Abstract
Rationale
Nicotine replacement is commonly used to treat tobacco use in heavy-drinking smokers. However, few studies have examined the effect of nicotine replacement on subjective and physiological responses to alcohol and alcohol drinking behavior.
Objective
The primary aim of this within-subject, double-blind study was to examine whether transdermal nicotine replacement (0 mg vs 21 mg/day) altered response to a low-dose priming drink and subsequent ad libitum drinking behavior.
Materials and methods
Subjects (n=19) were non-treatment-seeking, non-dependent heavy drinkers who were daily smokers. Six hours after transdermal patch application, subjective and physiological responses to a priming drink [designed to raise blood alcohol levels (BALs) to 0.03 g/dl] were assessed. This was followed by a 2-h self-administration period where subjects could choose to consume up to eight additional drinks (each designed to raise BALs by 0.015 g/dl) or to receive monetary reinforcement for drinks not consumed.
Results
We found that 6 h after patch application, tobacco craving associated with withdrawal relief was decreased, and systolic blood pressure and heart rate were increased in the active patch condition compared to the placebo patch condition. Subjective intoxication in response to the priming drink was attenuated in the active nicotine patch condition compared to 6 h of nicotine deprivation (i.e., placebo patch). During the self-administration period, subjects had longer latencies to start drinking and consequently appeared to consume fewer drinks when administered the active patch compared to the placebo patch.
Conclusions
In heavy drinkers, transdermal nicotine replacement compared to mild nicotine deprivation attenuated subjective and physiological alcohol responses and delayed the initiation of drinking.
Keywords: Transdermal nicotine replacement, Alcohol, Drinking, Self-administration, Craving, Monetary reinforcement
Introduction
Given the substantial health risks associated with concurrent alcohol and tobacco use (Hurt et al. 1996; Rosengren et al. 1993; Blot et al. 1988; Klatsky and Armstrong 1992; Vaillant et al. 1991), there has been a growing effort to address smoking cessation in those experiencing alcohol problems. Most studies have been conducted with alcohol-dependent drinkers (Burling et al. 1991; Joseph et al. 1990; Hurt et al. 1994), and less is known about how to promote smoking cessation in non-dependent heavy drinkers who represent the majority of those with problems resulting from alcohol consumption (Institute of Medicine 1990). Moreover, little is known about what effects standard smoking cessation treatments have on alcohol consumption in non-dependent heavy drinking smokers. Alcohol use has been shown to promote smoking relapse across studies using different methodologies (Baer and Lichenstein 1988; McKee et al. 2006; Shiffman 1986; Shiffman et al. 1996; Zimmerman et al. 1990). One way in which smoking cessation medications could promote quitting behavior in this population is by reducing alcohol responses and consumption, thereby attenuating the ability of alcohol to prompt smoking relapse.
While a number of studies document that alcohol reliably increases smoking behavior (e.g., Glautier et al. 1996; Griffiths et al. 1976; Mintz et al. 1985; Mitchell et al. 1995; Shiffman et al. 1994) and potentiates nicotine reward (Rose et al. 2004), relatively fewer studies examine the impact of nicotine on alcohol self-administration. In preclinical studies, acute administrations of nicotine decreased alcohol self-administration behavior (Katner et al. 1997; Nadel et al. 1998), whereas chronic administration of nicotine increased alcohol intake in alcohol-experienced rats (Blomqvist et al. 1996; Clark et al. 2001; Lê et al. 2000; Smith et al. 1999; Soderpalm et al. 2000). Nicotine has also been shown to facilitate the acquisition of alcohol self-administration behavior in rats (Smith et al. 1999) and reinstate alcohol seeking behavior (Lê et al. 2003).
Human laboratory studies have examined the effect of smoked tobacco on alcohol use (Barrett et al. 2006; Madden et al. 1995; Mello et al. 1980, 1987) as well as the effect of nicotine deprivation on the reinforcing value of alcohol (Colby et al. 2004; Cooney et al. 2003; Palfai et al. 2000; Perkins et al. 2000a). Fewer studies have tested the effect of nicotine replacement on subjective alcohol responses (Cooney et al. 2001; Kouri et al. 2004; Perkins et al. 1995) and alcohol self-administration behavior (Acheson et al. 2006). Cooney et al. (2001) found that in a sample of abstinent alcoholics who had quit smoking, those receiving nicotine patch had lower cravings for alcohol compared to those not receiving nicotine patch. Perkins et al. (1995) examined the acute effects of fixed doses of alcohol (0, 0.5 g/kg) and nicotine nasal spray (0, 1.3 mg/kg) on subjective intoxication and cardiovascular responses in daily smokers who were light to moderate drinkers after overnight abstinence. Nicotine was found to attenuate the effect of alcohol on intoxication ratings and produced additive effects with regard to cardiovascular responses. Kouri et al. (2004) examined the effect of transdermal nicotine patch (21 mg/day), compared to overnight nicotine deprivation, on responses to acute doses of alcohol (0.4, 0.7 mg/kg) in moderate to heavy-drinking daily-smoking males. Nicotine and alcohol additively increased heart rate response, similar to findings produced by others (Benowitz et al. 1986; Michel and Battig 1989; Perkins et al. 1995). However, in contrast to findings produced by Perkins et al. (1995), nicotine was found to increase ratings of subjective intoxication, alcohol craving, and tobacco craving.
The only study to examine the effect of nicotine replacement on alcohol self-administration behavior did so in light-smoking social drinkers. Acheson et al. (2006) examined the effect of transdermal nicotine replacement (0, 7, 14 mg/day) on responses to an initial priming drink (0.2 g/kg) and subsequent alcohol self-administration behavior in light-smoking social drinkers (one to ten cigarettes per day; at least four drinks per week). The 2-h self-administration procedure allowed subjects to purchase up to eight additional beverages (0.1 g/kg each), using an individually determined monetary amount, equivalent to a standard drink (see Young et al. 2005). Testing occurred in small groups, and results demonstrated that the priming drink increased heart rate and ratings of desire for additional alcohol regardless of nicotine condition. However, during the self-administration phase, nicotine replacement increased drinking behavior in men, but decreased drinking behavior in women.
Based on these findings, the relationship between nicotine replacement, alcohol responses, and self-administration behavior is complex and appears to be influenced by the degree of experience with alcohol and nicotine, type and dose of nicotine replacement, dose of alcohol, gender, and availability of alternate reinforcers. For the current study, we were interested in examining the effect of transdermal nicotine replacement on subjective and physiological responses to a low-dose priming drink of alcohol and subsequent self-administration behavior in a sample of heavy-drinking daily smokers. While alcohol–nicotine interactions have been documented in lighter users (e.g., Barrett et al. 2006), it has been suggested that the nature of these interactions may be more pronounced in those with heavier use (e.g., Henningfield et al. 1984; Mello et al. 1987). We enrolled individuals with heavier patterns of alcohol and cigarette use (15–25 cigarettes per day; at least nine drinks per week for females; at least 12 drinks per week for males) than the Acheson et al. (2006). Similar to the Acheson et al. (2006) we used an alcohol self-administration paradigm that has demonstrated sensitivity for detecting medication effects (see O’Malley et al. 2002), that includes money as an alternative reinforcer to provide some incentive for not drinking and to enhance the likelihood that the effects of nicotine on the relative reinforcing value of alcohol would be detected (see Higgins 1997; Rodefer et al. 1997).
