Abstract
Objective
Hyperplastic polyposis is a colonic polyposis condition of unknown aetiology. The purpose of this study was to examine the spectrum of phenotypic variation in patients with multiple serrated polyps as a basis for gene discovery.
Methods
One hundred and twenty-six patients with multiple (≥5) serrated polyps were recruited to the study. Polyp counts were extracted from histology and colonoscopy reports. Ethnicity was self-reported. Family history of cancer data were derived from pedigrees. Ascertainment status was classified as either index case or identified by screening.
Results
The average reported polyp count was 39. Patients with highest polyp numbers were more likely to be male (P = 0.02). Colorectal cancer (CRC) was identified in 49 of 119 patients (41%) and 28% of these patients had multiple CRC. Young onset patients had higher polyp numbers (P = 0.03) and were more likely to have their CRC in the distal colon (P = 0.02). CRC was significantly associated with the presence of adenomas (P = 0.03). Patients were divided into moderate polyposis (5-79 serrated polyps) and dense polyposis (80 or more) categories. The dense polyposis category was associated with a lack of family history for CRC (P = 0.034) and male gender (P = 0.014), independent of ascertainment status and recruitment site.
Conclusion
Multiple serrated polyps were associated with an increased personal risk of CRC. A subset of patients with the highest polyp numbers was more likely to be male and to have no family history of CRC. This result suggests heterogeneous modes of inheritance and has implications for studies investigating the genetic basis of multiple serrated polyps.
Keywords: Hyperplastic polyposis, Serrated neoplasia, Smoking, Family history, Ethnicity
Introduction
The recognition of the serrated neoplasia pathway has facilitated investigation of the aetiology of the remaining unexplained portion of familial colorectal cancer (CRC) [1, 2]. Analogous to the multiple adenoma syndromes familial adenomatous polyposis and MUTYH-associated polyposis, individuals with hyperplastic polyposis syndrome (HPS) also develop CRC on a background of multiple polyps throughout the colon but in contrast, most of the polyps in HPS demonstrate serrated morphology. HPS was first described in 1977 by Spjut and Estrada [3], frequently presents with features consistent with a genetic predisposition to CRC [4], and is currently defined by the World Health Organization (WHO) criteria as
at least five histologically diagnosed hyperplastic polyps proximal to the sigmoid colon, two of which are greater than 10 mm in diameter OR
any number of hyperplastic polyps occurring proximal to the sigmoid colon in an individual who has a first-degree relative with hyperplastic polyposis OR
more than 30 hyperplastic polyps of any size but distributed throughout the colon [5].
The WHO criteria were originally introduced to distinguish HPS from the common observation of satellite hyperplastic polyps around rectal cancers and diminutive distal colon lesions, and to highlight observations that even small numbers of larger hyperplastic polyps in the proximal colon may be associated with increased malignant potential [6]. However, because such definitions are necessarily stringent and somewhat arbitrary, many individuals and families with multiple hyperplastic polyps will fall short of these criteria [7]. Since the publication of the criteria in 2000, several investigators have proposed modifications to address these limitations. Higuchi and Jass have suggested that atypical serrated polyps (these include sessile serrated adenomas, serrated adenomas and mixed polyps) be included in the total polyp count which can be cumulative over time [8]. Variations on the threshold polyp count have been proposed, with Chow et al. [9], Rashid et al. [10], Hyman et al. [11], and Boparai et al. [12], all favouring a count of 20 hyperplastic polyps, and Carvajal-Carmona [13] using ten, instead of a count of 30 as described by the criteria.
As reflected in the WHO criteria, HPS presents with extensive phenotypic heterogeneity not only with respect to the number and size of polyps, but also with regard to presence of CRC, polyp histology, sex ratios, age of onset, and the presence of a family history of CRC [10, 14–22] (Table 1). The underlying basis for the observed phenotypic heterogeneity is currently unclear; however, an analysis of phenotype in a large series of high-risk patients with multiple serrated polyps may contribute to our understanding of this heterogeneity by identifying patterns of inheritance and relationships between clinical features. In this, the largest such patient group studied to date, we examined the spectrum of presentations as an initial step to establishing a modern classification for this condition upon which to base gene discovery programmes, a procedure best carried out in a high-risk population.
Table 1.
