Abstract
Presbycusis is a complex of high frequency hearing loss and disproportionate loss of speech discrimination that is seen concomitantly with physical signs of aging. Among the most extensively characterized strains of mice that show an early hearing loss is the C57B16/J strain, a strain that shows early onset of high frequency hearing loss at age 6 months and complete hearing loss by 1 year of age. The histopathology of this strain consists of loss of hair cells and spiral ganglion neurons in the basal turn, with a progression of loss of hair cells and ganglion neurons towards the apical portion of the cochlea as the animal ages. The process of aging has been extensively studied and although details differ in various organisms the consensus today is that oxidative stress, i.e. free radical-mediated tissue damage, is one of the core mechanisms of aging. Aerobic metabolism results in the creation of hydrogen peroxide and reactive oxygen species. These are normally detoxified by a variety of enzymes and free radical scavengers, including superoxide dismutase (SOD), catalase and glutathione. To determine whether oxidative stress plays a role in the pathophysiology of hearing loss in this mouse model of presbycusis we determined the relative change in mRNA production for selected free radical detoxifying enzymes in the C57B16/J mouse cochlea. Using semi-quantitative RT-PCR with tubulin mRNA as a control, relative levels of antioxidant enzyme mRNAs were determined. There was an overall increase in SOD1 mRNA levels when comparing 1 and 9 month time points, and a transient increase in the expression level of catalase mRNA. B6.CAST+Ahl mice, which carry the C57B16/J genome but receive their Ahl gene from CAST mice, do not show these alteractions in antioxidant enzyme production. Our results suggest that at an age of 9 months, at which point significant hearing loss has developed, the C57B16/J mouse cochlea is exposed to increased levels of free radicals and that the Ahl gene of the C57B16/J mouse mediates this decrease in protective enzymes and therefore increase in levels of oxidative stress.
Keywords: aging, C57B16/J mouse, oxidative stress, presbycusis
INTRODUCTION
Presbycusis is a complex of high frequency hearing loss and loss of speech discrimination that is seen concomitantly with physical signs of aging. Age of onset, progression of hearing loss and degree of impairment vary with the individual, suggesting that numerous factors, both genetic and environmental (noise trauma and ototoxin exposure), play a role in the pathophysiology of this common problem (1–7). There are several animal models of age-related hearing loss, including aged Fisher rats, aged gerbils, aged chinchillas and several strains of mice that lose hearing at various rates (7). That study of mouse models of age-related hearing loss (AHL) combines several advantages. The animals have limited life spans, so the aging process may be observed over a fairly short time period, and a large number of inbred strains are available, allowing genetic manipulation and study. Additionally, sequences of numerous mouse genes are available, allowing the application of molecular biology techniques to the study of age-related hearing loss.
The C57B16/J mouse has been extensively studied as a model of both genetic hearing loss and AHL. This strain begins to lose hearing by 6 months of age and progresses to complete hearing loss by the time the animal is 1 year-old (7). Environment may play a significant role in aging; as some laboratories report that onset of hearing loss in C57B16/J mice occurs at a later age (8). At a pathological level, damage to the outer hair cells is initially seen at the basal turn by 3 months of age. Degeneration progresses in an apical direction and involves outer hair cells to a greater degree than inner hair cells (9). Additionally, there is a significant amount of spiral ganglion neuronal degeneration present by 6 months of age (7) that parallels the areas of hair cell loss. Hearing loss in this strain has been linked to the Ahl locus on mouse chromosome 10 (8). This strain also appears to be particularly sensitive to noise exposure and ototoxins, making it an attractive model for the study of presbycusis, which is thought to result from a combination of both genetic and environmental factors (2).