Heavy-drinking daily smokers completed two laboratory sessions (0, 21 mg/day transdermal nicotine patch) in which they were first exposed to a low-dose alcohol drink [designed to raise blood alcohol levels (BALs) to 0.03 g/dl and prime drinking behavior] followed by a 2-h self-administration period where subjects could choose to consume up to eight additional drinks (each designed to raise BALs by 0.015 g/dl) or to receive monetary reinforcement for drinks not consumed. Drinking topography and the number of drinks consumed during the self-administration period were our primary outcome measures. Secondary measures included alcohol and tobacco craving (Burton and Tiffany 1997; Cooney et al. 2001; Drobes et al. 2000: Mintz et al. 1991), subjective alcohol effects (Madden et al. 1995; Perkins et al. 1995), cardiovascular responses (Kouri et al. 2004; Perkins et al. 1995), and blood alcohol levels (to confirm drinking behavior). Given that nicotine replacement has been shown to attenuate responses to tobacco, and in some cases alcohol [e.g., Acheson et al. 2006 (females only); Cooney et al. 2001; Perkins et al. 1995], we predicted that transdermal nicotine replacement would reduce drinking behavior in heavy-drinking daily smokers. In light of the results of Acheson et al. (2006), we also examined the effect of gender on our primary outcome.
Materials and methods
Participants
Participants were eligible to enroll if they were 21 to 55 years of age, smoked between 15 and 25 cigarettes per day, drank alcohol at least 3 days per week, and drank at least four drinks per episode for men and at least three drinks per episode for women. Subjects were excluded if they were alcohol-dependent, using illicit drugs, currently seeking treatment for alcohol use or smoking behavior, presented with current severe psychiatric disorders, or had medical conditions that would contraindicate alcohol use or transdermal nicotine use. A total of 19 subjects completed the study (8 women, 11 men). The average age was 26.68 (SD=9.01), and participants were primarily white (61%; 39% African American), had at least some college education (68%; 16% high school graduates; 16% some high school), and were not married (89%). Participants smoked, on average, 18.98 (SD=4.13) cigarettes per day, had baseline carbon monoxide readings of 19.95 ppm (SD=6.96), baseline urine cotinine values of 1079.85 ng/ml (SD=645.79), and average Fagerstrom nicotine dependence scores (Heatherton et al. 1991) of 5.16 (SD=1.50; possible range 1–10). Participants consumed an average of 24.04 (SD=8.67) standard drinks per week, drank an average of 4.05 (SD=1.23) times per week, had average Alcohol Use Disorders Identification Test (Babor et al. 1992) scores of 10.63 (SD=3.32; scores of 8 or greater indicate problematic drinking), and 21% met DSM-IV criteria for current alcohol abuse.
Procedures
Intake sessions
Written informed consent was obtained at the start of the intake session. The Structured Clinical Interview for DSM-IV (First et al. 1995) was used to exclude individuals who met diagnostic criteria for alcohol dependence or other Axis I disorders. The Timeline Followback (Sobell and Sobell 1993) was used to assess past 30-day drinking and smoking behavior. A physical examination was conducted in addition to an electrocardiogram, urine toxicology, pregnancy tests for women, and basic blood chemistries including liver function tests.
Laboratory sessions
Each subject completed two 14-h laboratory sessions (0 vs 21 mg transdermal nicotine patch), which took place at the Yale General Clinical Research Center. The order of sessions was counterbalanced. For women, laboratory sessions were scheduled during the follicular phase of the menstrual cycle to control for potential menstrual cycle effects (e.g., Perkins et al. 2000b). The average time between laboratory sessions was 24.37 days (SD=13.97), and this interval did not differ across gender. Subjects were paid $120 to complete each laboratory session and received a $50 bonus for completing the study.
Baseline assessment period
Laboratory sessions started at 8:00 A.M. Subjects were asked to not consume alcohol 24 h before the session but were free to smoke as they normally would up until 8:00 A.M. of the morning of the laboratory session. An IV cannulae was inserted to obtain blood samples throughout the laboratory procedure. Baseline assessments of breath CO (MCO2 Monitor, MicroDirect, Auburn, ME, USA), breath alcohol (Alco-Sensor III, Intoximeter, St. Louis, MI, USA), physiologic measures (blood pressure, heart rate, skin temperature), plasma cotinine and nicotine levels, urine drug screen, urine pregnancy screen, height, and weight were obtained. All breath alcohol, pregnancy, and drug screens were required to be negative. Baseline CO readings indicated that all subjects had smoked before the laboratory sessions (CO≥8 ppm).
Nicotine patch absorption period
At 9:00 A.M., a nicotine patch (0 mg or 21 mg Nicoderm CQ Transdermal System®, GlaxoSmithKline Consumer Healthcare) was applied to the subject’s upper arm. Both active and placebo patches were identical in appearance, and subjects and experimenters were blind as to the nicotine patch condition. The absorption period was 6 h so as to achieve peak nicotine levels for the active nicotine condition (Gupta et al. 1993). This time period also allowed for clearance of nicotine obtained through smoking before the laboratory session for the placebo nicotine condition. For the 6 h, subjects were able to watch TV and read. Lunch was provided at 12:00 P.M. to standardize the time and amount of last food intake. Assessments of side effects, plasma nicotine, nicotine withdrawal, alcohol craving, tobacco craving, and physiologic measures were obtained at the end of this period. Side effects associated with nicotine patch (i.e., skin irritation around patch, heart rate greater than 100, dizziness, nausea, and abdominal discomfort) were assessed on five-point scale (absent, minimal, mild, moderate, severe). There were no reports of skin irritation, nausea, or abdominal discomfort. There was one event of heart rate >100 (placebo patch group), and three reports of dizziness (minimal or mild ratings; two in placebo group, one in nicotine group).
Priming dose
The alcohol priming drink was administered at 3:00 P.M. and consisted of one part 80-proof liquor of the subject’s choosing to three parts mixer chosen from a selection of equicaloric, noncaffeinated, non-carbonated drinks. The amount of alcohol was designed to raise blood alcohol levels to 0.03 g/dl and was based on a formula that takes into account the gender, age, and weight of each subject (Watson 1989). This priming dose approximates a single standard alcoholic drink and has been successfully used to prime further drinking behavior (see O’Malley et al. 2002). Subjects were instructed to consume the beverage within 5 min. Blood alcohol level, alcohol craving, tobacco craving, and physiologic measures were assessed 15 min before and 10, 20, 30, and 40 min after the consumption of the priming drink in the order listed. Subjective effects of alcohol were assessed at baseline and 20 and 40 min after priming drink consumption. Using this procedure, we were able to monitor changes in subjective and physiological responses to alcohol during the ascending limb and the beginning of the descending limb of the blood alcohol curve.