Published series of HPS cases where n was greater than five individuals, highlighting the phenotypic diversity of HPS
| Author | Year | Cases HPS (n) | Mean age at diagnosis (years) | Males (%) | Number of polyps observed | CRC (%) | CRC in proximal colon (%) | Family history of CRC (%) |
|---|---|---|---|---|---|---|---|---|
| Buchanana | 2009 | 126 | 49 | 50 | 5-150 | 40 | 59 | 59 |
| Boparai [12] | 2009 | 77 | 56 | NS | 2-53 | 35 | NS | NS |
| Chow [9] | 2006 | 38 | 44 | 55 | Multiple | 26 | 40 | 50 |
| Carvajal-Carmona [13] | 2007 | 32 | 46 | 66 | multiple | 25 | NS | 59 |
| Renaut [19] | 2001 | 28 | 58 | 54 | Multiple | 29 | NS | 39 |
| Yeoman [40] | 2007 | 24 | 61 | 42 | Multiple | 54 | 84 | 17 |
| Ferrandez [15] | 2004 | 15 | 53 | 66 | Multiple | 0 | NS | 0 |
| Lage [16] | 2004 | 14 | 58 | NS | 19-100 | 43 | 67 | 33 |
| Hyman [11] | 2004 | 13 | 62 | 38 | Multiple | 54 | 71 | 38 |
| Rashid [10] | 2000 | 13 | 58 | 54 | Multiple | 77 | NS | 38 |
| Leggett [17] | 2001 | 12 | 57 | 42 | 30 to >100 | 58 | NS | 17 |
| Rubio [20] | 2006 | 10 | 61 | 80 | 6-159 | 70 | 43 | 10 |
| Spjut [3] | 1977 | 9 | 53 | NS | Multiple | 11 | NS | NS |
| Williams [21] | 1980 | 7 | 37 | 86 | 50-150 | 0 | NS | 14 |
| Torlakovic [30] | 1996 | 6 | 57 | 83 | 50-100 | 67 | NS | NS |
| Place [18] | 1999 | 6 | 60 | 100 | 50-100 | 50 | 100 | 14 |
NS not specified; multiple upper limit of polyp numbers not specified
aCurrent manuscript data
Patients and methods
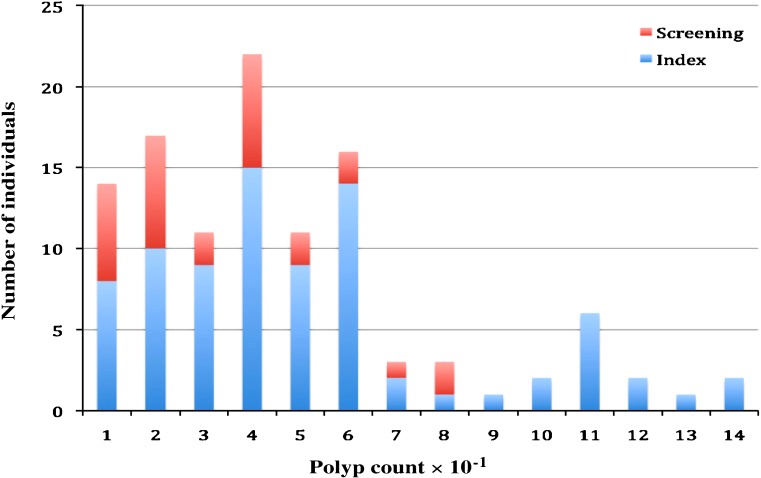
The use of the term serrated polyp in this report encompasses any polyp with serrated architecture [23], and includes both common (hyperplastic or metaplastic) and advanced (serrated adenoma, sessile serrated adenoma, and mixed polyp) lesions. Due to the limitations of the WHO criteria, this cross-sectional study comprised 126 patients with multiple serrated polyps (five or more) recruited from genetics clinics in Australasia (n = 94) and North America (n = 32) regardless of family history of polyps and cancer. Using this approach which targets high-risk patients, we are unlikely to have recruited elderly patients with common distal serrated polyps, whilst at the same time recruiting patients with clinically significant disease who may have fallen short of the current WHO criteria. Thirty-eight patients from Australasia and ten from North America have been reported previously [9, 24]. Clinical and pathology data were extracted from histology reports, minimum polyp counts were derived from serial colonoscopy reports, where available, accounting for polyps removed at each procedure. Patients with polyps demonstrating hamartomatous features were not included in the study. Smoking status was recorded as never/ever and note was made of whether the participants were currently smoking. Patients gave written informed consent to participate in the research. The study was approved by the Human Research Ethics Committee (HREC) of Queensland Institute of Medical Research under the Genetics of Serrated Neoplasia project (QIMR HREC Protocol P912). Self-reported paternal and maternal ethnicity was recorded where available. Colorectal cancers were verified in first-degree relatives (parents, children, and siblings of the proband) using histology reports. Where more than one instance of multiple serrated polyps was present in a family, family history used the youngest proband as a point of reference (n = 6). In 118 families, we were able to classify patients as either index cases (the initial presenting family member n = 89) or screening cases (where multiple serrated polyps were identified as a result of screening due to family history of colonic neoplasia, n = 32). All but two index cases presented due to abdominal symptoms (bleeding, pain, or change in habit). The remaining two cases were diagnosed as an incidental finding during investigations for a hernia repair and an incidental finding at population screening due to age, respectively. Polyp counts in the entire study group, as well as in index cases showed a bimodal distribution and fell into two groups (Fig. 1) about a cut-point polyp count of 80, and were classified as either moderate polyposis (<80 polyps) or dense polyposis (≥80 polyps). All screening cases were observed to be in the moderate polyposis group.
Fig. 1.
Frequency of polyp count numbers showing the distribution of polyp counts in a bimodal pattern about a polyp count of 80 and a concentration of screening cases below this number
Statistical analysis
The associations between categorical predictor variables were assessed for statistical significance using an exact chi-squared test and odds ratios where appropriate. Comparison of means for age and minimum polyp count data were made using t tests. Missing data were excluded from the analysis. Multivariate analysis was used for estimating the odds ratios and 95% confidence intervals for the association between variables and two categorical outcomes of polyp number (moderate versus dense polyp count). All P values were two-sided and a P value <0.05 was considered as statistically significant. SPSS version 17 was used for all statistical analysis. In order to limit the effects of ascertainment bias, any significant findings were re-analysed in the subset of index cases.
