes λ lysozyme an interesting system for studies of protein folding. A comparison of the folding properties of λ lysozyme and hen lysozyme will provide important insights into the role that disulfide bonds play in the refolding pathway of the latter protein. Here we report the 1H, 13C and 15N backbone resonance assignments for λ lysozyme by heteronuclear multidimensional NMR spectroscopy. These assignments provide the starting point for detailed investigation of the refolding pathway using pulse-labelling hydrogen/deuterium exchange experiments monitored by NMR.
Keywords: Backbone resonance assignments, Bacteriophage lambda, Heteronuclear NMR, Lysozyme, Protein folding
Biological context
Lysozymes are widespread in nature and have been used as model systems to study many aspects of protein structure and function, including the mechanism of protein folding and the determinants of protein stability. On the basis of sequence alignments, several classes of lysozymes have been defined among which the best known are the c-type (chicken) and v-type (virus) proteins. Hen egg-white and T4 lysozymes have been studied in detail as typical representatives of the c- and v-type lysozymes, respectively. Despite their lack of statistically significant sequence identity (Matthews et al. 1981) and their different mechanisms (Kuroki et al. 1995), the two enzymes display clear structural similarities and catalyze the same reaction; therefore, they are presumed to have evolved from a common ancestor and they constitute a classical example of divergent evolution (Kuroki et al. 1995; Matthews 1996; Matthews et al. 1981). Matthews and co-workers have studied hundreds of T4 lysozyme mutants, providing an in-depth characterization of the structural and functional properties of this protein (Matthews 1996). Hen lysozyme, on the other hand, has been used as a model system in studies of protein folding using a range of biophysical techniques (Matagne et al. 2000; Miranker et al. 1993; Radford et al. 1992). These studies have provided important insights about the general mechanism of protein folding (Dobson et al. 1994; Matagne and Dobson 1998). Hen lysozyme contains four disulfide bonds and most refolding experiments have been carried out with these bonds intact. Indeed, refolding from the fully reduced form of the protein produces large quantities of aggregated species (Goldberg et al. 1991; van den Berg et al. 1999). Therefore, most of the in vitro experiments with hen lysozyme do not reproduce correctly the process as it occurs in vivo, starting from the fully reduced form of the protein (Gething and Sambrook 1992).
Lysozyme from lambda bacteriophage (λ lysozyme) is composed of 158 amino acid residues (17825 Da). The three-dimensional structure of λ lysozyme, determined by X-ray crystallography (Evrard et al. 1998; Leung et al. 2001), contains some of the structural features common to all lysozymes; in particular, it consists of two structural domains connected by a helix, and has its catalytic residues located at the interface between these two domains. However, sequence alignments indicate only weak local similarities with v- and c-types lysozymes. In common with other lysozymes, λ lysozyme catalyses the cleavage of the glycosidic bond between the C1 of N-acetyl muramic acid (NAM) and the C4 of N-acetyl glucosamine (NAG) in the bacterial peptidoglycan. However, the mechanism is different from that of the other lysozymes; breakage of the β-1,4 bond between the C1 of the NAM and the C4 of the NAG results in transglycosylation and not in hydrolysis, as is usually the case. Another interesting difference between λ and hen lysozyme is the lack of disulfide bridges in λ lysozyme. Therefore, a comparison of the folding properties of λ lysozyme and the already well-characterized hen lysozyme will be of significant interest and will provide important insights into the role that disulfide bonds play in the refolding pathway of the latter protein. Here we report the 1H, 13C, and 15N backbone resonance assignments for λ lysozyme obtained using heteronuclear multidimensional NMR spectroscopy. These serve as the starting point for comparative studies of the refolding of λ and hen lysozyme, using a combination of techniques including pulse-labelling hydrogen/deuterium exchange experiments monitored by NMR.
