Abstract
Long-term resistance training (RT) may result in a chronic increase in 24-hour energy expenditure (EE) and fat oxidation to a level sufficient to assist in maintaining energy balance and prevent weight gain. However, the impact of a minimal RT program on these parameters in an overweight college age population, a group at high risk for developing obesity, is unknown.
Purpose
We aimed to evaluate the effect of 6-months of supervised minimal RT in previously sedentary, overweight (mean±SEM, BMI=27.7±0.5kg/m2) young adults (21.0±0.5yrs) on 24-hr EE, resting metabolic rate (RMR), sleep metabolic rate (SMR) and substrate oxidation using whole room indirect calorimetry 72-h after the last RT session.
Methods
Participants were randomized to RT (1 set, 3 d/wk, 3–6 repetition maximum, 9 exercises) (N=22) or control (C, N=17) groups and completed all assessments at baseline and 6 months.
Results
There was a significant (P<0.05) increase in 24-hr EE in the RT (527 ± 220kJ/d) and C (270 ± 168kJ/d) groups, however, the difference between groups was not significant (P=0.30). Twenty-four hour fat oxidation (g/day) was not altered after RT, however; reductions in RQ assessed during both rest (P<0.05) and sleep (P<0.05) suggested increased fat oxidation in RT compared with C during these periods. SMR (8.4±8.6%) and RMR (7.4±8.7%) increased significantly in RT (P<0.001) but not in C, resulting in significant (P<0.001) between group differences for SMR with a trend for significant (P=0.07) between group differences for RMR.
Conclusion
A minimal RT program that required little time to complete (11 min per session) resulted in a chronic increase in energy expenditure. This adaptation in energy expenditure may have a favorable impact on energy balance and fat oxidation sufficient to assist with the prevention of obesity in sedentary, overweight young adults, a group at high risk for developing obesity.
INTRODUCTION
The continued increase in the development of overweight and obesity, combined with the difficulty in treating these conditions, suggests that innovative strategies for obesity prevention need to be developed and evaluated (17). The college age population represents an important group of individuals at high risk of developing obesity (43, 45). Reports by many health organizations have recommended resistance exercise training (RT) as an integral part of adult fitness programs (4, 12, 17, 33, 42). Nevertheless, despite the plethora of investigations that have examined the acute effects of RT on energy expenditure and substrate oxidation, there have been limited investigations of long-term (e.g., 6 months or longer) RT on the chronic adaptations of energy expenditure and substrate oxidation in young adults.
Unlike aerobic exercise, which results in significant increases in energy expenditure during, and for a short time following cessation of the activity, the energy expenditure during RT is relatively low (29, 31), but the increase in energy expenditure after the cessation of the activity may be elevated (7, 29, 38). To this end, while there is a plausible basis for using RT as a weight control strategy, results from investigations designed to determine the effect of low volume RT on RMR (2, 5, 22, 36, 39), twenty-four hour energy expenditure (24-hr EE) (16, 32) and substrate oxidation (40) are scarce and inconsistent. Since, resting metabolic rate (RMR) accounts for the largest proportion of total daily energy expenditure; small changes in RMR could have long-term benefits for weight management (6, 16, 25, 34). However, RT studies that measured energy expenditure and substrate oxidation within 24-h after the last RT session determined an acute response to RT. Using this method reduces our understanding if an adaptation to RT resulting in a chronic elevation of energy expenditure and fat oxidation has occurred. Since many organizations (4, 12, 17, 33, 42) recommend performing RT sessions 48–72 hours apart it is important to determine the chronic effect of RT on energy expenditure and fat oxidation. Additionally, previous studies have used a greater volume of RT consisting of multiple sets (2–4) than recommended by the American College of Sports Medicine (1 set, 8–12 repetitions, 8–10 exercises) to develop and maintain muscular fitness in untrained individuals (13, 21). Higher volume, multiple set RT programs may not be attractive or adhered to by sedentary overweight individuals in need of weight management.
Another consideration is that few studies have controlled both pre-measurement energy intake and exercise. This is important for ensuring that subjects are in a similar state of energy balance and fuel repletion at the time of testing, because over and underfeeding have profound effects on energy expenditure and substrate oxidation. The most effective method to evaluate the chronic effects of RT on energy expenditure and substrate oxidation is to study subjects over a 24-h period by using whole room indirect calorimetry, with the application of suitable pre-measurement dietary and exercise controls.
The primary objective of this investigation was to evaluate the impact of a supervised 6 month minimal RT program, [1 set, 3-day per week, 9 exercises, 3–6 repetition maximum (RM)] in sedentary young adults on 24-hr EE, RMR, sleep metabolic rate (SMR) and substrate oxidation assessed by whole room indirect calorimetry 72-h after the last RT session. We hypothesized that the minimal RT program would result in an increase in 24-h EE, RMR, SMR, and fat oxidation compared to the non-exercising controls.