Alcohol self-administration
The self-administration procedures were similar to those used in our previous study (see O’Malley et al. 2002). Fifty minutes after the priming drink, subjects were exposed to two consecutive, 1-h ad-lib drinking periods (hour 1 started at +50 min and ended at +110 min; hour 2 started at +120 min and ended at +180 min). During each 1-h period, subjects were permitted to drink up to four alcoholic drinks each designed to raise BALS by 0.015 g/dl or to receive monetary reinforcement ($3 per drink) for each drink not consumed. At the beginning of each 1-h choice period, subjects were presented with four prepared drinks along with a “tab” sheet worth $12. Subjects were informed that these four drinks were available for the next 60 min and that they could choose to consume the beverages or to keep the money (up to $12). At the end of the first hour, any remaining drinks were removed before the start of the second drinking hour in which four additional drinks (and $12 tab) were presented. Thus, subjects could choose to consume up to eight additional drinks over a 2-h period or to receive up to $24. Any unspent drinking tab was paid to the subjects at the end of each laboratory session.
During the two 1-h drinking periods, assessments of blood alcohol levels, alcohol craving, and tobacco craving were collected at the following timepoints in the order listed [+40 min (collected just before the start of the first drinking hour); hour 1 timepoints +80 min, +110 min; hour 2 timepoints +150 min, +180 min]. Subjective ratings of alcohol effects, nicotine withdrawal, and physiologic measures were collected before the start of the first drinking hour and end of each 1-h drinking period (timepoints +40 min, +110 min; +180 min). Drinking behavior was videotaped for later analysis. The timing of assessments was limited to avoid interfering with the evaluation of drinking behavior. Subjects were discharged at 10:00 P.M. at which time their breath alcohol levels had fallen below 0.02%.
Outcome measures
Subjective measures
The Alcohol Urge Questionnaire (AUQ; Bohn et al. 1995) was used to assess alcohol craving. This eight-item measure was designed to assess an individual’s desire to drink alcohol right now [Visual Analogue Scale (VAS), range 1–100). Tobacco craving was assessed with the Tiffany Questionnaire of Smoking Urges–Brief (QSU-Brief; Cox et al. 2001), which consists of ten items to evaluate urges to smoke in response to positive (factor 1) or negative (factor 2) reinforcement (VAS scale, range 1–100). The Biphasic Alcohol Effects Scale (BAES; Martin et al. 1993) is a 14-item self-report, unipolar adjective rating scale used to measure the stimulant and sedative effects of alcohol (VAS scale, range 1–100). The Alcohol Effects Scale (AES) assessed subjective alcohol effects with five items (high, like, rush, feel-good, intoxicated). Participants indicated on a visual analogue scale (range 1–100) how much of an alcohol effect they were experiencing. This scale was adopted from others found in the literature (Schuckit 1984). A mean score of these five items was calculated. DSM-IV symptoms of nicotine withdrawal were assessed with the eight-item Minnesota Nicotine Withdrawal Scale (MNWS; Hughes and Hatsukami 1986). Instructions were worded to assess current symptoms of withdrawal (range 0–32).
Physiologic measures
Blood pressure (systolic and diastolic), heart rate, and skin temperature were assessed using a Dinamap oscillometric monitor (Critikon, Tampa, FL, USA). For each timepoint assessed, two measurements were obtained and averaged.
Nicotine plasma levels
Plasma nicotine was measured by reversed-phase high-performance liquid chromatography with UV detection modified from the literature (Hariharan et al. 1988) to include a micro acid back extraction cleanup step, which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4 ng/ml.
Blood alcohol levels
Blood samples for BALs taken during the laboratory session were collected in 400-μl microfuge tubes coated with Heparin. After microfuging, the plasma was transferred to microtubes and stored at −20°C. Samples were analyzed within 2 months using an AM1 analyser (Analox Instruments LTD, London, England). BALs were collected as a confirmation of drinking behavior and were not intended for pharmacokinetic analyses.
Drinking behavior
Videotaped drinking behavior was scored for each of the two drinking hours to determine the percent of subjects remaining abstinent, number of drinks consumed, latency to start drinking, number of sips per drink, inter-sip interval per drink, and inter-drink interval (i.e., time between the end of one drink and the start of another, within hour). Latency to start drinking was the number of minutes until the first sip during a given hour of the self-administration phase. Data for subjects who did not drink were handled in two ways. First, abstainers within a given hour were given a score of 60 min on latency to the first sip. In the second approach, these subjects were treated as missing for analyses of latency to the first drink.
Statistical analysis
Data analyses were conducted separately for the nicotine patch absorption period, the priming drink period, and the self-administration period. At the end of the 6-h nicotine patch absorption period, paired t tests were used to examine nicotine plasma levels, subjective measures (AUQ, QSU-B, MNWS), and physiologic measures (blood pressure, heart rate, skin temperature) within nicotine patch condition (0 vs 21 mg). Separate repeated measures multivariate analyses of variance were conducted for the priming drink and self-administration periods to examine BALs, subjective measures (AUQ, QSU-B, MNWS, AES, BAES), and physiologic measures within nicotine patch condition (0 vs 21 mg) and within time.
For the self-administration period, repeated measures analysis of variance (Hour 1, Hour 2) were conducted to examine the primary outcomes of drinking behavior (number of drinks consumed, latency to start drinking, sips per drink, inter-sip interval, and inter-drink interval) within nicotine patch conditions (0 vs 21 mg). Paired comparisons of available data were conducted within each hour by nicotine patch conditions. We also examined percentages of subjects remaining abstinent across each hour by patch conditions using McNemar tests of dependent proportions. Further, we examined gender and order of nicotine patch condition as between subject factors in our analyses involving drinking behavior. We found no significant effects of gender or order on our primary outcomes.
