Abstract
Rationale
The cardiac venous pole is a common focus of congenital malformations and atrial arrhythmias, yet little is known about the cellular and molecular mechanisms that regulate its development. The systemic venous return myocardium (sinus node and sinus horns) forms only late in cardiogenesis from a pool of pericardial mesenchymal precursor cells.
Objective
To analyze the cellular and molecular mechanisms directing the formation of the fetal sinus horns.
Methods and Results
We analyzed embryos deficient for the Wilms tumor 1 gene (Wt1) and observed a failure to form myocardialized sinus horns. Instead, the cardinal veins become embedded laterally in the pleuropericardial membranes (PPMs) that remain tethered to the lateral body wall by the persisting subcoelomic mesenchyme, a finding that correlates with decreased apoptosis in this region. We show by expression analysis and lineage tracing studies that Wt1 is expressed in the subcoelomic mesenchyme surrounding the cardinal veins, but that this Wt1-positive mesenchyme does not contribute cells to the sinus horn myocardium. Expression of the aldehyde dehydrogenase family 1, subfamily A2 gene (Raldh2) was lost from this mesenchyme in Wt1−/− embryos. Phenotypic analysis of Raldh2-mutant mice rescued from early cardiac defects by retinoic acid (RA) food supply revealed defects of the venous pole and pericardium highly similar to those of Wt1−/− mice.
Conclusions
Pericardium and sinus horn formation are coupled and depend on the expansion and correct temporal release of PPMs from the underlying subcoelomic mesenchyme. Wt1 and downstream Raldh2/RA-signaling are crucial regulators of this process. Thus, our results provide novel insight into the genetic and cellular pathways regulating the posterior extension of the mammalian heart and the formation of its coelomic lining.
Keywords: sinus horn, venous pole, sinoatrial node, Tbx18, Raldh2, retinoic acid
Introduction
The systemic venous return of the mature mammalian heart, which terminates in the right atrium, consists of multiple anatomical components including the myocardial sleeves of the right superior and inferior caval veins, the sinoatrial node (SAN), the coronary sinus (persisting left caval vein in the mouse), and the sinus venarum.1 The systemic venous return is a focus of congenital malformations and atrial arrhythmias,2,3 necessitating insight into the cellular and molecular programs by which it arises during cardiac development. Most myocardial components of the heart are not represented in its initial anlage, but are continuously added by recruitment and differentiation of precursor cells.4 The sinus horns, the myocardial parts of the common cardinal veins upstream of the venous valves that bulge into the pericardial cavity, form from pericardial precursors that differentiate into sinus venosus myocardium around the systemic venous connection to the atrium.5 They form only after embryonic day (E) 9.5, when outflow tract, left and right ventricle and the common atrium have already been established. In adults, most of the right sinus horn myocardium is incorporated into the right atrium to form the sinus venarum. In humans, the left sinus horn will lose its connection to the body and form the coronary sinus, whereas in mouse it will persist as the left superior caval vein.
Few genes regulating venous pole development have been characterized, including the T-box transcription factor 18 gene (Tbx18) that marks the sinus horn lineage.5,6 Wt1 was initially identified as a tumor suppressor gene involved in the etiology of Wilms’ tumor in humans. Genetic analyses in the mouse subsequently uncovered essential roles for the transcription factor Wt1 in the development of numerous organ systems.7 In the heart, Wt1 is required for the mesenchymal transition of epicardial cells. Failure to provide cellular constituents to the developing coronary vessels results in pericardial bleeding and midgestational lethality.8,9 Here, we present data demonstrating an additional function of Wt1 in the formation of the common cardinal veins and the PPMs. We implicate RA-signaling in the etiology of these defects and show a primary requirement of Wt1 and Raldh2 in the subcoelomic mesenchyme from which the PPMs are released.
Materials and Methods
Mice
Animal care was in accordance with national and institutional guidelines.