SUBJECTS AND METHODS
Participants/Design
Sixty-three overweight (BMI ± 25 kg/m2), young adult men and women volunteered to participate in this study after providing written informed consent. Participants were monetarily compensated for participation. Potential participants who used tobacco products, had a history of chronic disease (i.e. diabetes, heart disease, etc.), elevated blood pressure (>140/90mmHg), lipids (cholesterol > 240 mg·L−1; triglycerides >500 mg·L−1) (9), fasting glucose (>126 mg·dl−1) (11) or were physically active (>2100 kJ·week−1 on the Minnesota Leisure Time Physical Activity Questionnaire (35) were excluded from the study. Participants were matched for fat-free-mass (FFM) (± 0.5kg) and randomized at approximately a 1.5:1 ratio to RT (n = 37) or non- exercise control (C, n = 26). This assignment ratio was in anticipation of greater attrition in the RT group compared to the C group. Both groups were instructed to maintain their normal ad-libitum diet, and normal activities of daily living. Eight participants failed to complete the study protocol (n= 5 RT, n = 3 C). Of the 55 participants who completed the study (n=32 RT, n = 23 C) 39 participants volunteered to complete the whole room calorimeter protocol (n = 22 RT, n = 17 C) and these data are reported in this paper. Twenty-four hour EE, RMR, SMR, substrate oxidation, muscular strength, and body composition were assessed at baseline and after completion of a 6 month supervised RT program. In addition, dietary intake was assessed monthly throughout the RT program. The study protocol was approved by the Institutional Review Board at the University of Kansas-Lawrence.
Procedures
Resistance Training Protocol
RT was performed on 3 non-consecutive days per week for 6 months using Paramount weight stack resistance equipment (Paramount Fitness Corporation, Los Angeles, CA) located in the Energy Balance Laboratory at the University of Kansas-Lawrence. To ensure compliance with the RT protocol all sessions were supervised on a one-on-one basis by experienced laboratory technicians. Participants were informed that an adherence rate of greater than 90% of scheduled sessions was required to remain in the study. Detailed records regarding attendance, number of repetitions performed, resistance used, and the total amount of weight lifted per session were maintained by the training supervisor. Participants performed 1 set of 9 exercises designed to train all major muscle groups (chest press, back extension, lat pull down, triceps extension, shoulder press, leg press, calf raise, leg curl, and abdominal crunch) using a resistance of 3–6 1RM, approximately equal to 85–90% of 1RM. We selected the higher intensity RT program in order to maximize the potential effect on fat-free mass (FFM) and therefore also RMR, SMR and 24-h EE. During the first training session participants were familiarized with the equipment and instructed on proper lifting techniques for all exercises using minimal resistance (~12 – 15 RM). On the fourth training day, a resistance eliciting 3–6 RM was determined based on the participant's performance during the initial 3 training periods. All lifts were conducted at two-second concentric and four-second eccentric movements (33) to decrease the likelihood that momentum was being used to perform the lift and to ensure loading throughout the full range of motion. When more than 6 repetitions were completed using good form for two consecutive exercise sessions, the resistance was increased by approximately 2.25 kg to reduce the maximum number of repetitions to between 3 and 6. The order of exercises (as listed previously) was the same for all training sessions. Each exercise was preceded by a 5 min of upper and lower body stretching, and followed by a 5 min cool down/stretching period.
Assessments
With the exception of dietary intake, all assessments were conducted at the same time of day in both RT and C groups at baseline and following the 6 month intervention period. Post-training assessments for the RT group were conducted 72 hours following completion of the final RT session.
Body Mass/BMI
Body mass was assessed at baseline and 6 months between the hours of 7 and 9 a.m. using a digital scale accurate to ± 0.1 kg (Befour Inc., Model #PS6600, Saukville, WI). The participants were weighed prior to breakfast and after attempting to void and wore a standard hospital gown at the time of weighing. Height was assessed using a wall-mounted stadiometer (Model PE-WM-60-84, Perspective Enterprises, Portage, Mi). BMI was calculated as weight (kg)/height (m2).
Body Composition
Fat-free mass (FFM), fat mass (FM), and percent body fat were assessed using dual energy x-ray absorptiometry (DXA–Lunar DPX-IQ, Madison, WI). DXA exams were performed during the afternoon with the participants clothed in a hospital gown. All females completed a pregnancy test prior to DXA evaluations. DXA is capable of detecting changes in fat-free mass of approximately 1.6 – 3.8 % (20).
Maximal Strength Testing
Prior to beginning the 4th training session a 1RM strength test for the chest press and leg press, was performed according to the protocol described by Lemmer et al.(25). Briefly, participants performed a light warm-up of 10 repetitions of the exercise to be evaluated, against minimal resistance. A resistance estimated to be just below the subject's 1RM strength was chosen and the participant was asked to lift the weight one time. If the lift was completed successfully through the full range of motion, the resistance was increased (minimum increment 1.1kg) and another attempt made following a rest period of at least 1 minute. This process was continued until the subject was unable to lift the prescribed resistance. The highest weight lifted was recorded as the 1RM. The 1RM was determined within 5 attempts for each exercise. The test-retest reliability (intraclass correlation) for maximal strength assessment was 0.95 for chest press and 0.94 for leg press.
Twenty-four Hour Energy Expenditure –Room Calorimeter
Room Description
The calorimeter is a small room measuring 2.6m wide, 3.4m long, and 2.58m high, with a total volume of 17,500 liters and is equipped with a couch that folds into a bed, a desk, toilet, entertainment facilities (TV, VCR, computer), telephone, and a stationary bicycle, reducing the volume to 16,300 liters. An airlock (78.1 × 33.7 cm) allows for the passage of food and materials to the subject while inside the room.