One of the key protagonists in the pathophysiology of aging in the central nervous system is oxidative stress. This occurs due to the accumulation of damage from reactive oxygen species (ROS), a natural byproduct of aerobic metabolism (10). Key enzymes that detoxify free radical cascade include superoxide dismustase (SOD), catalase and the enzymes controlling the synthesis and maintenance of glutathione. Studies in the CNS have shown that the accumulation of the free radical damage (e.g. lipid and protein peroxidation, accumulation of mitochondrial mutations) is proportional to aging. Decreases in levels of antioxidants or overwhelming exposure to ROS may accelerate the process of age-related oxidative damage (10). In the auditory system, numerous studies have begun to demonstrate the role of oxidative stress in such diverse processes as sound trauma and ototoxicity (6, 11, 12). Several studies have also demonstrated an accumulation of mitochondrial mutations with age in the auditory systems of both mice and humans (13). To determine if oxidative stress may play a role in presbycusis we examined levels of SOD (Cu/Zn and Mn), catalase and glutathione peroxidase in aging C57B16/J mice using semi-quantitative RT-PCR (SQRT-PCR). As controls we examined age-matched CBA/J mice which do not show signs of hearing loss until an advanced age (i.e. 20 months). The SQRT-PCR technique was chosen for case of application and the ability to process small samples. Immunohistochemistry for SOD, glutathione-S-transferase and 4-hydroxynonenal, a mediator of oxidative damage (12), was carried out on age-matched C57B16/J and CBA/J mice. B6.CAST+Ahl mice, in which the Ahl gene locus from CAST mice which have normal hearing has been crossbred to C57B16/J mice, were also tested to examine the effect of the Ahl gene on the level of antioxidant enzyme expression.
MATERIALS AND METHODS
Animals
All protocols were carried out as per NIH mandated guidelines for animal experimentation and were approved by the Massachusetts Eye and Ear Infirmary Animal Care Committee. One, 3-, 6- or 9-month old C57B16/J mice, and CBA/J mice were painlessly euthanized. The animals were decapitated and the cochleae removed in phosphate-buffered saline (PBS). All extra cochlear tissue was removed. Specimens were then processed either for immunohistochemistry or for SQRT-PCR. B6.CAST+Ahl mice were obtained from Jackson Labs (Bar Harbor, ME). Eleven-month-old mice were matched with 11-month-old C57B1/6J mice that had been raised in the same facility. The animals were then painlessly euthanized and the cochleae removed and processed for SQRT-PCR.
Semi-quantitative RT-PCR
Whole cochleae were crushed and mRNA extracted using 1 cm3 of TRIZOL reagent (Gibco). Concentrations of mRNA were checked, samples were treated with DNAse and reverse transcription (MMLV-RT) using oligo dT was carried out.
Quantification of amplification using the ABI Prism 7700 sequence detector (Taqman)
Messenger RNA was prepared as described above and RT was carried out using MMLV reverse transcriptase (Gibco/BRL). Five cochleae from different animals were analyzed by three PCR runs for all final results. In each run the particular age group of interest was run as three repeat amplifications for tubulin, SOD1, SOD2, catalase and glutathione peroxidase. Four time points were tested for C57B16/J mice: 1, 3, 6 and 9 months of age. One-, 3-, 6- and 9-month-old CBA/CaJ mice served as controls. Amplification was carried out using the Syber Green kit (Perkin Elmer) using a 25 μl reaction volume. Thirtyfive cycles were run under the following reaction conditions: 94°C for 10 min, 92°C for 1 min, 58°C for 1 min and 72°C for 1 min. The ABI Prism determined the cycle point at which amplification product became detectable. Experiments with detection at >30 cycles were rejected due to the possibility of detecting dimerized primers. The presence of amplification product was confirmed by running the reaction mix on a standard agarose gel. Controls for each reaction included reaction mix alone, and reaction mix with individual primer sets. Analysis of relative quantities of reaction product were carried out using the following method. The C(t) (cycle of detection threshold) for each primer set at each age point was determined. Change in cycle of detection threshold ΔC(t)=C(t) for the amplification product of interest–C(t) for the reference amplification product (tubulin) within one individual run. This was then compared to ΔC(t) for the reference tissue (3-month-old CBA/J mouse cochleae) for the primer set of interest to yield ΔΔC(t). The amount of amplification product relative to the baseline tissue can then be expressed as 2−ΔΔC(t).