Mice carrying a null allele of Wt1 (Wt1tm1Jae)10, Tbx18GFP (Tbx18tm2Akis)5 and Raldh2 (Aldh1a2tm1Ipc)11, R26lacZ and tetObiLacZ-GFP reporter mice12,13, and the RARE-Hsp68LacZ (synonym: Tg(RARE-Hspa1b/lacZ)12Jrt/J) reporter transgenic line,14 which harbors a tetrameric repeat of the RARβ2 RARE linked to the Hsp68 minimal promoter used to determine RA-signaling, were all described before. For the Wt1BAC-IRES-EGFPCre transgenic line, the BAC clone RP23-266M16 was modified by inserting an IRES/EGFP-Cre cassette 17bp downstream of the translation stop site of the Wt1 gene (The generation and evaluation of this line will be described elsewhere). In Wt1tTA mice, the coding sequence of an improved tetracycline-dependent transactivator tTA2S15 was introduced into the Wt1 locus by gene targeting (Lausch et al., manuscript in preparation). All mouse lines were maintained on an outbred (NMRI or FvB) background.
An expanded Materials and Methods section is available in the online data supplement.
Results
Defects in the systemic venous return of Wt1-deficient hearts
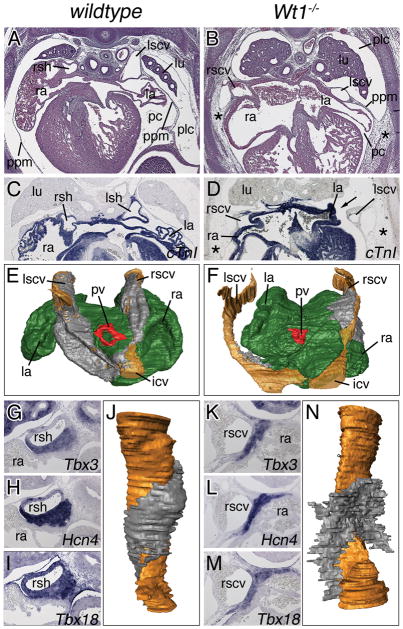
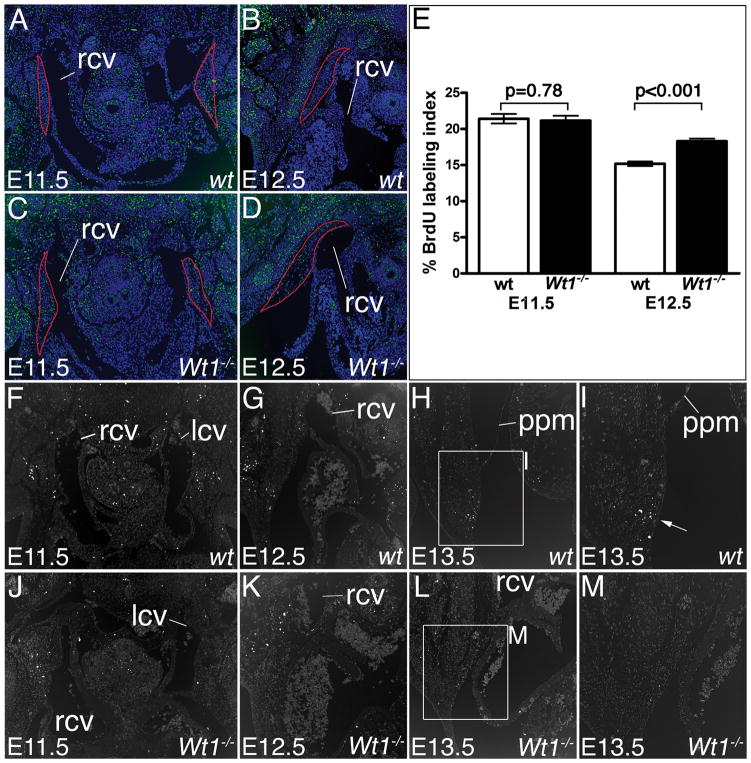
Wt1−/− mice maintained on an NMRI outbred background died during midgestation presenting defects reported on earlier, including lack of kidneys, diaphragmatic hernia and defects in coronary vessel formation the latter of which may underlie embryonic lethality. Yet, histological inspection of surviving embryos at E14.5 revealed an undescribed variation in the systemic venous return that prompted us to analyze the cardiac venous pole more carefully (Figure 1). In wildtype embryos of this stage, the sinus horns bulged into the pericardial cavity (Figure 1A). These sinus horns were completely myocardialized (cardiac Troponin I-positive; Figure 1C). Pericardial and pleural cavities were separated by the PPMs that were stretched out as thin tissue layers between their roots in the lung bud and their distal attachment points in the ventro-lateral body wall (Figure 1A,C). In contrast, Wt1-mutant hearts featured thinner, unmyocardialized cardinal veins (Figure 1B,D). These occupied an abnormal lateral position within the PPMs that also tethered the atrial roofs to the body wall. The pleural cavity was not fully expanded, leaving the pericardium ventrally attached to the body wall by a mesh of loose mesenchymal cells (Figure 1B). More anteriorly on the left side, pleural and pericardial cavities communicated by a thin canal leaving space for the atria to touch the lung (arrow in Figure 1D). Since sections planes between different embryos were sometimes difficult to match, we performed serial sections and subsequent 3-dimensional (3D)-reconstruction analysis. This confirmed our histological findings and showed that in Wt1−/− hearts, the non-myocardialized superior cardinal veins were thin and lying lateral to the atria near the body wall. The defects were restricted to the sinus horns, as the myocardium of pulmonary vein and the dorsal atrial wall myocardium appeared unchanged (Figure 1F).