Dietary Control for Calorimeter Stay
Three days prior to the calorimeter stay, participants were provided a diet (3 meals and 2 snacks per day) estimated to meet free-living energy requirements in order to maintain weight stability and standardize macronutrient intake. Twenty-four hour energy requirements (kJ/day) were estimated as 2065.16 − (12.90*height in cm) + (13.49*body mass in kg) + (0.03418*grams of lean tissue measured from DXA)*4.186 (8). The composition of the diet was 30% of energy from fat, 15% from protein, and 55% from carbohydrate. All menus were planned by a registered dietitian and all food was prepared and served at the university cafeteria. Weigh and measure techniques (41) were utilized to document the macronutrient and energy composition of the diet. The two daily between meal snacks contained the same macronutrient composition as the 3 meals. To confirm weight stability (× 1kg), individuals were measured in a standard hospital gown, after voiding and before consumption of breakfast, three days prior to the calorimeter stay, and immediately before entering the calorimeter. During the 3 days prior to the calorimeter stay participants were instructed to maintain their normal daily physical activity patterns and to refrain from performing any RT or aerobic exercise 72 hours prior to entering the calorimeter. This protocol was done specifically to reduce the effect of the last RT session on post-training assessments of energy expenditure and substrate oxidation in the RT group (28). Participants spent the night before the calorimeter stay in our laboratory dormitory under supervision of the research staff.
Calorimeter stay
Participants entered the calorimeter at 8:00 AM and exited at 7:00 AM the following day. The data was extrapolated to 24-h values. During each stay in the calorimeter, participants consumed a diet with a caloric content designed to achieve energy balance and with the same macronutrient composition as described above. Participants were provided breakfast (8:30AM), lunch (12:30PM), snack (3:00PM), dinner (5:30PM) and a second snack (8:00PM) during the 23-h stay. All food for the calorimeter stay was prepared in the metabolic kitchen registered dietitians. To account for energy expended in normal activities of daily living, an individualized cycle ergometer protocol (75 Watts) designed to expend ~837 kJ per session was performed at 10:30AM, 4:00PM, and 7:00 PM. The number of minutes of exercise required to expend ~837 kJ was calculated using the ACSM metabolic equations (1). Participants were free to move about the calorimeter during other times of the day. However, free time was primarily spent in sedentary activities such as reading, writing, watching TV, or using the computer. Participants were instructed to remain awake and not to nap or perform any exercise other than the cycle ergometer exercise prescribed by the protocol. Participants went to bed at the same time (± 30 min) during each calorimeter stay.
Assessments/Calibration
Total 24-h EE, RMR, SMR and substrate oxidation were determined from oxygen consumption and carbon dioxide production. Gas volumes (SPTD) were assessed from the flow rate and the differences in carbon dioxide and oxygen concentrations between air entering and exiting the calorimeter using Siemens Ultramat/Oxymat 6 (Siemens, Karlsruhe, Germany) oxygen and carbon dioxide analyzer which is calibrated prior to each test. The oxygen and carbon dioxide analyzers were calibrated with gasses of known concentration prior to each calorimeter stay.
The accuracy and precision of the whole room calorimeter was evaluated monthly by burning propane at variable rates. We consistently observed 97–98% recovery of the predicted values for oxygen consumption and carbon dioxide production. Repeated measures under identical conditions over a short period of time have not been previously completed in our calorimeter. However, in a separate study, when examining repeated measures performed under separate but similar conditions approximately 6 weeks apart, intra-class correlations for 24hr EE, SMR, and RMR was 0.804, 0.718, and 0.70, respectively. Coefficients of variation (CV) for 24hr EE, SMR, and RMR were 4.1, 7.9, and 7.3, respectively. Although the CV values are slightly higher than what has been previously reported, the sample size is considerably larger (n=26), and the time between tests is ~ 6 weeks. Both of which could possibly explain the larger CV for our repeated measures
The operation of the calorimeter was computer controlled. Data were collected continuously and averaged over 15-minute intervals and recorded to a data file. Sleeping metabolic rate was assessed as the average metabolic rate obtained for 1:00 to 5:00 AM. Resting metabolic rate was measured between 6:00 and 6:45 AM with the participant awake, lying quietly on their back. All participants were monitored by research assistants during the calorimeter stay. Urine was collected throughout the calorimeter stay for determination of total nitrogen concentration (37) which was used to assess 24-hr protein oxidation (27). Energy expenditure and substrate oxidation were calculated from measured oxygen consumption and respiratory quotient (RQ) using the equations of Jequier et al.(18).
Dietary Intake
Dietary intake was assessed monthly over the course of the intervention using 24-h dietary recalls and 3 day food records (15) performed on one randomly selected day each month. Research assistants were trained by a registered dietitian to conduct standardized, structured interviews using neutral probing questions (10). To promote accurate reporting of portion sizes, subjects were given 3-dimentional food models (10). Nutrient calculations were performed using the Nutrition Data System for Research software (Version 4.03, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, Food and Nutrient Database 31, released 2000).