Immunohistochemistry
Cochleae were fixed in PBS-buffered 4% paraformaldehyde for 6 h at 4°C and subsequently decalcified in CalEx solution (Fisher). After embedding in paraffin, 7-μm sections were cut and mounted on silanated slides. Sections were deparaffinized and hydrated.
Immunohistochemistry was carried out with a streptavidin–biotin–peroxidase reaction (Biomeda). Antibodies for SOD (Sigma), glutathione-S-transferase (Upstate Biotech) and 4-hydroynonenal were used. Incubation times were standardized for all four experimental conditions. Photomicrographs were obtained using a 100× oil immersion objective.
RESULTS
Immunohistochemistry
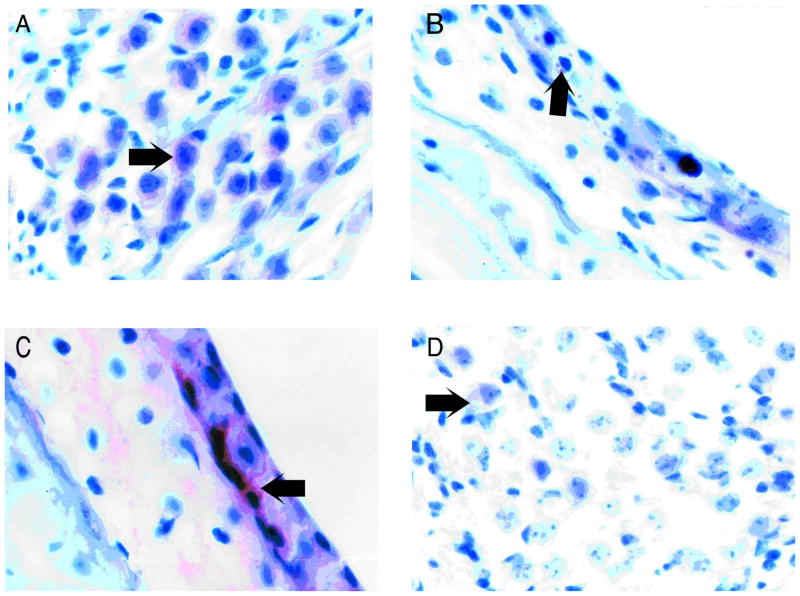
Immunohistochemical staining for Cu/Zn SOD was carried out on sections of cochleae from 3- and 9-month-old C57B16/J and CBA/J mice. Accumulation of reaction product was seen predominantly in the auditory neurons (Fig. 1A) and in the lateral wall of the cochlea, with some staining in the organ of Corti. Intensity of staining was compared using a five-step intensity scale. Staining for SOD1 was most intense in the lateral wall (stria vascularis and spiral ligament) of the cochleae of C57B16/J mice. Staining for SOD1 was distributed evenly at a lower level throughout the inner ear (Table I). Staining for glutathione-S-transferase yielded a more complex pattern. Three-month-old C57B16/J mice showed the most intense staining pattern, with greatest staining in the lateral wall (stria vascularis and spiral ligament). In particular the interdentate cells of the spiral limbus were stained (Fig. 1C). There was also light immuno-staining in the organ of Corti. Auditory neurons did not stain at any time point or for any strain (Table II). Staining for 4-hydroxynonenal was localized predominantly to the lateral wall of the cochlea. However, 3-month-old C57B16/J mice also showed accumulation of reaction product in the neurons of the spiral ganglion (Fig. 1D) and in the organ of Corti (Table III).
Fig. 1.
(A) Immunohistochemical staining for SOD1 in the spiral ganglion of a 3-month-old mouse. The arrow demonstrates accumulation of reaction product in the spiral ganglion neuron. (B) SOD1 staining in the lateral wall of a 9-month-old CBA mouse. There is minimal accumulation of immuno-stain in the stria vascularis. Note also the accumulation of lipofuscin pigment (arrow). (C) Immuno-staining for glutathione-S-transferase in a 3-month-old C57B16/J stria. The interdentate cells (arrow) are stained. (D) Immuno-staining for 4-hydroxynonenal in the spiral ganglion of a 9-month-old C57B16/J mouse. There is weak staining seen in the neuronal cell bodies (arrow).