Results
Characteristics of multiple serrated polyp patients
Table 2 shows that approximately one-half of patients were female (one patient unreported gender), and almost all were of Northern European ancestry, predominantly English, Irish, Scots, Welsh, French or French-Canadian, German, Swedish and Dutch. Six patients (5%) reported Italian, Greek or Middle-eastern ancestry (two of each). Two patients reported Anglo-Maori and Anglo-Middle-eastern ethnicity, respectively, and a third patient had a Jamaican parent. Two patients from a single family reported European-Russian-Jewish ancestry.
Table 2.
Baseline characteristics of the participants in the study
| Characteristic | Number/mean (SD) | Total | Percentage/range |
|---|---|---|---|
| White Ethnicity (Northern European) | 114 | 120 | 95.0% |
| Sex female | 62 | 125 | 49.6% |
| Age at presentation (years) | 49.0 (13.60) | 124 | 18-86 |
| <50 years | 61 | 124 | 49% |
| ≥50 years | 63 | 124 | 51% |
| Currently smoking | 21 | 87 | 24% |
| Ever smoking | 51 | 88 | 58% |
| Colorectal cancer | 49 | 119 | 41% |
| Proximal CRC | 24 | 41 | 59% |
| Mean reported polyp number | 39 (31.61) | 116 | 5-150 |
| Adenomas | 98 | 115 | 85% |
| Advanced serrated polyps | 47 | 70 | 67% |
| Family history of 1st degree relative with CRC | 63 | 107 | 59% |
| Family history of first or second degree relative with CRC | 66 | 91 | 73% |
Patients from North America did not differ significantly from patients from Australia with respect to rates of CRC. CRC was diagnosed in 41% of patients, the majority of which were proximal (CRC site could not be confirmed in eight patients). Nine of the 24 patients (38%) with a proximal CRC had multiple (two to four) CRCs in the proximal colon and all but one of these were synchronous (Table 3). Multiple proximal CRCs were divided equally between males and females. No multiple CRCs were seen within in the distal colon; however, a further two patients had both a proximal and a distal CRC. Overall, 11 of 40 patients had multiple CRC (28%). Females with CRC were more likely to present with proximal malignancy than with distal CRC, whilst males with CRC were evenly divided between sites.
Table 3.
Univariate analyses of phenotypic features and risk factors in 126 patients
| Exposure | Number/mean (SD) | Total | Percentage/range | Significance (P value) |
|---|---|---|---|---|
| Age at presentation (years) | ||||
| Females | 49 (45.16) | 62 | 19-86 | 0.98 |
| Males | 49 (45.76) | 61 | 18-67 | |
| With CRC | 51(46.50) | 49 | 18-86 | 0.91 |
| Without CRC | 48 (44.89) | 68 | 19-67 | |
| Colorectal cancer | ||||
| Females | 23 | 59 | 39% | 0.58 |
| Males | 26 | 59 | 44% | |
| CRC proximal | 24 | 41 | 59% | |
| Multiple proximal CRC | 9 | 24 | 38% | 0.04 |
| Multiple distal CRC | 0 | 18 | 0% | |
| Female CRC—proximal | 13 | 18 | 72% | 0.12 |
| Male CRC—proximal | 11 | 23 | 48% | |
| CRC Distal | ||||
| <50 years of age | 12 | 20 | 60% | 0.02 |
| ≥50 years of age | 4 | 19 | 22% | |
| Mean reported polyp number | 39 (31.61) | 116 | 5-150 | |
| With CRC | 47 (36.09) | 43 | 5-150 | 0.13 |
| Without CRC | 35 (28.50) | 68 | 5-150 | |
| Females | 34 (27.76) | 56 | 5-150 | 0.44 |
| Males | 44 (34.66) | 59 | 5-150 | |
| Age group <50 years | 45 (36.53) | 55 | 5-150 | 0.03 |
| ≥50 years | 34 (25.84) | 59 | 5-129 | |
| With first degree relative with CRC | 32 (25.65) | 60 | 5-150 | 0.008 |
| Without first degree relative with CRC | 53 (41.29) | 42 | 11-150 | |
| With any degree relative with CRC | 36 (29.78) | 64 | 5-150 | 0.003 |
| Without any degree relative with CRC | 58 (36.58) | 25 | 11-129 | |
| Smoking | ||||
| Current | 51 (37.95) | 21 | 5-129 | 0.43 |
| Not currently smoking | 34 (28.41) | 66 | 5-150 | |
| Ever | 41 (31.53) | 52 | 5-129 | 0.56 |
| Never | 32 (30.52) | 37 | 5-150 | |
| Adenomas | ||||
| CRC | 42 | 43 | 98% | 0.03 |
| Without CRC | 54 | 67 | 81% | |
| Advanced serrated polyps | ||||
| CRC | 19 | 28 | 68% | 0.92 |
| Without CRC | 26 | 39 | 67% | |
| Females | 28 | 38 | 74% | 0.44 |
| Males | 21 | 33 | 64% | |
| <50 years of age | 19 | 31 | 51% | 0.31 |
| ≥50 years of age | 27 | 37 | 73% | |
There was no significant difference in mean age at presentation between the sexes (P = 0.98), or between patients with and without CRC (P = 0.91), however, patients presenting with CRC before age 50 were significantly more likely to have a distal cancer than were patients who presented with a CRC at 50 years or older (60% vs. 22%; P = 0.022), and patients presenting before age 50 had significantly more polyps (45 vs. 34; P = 0.03). Approximately three-quarters of patients had a first- or second-degree relative with CRC. Fifty-nine percent of the patients had a first-degree relative with CRC (60% of females and 59% of males; P = 0.90). Fifty-eight percent of participants had smoked for more than 3 months, and 24% of the participants were currently smoking. There was no difference between the sexes for current smoking status, with 22% of males and 30% of females continuing to smoke.