Figure 1.
Sinus horn defects in Wt1-deficient hearts. Histological, molecular and morphological analyses of sinus horns (A–F) and the SAN (G–N) were carried out on transverse sections of the venous pole region of E14.5 wildtype and Wt1−/− hearts. A–F, Histological stainings with haematoxylin and eosin (A,B), in situ hybridization analysis of cTnI expression (C,D), and 3D-reconstructions of serial sections stained for cTnI in a dorsal-posterior view. Atrial myocardium is shown in green, cardinal vein myocardium in grey, the lumen of the cardinal veins in brown, and the pulmonary vein myocardium in red (E,F). Asterisks (B,D) mark persisting subcoelomic mesenchyme. G–N, In situ hybridization analyses of sections through the base of the cardinal veins for SAN marker genes with probes as indicated (G–I,K–M), and 3D-reconstructions of serial sections stained for Tbx3 expression through the cardinal veins (in brown) to visualize the SAN (in grey) (J,N). icv, inferior cardinal vein; la, left atrium; lu, lung; lscv, left superior cardinal vein; pc, pericardial cavity; plc, pleural cavity; ppm, pleuropericardial membrane; pv, pulmonary vein; ra, right atrium; rscv, right superior cardinal vein; rsh, right sinus horn.
The SAN is an elongated “comma shaped” structure at the junction of the right venous entrance and the atrium expressing Tbx3, the hyperpolarization-activated, cyclic nucleotide-gated K+ 4 gene (Hcn4) and Tbx18 (Figure 1G–I).6 In Wt1−/− hearts, these markers were detected at E14.5 but the domain of expression protruded away from the right superior cardinal vein (Figure 1K–M). 3D-reconstruction of the SAN from serial sections stained for Tbx3 expression confirmed that the SAN myocardium was not wrapped around the sinus horn as in the wildtype (Figure 1J) but had an open “wing”-like structure (Figure 1N). Volume measurements revealed that the SAN cell mass was not significantly reduced in Wt1-embryos (data not shown) arguing that SAN morphology rather than myocardial differentiation and growth are affected by loss of Wt1.