Table I.
Immunohistochemical distribution of SOD in 3- and 9-month -old C57BL/6J and CBA/CaJ mice. Levels of staining are represented as 0=none, with +, ++ and +++ for increasing intensity
| Strain | Age (months) | Neurons | Organ of Corti | Lateral wall |
|---|---|---|---|---|
| C57BL | 3 | ++ | + | +++ |
| C57BL | 9 | + | + | + |
| CBA | 3 | + | + | + |
| CBA | 9 | + | + | + |
Table II.
Immunohistochemical distribution of gamma glutamyl transferase in 3- and 9-month old C57BL/6J and CBA/CaJ mice. Levels of staining as in Table I
| Strain | Age (months) | Neurons | Organ of Corti | Lateral wall |
|---|---|---|---|---|
| C57BL | 3 | − | + | +++ |
| C57BL | 9 | − | − | ++ |
| CBA | 3 | − | + | + |
| CBA | 9 | − | + | + |
Table III.
Immunohistochemical distribution of 4-hydroxynonenal in 3- and 9-month old C57BL/6J and CBA mice. Levels of staining as in Table I
| Strain | Age (months) | Neurons | Organ of Corti | Lateral wall |
|---|---|---|---|---|
| C57BL | 3 | + | + | + |
| C57BL | 9 | ± | − | ++ |
| CBA | 3 | − | − | + |
| CBA | 9 | − | − | + |
Experiments were carried out with the ABI Prism Taqman system (ABI Prism 7700 sequence detector) using Syber green as a reference dye. Relative levels of mRNA expression for SOD1, SOD2, glutathione peroxidase and catalase compared to tubulin were determined at 1, 3, 6 and 9 months of age in cochlear specimens from C57B16/J mice. Three- and 9-month old CBA/CaJ mice served as controls. There was no statistically significant difference in amplification of glutathione peroxidase in any of the age groups studied. There was a mild elevation in the expression of SOD2 mRNA at the 9-month time point in the C57B16/J mice (Table IV). Levels of SOD1 rose slightly and peaked at 9 months of age with an average 699-fold higher expression of SOD1 mRNA compared to 3-month-old CBA/CaJ control mice (p < 0.001). Nine-month-old CBA/CaJ mice showed an average change in expression of SOD1 of 0.39, which was within one standard deviation. Levels of catalase expression increased significantly and peaked at 6 months of age in the C57B16/J mice and then declined, with a maximum expression of 55-fold elevation at 6 months. CBA/CaJ mice showed a 130-fold elevation of catalase mRNA levels above baseline at 9 months of age, which was statistically significant. B6.CAST+Ahl and C57B16/J mice were analyzed at 11 months of age. Compared to 3-month-old CBA/CaJ mice, B6.CAST+Ahl mice showed 25.28-fold higher expression of SOD1. Levels of catalase expression were 2.7-fold higher (p = 0.1). Levels of SOD2 and glutathione peroxidase expression were similar in 3-month-old CBA/CaJ and 11-month-old B6.CAST+Ahl mice.
Table IV.
Expression of antioxidant enzyme mRNA in C57BL/6J mice at 1, 3, 6 and 9 months of age. Expression levels were determined by comparing levels of enzyme mRNA expression to tubulin mRNA and relating that to a baseline normal tissue (3-month -old CBA/CaJ mouse cochlea). Data for expression levels of antioxidant enzyme mRNA are also shown for 9-month-old CBA/CaJ mice
| Age of C57BL6/J mice (months) |
CBA-9 (n=6) | ||||
|---|---|---|---|---|---|
| 1 (n=6) | 3 (n=20) | 6 (n=6) | 9 (n=20) | ||
| SOD (Cu/Zn) | 0.82 ± 0.2 | 47.5 ± 0.1* | 23.7 ± 1.58* | 699 ± 0.14* | 0.39 ± 0.6 |
| SOD (Mn) | 0.27 ± 1.35 | 1.24 ± 0.2 | 0.25 ± 1.49 | 4.69 ± 0.2* | 0.51 ± 0.45 |
| Catalase | 0.22 ± 0.1 | 7.8 ± 0.3* | 55.7 ± 0.25* | 13.4 ± 0.3* | 130 ± 0.6* |
| Glutathione peroxidase | 0.15 ± 0.9 | 1.33 ± 0.5 | 0.7 ± 0.64 | 0.675 ± 0.1 | 0.6 ± 0.3 |
p < 0.05.