Methods and experiments
Protein expression and purification
The gene coding for wild-type λ lysozyme (the R gene), kindly provided by Dr. Patrice Soumillion (Université Catholique de Louvain, Belgium), was cloned in a pET22b expression vector (containing the ampicillin resistance gene), for which expression of the recombinant gene is IPTG inducible. The pET22b plasmid was then transformed into an Escherichia coli BL21 (DE3) expression strain (Novagen, WI, USA). Both the uniformly 13C/15N and 15N isotopically enriched protein samples were prepared by growing the bacteria in minimal media, containing 15NH4Cl, with either 13C6-glucose or unlabeled glucose. Purification of λ lysozyme was achieved using a DEAE Sepharose column (GE Healthcare), previously equilibrated with 30 mM phosphate buffer, pH 7.3. The enzyme was eluted in the column flow-through and the fractions containing λ lysozyme were pooled and loaded on an SP Sepharose HP column (GE Healthcare), previously equilibrated with 10 mM HEPES buffer, pH 7 (buffer A). The column was washed with 300 ml of buffer A, and proteins were eluted with buffer A and a linear NaCl gradient (0 to 1 M). The lysozyme-containing fractions were pooled and stored at −20°C in buffer A.
NMR spectroscopy
NMR samples contained ~1 mM protein in 95% H2O/5% D2O at pH 5.45. All NMR spectra were acquired at 293 K using a home-built 750 MHz spectrometer which is controlled with GE/Omega software and is equipped with a home-built triple-resonance pulsed-field-gradient probehead. Sequential assignments were carried out initially using 15N-labelled λ lysozyme and 3D 15N-edited TOCSY-HSQC, NOESY-HSQC and HSQC-NOESY-HSQC experiments; analysis of these spectra resulted in nearly complete assignment of the 1HN and 15N resonances. The sequential assignments obtained from the 15N-labelled sample were confirmed with a 3D HNCA experiment and further 13C and 1H assignments were obtained using 3D HNCO, (H)CC(CO)NH and HCCH-TOCSY experiments.
Extent of assignments and data deposition
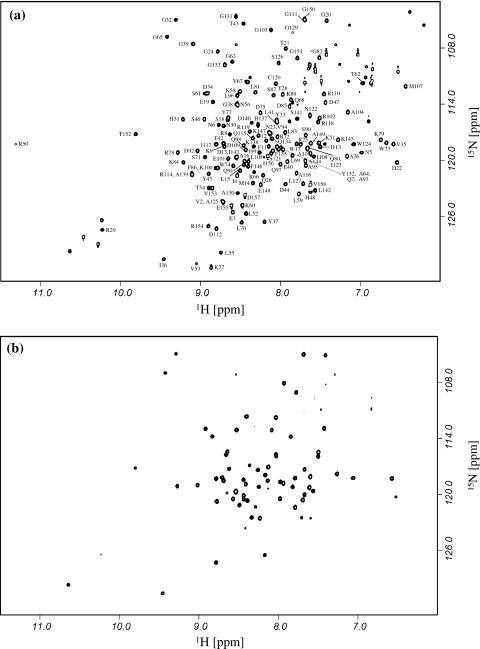
Figure 1a shows the 1H-15N HSQC spectrum of λ lysozyme. 1HN and 15N backbone assignments for all residues except E136 are indicated in Fig. 1a. 15N assignments for the N-terminal residue, M1, and for the 5 proline residues have not been obtained. A total of 95% of the 1Hα, 97% of the 13Cα and 94% of the 13C′ resonances were also assigned; most of the missing assignments correspond to proline residues or residues adjacent to prolines. Figure 2 shows the consensus chemical shift index analysis of Wishart and Sykes (1994) for λ lysozyme; the protein contains both α-helical and β-sheet secondary structure in solution.
Fig. 1.
750 MHz 1H-15N HSQC spectra of 15N-labelled λ lysozyme at pH 5.45, 293K. a Spectrum collected for λ lysozyme in 95% H2O/5% D2O. Peak assignments for backbone amides are indicated. b Spectrum collected for λ lysozyme in 99.9% D2O. The observed peaks correspond to amides that are protected from exchange due to stable hydrogen bonds or significant burial from solvent
Fig. 2.
Consensus chemical shift index (Wishart and Sykes 1994) for λ lysozyme derived from the 1Hα, 13Cα, 13 C′ and a subset of 13Cβ chemical shifts. Regions identified to have α-helical and β-strand secondary structure are indicated
Figure 1b shows the 1H-15N HSQC spectrum of λ lysozyme following exchange of the protein from H2O to D2O. The ~65 backbone amide peaks that are observed in this spectrum correspond to amides that are protected from hydrogen/deuterium exchange as a result of stable hydrogen bonds or significant burial from solvent. The observation of a large number of protected amides, distributed throughout the structure of λ lysozyme, will serve as the basis for residue-specific studies of the folding pathway of λ lysozyme using pulse-labelling hydrogen/deuterium exchange methods.