Sinus horn defects of Wt1−/− embryos arise early in development
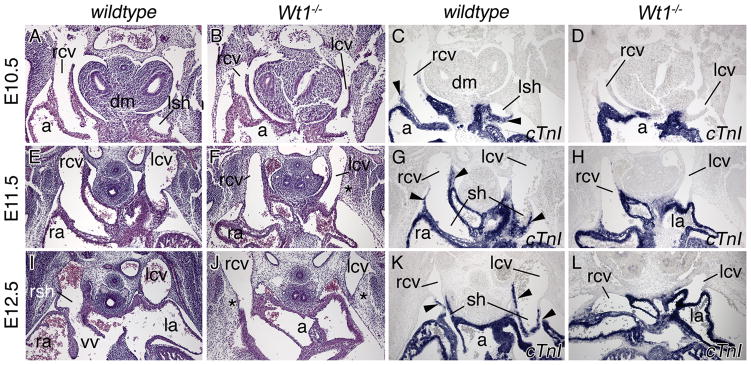
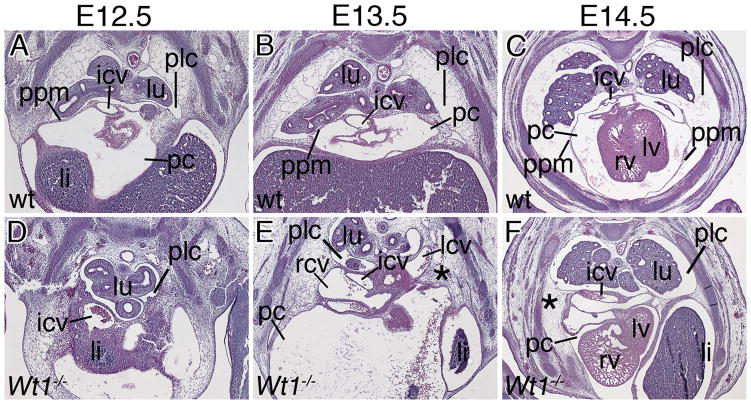
To determine the onset of venous pole defects in Wt1-deficient hearts, we analyzed embryos at earlier developmental time-points (Figures 2 and 3). In Wt1−/− embryos the venous pole region appeared unchanged at E9.5 (data not shown). At E10.5, protrusions of the cardinal veins into the pericardial cavity generated short sinus horns that, however, failed to expand and myocardialize at subsequent stages (Figure 2A–L). Analysis of Hcn4 expression indicated presence of dispersed pacemaker tissue at E12.5 (Online Figure I). The subcoelomic mesenchyme around the common cardinal veins was present but loosely organized at E11.5 and 12.5 (asterisks in Figure 2F,J). At E12.5, a mesothelial lining separated the posterior pleural and pericardial cavities in the wildtype (Figure 3). These PPMs originated laterally in the body wall at the height of the developing ribs, and projected medially to connect to the hilus of the lung bud at more anterior levels (Figure 3A). In subsequent stages the lateral insertion points moved ventrally following the distal extension of the ribs to become loosely connected to the sternum at E14.5. During this growth phase the PPMs were stretched out to thin epithelial sheets. The pleural cavity dramatically gained in volume. Loose mesenchymal tissue initially filling the pleural cavity at the onset of its expansion, was completely cleared by E14.5 (Figure 1A, Figure 3B,C). In Wt1−/− embryos, PPMs were not apparent as distinct mesothelial linings separating the two cavities at E12.5 and E13.5. Instead, the pericardium was tethered to the atrial roof and cardinal veins on one side, and to a mesenchymal mesh that was continuous with the lateral body wall on the other side (Figure 3D,E). Inflation of this subcoelomic mesenchyme was delayed and clearance not achieved by E14.5 (Figure 1B and 3F). Thus, defects in sinus horn formation and PPM/subcoelomic mesenchyme organization arise early in sinus horn/caval vein development and may be linked.
Figure 2.
Developmental onset of sinus horn defects in Wt1−/− hearts. Histological and molecular analyses of sinus horn development were carried out on transverse sections of the venous pole region of E10.5 to E12.5 wildtype and Wt1−/− hearts as indicated. Haematoxylin and eosin stainings (A,B,E,F,I,J), in situ hybridization analysis of cTnI expression on adjacent sections (C,D,G,H,K,L). Arrowheads point to myocardialized sinus horns. Asterisks mark subcoelomic mesenchyme in the mutant. a, common atrium; dm, dorsal mesocardium; la, left atrium; lcv, left common cardinal vein; lsh, left sinus horn; ra, right atrium; rcv, right common cardinal vein; rsh, right sinus horn; sh, sinus horn; vv, venous valves.
Figure 3.
Developmental onset of mesothelial defects in Wt1−/− hearts. Histological analysis by haematoxylin and eosin staining of PPM development was carried out on transverse sections of the posterior venous pole region of E12.5 to E14.5 wildtype and Wt1−/− hearts as indicated. li, liver; other abbreviations are as in Figures 1 and 2.