Statistics and Data Analysis
A two-factor (group × time) repeated measures (time) analysis of variance (ANOVA) was used for main effects. If there was a significant interaction effect of group or time, we employed the Tukey post-hoc test. Student's t-test was used to determine differences for change scores. Statistical significance was defined at P <0.05 for all tests. All values are expressed as mean ± standard error of mean (SEM). Data were analyzed using SAS (8.2, Cary, NC). Using an α level of 0.05 and a sample size of 22 in the RT and 17 in the C group the results provided a 72% statistical power for the interaction effects in 24-h EE and 82% in RMR.
RESULTS
Thirty nine overweight (BMI = 27.7 ± .0.5 kg/m2) adults [N = 22 RT (16 Male, 6 Female); N = 17 C (11 Male, 6 Female)], 85% white, mean age (21.0 ± 0.5 yrs) completed the 6 month study and all baseline and end-study assessments. Adherence to the RT protocol was excellent with participants completing 96 ± 1% of the total prescribed exercise sessions. No participant fell below the 90% adherence rate required to be considered compliant with the RT protocol. The average time to complete each exercise session was 11 ± 1 minutes. No major adverse events occurred during the study in either the RT or C groups. There were no significant baseline differences between groups for any variables assessed. Also, there was no difference between subjects who dropped out of the study and those who completed the study.
Strength (1RM)
As expected, significant between group differences for change in both chest (RT=+ 47.5±4.9%) and leg press strength (RT=+53.7±8.5%) were observed (P<0.001). No change was observed in the C group.
Body mass and composition (Table 1)
Table 1.
Body weight and composition at baseline and 6 months.
| Variable | Group | Baseline | 6 months | Change | p for group X time interaction |
|---|---|---|---|---|---|
| Body Weight (kg) | C1 | 82.2 ± 2.83 | 84.6 ± 3.1 | 2.4 ± 0.64 | 0.87 |
| RT2 | 86.7 ± 2.8 | 89.2 ± 2.6 | 2.5 ± 0.74 | ||
|
| |||||
| BMI (kg/m2) | C | 27.6 ± 0.6 | 28.3 ± 0.7 | 0.7 ± 0.94 | 0.36 |
| RT | 27.8 ± 0.7 | 28.7 ± 0.7 | 0.8 ± 1.64 | ||
|
| |||||
| Fat Mass (kg) | C | 26.0 ± 1.8 | 28.3 ± 1.7 | 2.3 ± 0.64 | 0.12 |
| RT | 27.2 ± 1.9 | 28.2 ± 1.7 | 0.9 ± 0.6 | ||
|
| |||||
| Fat Free Mass (kg) | C | 52.7 ± 2.6 | 52.4 ± 2.5 | −0.3 ± 0.2 | 0.003 |
| RT | 55.3 ± 1.9 | 56.8 ± 2.0 | 1.5 ± 0.54 | ||
|
| |||||
| Body Fat (%) | C | 33.2 ± 2.2 | 35.3 ± 1.8 | 2.1 ± 0.64 | 0.002 |
| RT | 32.7 ± 1.8 | 33.0 ± 1.7 | 0.3 ± 0.5 | ||
C =Control (n = 17),
RT = Resistance Trained (n = 22)
Values are means ± SE.
Significantly different within group, p<0.05.
Over the 6 month intervention, body mass and BMI increased significantly (P < 0.05) in both RT (weight = 2.9%, BMI = 2.9%) and C groups (weight = 2.9%, BMI = 2.5%), however; differences in change in body mass and BMI between groups were not statistically significant. RT had a significant impact on body composition. The increase in FFM was significantly greater (P< 0.01) in RT (+2.7%) compared with C (−0.6%). Although the between group difference for change in FM was not statistically significant, we observed a significant increase in FM (8.8%, P<0.05) in C and a non-significant increase (3.3%) in RT. The combination of similar increases in body mass, and greater increases in FFM and reduced increase in FM favoring RT compared with C, resulted in a significant between group difference (P <0.05) for change in percent body fat (RT = 0.9%, C = 6.3%).
24-hr EE, RMR, and SMR (Table 2)
Table 2.
Twenty-four-hour, rest and sleep energy expenditure at baseline and 6 months
| Variable | Group | Baseline | 6 months | Change | p for group X time interaction |
|---|---|---|---|---|---|
| 24-hr EE (kJ/d) | C1 | 12835 ± 4793 | 13105 ± 479 | 270 ± 168 | 0.30 |
| RT2 | 13091 ± 358 | 13618 ± 383 | 527 ± 2204 | ||
|
| |||||
| RMR (kJ/d) | C | 9448 ± 371 | 9683 ± 432 | 235 ± 168 | 0.07 |
| RT | 9236 ± 1327 | 9915 ± 268 | 679 ± 1735 | ||
|
| |||||
| SMR (kJ/d) | C | 9166 ± 322 | 9258 ± 340 | 92 ± 177 | 0.01 |
| RT | 9143 ± 283 | 9844 ± 234 | 701 ± 1625 | ||
C =Control (n = 17)
RT = Resistance Trained (n = 22)
Values are means ± SE
Significantly different within group, p<0.05
Significantly different within group, p<0.001
There was no between group difference for change in 24-hr EE (P=0.30), however; the increase in 24-hr EE in RT (P<0.05) was double that of the increase in the C group. RT resulted in a significant increase (P< 0.001) in RMR, with a trend for time X group interaction for RMR (P =0.07). SMR increased significantly in RT (P < 0.001) but not in C resulting in a significant between group difference (P<0.05). Change in FFM was positively associated with change in 24-hr EE (r=0.44, P=0.04), RMR (r=0.37, P=0.08) and SMR (r=0.29, P=0.18) in the RT group. Likewise the regression approach (24) was also used to examine the effects of the change in FFM with 24-EE, RMR, and the results of these analyses were similar to those using the ratio method.