Many patients (85%) had at least one polyp clearly reported as an adenoma, and CRC was significantly associated with the presence of these lesions (P = 0.03). Specialist pathology review was carried out by two gastrointestinal pathologists (JRJ and NIW) and serrated polyp sub-classification (common vs. advanced serrated polyps) was available on 70 patients. Approximately two-thirds had at least one advanced serrated polyp, and this proportion did not differ between patients with and without CRC. Though advanced serrated polyps were more likely to arise in females and those presenting at 50 years or older, the differences did not attain statistical significance.
Number of serrated polyps
The mean reported number of hyperplastic polyps per patient was 39 (Table 2). We found no difference in mean reported polyp number by sex, CRC, between patients with and without advanced serrated polyps, (Table 3) or between patients from Australia and North America. Mean reported polyp count was 40% lower in patients with a first-degree relative with CRC (32, 95% CI 26-39) compared to patients without a first-degree relative with CRC (53, 95% CI 41-64; P = 0.008). Moreover, there was also a significant difference in the mean reported polyp count between the patients with any first- or second-degree relative with CRC (36, 95% CI 29-44) and without any relative with CRC (58, 95% CI 43-73; P = 0.003). Current smokers had a higher reported polyp count (51 vs. 34; P = 0.043).
Ascertainment issues
Where information could be obtained, index cases comprised 89/122 multiple serrated polyp patients (73%) and the characteristics of these patients and those identified due to screening (n = 32, 27%) are shown in Table 4. When gender and family history of CRC were analysed in the first presenting patient in a family, mean reported polyp count was significantly lower in females (39 vs. 48; P = 0.047) and in patients with a first-degree relative with CRC (34 vs. 55; P = 0.026) though in both cases, the variance was large. Consistent with these findings, the mean reported polyp count in individuals screened because of family history was low, as was the rate of CRC in these asymptomatic patients. In three screening cases, patients were examined due to non-malignant colonic neoplasia in a relative, and all of these had relatively high polyp counts.
Table 4.
Comparison of case characteristics according to ascertainment (index vs. screening cases)
| Variable | Number (%) Index case | P value | Number (%) Screening case | P value | |
|---|---|---|---|---|---|
| Gender | Male | 49 (55) | 13 (41) | ||
| Female | 40 (45) | NA | 19 (59) | 0.22 | |
| CRC | Yes | 37 (43) | 09 (31) | ||
| No | 48 (57) | NA | 20 (69) | 0.28 | |
| FDR with CRC | Yes | 36 (48) | 24 (88) | ||
| No | 39 (52) | NA | 03 (12)a | 0.0002b | |
| Variable | Mean (SD) | Mean (SD) | |||
| Age | 48 (14) | NA | 50 (12) | 0.49 | |
| Mean polyp count | 44 (34) | NA | 27 (21) | 0.048 | |
| Mean polyp count | Male | 48 (36) | 0.047 | 27 (20) | 0.82 |
| Mean polyp count | Female | 39 (30) | 26 (22) | ||
| Mean polyp count | FDR with CRC | 34 (28) | 0.026 | 26 (19) | 0.49 |
| Mean polyp count | No FDR with CRC | 55 (37) | 43 (29) | ||
aCases screened due to family history of colonic neoplasia (non-malignant, n = 3)
bP value from two-sided Fisher’s exact test
In 47 families, Lynch syndrome could be excluded by immunohistochemistry for mismatch repair genes coupled with BRAF mutation testing. In two families, both Lynch syndrome and multiple serrated neoplasms segregated independently, and therefore family history of cancer information from these two families was not considered in this study. These two families have been described in detail as case reports elsewhere [25].
Polyp number category
Seventy-three percent (n = 66) had a reported number of between five and 79 serrated polyps (moderate polyposis), and the remainder had 80 or more (dense polyposis; Table 5). Females were significantly under-represented in the dense polyposis group (P = 0.02). Family history of CRC was inversely associated with polyp count category with the dense polyposis group having significantly less family history than those with moderate polyposis (P = 0.049). Though the rate of CRC did not differ between the two groups, patients presenting with CRC in the dense polyposis group were more likely to have a distal CRC. In contrast, proximal CRC predominated in the moderate polyposis group. Patients who met WHO criterion 3 for HPS (>30 hyperplastic polyps) were also significantly less likely to have a family history of CRC (P = 0.04), though no male predominance was seen, nor were distal CRC more common. Current smokers were more prevalent in the higher polyp count categories for both classifications but this was not statistically significant (P = 0.39 and 0.44). Multivariate analysis indicated that both the male predominance and the lack of family history of CRC were independent predictors of dense polyposis (Table 6).