Results
Nicotine patch absorption period
Across the two laboratory sessions, subjects had equivalent baseline nicotine plasma levels at the start of the laboratory sessions (p=0.48; 0 mg nicotine patch mean=9.66 ng/ml, SE=0.75; 21 mg nicotine patch=9.49 ng/ml, SE=1.11). At the end of the 6-h absorption period, as expected, active patch demonstrated greater nicotine levels [t(18)=14.57, p<0.0005; 0 mg patch mean=0.54 ng/ml, SE=0.37; 21 mg patch mean=18.58 ng/ml, SE=1.48]. Additionally, active patch decreased QSU-B factor 2 scores [t(18)=2.25, p<0.05; 0 mg patch mean=26.89, SE=6.10; 21 mg patch mean=16.72, SE=4.37], increased measures of systolic blood pressure [t(18)=3.23, p<0.01; 0 mg patch mean= 115.16 mmHg, SE=3.83; 21 mg patch mean=124.45 mmHg, SE=3.78], and increased heart rate [t(18)=3.24, p<0.01; 0 mg patch mean=68.66 bpm, SE=2.37; 21 mg patch mean=75.68 bpm, SE=2.79]. Diastolic blood pressure (mean=66.75 mmHg), skin temperature (mean= 85.84), AUQ scores (mean=27.08), QSU-B factor 1 scores (mean=54.43), and nicotine withdrawal scores (mean= 0.63) did not significantly differ.
Priming drink period
Blood alcohol levels
BALs increased over time (F=135.27, p<.0005; +10 timepoint mean=.0148, SE=.002; +20 time-point mean=.0224, SE=.002; +30 timepoint mean=.0218, SE=.002; +40 timepoint mean=.0203, SE=.002) and did not differ by patch condition.
Subjective measures
AUQ scores increased over the priming drink period (F=14.97, p<0.001; −15 min mean=27.08, +40 min mean=36.81), but there was no significant interaction of patch condition by time (p=0.08). There were significant interactions of patch condition by time for tobacco craving for positive reinforcement (QSU-B factor 1; F=8.08, p<0.01) and negative reinforcement (QSU-B factor 2; F=2.72, p<0.04]. Active patch decreased tobacco craving for positive reinforcement 10 min after the consumption of alcohol, as evidenced by paired comparisons (0 mg patch mean=67.65, SE=7.44; 21 mg patch mean=58.27, SE= 7.89; p<0.05). Active patch also decreased ratings of tobacco craving for negative reinforcement before the consumption of alcohol and continued to be lower 10 min (0 mg patch mean=33.70, SE=6.63; 21 mg patch mean=20.11, SE=4.75; p<0.05) and 20 min (0 mg patch mean= 32.44, SE=6.67; 21 mg patch mean=17.37, SE=4.49; p< 0.05) after the consumption of the priming drink. Given the pre-alcohol differences in QSU-B factor 2 craving scores, we compared patch conditions using change scores calculated as differences from the −15-min timepoint. Change in QSU-B factor 2 craving scores were not significantly different across patch conditions at the +10- and +20-min timepoints.
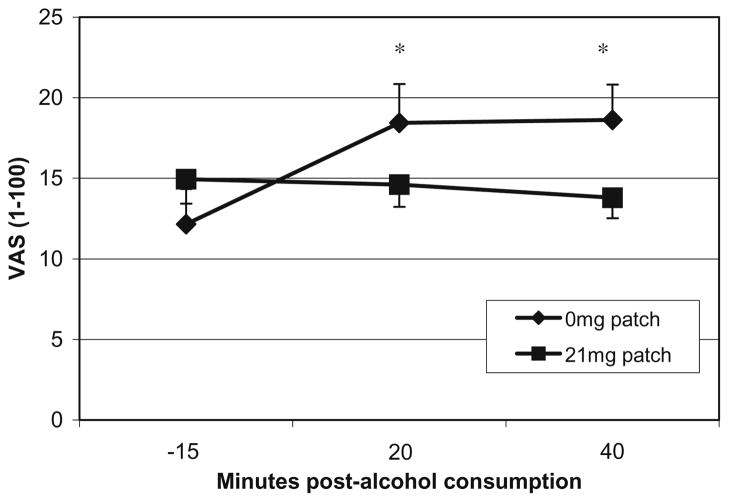
Placebo patch increased ratings of alcohol effects (mean score of high, like, rush, feel-good, intoxicated) after the consumption of alcohol (patch × time; F=7.39, p<0.02; Fig. 1) with paired comparisons demonstrating differences at +20 and +40 min. BAES stimulation scores decreased over the priming drink period (F=5.20, p<0.04; 0 min mean=1.77, +40 min mean=1.39). BAES sedation scores did not differ. Nicotine withdrawal scores increased over the priming drink period (F=9.89, p<0.01; −15 min mean= 0.63, +40 min mean=0.82) and did not differ by patch condition.
Fig. 1.
Mean visual analogue scale ratings (VAS 1–100) for AES during the priming drink period for each transdermal nicotine replacement condition (0 vs 21 mg/day). AES is a mean score of high, like, rush, feel-good, intoxicated. *p<0.05 paired comparisons for 0 mg vs 21 mg/day nicotine patch within a timepoint
Physiologic measures
Systolic blood pressure decreased over the priming drink period (F=10.60, p<0.01, +0 min mean=119.74 mmHg, +40 min mean=115.02 mmHg). Diastolic blood pressure demonstrated a significant quadratic effect of time (F=9.02, p<0.01), with values increasing 10 min post-alcohol consumption (+10 min mean=69.78 mmHg) and then decreasing over the priming drink period (+40 min mean=63.44 mmHg). Active patch increased heart rate (F=6.40, p<0.05; 0 mg patch mean=71.55 bpm, SE=2.76; 21 mg patch mean=77.01, SE=2.67). Heart rate also increased over the priming drink period (F=10.98, p<0.01; 0 min mean=73.19 bpm, +40 min mean=75.82 bpm). Active nicotine patch decreased skin temperature (patch × time; F=5.93, p<0.03) 10 min after the consumption of the priming drink (0 mg patch mean=88.61, SE=0.87; 21 mg patch mean=85.62, SE=1.60).
Self-administration period
Drinking behavior
While slightly more subjects remained abstinent during the active (Hour 1 n=5; Hour 2 n=6) vs placebo patch condition (Hour 1 n=2; Hour 2 n=3), across each hour, the differences in proportions were not significant (see Table 1). The active patch increased the latency to start drinking (F=6.43, p<0.02) across the 2-h self-administration period when latencies were coded as 60 min for subjects who abstained from drinking during a given hour of the self-administration period. In the subset of subjects who drank, patch condition did not alter latency to drink, number of sips, inter-sip interval, or inter-drink interval. Drinking behavior did not differ between Hour 1 and Hour 2, as there were no significant multivariate effects of time found for any of the drinking measures. As a probable consequence of delayed drinking onset, the total number of drinks consumed during the 2-h self-administration period was significantly lower in the active patch compared to the placebo patch condition (F=9.80, p<0.01).
Table 1.