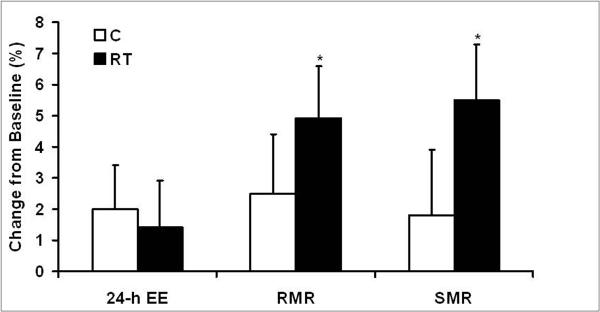
There were no significant within or between group differences for change in 24-hr EE/kg FFM. RT resulted in significant increases (P<0.05) in both RMR/kgFFM and SMR/kg FFM, however; the between group differences in change for both of these outcomes were not statistically significant (Figure 1).
Figure 1.
Relative change in twenty-four hour energy expenditure (24-h-EE), resting metabolic rate (RMR), and sleeping metabolic rate (SMR) expressed per kilogram of fat-free mass.
Values are means ± SE. *Significantly different within group, P<0.05.
Substrate oxidation (Table 3, Figure 2)
Table 3.
Substrate oxidation at baseline and 6 months.
| Variable | Group | Baseline | 6 months | Change | p for group X time interaction |
|---|---|---|---|---|---|
| Carbohydrate oxidation (g/day) | C1 | 473.1 ± 21.83 | 502 ± 25.3 | 28.9 ± 19.6 | 0.57 |
| RT2 | 463.1± 14.1 | 488.9 ± 20.9 | 25.8 ± 17.3 | ||
|
| |||||
| Fat oxidation (g/day) | C | 85.4± 9.0 | 74.4 ± 7.1 | −11.0 ± 8.5 | 0.67 |
| RT | 91.9 ± 38.5 | 93.1 ± 8.2 | 1.2 ± 8.2 | ||
|
| |||||
| Protein oxidation (g/day) | C | 66.1 ± 4.7 | 77.1 ± 6.5 | 11.0 ± 5.4 | 0.27 |
| RT | 75.3 ± 5.1 | 77.3 ± 5.3 | 2.0 ± 6.1 | ||
C =Control (n = 17)
RT = Resistance Trained (n = 22)
Values are means ± SE.
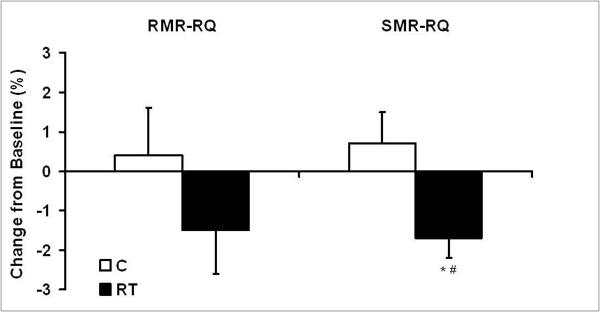
Figure 2.
Relative change in respiratory quotient measured during assessment of resting (RMR-RQ) and sleeping metabolic rate (SMR-RQ).
Values are means ± SE. *Significantly different within group, #between groups, P<0.05.
There were no significant between or within group differences for change in protein, fat or carbohydrate oxidation (g/day). However, changes in RQ assessed during both rest and sleep suggested increased fat oxidation in RT compared with control. SMR-RQ increased slightly in C (0.5%) and decreased significantly (P<0.05) in RT (−1.7%) resulting in a significant difference between groups (P<0.05, Figure 2). RMR-RQ was not significantly different between groups, however, RMR-RQ decreased in RT (−1.5%, p=0.15) and increased in C (0.4%) suggesting a favorable trend for increased fat oxidation as a result of RT.
Dietary intake
Differences in reported dietary intake (total energy, carbohydrate, fat, protein) were not significant between the baseline and intervention periods for either RT or C, or between the 2 groups during the intervention. The mean intakes for total energy, and percent of dietary carbohydrate, fat and protein were 9538 kJ/day, 50%, 34%, and 16%, respectively. There was no difference for either group at baseline and 6 months between energy and macronutrient intake during the three days of standardized food prior to or during the calorimeter stay.
DISCUSSION
To our knowledge this is the first study to use a whole room indirect calorimeter to measure changes in 24-h EE, RMR, SMR and substrate oxidation 72-h after the last RT session in response to a long term (6 months), low volume RT program in young overweight adults. Results showed a favorable impact of RT on body composition corresponding to a chronic adaptation of both energy expenditure and fat oxidation.