DISCUSSION
The C57B16/J mouse has been widely studied and has been used as a mouse model of presbycusis (7, 14) or non-syndromic late-onset sensorineural hearing loss. Hearing loss is first noted in the high frequencies as early as 3 months of age and progresses to severe impairment by the time the mouse is 1 year old (7). Age of onset of hearing loss in CBA C57B16/J mice has been found to vary between different laboratories and has been shown to start as late as 10 months of age (8). This may be due to variations in the ambient sound levels of an animal’s environment or to how the extent of hearing loss is measured. Recent studies have defined the progression of both hair cell degeneration and loss of auditory neurons. Hair cells are damaged and lost in base-to-apex pattern, with degeneration of both hair cells and spiral ganglion neurons (7). Genetic analysis has been carried out, resulting in the identification of an Ahl locus on mouse chromosome 10 (8). The actual mechanism or genetic defect of hearing loss in these mice has not been identified. Studies of human presbycusis have also suggested that there is a strong genetic component to age in this disorder (6), combined with environmental exposure, making this mouse an interesting model for examining the effect of genotype on susceptibility to environmental stressors.
Oxidative stress has been implicated in a number of age-related degenerative processes in the central nervous system (10) and has also been implicated in the pathophysiology of age-related hearing loss (10, 14, 15). Recent studies of mice carrying a null mutation of SOD gene show that these animals are more susceptible to noise-induced hearing loss and also that they have early onset hearing loss (9, 16). The impact of oxidative stress has not been directly studied in the C57B16/J strain. Li and Hultcrantz (17) have shown that C57B16/J mice are more susceptible to noise-induced hearing loss (7), which is thought to be at least partially mediated through an increase in oxidative stress. Limited experiments using caloric restriction have also been carried out in these mice, with interesting results. Caloric restriction, with is thought to reduce levels of oxidative stress, reduced the rate of AHL for C57B16/J mice (14). This suggests that at least part of the pathologic auditory degeneration seen in this mouse may be ascribed to increased oxidative stress. Some previous studies have also suggested that the C57B16/J strain expresses a less active form of the enzyme catalase than other strains, thus further predisposing the cochlea of this strain of mouse to auditory damage induced by the Ahl gene (18).
To understand the role of oxidative stress in the pathophysiology of hearing loss in these mice we designed a series of assays that would test whether or not there is a change in the regulation of antioxidant enzymes during the period of hearing loss in the C57B16/J mouse (7). As previously described, loss of hair cells at the basal turn of the cochlea could already be seen in 3-month-old mice and high frequency hearing loss developed at between 3 and 6 months of age in animals kept in the animal quarters at our institution (data not shown). We targeted the age groups at which maximum hearing loss was occurring as measuring antioxidant levels at extremes of age could result in erroneous measurements because, at that point in the process of AHL, a significant number of most sensory cells in the C57B16/J mouse have been lost. Several different approaches can be used to document the antioxidant status of the inner ear. Immunohistochemistry for antioxidant enzymes can demonstrate and localize the presence of individual antioxidant enzymes within the cochlea, but these results are difficult to quantify. In our immunostain label studies, staining for SOD1 was increased in the C57B16/J mice vs the CBA/CaJ mice, suggesting either increased oxidative stress and subsequent upregulation of SOD1 or overexpression of a poorly functional SOD1. Of additional importance in these studies is the involvement of the lateral wall of the cochlea. The stria vascularis and spiral ligament are being increasingly recognized as playing a crucial role in the maintenance of cochlear homeostasis. These areas are also rich in mitochondria and thus represent a potential source of oxidative stress. Interestingly, staining for glutathione-S-transferase, the rate-limiting enzyme in the synthesis of glutathione, was strongly localized to the spiral limbus cells of the lateral wall (19).