Drinking behavior measures [% or mean (SE)] collected during the 2-h self-administration phase for transdermal nicotine replacement conditions (0 vs 21 mg/day)
| Drinking measure | Choice block no. 1 (60 min, four drink options) |
Choice block no. 2 (60 min, four drink options) |
||
|---|---|---|---|---|
| 21 mg Patch | 0 mg Patch | 21 mg Patch | 0 mg Patch | |
| Percent abstinent | 26% (5/19) | 11% (2/19) | 32% (6/19) | 16% (3/19) |
| Drinks consumed | 1.90 (0.35)* | 2.53 (0.30) | 1.68 (0.36)* | 2.32 (0.39) |
| Latency to start (min)a | 20.24 (5.88)* | 9.60 (4.24) | 27.89 (5.77)* | 15.27 (5.08) |
| Latency to start (min)b | 6.04 (2.47)** | 3.67 (1.32) | 13.07 (3.87)** | 6.88 (2.67) |
| Sips per drink | 5.90 (0.80) | 5.49 (0.68) | 5.10 (0.77) | 5.23 (0.62) |
| Inter-sip interval (min) | 1.97 (0.37) | 2.23 (0.51) | 2.59 (0.47) | 1.92 (0.28) |
| Inter-drink interval (min) | 10.02 (2.07) | 7.87 (1.43) | 13.79 (4.44)** | 6.01 (1.06) |
Mean values reflect all available data within each hour and patch condition.
With abstinent subjects coded as 60 min
With abstinent subjects coded as missing
p<0.05 paired comparisons within hour for 0 mg vs 21 mg/day nicotine patch
p<0.10 paired comparisons within hour for 0 mg vs 21 mg/day nicotine patch
We also conducted exploratory analyses to examine potential predictors of significant drinking effects (i.e., mean latency to drink, and drinks consumed). For those variables which demonstrated some indication of nicotine patch effects during the priming drink period (i.e., alcohol craving, tobacco craving, alcohol effects, skin temperature with p<0.10), their +40 min values (end of the priming drink period) were correlated with average latency to drink and total drinks consumed across both hours within each patch condition. Alcohol craving was positively associated with drinks consumed (0 mg patch r=0.48, p<0.05; 21 mg patch r=0.65, p<0.01) and negatively associated with latency to drink (0 mg patch r=−0.49, p<0.05; 21 mg patch r=−0.46, p<0.05) for both active and placebo patch conditions. For the placebo patch condition only, tobacco craving for positive (r=−0.65, p<0.01) and negative reinforcement (r=−0.53, p<0.05) were negatively associated with latency to drink. No significant effects were demonstrated for alcohol effects or skin temperature.
Blood alcohol levels
Compared to the placebo patch condition, subjects in the active patch had lower blood alcohol levels at certain timepoints (condition × time interaction; F=3.55, p<0.01), consistent with the increased latency to begin consuming alcohol after nicotine. Paired comparisons of mean BALs for active and placebo patch indicate that BALs were lower halfway through each of the drinking hours [midpoint of the first drinking hour (+80 timepoint: 0 mg patch mean=0.034 mg%, SE=0.004; 21 mg patch mean=0.024 mg% SE=0.004); midpoint of the second drinking hour (+150 timepoint: 0mg patch mean=0.050 mg%, SE=0.008; 21 mg patch mean=0.034 mg%, SE=0.008)], but that BALs did not differ by patch condition at other times [before the start of the first drinking hour (+40 timepoint mean=0.020 mg%), end of the first drinking hour (+110 timepoint mean=0.034 mg%), or at the end of the second drinking hour (+180 timepoint mean=0.050 mg%)].
Subjective measures
Alcohol craving (AUQ: F=6.16, p<0.03) decreased, whereas alcohol effect scores (AES: F=5.12, p<0.04) increased over the ad-lib period (see Table 2). Given the differences in AES scores between the patch conditions before the self-administration phase, we analyzed AES change scores calculated as the difference from the +40 min timepoint by patch condition. The time effect for AES was no longer significant. Tobacco craving for positive reinforcement (QSU-B factor 1 scores; F=8.86, p<0.01) and negative reinforcement (QSU-B factor 2 scores; F=12.19, p<0.01) increased over the ad-lib period. Nicotine withdrawal scores did not significantly differ. The active patch increased BAES stimulation scores by the end of the 2-h drinking period (patch × time; F=4.57, p<0.05).
Table 2.
Mean (SE) subjective and physiological effects during the alcohol self-administration phase for transdermal nicotine replacement conditions (0 vs 21 mg/day)
| Condition | Alcohol self-administration period |
|||||
|---|---|---|---|---|---|---|
| +40 mina | +80 min | +110 min | +150 min | +180 min | ||
| Subjective measures | ||||||
| AUQb | 0 mg patch | 37.65 (5.28) | 31.41 (4.97) | 31.79 (5.36) | 36.26 (6.43) | 28.07 (5.74) |
| 21 mg patch | 37.23 (6.32) | 35.31 (7.10) | 34.78 (6.90) | 34.82 (6.23) | 29.26 (6.51) | |
| QSU-B factor 1b | 0 mg patch | 63.08 (7.22) | 67.04 (6.66) | 71.326 (6.52) | 70.45 (6.77) | 69.04 (6.87) |
| 21 mg patch | 61.44 (7.90) | 61.86 (8.02) | 64.76 (8.02) | 64.12 (7.98) | 67.51 (7.79) | |
| QSU-B factor 2b | 0 mg patch | 30.14 (5.63) | 33.74 (6.26) | 34.60 (7.04) | 36.86 (6.76) | 36.98 (7.23) |
| 21 mg patch | 28.67 (6.27) | 28.54 (4.95) | 28.46 (5.87) | 31.02 (5.62) | 39.75 (8.10) | |
| AES | 0 mg patch | 20.03 (2.52) | – | 26.99 (6.29) | – | 27.11 (4.76) |
| 21 mg patch | 14.00 (1.23) | – | 20.60 (4.17) | – | 25.27 (6.01) | |
| MNWS | 0 mg patch | 0.91 (0.16) | – | 1.07 (0.16) | – | 1.00 (0.19) |
| 21 mg patch | 0.76 (0.13) | – | 0.86 (0.15) | – | 1.00 (0.19) | |
| BAES-stimulationd | 0 mg patch | 1.50 (0.28) | – | 1.77 (0.44) | – | 1.37 (0.36) |
| 21 mg patch | 1.39 (0.26) | – | 1.68 (0.48) | – | 1.93 (0.46) | |
| BAES-sedation | 0 mg patch | 2.14 (0.46) | – | 1.70 (0.68) | – | 2.03 (0.47) |
| 21 mg patch | 1.59 (0.38) | – | 1.75 (0.35) | – | 2.03 (0.51) | |
| Physiological measures | ||||||
| Heart rateb,c | 0 mg patch | 72.47 (2.87) | – | 74.95 (3.03) | – | 76.21 (3.20) |
| 21 mg patch | 76.90 (2.78) | 79.63 (3.25) | 79.97 (3.20) | |||
| Systolic BP | 0 mg patch | 112.87 (3.64) | – | 114.45 (4.24) | – | 115.24 (2.74) |
| 21 mg patch | 117.26 (3.65) | – | 118.61 (4.74) | – | 116.97 (3.36) | |
| Diastolic BP | 0 mg patch | 63.56 (2.43) | – | 65.94 (2.48) | – | 67.58 (2.51) |
| 21 mg patch | 64.00 (2.18) | – | 64.42 (3.41) | – | 63.64 (1.76) | |
| Skin tempb | 0 mg patch | 90.23 (1.17) | – | 88.10 (0.95) | – | 88.59 (1.12) |
| 21 mg patch | 89.80 (1.07) | – | 88.22 (1.37) | – | 88.01 (1.35) | |
AUQ Alcohol Urge Questionnaire, QSU-B factor 1 Questionnaire of Smoking Urges Positive Reinforcement, QSU-B factor 2 Questionnaire of Smoking Urges Negative Reinforcement, AES Alcohol Effects Scale, BAES Biphasic Alcohol Effects Scale, MNWS Minnesota Nicotine Withdrawal Scale
The priming drink period ended at +40 min and the self-administration period started +50 min.