Table 5.
Univariate analysis of risk variables for a moderate or dense serrated polyp count in the index cases
| Moderate (5-79 polyps) | Dense (80 polyps or more) | ||
|---|---|---|---|
| n = 66 | n = 14 | ||
| Mean (SD) | Mean (SD) | P value | |
| Age (years) | 48.2 (13.6) | 44.5 (15.1) | 0.36a |
| N (%) | N (%) | OR (95% CI), P value | |
| Gender | |||
| Female | 34 (50%) | 2 (14%) | 6.0 (1.25-28.86), |
| Male | 34 (50%) | 12 (86%) | 0.018b |
| CRC | |||
| No | 41 (64%) | 6 (43%) | 2.38 (0.73-7.7) |
| Yes | 23 (36%) | 8 (57%) | 0.23b |
| CRC Site | |||
| Proximal | 13 (62%) | 1 (17%) | 8.13 (0.79-82.73) |
| Distal | 8 (38%) | 5 (83%) | 0.077b |
| First degree relative with CRC | |||
| No | 27 (47%) | 10 (77%) | 0.26 (0.07-1.05) |
| Yes | 31 (53%) | 3 (23%) | 0.066b |
| First or second degree relative with CRC | |||
| No | 16 (29%) | 8 (62%) | 0.25 (0.07-0.88) |
| Yes | 40 (71%) | 5 (38%) | 0.049b |
aIndependent samples t test for comparison of age at presentation between moderate and dense polyp groups
bP value from two-sided Fisher’s exact test
Table 6.
Logistic regression analysis of putative predictors of a dense serrated polyp count in the index cases only
| Dense polyp group (80-150 polyps) | OR | 95% Confidence interval | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Male gender | 9.5 | 1.58 | 57.12 | 0.014 |
| First degree relative with CRC | 0.17 | 0.033 | 0.87 | 0.034 |
| Occurrence of CRC | 1.94 | 0.44 | 8.56 | 0.38 |
The moderate polyp group (5-79 polyps) was the reference group with age, ethnicity, and recruitment site included as co-variates in the final analysis of main effects
Discussion
In this study, we examined the spectrum of phenotypic features in a large group of patients with multiple serrated polyps recruited from genetics clinics. Our results demonstrate significant phenotypic heterogeneity and suggest that both personal and familial risks of CRC in these patients are associated with multiple factors.
Multiple serrated polyp patients demonstrated a high level of CRC risk, with 41% of participants presenting with CRC, a figure consistent with the findings of multiple reports [9–11, 16, 17, 19, 20, 22]. In contrast, two reports have suggested that HPS is not associated with an increased risk of CRC [15, 21]. This may reflect variation in the prevalences and exposures of genetic and environmental risk factors, respectively, within these local populations. Though polyp numbers were higher in patients with CRC [12], neither polyp numbers, nor the presence of advanced serrated polyps significantly determined whether a patient presented with CRC. Consistent with a previous report however, CRC was significantly associated with the presence of adenomas [20]. Dysplasia in co-existing polyps has also been reported previously as a risk factor for CRC [17]. Adenomas are common in the general population and it is likely that the factors which contribute to the growth and development of serrated polyps in these patients also act to increase the growth of a limited number of micro-adenomas [26].
CRC arose in the proximal colon more often than would be expected [27], with proximal cancers arising more frequently in females. A high rate of multiple synchronous CRCs was observed, with multiplicity significantly more common in the proximal colon when compared to the distal colon. In CRC arising via the serrated pathway, multiplicity and proximal location have been previously reported as associations [27, 28]. However, in patients less than 50 years, CRCs were more likely to be distal, which has been reported recently as a feature of young-onset CRC in general [29]. One of the largest HPS series reported to date (38 patients with a mean age at presentation of 44 years) found that 60% of CRC arose in distal sites [9]. This is an important finding as the emphasis placed on the proximal colon by the current WHO criteria may serve to distract from the risk of CRC in the young onset patient with multiple distal lesions whose presentation falls short of the current criteria.
A slight male preponderance of 58% can be calculated overall from the published reports on HPS [9–11, 13, 14, 16–22, 30], and notably the St. Mark’s study of 1980 suggested that HPS was a disorder of young males [21]. No evidence of a male bias for multiple serrated polyps was observed in our 126 patients, displaying an approximately equal sex ratio when analysed overall. However, considerable differences were observed when this group of multiple serrated polyp patients was stratified. The group with the highest polyp numbers and the fewest relatives affected with CRC was predominantly male, and this is consistent with at least two previous reports, and may explain the results of the St. Mark’s series of 1980 [21]. The question arises as to whether these two results regarding gender and family history of CRC might reflect ascertainment bias. It could be postulated that individuals screened because of CRC in a relative may be more likely to have a lower polyp count because they are asymptomatic, and females may be more likely to present for screening. However, relationships between polyp count and both gender and family history of CRC demonstrated this same pattern when only index cases were examined. As would therefore be expected, asymptomatic cases screened because of family history of colonic neoplasia demonstrated lower polyp counts and a low rate of CRC.