The favorable results for 24-h energy expenditure suggests that a 6 month minimal RT may have a significant impact on daily energy expenditure. In fact, increases in 24-h EE may potentially result in a negative energy balance of sufficient magnitude to prevent increases in fat mass (25, 34, 40). Hill et al.(14) estimated that an “energy gap” of only 420KJ per day may be responsible for weight gain in 90% of the population. Reduction or elimination of this energy gap could prevent or reduce body fat gain in the majority of Americans. In the current study subjects in the RT group increased their 24-h EE by ~500kJ and although both groups gained a similar amount of body mass, fat mass increased in C but not in RT. These results suggest that a minimal RT program, such as the one performed in the current study, may increase 24-h EE sufficiently to prevent gains in body fat, despite an increase in body mass resulting from increased FFM. It also is encouraging that the increase in 24-h EE, measured greater than 72-h after the last RT session, suggests a chronic adaptation, rather than an acute effect of RT. Since RT is routinely recommended by health organizations to be performed every other day the fact that even a low volume RT program can provide a sustained increase in energy expenditure may be important for weight management.
Despite the fact the increase in 24-h EE in the RT group was double that of the increase in the C group, the lack of significance between groups may be attributable to the individual variable response to RT for RT (range −1547 to 3009kJ/d) and C (range −1117 to 1356kJ/d). These results may suggest individuals respond differently to RT and that some individuals may require greater volumes of RT to increase energy expenditure. Further, studies evaluating these individual responses to RT are warranted. Another consideration for the lack of difference between groups may be due to the increase energy expenditure in the C as a result of an increase in fat mass. Nevertheless, the fact that a minimal amount of RT can have a meaningful impact on energy expenditure which translates to favorable improvements in body composition supports recommendations by various health organizations to include RT as part of a healthy lifestyle. Additionally, subjects gained weight despite a ~4000kJ difference between reported energy intake (9,538 kJ/d) and measured energy expenditure (~13,330kJ/d). These results support previous research(23, 26) that overweight subjects underreport their energy intake.
The minimal RT protocol described in this study may provide an attractive alternative to either aerobic exercise or multiple set RT programs for weight management in busy young adults, due to the minimal time commitment (11 min/session) and the fact that participants did not need to change clothes or shower. In fact, compared to the energy expenditure of the American College of Sports Medicine recommended 30 min endurance exercise (~1500kJ)(8) a session of RT is relatively low (~650kJ) (30). However, the long-term adaptation to RT through increases in FFM and reductions in FM as seen in the current study provide an alternative approach to managing fat mass gain often seen in the college aged population, a group at high risk for developing obesity.
The ~7% increase in RMR and SMR are in agreement with other studies using single (25, 34) and multiple (3, 6, 16) sets. Further, increased energy expenditure as a result of RT observed in this study is at least partially a function of increased FFM, as indicated by the positive correlation for change in FFM and change in 24-hr EE, RMR and SMR. However, RMR and SMR both increased as a result of RT after adjustment for FFM, suggesting that other factors may also be contributing to the increase. Although not measured in this study, myofibrillar protein turnover (19) could increase both SMR and RMR and has been shown to account for as much as 20% of RMR (44). Additionally, sympathetic nervous system activity may be related to changes in SMR and RMR. For example, low volume RT has been shown to increase muscle sympathetic nerve activity (34) and to elevate rates of muscle protein synthesis and breakdown up to 48-h post-exercise.
Twenty-four hour fat oxidation (g/day) increased slightly with RT (1.3%) and decreased in C (−12.9%); however, neither between nor within group differences were statistically significant. The lack of significant change may be due to the smaller number of subjects or a need for a greater volume of exercise. Additionally, changes in fat oxidation may be effected by the energy and macronutrient balance in the indirect calorimeter. Every attempt was made to keep subjects in energy balance by having subjects eat a standardized diet three days prior to the chamber stay and during the chamber stay. Both groups were underfed ~8% at baseline and end study while the macronutrient oxidation rates in the chamber for fat (26%), carbohydrate (~65%) and protein (~10%) were slightly different than standardized diet of 30% fat, 55% carbohydrates, and 15% protein. These differences are most likely due to the underfeeding. Nevertheless, since both groups were fed identical standardized diets before and during the chamber with no difference between groups then the differences between groups for energy expenditure and substrate oxidation are most likely due to the intervention rather than composition of the diet. It is important to note, however, that the changes in both RMR-RQ and SMR-RQ (i.e. increased in C and decreased in RT) are consistent with the 24-hr fat oxidation data. Increased fat oxidation with RT may also be a result of increased sympathetic nervous system activity, as plasma norepinephrine has been shown to increase after RT in men (34). The positive influence of even a small amount of RT on fat oxidation suggests an important role of RT on body mass management.
In conclusion, a minimal RT program, performed over 6 months, produced significant increases in components of 24-hr EE (RMR and SMR) and a favorable increase in 24-hr EE. In addition, observed changes in both RMR-RQ and SMR-RQ suggested a potential for increased fat oxidation as a result of RT. Together these findings suggest that a minimal RT program may provide a sufficient stimulus to impact energy balance, and prevent long-term weight or body fat gain in sedentary, overweight, young adults. Further, the minimal RT protocol described in this study may provide an attractive alternative to either aerobic exercise or multiple set RT programs for weight management in busy young adults, due to the minimal time commitment. Studies with larger samples of both men and women are needed to assess potential gender differences in the energy expenditure and substrate oxidation response to minimal RT and to elucidate the impact of such programs on substrate oxidation and total and free living physical activity energy expenditure perhaps employing the use of doubly labeled water.