Main effect of time, p<0.05
Main effect of nicotine, p<0.05
Interaction of time by nicotine patch condition, p<0.05 for repeated measures analyses of variance
Physiologic measures
Active patch increased heart rate (F=4.81, p<0.05). Heart rate also increased over the 2-h drinking period (F=7.74, p<0.02), whereas skin temperature decreased (F=7.51, p<0.02). Blood pressure did not significantly differ.
Discussion
Using an established alcohol self-administration paradigm (Anton et al. 2004; O’Malley et al. 2002), we found that transdermal nicotine replacement increased the latency to the first drink and consequently appeared to reduce the number of drinks consumed during a 2-h self-administration period, compared to 6 h of nicotine deprivation (i.e., placebo patch), in heavy drinking daily smokers. Although not significantly different, during the active patch condition, a larger proportion of subjects declined all drinks within each hour. When drinks were chosen, however, the number of sips taken per drink, time between sips, and time between drinks were similar for the active and placebo conditions. Thus, we conclude that active nicotine replacement compared to placebo replacement delayed the onset of consumption. Although subjects on nicotine patch compared to placebo patch consumed fewer drinks in the time allotted, it is not possible to determine whether this effect would be maintained, enhanced, or reduced if the time period in which drinks could be chosen was not constrained.
Our drinking outcomes are consistent with Acheson et al. (2006) findings with women. Although Acheson et al. (2006) did not evaluate latency to drink, our results regarding reduced drinking (which are secondary to latency to drink) were similar. However, their study found that nicotine patch increased alcohol consumption in males, whereas we found that nicotine patch also reduced drinking by men. A number of procedural differences between the two studies may account for the differential findings. Acheson et al. (2006) examined light-smoking social drinkers and subjects were tested in small groups, whereas our subjects were heavier drinkers and smokers and were tested individually. In the Acheson et al. (2006) study, the self-administration phase commenced while the blood alcohol levels produced by the priming drink were ascending, while our self-administration phase began when the blood alcohol levels were descending. Alcohol–nicotine interactions have been shown to be sensitive to ascending and descending limb effects (King and Epstein 2005; McKee et al. 2006; Mitchell et al. 1995), and others have demonstrated that medication effects are sensitive to the interval between the priming drink and the self-administration phase (Anton et al. 2004). Additional work is needed to delineate how nicotine replacement interacts with temporal effects of the priming drink on inhibiting or promoting further alcohol consumption.
In the period before the priming drink, we found that active nicotine patch in comparison to 6 h of nicotine deprivation (i.e., placebo patch) attenuated tobacco craving for negative reinforcement, consistent with prior research (e.g., Tiffany et al. 2000). We also found increases in systolic blood pressure and heart rate consistent with the effects of transdermal nicotine replacement on cardiovascular responses (Zevin et al. 1998). Nicotine withdrawal scores, as measured by the MNWS, were minimal after 6 h of cigarette deprivation and were not influenced by nicotine patch condition (see Pickworth et al. 1996).
Our results suggest that treatment with the nicotine patch alters subjective and physiological responses to a standard dose of alcohol. Specifically, after the priming drink, the subjective effects of alcohol (e.g., intoxication ratings) were lower when subjects received active compared to placebo transdermal nicotine replacement. After consuming the priming drink, heart rate was increased and skin temperature was decreased by active nicotine patch. These results are consistent with prior studies which have examined the single and combined effects of alcohol and nicotine on cardiovascular responses (Benowitz et al. 1986; Michel and Battig 1989; Perkins et al. 1995). Pre-alcohol differences in tobacco craving persisted with transdermal nicotine replacement attenuating craving for both positive and negative reinforcement. These findings are counter to those produced by Kouri et al. (2004) after overnight tobacco abstinence. It is difficult to make cross-study comparisons, as Kouri et al. (2004) used stronger alcohol priming doses (0.4, 0.7 g/kg) and removed subjects who failed to have craving responses (2 subjects of 12 total subjects) from the analyses. However, others have demonstrated that nicotine replacement and smoked tobacco resulted in lowered intoxication ratings (Madden et al. 1995; Perkins et al. 1995) and lowered tobacco craving (Mintz et al. 1991) after a fixed dose of alcohol.
During the alcohol self-administration period, alcohol craving was found to decrease, while craving for tobacco increased irrespective of patch condition. Active nicotine patch increased stimulation scores as well as heart rate during this period. Others have found that nicotine replacement can attenuate some of the sedating effects produced by alcohol (Perkins et al. 1995).
We also conducted exploratory analyses to investigate potential mechanisms underlying any alterations in drinking behavior. Alcohol craving at the end of the priming drink period was negatively associated with latency to consume alcohol and positively associated with the number of drinks consumed in both the nicotine and placebo patch conditions, supporting prior findings (O’Malley et al. 2002). Additionally, we found that higher tobacco craving for both positive and negative reinforcement was associated with reduced latency to consume alcohol in the placebo patch condition, but not in the nicotine patch condition. Thus, after 6 h of nicotine deprivation, higher tobacco craving responses seen in the placebo patch condition during the priming drink period may underlie the shorter latency to consume alcohol. Similarly, Epstein et al. (2007) found that tobacco craving for positive reinforcement was associated with blood alcohol levels. While our findings are suggestive of possible mechanisms underlying the initiation of drinking behavior, it should be noted that given our limited sample size, we were unable to conduct a full mediational analysis (see Baron and Kenny 1986).