The chemical shift assignments for λ lysozyme have been deposited in the BioMagResBank (http://www.bmrb.wisc.edu) under the accession number 16664.
Acknowledgments
C.R. is funded by the Wellcome Trust (Grant number 079440). A.M. is funded by the Fonds de la Recherche Fondamentale et Collective (contract numbers 2.4.550.05 and 2.4.530.09) and by the Belgian program of Interuniversity Attraction Poles initiated by the Federal Office for Scientific Technical and Cultural Affairs (PAI n°P6/19). A.D.P. was the recipient of a FRIA (Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture) fellowship. His stay in Oxford was supported by short-term fellowships from the EMBO and the F.R.S.-FNRS (Belgium).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Dobson CM, Evans PA, Radford SE. Understanding how proteins fold: the lysozyme story so far. Trends Biochem Sci. 1994;19:31–37. doi: 10.1016/0968-0004(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Evrard C, Fastrez J, Declercq JP. Crystal structure of the lysozyme from bacteriophage lambda and its relationship with V and C-type lysozymes. J Mol Biol. 1998;276:151–164. doi: 10.1006/jmbi.1997.1499. [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Rudolph R, Jaenicke R. A kinetic study of the competition between renaturation and aggregation during the refolding of denatured-reduced egg white lysozyme. Biochemistry. 1991;30:2790–2797. doi: 10.1021/bi00225a008. [DOI] [PubMed] [Google Scholar]
- Kuroki R, Weaver LH, Matthews BW. Structure-based design of a lysozyme with altered catalytic activity. Nat Struct Biol. 1995;2:1007–1011. doi: 10.1038/nsb1195-1007. [DOI] [PubMed] [Google Scholar]
- Leung AK, Duewel HS, Honek JF, Berghuis AM. Crystal structure of the lytic transglycosylase from bacteriophage lambda in complex with hexa-N-acetylchitohexaose. Biochemistry. 2001;40:5665–5673. doi: 10.1021/bi0028035. [DOI] [PubMed] [Google Scholar]
- Matagne A, Dobson CM. The folding process of hen lysozyme: a perspective from the ‘new view’. Cell Mol Life Sci. 1998;54:363–371. doi: 10.1007/s000180050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matagne A, Jamin M, Chung EW, Robinson CV, Radford SE, Dobson CM. Thermal unfolding of an intermediate is associated with non-Arrhenius kinetics in the folding of hen lysozyme. J Mol Biol. 2000;297:193–210. doi: 10.1006/jmbi.2000.3540. [DOI] [PubMed] [Google Scholar]
- Matthews BW. Structural and genetic analysis of the folding and function of T4 lysozyme. Faseb J. 1996;10:35–41. doi: 10.1096/fasebj.10.1.8566545. [DOI] [PubMed] [Google Scholar]
- Matthews BW, Remington SJ, Grutter MG, Anderson WF. Relation between hen egg white lysozyme and bacteriophage T4 lysozyme: evolutionary implications. J Mol Biol. 1981;147:545–558. doi: 10.1016/0022-2836(81)90399-5. [DOI] [PubMed] [Google Scholar]
- Miranker A, Robinson CV, Radford SE, Aplin RT, Dobson CM. Detection of transient protein folding populations by mass spectrometry. Science. 1993;262:896–900. doi: 10.1126/science.8235611. [DOI] [PubMed] [Google Scholar]
- Radford SE, Dobson CM, Evans PA. The folding of hen lysozyme involves partially structured intermediates and multiple pathways. Nature. 1992;358:302–307. doi: 10.1038/358302a0. [DOI] [PubMed] [Google Scholar]
- van den Berg B, Chung EW, Robinson CV, Dobson CM. Characterisation of the dominant oxidative folding intermediate of hen lysozyme. J Mol Biol. 1999;290:781–796. doi: 10.1006/jmbi.1999.2915. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J Biomol NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]