Wt1 is not expressed in sinus horns but in mesothelia and the underlying mesenchyme
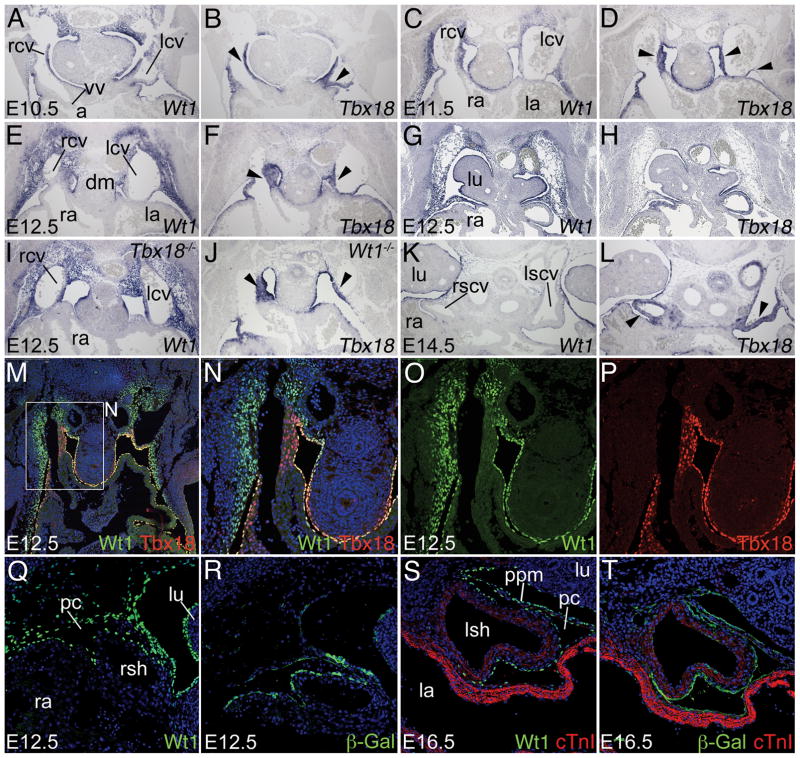
To determine the temporal and spatial requirement for Wt1 in sinus horn development more carefully, we analyzed expression of Wt1 from E10.5 to E14.5 in the venous pole region (Figure 4). We compared Wt1 expression to that of Tbx18, which is restricted to the mesenchyme and myocardium of developing sinus horns.5
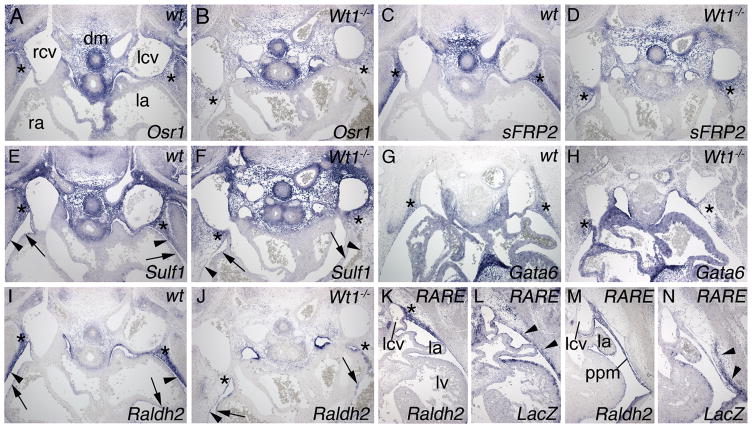
Figure 4.
Wt1 expression in the subcoelomic mesenchyme. A–L, Comparative in situ hybridization analysis of Wt1 and Tbx18 expression on transverse sections of the venous pole region of E10.5 to E14.5 hearts of wildtype (A–H,K,L), Tbx18−/− (I) and Wt1−/− (J) embryos. Black arrowheads point to Tbx18 expression in the mesenchyme and myocardium of the sinus horns. M–P, Immunofluorescence analysis of Wt1 and Tbx18 protein at the cardiac venous pole. Q–T, Comparative immunofluorescence analysis of Wt1 and β-galactosidase protein expression in the venous pole region in Wt1BAC-IRES-EGFPCre/+; Rosa26LacZ/+ embryos. cTnI immunofluorescence (red) marks sinus horn and atrial myocardium. Stages, genotypes and probes are as indicated. Abbreviations are as in Figures 1 and 2.