This study has several limitations. First, the sample size was modest and only generalizeable to the population of heavy-drinking daily smokers. Although our sample size was comparable to other laboratory studies examining the effect of nicotine replacement on alcohol responses (e.g., Acheson et al. 2006; Barrett et al. 2006; Perkins et al. 1995, 2000a), our analyses for gender comparisons were under-powered. Second, only a single priming dose was investigated. However, we have found that an alcohol dose designed to raise BALs by 0.03 g/dl is effective in priming drinking behavior (see O’Malley et al. 2002; Krishnan-Sarin et al. 2007). In the current study, we demonstrated that this priming dose was sensitive to the effects of transdermal nicotine on at least delaying further drinking behavior. Third, no alternative beverage was provided during the self-administration phase, and it is unknown whether reductions in alcohol consumption during the transdermal nicotine replacement condition were specific to alcohol. Fourth, only a single dose of transdermal nicotine replacement was investigated. However, the 21-mg/day dose is the dose of transdermal nicotine replacement most commonly used and most likely to be used by heavy drinkers attempting smoking cessation. Nonetheless, from a mechanistic standpoint, it would be worthwhile to conduct a dose-ranging study of transdermal nicotine replacement in this population of heavy-drinking smokers. Further, we did not include a placebo alcohol condition. As a result, we cannot determine whether the observed effects were a product of transdermal nicotine replacement or transdermal nicotine replacement interacting with alcohol. However, our results for the priming drink period (i.e., cardiovascular responses, subjective alcohol effects) were consistent with the only prior study to incorporate an active and placebo alcohol dose to examine the effect of nicotine replacement on responses to a fixed dose of alcohol (Perkins et al. 1995). Finally, the duration of the session limited our ability to determine whether nicotine also decreases alcohol consumption once it has been initiated or whether the primary effect of nicotine is to delay the initiation of drinking.
Given the substantial health risks associated with concurrent alcohol and tobacco use (Rosengren et al. 1993), addressing smoking cessation in those experiencing alcohol problems is an important area for research. Although some studies have found no differences in the ability to quit smoking on the basis of alcohol problems (e.g., Hayford et al. 1999; Hughes et al. 2003; Hurt et al. 1995; Sobell et al. 1995), many studies demonstrate that individuals with current or past histories of alcohol problems are less likely to be successful at quitting smoking compared to those without alcohol problems (e.g., Daeppen et al. 2000; Di Franza and Guerrera 1990; Hughes 1995). However, little is known about how to best promote smoking cessation in this population of drinkers who are at increased risk for alcohol-mediated tobacco relapse (Baer and Lichenstein 1988; Brandon et al. 1990; Sobell et al. 1990; Shiffman et al. 1994, 1996; Zimmerman et al. 1990).
In summary, we found that transdermal nicotine, compared to 6 h of nicotine deprivation, reduced subjective and physiological responses to a single alcohol drink, increased latency to initiate drinking, and consequently appeared to decrease the amount of alcohol subsequently consumed. It is possible that nicotine reduced the effects of nicotine deprivation on drinking. Some studies find that nicotine deprivation increases drinking behavior (e.g., Palfai et al. 2000), and theories of behavioral choice (Vuchinich and Tucker 1988) suggest that nicotine deprivation may increase the incentive value for alcohol because of the lack of availability of nicotine. In support of this hypothesis, subjects who reported greater tobacco craving in the placebo patch condition exhibited shorter latencies to drink. In addition, because alcohol consumption is a known precipitant of smoking relapse, it is conceivable that nicotine replacement could further facilitate smoking cessation by reducing or delaying drinking. Future laboratory and clinical studies should examine the effect of other smoking cessation pharmacotherapies on alcohol drinking to better understand the value of these treatments for this population of treatment-resistant smokers.
Acknowledgments
We would like to thank Dr. Peter Jatlow, PI of the Laboratory Core (P50AA015632) for analyzing the nicotine plasma samples. GlaxoSmithKline generously provided the active and placebo transdermal nicotine replacement patches.
Funding: R03AA013622; R01AA015596; P50DA13334, P50AA015632, K05AA014715, CTSA-UL1RR024139.
References
- Acheson A, Mahler SV, Chi H, de Wit H. Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology. 2006;186:54–63. doi: 10.1007/s00213-006-0338-y. [DOI] [PubMed] [Google Scholar]
- Anton RF, Dorbes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharmacology. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: the alcohol use disorders identification test: Guidelines for use in primary health care. World Health Organization; Geneva, Switzerland: 1992. [Google Scholar]
- Baer JS, Lichenstein E. Classification and prediction of smoking relapse episodes: an exploration of individual differences. J Consult Clin Psychol. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Tichauer M, Leyton M, Pihl RO. Nicotine increases alcohol self-administration in non-dependent male smokers. Drug Alcohol Depend. 2006;81:197–204. doi: 10.1016/j.drugalcdep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT, Jacob P., III Additive cardiovascular effect of nicotine and ethanol. Clin Pharmacol Ther. 1986;40:420–424. doi: 10.1038/clpt.1986.200. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Sodepalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Post-cessation cigarette use: the process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Burling TA, Marshall GD, Seidner A. Smoking cessation for substance abuse inpatients. J Subst Abuse. 1991;3:269–276. doi: 10.1016/s0899-3289(10)80011-2. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Clark A, Lindgren S, Brooks SP, Watson WP, Little HJ. Chronic infusion of nicotine can increase operant self-administration of alcohol. Neuropharmacology. 2001;41:108–117. doi: 10.1016/s0028-3908(01)00037-5. [DOI] [PubMed] [Google Scholar]
- Colby SM, Rohsensow DJ, Monti PM, et al. Effects of tobacco deprivation on alcohol cue reactivity and drinking among young adults. Addict Behav. 2004;29:879–892. doi: 10.1016/j.addbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Cooney NL, Litt MD, Cooney JL, Oncken CA, Pilkey DT, Findley J. Ecological momentary assessment of smoking cessation in alcoholic smokers. Paper Presented at the Annual Meeting of the Society for Research on Nicotine and Tobacco; Seattle, WA. 2001. [Google Scholar]
- Cooney JL, Cooney NL, Pilkey DT, Kranzler HR, Oncken CA. Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction. 2003;98:913–921. doi: 10.1046/j.1360-0443.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Daeppen J, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Ducholz KK, Raimo E, Schuckit MA The Collaborative Study Group on the Genetics of Alcoholism. Clinical correlates of cigarette smoking and nicotine dependence in alcohol dependent men and women. Alcohol Alcohol. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Beylotte F, Scott M, Saladin ME, Randell CL, Anton RF. Cross-reactivity to alcohol and smoking cues. Alcohol Clin Exp Res. 2000;24:147a. [Google Scholar]