AKNOWLEDGEMENTS
We would like to thank the participants who volunteered their time and effort for this study. Additionally, we thank the staff of the University of Kansas Energy Balance Lab for their time and expertise. The results of the present study do not constitute endorsement by American College of Sports Medicine.
This project was supported by The National Institute of Diabetes and Digestive and Kidney Diseases (NIHDK62832) and The American Heart Association (0410041Z).
Footnotes
Conflict of Interest None of the authors had a relevant financial or personal interest in any company or organization sponsoring this research. Additionally, the results of the present study do not constitute endorsement of any product(s) by the authors.
REFERENCES
- 1.American College of Sports Medicine . Resources Manual for Guidelines for Exercise Testing and Prescription. Williams and Wilkins; Baltimore: 2000. pp. 145–160. [Google Scholar]
- 2.Bosselaers I, Buemann B, Victor OJ, Astrup A. Twenty-four-hour energy expenditure and substrate utilization in body builders. Am J Clin Nutr. 1994;59:10–12. doi: 10.1093/ajcn/59.1.10. [DOI] [PubMed] [Google Scholar]
- 3.Byrne HK, Wilmore JH. The effects of a 20-week exercise training program on resting metabolic rate in previously sedentary, moderately obese women. IntJ Sport Nutr ExercMetab. 2001;11:15–31. doi: 10.1123/ijsnem.11.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Conley MS, Rozenek R. National Strength and Conditioning Association Position Statement: Health Aspects of Resistance Exercise and Training. Strength and Conditioning Journal. 2001;23:9–23. [Google Scholar]
- 5.Dionne IJ, Melancon MO, Brochu M, Ades PA, Poelhman ET. Age-related differences in metabolic adaptations following resistance training in women. Exp Gerontol. 2004;39:133–138. doi: 10.1016/j.exger.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Dolezal BA, Potteiger JA. Concurrent resistance and endurance training influence basal metabolic rate in nondieting individuals. JApplPhysiol. 1998;85:695–700. doi: 10.1152/jappl.1998.85.2.695. [DOI] [PubMed] [Google Scholar]
- 7.Dolezal BA, Potteiger JA, Jacobsen DJ, Benedict SH. Muscle damage and resting metabolic rate after acute resistance exercise with an eccentric overload. MedSciSports Exerc. 2000;32:1202–1207. doi: 10.1097/00005768-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Donnelly JE, Kirk EP, Jacobsen DJ, Hill JO, Sullivan DK, Johnson SL. Effects of 16 mo of verified, supervised aerobic exercise on macronutrient intake in overweight men and women: the Midwest Exercise Trial. Am J Clin Nutr. 2003;78:950–956. doi: 10.1093/ajcn/78.5.950. [DOI] [PubMed] [Google Scholar]
- 9.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection E, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 10.Gibson RS. Principles of nutrition assessment. Oxford University Press; Oxford: 1990. pp. 15–20. [Google Scholar]
- 11.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr., Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 12.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 13.Hass CJ, Feigenbaum MS, Franklin BA. Prescription of resistance training for healthy populations. Sports Med. 2001;31:953–964. doi: 10.2165/00007256-200131140-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 15.Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer- recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 2002;75:263–267. doi: 10.1093/ajcn/75.2.263. [DOI] [PubMed] [Google Scholar]
- 16.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000;89:977–984. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 17.Jakicic JM, Clark K, Coleman E, Donnelly JE, Foreyt J, Melanson E, Volek J, Volpe SL. American College of Sports Medicine position stand. Appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2001;33:2145–2156. doi: 10.1097/00005768-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 18.Jequier E, Acheson K, Schutz Y. Assessment of energy expenditure and fuel utilization in man. Annu Rev Nutr. 1987;7:187–208. doi: 10.1146/annurev.nu.07.070187.001155. [DOI] [PubMed] [Google Scholar]
- 19.Kim PL, Staron RS, Phillips SM. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training. J Physiol. 2005;568:283–290. doi: 10.1113/jphysiol.2005.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohrt WM. Preliminary evidence that DEXA provides an accurate assessment of body composition. J Appl Physiol. 1998;84:372–377. doi: 10.1152/jappl.1998.84.1.372. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer WJ, Volek JS, Clark KL, Gordon SE, Incledon T, Puhl SM, Triplett-McBride NT, McBride JM, Putukian M, Sebastianelli WJ. Physiological adaptations to a weight-loss dietary regimen and exercise programs in women. JApplPhysiol. 1997;83:270–279. doi: 10.1152/jappl.1997.83.1.270. [DOI] [PubMed] [Google Scholar]
- 23.Kretsch MJ, Fong AK, Green MW. Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc. 1999;99:300–306. doi: 10.1016/S0002-8223(99)00078-4. [DOI] [PubMed] [Google Scholar]