From E10.5 to E12.5, Wt1 was expressed in the developing mesothelia, including the epicardium, pericardium and pleura, and in the subcoelomic mesenchyme of the lateral body wall including the one surrounding the common cardinal veins, but surprisingly, not in the sinus horns and the SAN where Tbx18 was strongly expressed (Figure 4A–H). Co-immunofluorescence analysis of Wt1 and Tbx18 in the venous pole region at E12.5 revealed patterns of protein expression identical to mRNA domains, confirming mutual exclusion of the two expression domains (Figure 4M–P). Complementary expression of Wt1 in the mesenchyme surrounding the common cardinal veins and Tbx18 in the sinus horn myocardium, prompted us to examine the possibility of negative cross-regulation of these transcription factors. However, Tbx18 was not derepressed in the Wt1 mutant and vice versa (Figure 4I,J). At E14.5, Wt1 was maintained in the mesothelia whereas Tbx18 expression persisted in the sinus horn myocardium (Figure 4K,L).
The sinus horn defect in Wt1 mutants suggests that the Wt1-positive mesenchyme surrounding the common cardinal veins may provide precursor cells for the Wt1-negative sinus horn myocardium. To test this hypothesis, we used genetic lineage tracing systems to determine the fate of Wt1-expressing cells. First, we employed a binary system with mice carrying a knock-in of the tTA gene in the Wt1 locus and a reporter line with a tetObiLacZ-GFP transgene.13 Expression of tTA under Wt1 control elements leads to transcription of the LacZ gene and production of the β-galactosidase protein whose longevity provides a tool to trace cells initially expressing Wt1. We did not find β-galactosidase positive cells in the sinus horn myocardium at E12.5 and E14.5 using this system (Online Figure II). We next crossed a Wt1BAC-IRES-EGFPCre line, in which the expression of the Cre recombinase gene mimics that of the Wt1 gene, with R26LacZ reporter mice.12 This system irreversibly labels Wt1-expressing cells and their daughters by LacZ expression (β-galactosidase activity). Again, we did not find β-galactosidase positive cells in the sinus horn myocardium at E12.5 and E16.5 (Figure 4Q–T) providing further evidence that the Wt1-positive mesenchyme of the lateral body wall does not represent a cellular source for the sinus horn myocardium.
Collectively, these analyses suggest Wt1 is not required within the sinus horn mesenchyme like Tbx18 is, but that Wt1 expression in the mesothelia and/or subcoelomic mesenchyme controls sinus horn formation by an indirect process.
Cellular changes of the lateral body wall mesenchyme in Wt1−/− mice
We next analyzed whether changes of proliferation and apoptosis may underlie the failure of sinus horn formation and the persistence of the subcoelomic body wall mesenchyme. Cell proliferation appeared unaffected in Wt1−/− embryos at E11.5 (wildtype 0.214±0.0064 vs mutant 0.211±0.0067; P=0.78). At E12.5, we detected a significant but small increase of cell proliferation in the Wt1−/− lateral body wall mesenchyme (wildtype 0.151±0.0031 vs mutant 0.183±0.0035; P<0.0001) (Figure 5A–E). Using TUNEL assays, we did not detect changes of the low levels of apoptosis in the subcoelomic mesenchyme at the venous pole region at E11.5 (we analyzed three wildtype and three Wt1-mutant embryos) and E12.5 (four embryos of each genotype were analyzed) in Wt1−/− hearts (Figure 5F,G,J,K). At E13.5, we observed clusters of apoptotic cells in the mesenchyme underlying the PPM insertion point in the body wall in 8 of 11 tested wildtype embryos (Figure 5H,I). These clusters of apoptotic cells were not detected in Wt1−/− embryos (n=5) at this stage (Figure 5L,M). Thus, loss of apoptosis in this region may contribute to the non-release of the PPMs from the lateral body wall and the persistence of the underlying mesenchyme.
Figure 5.