- Epstein AM, Sher TG, Young AA, King AC. Tobacco chippers show robust increases in smoking urge after alcohol consumption. Psychopharmacology. 2007;190:321–329. doi: 10.1007/s00213-006-0438-8. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV, Patient Edition. American Psychiatric Press; Washington DC: 1995. [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behav Pharmacol. 1996;7:144–154. [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GR, Liebson I. Facilitation of human tobacco self-administration by ethanol: a behavioral analysis. J Exp Anal Behav. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Benowitz NL, Jacob P, Gorsline A. Bioavailability and absorption kinetics of nicotine following application of transdermal system. Br J Pharmacol. 1993;36:221–227. doi: 10.1111/j.1365-2125.1993.tb04221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariharan M, Van Noord T, Greden JF. A high performance liquid chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. Br J Psychol. 1999;174:173–178. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking volunteers without histories of alcoholism. Psychopharmacology. 1984;82:1–5. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacol Biochem Behav. 1997;57 (3):419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Fuller R, editors. Alcohol and tobacco: from basic science to policy, NIAAA research monograph 30. National Institute on Alcoholism and Alcohol Abuse; Rockville, MD: 1995. pp. 171–185. [Google Scholar]
- Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Callas PW High Dose Study Group. Past alcohol problems do not predict worse smoking cessation outcomes. Drug Alcohol Depend. 2003;71:269–273. doi: 10.1016/s0376-8716(03)00139-x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, et al. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcohol Clin Exp Res. 1994;4:867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Offord KP, Croghan IT, Hays JT, Gomez-Dahl L. Nicotine patch therapy for smoking cessation in recovering alcoholics. Addiction. 1995;90:1541–1546. doi: 10.1046/j.1360-0443.1995.9011154112.x. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton J. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Broadening the base of treatment for alcohol problems. National Academy Press; Washington, DC: 1990. [PubMed] [Google Scholar]
- Joseph AM, Nicol KL, Willenbring ML, Korn JE, Lysaght LS. Beneficial effects of treatment of nicotine dependence during an inpatient substance abuse treatment program. J Am Med Assoc. 1990;263:3043–3046. [PubMed] [Google Scholar]
- Katner SN, McBride WJ, Lumeng L, Li T-K, Murphy JM. Involvement of CNS cholinergic systems in alcohol drinking of P rats. Addict Biol. 1997;2:215–233. doi: 10.1080/13556219772769. [DOI] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcohol Clin Exp Res. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136:1248–1257. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug Alcohol Depend. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS. Family history of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry. 2007;62:694–697. doi: 10.1016/j.biopsych.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Lê AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotine receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lê AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Madden PA, Heath AC, Starmer GA, Whitfield JB, Martin NG. Alcohol sensitivity and smoking history in men and women. Alcohol Clin Exp Res. 1995;19:1111–1120. doi: 10.1111/j.1530-0277.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin K, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Sellers ML, Kuehnle JC. Effect of alcohol and marijuana on tobacco smoking. Clin Pharmacol Ther. 1980;27:202–209. doi: 10.1038/clpt.1980.32. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Michel C, Battig K. Separate and combined physiological effects of cigarette smoking and alcohol consumption. Psychopharmacology. 1989;97:65–73. doi: 10.1007/BF00443415. [DOI] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra VC, Jarvik MC. Alcohol increases cigarette smoking: a laboratory demonstration. Addict Behav. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Mintz J, Phipps C, Arruda MJ, Glynn SM, Schneider NG, et al. Combined use of alcohol and nicotine gum. Addict Behav. 1991;16:1–10. doi: 10.1016/0306-4603(91)90034-f. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, deWit H, Zacny JP. Effects of varying ethanol dose of cigarette consumption in healthy normal volunteers. Behav Pharmacol. 1995;6:359–365. [PubMed] [Google Scholar]
- Nadel R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Exp Clin Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo–pituitary–adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Monti PM, Ostafin B, Hutchison K. Effects of nicotine deprivation on alcohol-related information processing and drinking behavior. J Abnorm Psychology. 2000;109:96–105. doi: 10.1037//0021-843x.109.1.96. [DOI] [PubMed] [Google Scholar]
- Perkins KH, Sexton JE, Dimarco A, Grobe J, Scierka A, Stiller RL. Subjective and cardiovascular responses to nicotine combined with alcohol in male and female smokers. Psychopharmacology. 1995;119:205–212. doi: 10.1007/BF02246162. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Grobe JE. Sex differences in the acute effects of cigarette smoking on the reinforcing value of alcohol. Behav Pharmacol. 2000a;11:63–70. doi: 10.1097/00008877-200002000-00007. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D’Amico D, Miller A, Keins A, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol. 2000b;68:176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Butschky MF, Henningfield JE. Effects of transdermal nicotine delivery on measures of acute nicotine withdrawal. J Pharmacol Exp Ther. 1996;279:450–456. [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Drug Alcohol Depend. 1997;45:21–29. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine Tob Res. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Wilhelmsen L, Wedel H. Separate and combined effects of smoking and alcohol abuse in middle-aged men. ACTA Med Scand. 1993;223:111–118. doi: 10.1111/j.0954-6820.1988.tb15774.x. [DOI] [PubMed] [Google Scholar]
- Schuckit M. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psychiatry. 1984;41:879–885. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Shiffman S. A cluster-analysis classification of smoking relapse episodes. Addict Behav. 1986;11:292–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Fischer LA, Paty J, Gnys M, Hickcox M, Kassel JD. Drinking and smoking: a field study of their association. Annals Behav Med. 1994;16:203–209. [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology. 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. Humana Press; New Jersey: 1993. [Google Scholar]
- Sobell LC, Sobell MB, Kozlowski LT, Toneatto T. Alcohol or tobacco research versus alcohol and tobacco research. Br J Addict. 1990;85:263–269. doi: 10.1111/j.1360-0443.1990.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Kozlowski LT. Dual recoveries from alcohol and smoking problems. In: Fertig JB, Allen JP, editors. Alcohol and tobacco: from basic science to clinical practice. National Institutes of Health; Bethesda, MD: 1995. pp. 207–224. [Google Scholar]
- Soderpalm B, Reicson M, Olausson P, Blomqvist O, Engel JA. Nicotine mechanisms involved in dopaminergic activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Cox LS, Elash CA. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol. 2000;68:233–240. doi: 10.1037//0022-006x.68.2.233. [DOI] [PubMed] [Google Scholar]
- Vaillant GE, Schnurr PP, Baron JA, et al. A prospective study of the effects of cigarette smoking and alcohol abuse on mortality. J Gen Intern Med. 1991;6:299–304. doi: 10.1007/BF02597425. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Tucker JA. Contributions from behavioral theories of choice to an analysis of alcohol use. J Abnorm Psychology. 1988;97:181–195. doi: 10.1037//0021-843x.97.2.181. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt Rd, editors. Human metabolism of alcohol. I. CRC; Boca Raton, FL: 1989. pp. 41–56. [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- Zevin S, Jacob P, Benowitz NL. Dose-related cardiovascular and endocrine effects of transdermal nicotine. Clin Pharmacol Ther. 1998;64:87–95. doi: 10.1016/S0009-9236(98)90026-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman RS, Warheit GJ, Ulbrich PM, Auth JB. The relationship between alcohol use and attempts and success at smoking cessation. Addict Behav. 1990;15:197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]