- 24.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 25.Lemmer JT, Ivey FM, Ryan AS, Martel GF, Hurlbut DE, Metter JE, Fozard JL, Fleg JL, Hurley BF. Effect of strength training on resting metabolic rate and physical activity: age and gender comparisons. Med Sci Sports Exerc. 2001;33:532–541. doi: 10.1097/00005768-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Lichtman SW, Pisarska K, Berman ER, Pestone M, Dowling H, Offenbacher E, Weisel H, Heshka S, Matthews DE, Heymsfield SB. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med. 1992;327:1893–1898. doi: 10.1056/NEJM199212313272701. [DOI] [PubMed] [Google Scholar]
- 27.Livesey G, Elia M. Estimation of energy expenditure, net carbohydrate utilization, and net fat oxidation and synthesis by indirect calorimetry: evaluation of errors with special reference to the detailed composition of fuels. Am J Clin Nutr. 1998;47:608–628. doi: 10.1093/ajcn/47.4.608. [DOI] [PubMed] [Google Scholar]
- 28.Melanson EL, Sharp TA, Seagle HM, Horton TJ, Donahoo WT, Grunwald GK, Hamilton JT, Hill JO. Effect of exercise intensity on 24-h energy expenditure and nutrient oxidation. J Appl Physiol. 2002;92:1045–1052. doi: 10.1152/japplphysiol.00706.2001. [DOI] [PubMed] [Google Scholar]
- 29.Melby C, Scholl C, Edwards G, Bullough R. Effect of acute resistance exercise on postexercise energy expenditure and resting metabolic rate. J Appl Physiol. 1993;75:1847–1853. doi: 10.1152/jappl.1993.75.4.1847. [DOI] [PubMed] [Google Scholar]
- 30.Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol. 2002;80:1045–1053. doi: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- 31.Phillips WT, Ziuraitis JR. Energy cost of the ACSM single-set resistance training protocol. J Strength Cond Res. 2003;17:350–355. doi: 10.1519/1533-4287(2003)017<0350:ecotas>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Poehlman ET, DeNino WF, Beckett T, Kinaman KA, Dionne IJ, Dvorak R, Ades PA. Effects of endurance and resistance training on total daily energy expenditure in young women: a controlled randomized trial. J Clin Endocrinol Metab. 2002;87:1004–1009. doi: 10.1210/jcem.87.3.8282. [DOI] [PubMed] [Google Scholar]
- 33.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, Limacher M, Pina IL, Stein RA, Williams M, Bazzarre T. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101:828–833. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- 34.Pratley R, Nicklas B, Rubin M, Miller J, Smith A, Smith M, Hurley B, Goldberg A. Strength training increases resting metabolic rate and norepinephrine levels in healthy 50- to 65-yr-old men. J Appl Physiol. 1994;76:133–137. doi: 10.1152/jappl.1994.76.1.133. [DOI] [PubMed] [Google Scholar]
- 35.Richardson MT, Leon AS, Jacobs DR, Jr., Ainsworth BE, Serfass R. Comprehensive evaluation of the Minnesota Leisure Time Physical Activity Questionnaire. J Clin Epidemiol. 1994;47:271–281. doi: 10.1016/0895-4356(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 36.Roth SM, Martel GF, Ivey FM, Lemmer JT, Tracy BL, Hurlbut DE, Metter EJ, Hurley BF, Rogers MA. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J Appl Physiol. 1999;86:1833–1840. doi: 10.1152/jappl.1999.86.6.1833. [DOI] [PubMed] [Google Scholar]
- 37.Skogerboe KJ, Labbe RF, Rettmer RL, Sundquist JP, Gargett AM. Chemiluminescent measurement of total urinary nitrogen for accurate calculation of nitrogen balance. Clin Chem. 1990;36:752–755. [PubMed] [Google Scholar]
- 38.Taaffe DR, Pruitt L, Reim J, Butterfield G, Marcus R. Effect of sustained resistance training on basal metabolic rate in older women. J Am Geriatr Soc. 1995;43:465–471. doi: 10.1111/j.1532-5415.1995.tb06090.x. [DOI] [PubMed] [Google Scholar]
- 39.Treuth MS, Hunter GR, Figueroa-Colon R, Goran MI. Effects of strength training on intra-abdominal adipose tissue in obese prepubertal girls. Med Sci Sports Exerc. 1998;30:1738–1743. doi: 10.1097/00005768-199812000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Treuth MS, Hunter GR, Weinsier RL, Kell SH. Energy expenditure and substrate utilization in older women after strength training: 24-h calorimeter results. J Appl Physiol. 1995;78:2140–2146. doi: 10.1152/jappl.1995.78.6.2140. [DOI] [PubMed] [Google Scholar]
- 41.United States Department of Agriculture . General guidelines for determining food acceptability (procedures for plate waste studies) USDA, Food and Nutrition Service; Washington, D.C.: 1975. pp. 1–10. [Google Scholar]
- 42.US. Department of Health and Human Services . The surgeon general's call to action to prevent and decrease overweight and obesity 2001. U.S. Department of Health and Human Services; Rockville: 2001. pp. 25–35. [Google Scholar]
- 43.U.S. Department of Health and Human Services U.S. Government Printing Office; Washington, D.C.: United States Department of Health and Human Services: Healthy People 2010: Understanding and improving health. 2000:15–20.
- 44.Welle S, Nair KS. Relationship of resting metabolic rate to body composition and protein turnover. Am J Physiol. 1990;258:E990–998. doi: 10.1152/ajpendo.1990.258.6.E990. [DOI] [PubMed] [Google Scholar]
- 45.World Health Organization . WHO Technical Report Series. World Health Organization; Geneva, Switzerland: 2000. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; p. 894. [PubMed] [Google Scholar]