In this study, we found a significant increase in polyp numbers associated with direct current exposure to cigarette smoke, and suggests that rather than having a causal role in HPS, smoke exposure may be associated with modulation of phenotype, as manifested by increased polyp numbers. This enhancement of phenotype was not unexpected as population-based studies have consistently shown that the association between smoking and colorectal cancer was largely due to the strong effect of smoking on the subset of CRC which arose through the serrated neoplasia pathway [10, 31–33]. Smokers are more likely to present with hyperplastic polyps, even in asymptomatic individuals [34], with the most significant association between smokers and serrated neoplasia in the population having both hyperplastic and adenomatous polyps [35–37], a phenotype shared with individuals with HPS. As has been demonstrated in this report, multiple serrated polyps and CRC in a background of multiple serrated polyps, both can occur in the absence of smoking further supporting the premise that smoking is likely to be a modifier of phenotype rather than a causal factor. The two most significant predictors of increased polyp number were male sex and lack of family history of CRC, and this was independent of smoking status.
A family history of CRC in first-degree relatives has been reported in HPS by several authors [9–11, 19]. In our study, patient recruitment was effected through high-risk clinics, rather than through population-based screening, and even though our findings for the rate of first-degree relatives with CRC however are completely consistent with an independent large series from the UK describing 32 HPS patients [13], this method of recruitment is likely to have inflated the risk of having affected relatives. Importantly, 40% of the patients in this study did not have a first-degree relative with CRC, suggesting that patients with multiple serrated polyps from genetics clinics are equally likely to be referred in the absence of a first-degree family history of CRC. Our current study demonstrated that family history of CRC was relatively low in the group of patients with very high polyp counts, thus highlighting the possibility of genetic heterogeneity as a basis for the phenotypic variation observed in this condition. Though this finding emerged from a stratified analysis, separation of the predispositions was incomplete; suggesting that predisposition to serrated neoplasia may be a complex genetic condition.
In summary, our data support the notion, previously proposed by multiple authors [6, 10, 30, 38, 39], that patients with multiple serrated polyps have a heterogeneous condition. The clinical importance in identifying HPS is highlighted by the observation that 40% of HPS patients in our cohort presented with CRC, frequently in the proximal colon with an increased risk of multiplicity. Also of clinical importance is the risk of CRC in relatives which is likely to exceed that of the population, though quantitation of this risk remains problematical [6, 39]. The study demonstrated that young-onset patients with multiple serrated polyps are more likely to develop a distal CRC despite the emphasis placed on proximal disease by the WHO criteria. Importantly, the study has presented evidence for consideration potentially highlighting heterogeneity in the mode of inheritance of serrated polyp predisposition, with the identification of a younger-onset group with highest polyp counts, male gender and little family history of CRC, features alluded to in an influential publication in 1980 [21]. The novel finding that family history varies inversely with polyp count and gender, is unlikely to be confounded by age, and is independent of ascertainment and smoking, and suggests that patients with multiple serrated polyps have a complex condition modified by a multitude of genetic, environmental, and sex-specific factors. The development of new clinical criteria with a biological basis [13] to better define this predisposition and to identify those with the highest personal and familial risks of CRC are warranted.
Acknowledgments
This work was supported by grants from the Cancer Council Queensland, the Hicks Foundation in Victoria, and the National Cancer Institute 1R01CA123010 (Genetics of Serrated Neoplasia). The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute nor does mention of trade names, commercial products, or organisations imply endorsement by the US Government. JY is a Cancer Council Queensland Senior Research Fellow. MD and EC were Hicks Foundation in Victoria Fellows.
Declaration
The authors have no conflict of interest to declare with respect to this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
An erratum to this article is available at http://dx.doi.org/10.1007/s00384-010-1055-x.
References
- 1.Kampman E. A first-degree relative with colorectal cancer: what are we missing? Cancer Epidemiol Biomarkers Prev. 2007;16:1–3. doi: 10.1158/1055-9965.EPI-06-0984. [DOI] [PubMed] [Google Scholar]