4-Hydroxynonenal may be a toxic mediator of oxidative stress in the ear (12). Staining for this aldehydic adduct of membrane lipid peroxidation was pronounced in 3-month-old C5716/J mice. Additionally it was present in the lateral wall of the cochlea for all animals tested, indicating that this is a metabolically active area and an area of high oxidative stress. As staining within the cochlea of C57B16/J animals was already positive at 3 months of age, some oxidative damage has probably already occurred by this time. This is confirmed by histological studies that show loss of hair cells by 3 months of age (7) and auditory brainstem response threshold data that show loss of high frequency hearing.
We found that both catalase and SOD were upregulated during the time when hearing loss is occurring in the cochlea of the C57B16/J mice. Catalase expression peaked at 6 months of age and then declined slightly. This is the time point at which high frequency hearing loss is rapidly progressing (7) and also a time at which significant degeneration of basal sensory cells is taking place. Nine-month-old CBA/CaJ mice also showed an elevation of catalase. The accumulation of lipofuscin pigment in the lateral wall of CBA/J mice (Fig. 1B) is an indication that this is an area undergoing oxidative stress in strains that do not show a significant hearing loss at that age. Previous studies have examined antioxidant activities in a number of strains of mice. The activity of C57B16/J catalase was found to be significantly lower compared to that of other inbred strains of mice, such as BALB/c (18). It is plausible that inter-strain differences in antioxidant status of the inner ear could influence the rate of degeneration of hearing due to a gene mutation such as Ahl. SOD1 and 2 both showed significant signs of elevation, that continued up to 9 months of age in the C57B16/J mice. This is consistent with the results of immunolabeling of these cochlea. Previous immunohistochemical studies have localized SOD in the lateral wall of the cochlea as well as in the organ of Corti, which is in agreement with our findings (20), with the exception of pronounced staining for SOD1 in the spiral ganglion (Fig. 1A).
Other models of aging in the auditory system have suggested that the glutathione pathway provides an important axis for free radical detoxification (11). We did not detect any upregulation of glutathione peroxidase; however, in this series of experiments we did not test the remaining members of the glutathione cycle (i.e. glutathione synthetase). Furthermore, we did not extend our observations to extreme old age in the mice, as was the cases with the rats that were used as the experimental model for the glutathione studies.
The Ahl gene does appear to influence the expression of antioxidant genes irrespective of the overall genetic background of the C57B16/J strain. A limited number of congenic C57B16/J animals carrying the Ahl gene from CAST mice (B6.CAST+Ahl) were tested, suggesting that substitution of the Ahl gene from a different strain without hearing loss does appear to influence expression of SOD1 and SOD2.
The hearing loss that is the result of the Ahl defect is probably not a primary defect of the antioxidant system but rather the Ahl defect results in an increased exposure to oxidative stress. This in part may also explain this strain’s increased susceptibility to noise damage, which has also been linked to free radical generation, as well as the observation that caloric restriction can ameliorate the damage caused by the presence of the Ahl gene. The results of our study suggest that changes in expression levels of antioxidant enzyme mRNA within the cochlea are dynamic and that these levels change over time in both C57B16/J and CBA/CaJ mice. The presence of the Ahl gene does appear to have a significant impact on the expression of antioxidant genes and future studies will need to address how this system is regulated and how Ahl influences the antioxidant status of the cochlea.
Acknowledgments
We thank Dr. K. R. Johnson, The Jackson Laboratory (TJL), for his careful review of the manuscript. This work was supported by grant T32 DC00020, DC62108 from the National Institutes of Health, the National Institute on Deafness and Other Communication Disorders and by a grant from the American Hearing Research Foundation.