- 2.Sinicrope FA. Insights into familial colon cancer: the plot thickens. Clin Gastroenterol Hepatol. 2005;3:216–217. doi: 10.1016/S1542-3565(04)00719-0. [DOI] [PubMed] [Google Scholar]
- 3.Spjut H, Estrada RG. The significance of epithelial polyps of the large bowel. Pathol Annu. 1977;12(Pt 1):147–170. [PubMed] [Google Scholar]
- 4.Young J, Jenkins M, Parry S, Young B, Nancarrow D, English D, Giles G, Jass J. Serrated pathway colorectal cancer in the population: genetic consideration. Gut. 2007;56:1453–1459. doi: 10.1136/gut.2007.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt R, Jass JR. Hyperplastic polyposis. In: Hamilton SR, Aaltonen LA, editors. Pathology and genetics of tumours of the digestive system. Lyon: IARC Press; 2000. pp. 135–136. [Google Scholar]
- 6.Jass JR. Colorectal polyposes: from phenotype to diagnosis. Pathol Res Prac. 2008;204(7):431–447. doi: 10.1016/j.prp.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Young J, Barker MA, Simms LA, Walsh MD, Biden KG, Buchanan D, Buttenshaw R, Whitehall VL, Arnold S, Jackson L, Kambara T, Spring KJ, Jenkins MA, Walker GJ, Hopper JL, Leggett BA, Jass JR. Evidence for BRAF mutation and variable levels of microsatellite instability in a syndrome of familial colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:254–263. doi: 10.1016/S1542-3565(04)00673-1. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi T, Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682–686. doi: 10.1136/jcp.2003.015230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow E, Lipton L, Lynch E, D’Souza R, Aragona C, Hodgkin L, Brown G, Winship I, Barker M, Buchanan D, Cowie S, Nasioulas S, du Sart D, Young J, Leggett B, Jass J, Macrae F. Hyperplastic polyposis syndrome: phenotypic presentations and the role of MBD4 and MYH. Gastroenterology. 2006;131:30–39. doi: 10.1053/j.gastro.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 10.Rashid A, Houlihan PS, Booker S, Petersen GM, Giardiello FM, Hamilton SR. Phenotypic and molecular characteristics of hyperplastic polyposis. Gastroenterology. 2000;119:323–332. doi: 10.1053/gast.2000.9361. [DOI] [PubMed] [Google Scholar]
- 11.Hyman NH, Anderson P, Blasyk H. Hyperplastic polyposis and the risk of colorectal cancer. Dis Colon Rectum. 2004;47:2101–2104. doi: 10.1007/s10350-004-0709-6. [DOI] [PubMed] [Google Scholar]
- 12.Boparai KS, Mathus-Vliegen EM, Koornstra JJ, Nagengast FM, van Leerdam M, van Noesel CJ, Houben M, Cats A, van Hest LP, Fockens P, Dekker E (2009) Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut [DOI] [PubMed]
- 13.Carvajal-Carmona LG, Howarth KM, Lockett M, Polanco-Echeverry GM, Volikos E, Gorman M, Barclay E, Martin L, Jones AM, Saunders B, Guenther T, Donaldson A, Paterson J, Frayling I, Novelli MR, Phillips R, Thomas HJ, Silver A, Atkin W, Tomlinson IP. Molecular classification and genetic pathways in hyperplastic polyposis syndrome. J Pathol. 2007;212:378–385. doi: 10.1002/path.2187. [DOI] [PubMed] [Google Scholar]
- 14.Bengoechea O, Martinez-Penuela JM, Larrinaga B, Valerdi J, Borda F. Hyperplastic polyposis of the colorectum and adenocarcinoma in a 24-year-old man. Am J Surg Pathol. 1987;11:323–327. doi: 10.1097/00000478-198704000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ferrandez A, Samowitz W, DiSario JA, Burt RW. Phenotypic characteristics and risk of cancer development in hyperplastic polyposis: case series and literature review. Am J Gastroenterol. 2004;99:2012–2018. doi: 10.1111/j.1572-0241.2004.30021.x. [DOI] [PubMed] [Google Scholar]
- 16.Lage P, Cravo M, Sousa R, Chaves P, Salazar M, Fonseca R, Claro I, Suspiro A, Rodrigues P, Raposo H, Fidalgo P, Nobre-Leitao C. Management of Portuguese patients with hyperplastic polyposis and screening of at-risk first-degree relatives: a contribution for future guidelines based on a clinical study. Am J Gastroenterol. 2004;99:1779–1784. doi: 10.1111/j.1572-0241.2004.30178.x. [DOI] [PubMed] [Google Scholar]
- 17.Leggett BA, Devereaux B, Biden K, Searle J, Young J, Jass J. Hyperplastic polyposis: association with colorectal cancer. Am J Surg Pathol. 2001;25:177–184. doi: 10.1097/00000478-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Place RJ, Simmang CL. Hyperplastic-adenomatous polyposis syndrome. J Am Coll Surg. 1999;188:503–507. doi: 10.1016/S1072-7515(99)00019-8. [DOI] [PubMed] [Google Scholar]
- 19.Renaut AJ, Douglas PR, Newstead GL. Hyperplastic polyposis of the colon and rectum. Colorectal Dis. 2002;4:213–215. doi: 10.1046/j.1463-1318.2002.00354.x. [DOI] [PubMed] [Google Scholar]
- 20.Rubio CA, Stemme S, Jaramillo E, Lindblom A. Hyperplastic polyposis coli syndrome and colorectal carcinoma. Endoscopy. 2006;38:266–270. doi: 10.1055/s-2006-925026. [DOI] [PubMed] [Google Scholar]
- 21.Williams GT, Arthur JF, Bussey HJ, Morson BC. Metaplastic polyps and polyposis of the colorectum. Histopathology. 1980;4:155–170. doi: 10.1111/j.1365-2559.1980.tb02909.x. [DOI] [PubMed] [Google Scholar]