References
- 1.Ding DL, McFadden S, Wang J, Hu BH, Salvi RJ. Age and strain-related differences in dehydrogenase activity and glycogen levels in CBA and C57 mouse cochleas. Audiol Neurol Otol. 1999;4:55–63. doi: 10.1159/000013822. [DOI] [PubMed] [Google Scholar]
- 2.Erway LC, Shiau YW, Davis RR, Krieg EF. Genetics of age-related hearing loss in mice. III. Susceptibility of inbred and F1 hybrid strains to noise induced hearing loss. Hear Res. 1996;93:181–7. doi: 10.1016/0378-5955(95)00226-x. [DOI] [PubMed] [Google Scholar]
- 3.Gabaizadeh R, Staecker H, Liu W, et al. Protection of both auditory hair cells and neurons from cisplatin induced damage. Act Otolaryngol. 1997;117:232–8. doi: 10.3109/00016489709117778. [DOI] [PubMed] [Google Scholar]
- 4.Gates GA, Couropmitree NN, Myers RH. Genetic association in age-related hearing thresholds. Arch Otolaryngol Head Neck Surg. 1999;125:654–9. doi: 10.1001/archotol.125.6.654. [DOI] [PubMed] [Google Scholar]
- 5.Guegan C, Ceballos-Picot I, Chevalier E, Nicole A, Onteniente B, Sola B. Reduction of ischemic damage in NGF-transgenic mice: correlation with enhancement of antioxidant enzyme activities. Neurobiol Dis. 1999;6:180–9. doi: 10.1006/nbdi.1999.0240. [DOI] [PubMed] [Google Scholar]
- 6.Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–8. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 7.Spongr D, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57BL/6J mice throughout their life spans. J Acoust Soc Am. 1997;101:3546–53. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/s0378-5955(97)00155-x. [DOI] [PubMed] [Google Scholar]
- 9.Ohlemiller KK, McFadden SL, Ding D, et al. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (SOD1) increases susceptibility to noise-induced hearing loss. Audiol Neurol Otol. 1999;4:237–46. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- 10.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lautermann J, Crann S, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res. 1997;144:75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 12.Stupak H, Zur K, Rho M, Van De Water TR. A product of lipid peroxidation, 4-hydroxynonenal, is a mediator of cisplatin ototoxicity. ARO Midwinter Meeting; 1998. [Google Scholar]
- 13.Seidman M, Bai U, Khan M, Quirk W. Mitochondrial DNA deletions associated with aging and presbycusis. Arch Otolaryngol Head Neck Surg. 1997;123:1039–45. doi: 10.1001/archotol.1997.01900100009001. [DOI] [PubMed] [Google Scholar]
- 14.Willott JF, Erway LC. Genetics of age-related hearing loss in mice. IV. Cochlear pathology and hearing loss in 25 BXD recombinant inbred mouse strains. Hear Res. 1998;119:27–36. doi: 10.1016/s0378-5955(98)00029-x. [DOI] [PubMed] [Google Scholar]
- 15.Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82–90. doi: 10.1016/s0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- 16.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 17.Li HS, Hultcrantz M. Age-related degeneration of the organ of Corti in two genotypes of mice. ORL J Otorhinolaryngol Relat Spec. 1994;56:61–7. doi: 10.1159/000276611. [DOI] [PubMed] [Google Scholar]
- 18.Schisler NJ, Singh SM. Inheritance and expression of tissue-specific catalase activity during development and aging in mice. Genome. 1987;29:748–60. doi: 10.1139/g87-127. [DOI] [PubMed] [Google Scholar]
- 19.Usami S, Hjelle OP, Ottersen OP. Differential cellular distribution of glutathione—an endogenous antioxidant in the guinea pig inner ear. Brain Res. 1996;743:337–40. doi: 10.1016/s0006-8993(96)01090-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen L, Segal DM, Mash DC. Semi-quantitative reverse-transcriptase polymerase chain reaction: an approach for the measurement of target gene expression in human brain. Brain Res Brain Res Protoc. 1999;4:132–9. doi: 10.1016/s1385-299x(99)00009-4. [DOI] [PubMed] [Google Scholar]