- 22.Yeoman A, Young J, Arnold J, Jass J, Parry S. Hyperplastic polyposis in the New Zealand population: a condition associated with increased colorectal cancer risk and European ancestory. NZMJ. 2007;120:31–39. [PubMed] [Google Scholar]
- 23.Jass JR. Serrated adenoma of the colorectum and the DNA-methylator phenotype. Nat Clin Prac. 2005;2:398–405. doi: 10.1038/ncponc0248. [DOI] [PubMed] [Google Scholar]
- 24.Sweet K, Willis J, Zhou XP, Gallione C, Sawada T, Alhopuro P, Khoo SK, Patocs A, Martin C, Bridgeman S, Heinz J, Pilarski R, Lehtonen R, Prior TW, Frebourg T, Teh BT, Marchuk DA, Aaltonen LA, Eng C. Molecular classification of patients with unexplained hamartomatous and hyperplastic polyposis. Jama. 2005;294:2465–2473. doi: 10.1001/jama.294.19.2465. [DOI] [PubMed] [Google Scholar]
- 25.Walsh MD, Buchanan DD, Walters R, Roberts A, Arnold S, McKeone D, Clendenning M, Ruszkiewicz AR, Jenkins MA, Hopper JL, Goldblatt J, George J, Suthers GK, Phillips K, Young GP, Macrae F, Drini M, Woods MO, Parry S, Jass JR, Young JP. Analysis of families with Lynch syndrome complicated by advanced serrated neoplasia: the importance of pathology review and pedigree analysis. Fam Can. 2009;8(4):313–323. doi: 10.1007/s10689-009-9238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jass JR. Relation between metaplastic polyp and carcinoma of the colorectum. Lancet. 1983;1:28–30. doi: 10.1016/S0140-6736(83)91564-7. [DOI] [PubMed] [Google Scholar]
- 27.Jass JR, Do KA, Simms LA, Iino H, Wynter C, Pillay SP, Searle J, Radford-Smith G, Young J, Leggett B. Morphology of sporadic colorectal cancer with DNA replication errors. Gut. 1998;42:673–679. doi: 10.1136/gut.42.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeevaratnam P, Cottier DS, Browett PJ, Van De Water NS, Pokos V, Jass JR. Familial giant hyperplastic polyposis predisposing to colorectal cancer: a new hereditary bowel cancer syndrome. J Pathol. 1996;179:20–25. doi: 10.1002/(SICI)1096-9896(199605)179:1<20::AID-PATH538>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Yantiss RK, Goodarzi M, Zhou XK, Rennert H, Pirog EC, Banner BF, Chen YT. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol. 2009;33:572–582. doi: 10.1097/PAS.0b013e31818afd6b. [DOI] [PubMed] [Google Scholar]
- 30.Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748–755. doi: 10.1053/gast.1996.v110.pm8608884. [DOI] [PubMed] [Google Scholar]
- 31.Samowitz WS, Albertsen H, Sweeney C, Herrick J, Caan BJ, Anderson KE, Wolff RK, Slattery ML. Association of smoking, CpG island methylator phenotype, and V600E BRAF mutations in colon cancer. J Natl Cancer Inst. 2006;98:1731–1738. doi: 10.1093/jnci/djj468. [DOI] [PubMed] [Google Scholar]
- 32.Slattery ML, Curtin K, Anderson K, Ma KN, Ballard L, Edwards S, Schaffer D, Potter J, Leppert M, Samowitz WS. Associations between cigarette smoking, lifestyle factors, and microsatellite instability in colon tumors. J Natl Cancer Inst. 2000;92:1831–1836. doi: 10.1093/jnci/92.22.1831. [DOI] [PubMed] [Google Scholar]
- 33.Slattery ML, Curtin K, Sweeney C, Levin TR, Potter J, Wolff RK, Albertsen H, Samowitz WS. Diet and lifestyle factor associations with CpG island methylator phenotype and BRAF mutations in colon cancer. Int J Cancer. 2007;120:656–663. doi: 10.1002/ijc.22342. [DOI] [PubMed] [Google Scholar]
- 34.Peppone LJ, Mahoney MC, Cummings KM, Michalek AM, Reid ME, Moysich KB, Hyland A. Colorectal cancer occurs earlier in those exposed to tobacco smoke: implications for screening. J Cancer Res Clin Oncol. 2008;134(7):743–751. doi: 10.1007/s00432-007-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji BT, Weissfeld JL, Chow WH, Huang WY, Schoen RE, Hayes RB. Tobacco smoking and colorectal hyperplastic and adenomatous polyps. Cancer Epidemiol Biomarkers Prev. 2006;15:897–901. doi: 10.1158/1055-9965.EPI-05-0883. [DOI] [PubMed] [Google Scholar]
- 36.Morimoto L, Newcomb P, Ulrich C, Bostick R, Lais C, Potter J. potential? Rffhaapefm. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 37.Shrubsole MJ, Wu H, Ness RM, Shyr Y, Smalley WE, Zheng W. Alcohol drinking, cigarette smoking, and risk of colorectal adenomatous and hyperplastic polyps. Am J Epidemiol. 2008;167(9):1050–1058. doi: 10.1093/aje/kwm400. [DOI] [PubMed] [Google Scholar]
- 38.Burt RW, Samowits WS. Serrated adenomatous polyposis: a new syndrome? Gastroenterology. 1996;110:950–952. doi: 10.1053/gast.1996.v110.agast960950. [DOI] [PubMed] [Google Scholar]
- 39.Jass JR. Gastrointestinal polyposes: clinical, pathological and molecular features. Gastroenterol Clin North Am. 2007;36:927–946. doi: 10.1016/j.gtc.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 40.Yeoman A, Young J, Arnold J, Jass J, Parry S. Hyperplastic polyposis in the New Zealand population: a condition associated with increased colorectal cancer risk and European ancestry. N Z Med J. 2007;120:U2827. [PubMed] [Google Scholar]