Analysis of cellular changes in the Wt1−/− lateral body wall. A–D, Analysis of cell proliferation in the Wt1-positive mesenchyme of the lateral body wall surrounding the common cardinal veins performed on transverse sections through the venous pole by BrdU immunohistochemistry. Dorsal is oriented up. E, Proliferation rates (% BrdU labeling index) of red encircled regions in genotypes as shown in A–D. F–M, TUNEL staining for apoptosis in Wt1-expressing subcoelomic mesenchyme on transverse embryo sections. The white arrow in (I) points to a cluster of apoptotic cells in E13.5 wildtype embryos. Stages and genotypes are as stages as indicated. Abbreviations are as in Figure 1 and 2.
Loss of Raldh2 expression in the lateral body wall mesenchyme
To identify molecular changes that may underlie and explain the defects of the venous pole in Wt1-mutant hearts, we analyzed expression of genes that represent markers for the myocardium of the atria, the SAN and the sinus horns. Expression of these markers was unchanged in the Wt1−/− cardiac venous pole region arguing that regionalization and cellular contributions to SAN and atrial myocardium occurred normally (Online Figure III). Tbx18 expression was found in the small region of SAN myocardium present (Figure 1M,4J) arguing that Wt1 controls formation of sinus horn but not of SAN myocardium.
Our analysis uncovered a number of novel markers for the subcoelomic mesenchyme of the lateral body wall that were co-expressed with Wt1 in this region (Figure 6). The odd-skipped related 1 gene (Osr1), the gene encoding secreted frizzled-related protein 2 (sFRP2) and sulfatase 1 (Sulf1) were additionally expressed in the dorsal mesocardium (Figure 6A,C,E), whereas Sulf1 showed additional expression in epicardium (black arrow Figure 6E) and pericardium (black arrowhead in Figure 6E). Expression of Osr1, sFRP2, Sulf1, and GATA binding protein 6 (Gata6) was maintained (albeit at seemingly lower levels) in the loose Wt1−/− lateral body wall mesenchyme, independently confirming the persistence of cells formerly expressing Wt1 in this region (asterisks in Figure 6A–H). Raldh2 was coexpressed with Wt1 in the epicardium, pericardium and subcoelomic mesenchyme from E11.5 to E14.5 in the entire pericardial cavity (Online Figure IV). Intriguingly, expression of Raldh2 was specifically lost in the lateral body wall mesenchyme (asterisks in Figure 6J) but persisted in the epicardium (black arrow in Figure 6J) and pericardium (black arrowhead in Figure 6J). Raldh2 is an enzyme that converts retinaldehyde to RA. To study which tissues respond to this compound in this region, we analyzed LacZ expression of a transgenic reporter line for RA-signaling (RARE-Hsp68LacZ).14 LacZ expression was detected in a dorsal to latero-ventral wave in the subcoelomic mesenchyme of the lateral body wall at E12.5 and E13.0 strongly arguing that RA-signaling does not act onto the developing sinus horns (Figure 6K–N).
Figure 6.
Absence of Raldh2 expression in the Wt1−/− lateral body wall mesenchyme. A–J, in situ hybridization analysis of marker expression carried out on transverse sections of the venous pole region of E12.5 wildtype and Wt1−/− hearts. Genotypes and probes are as indicated. Asterisks (A–K) mark the subcoelomic lateral body wall mesenchyme. Black arrows and arrowheads (E,F,I,J) mark epicardium and pericardium, respectively. J, loss of Raldh2 expression in the subcoelomic mesenchyme of Wt1−/− embryos. K–N, in situ hybridization analysis of Raldh2 and LacZ expression carried out on transverse sections of the venous pole region of E12.5 (K,L) and E13.0 (M,N) hearts of RARE-Hsp68LacZ/+ transgenic embryos (RARE). Arrowheads in L and N mark RA-signaling. Abbreviations are as in Figure 1 and 2.
Because of the similarity of the cardinal vein phenotypes in Wt1−/− and Tbx18−/− mice, we tested whether loss of Tbx18 affects expression of marker genes in the adjacent subcoelomic mesenchyme. However, unchanged expression of these genes (including Raldh2) in Tbx18−/− embryos excluded a paracrine Tbx18-dependent signal from the sinus horn myocardium/mesenchyme onto the subcoelomic mesenchyme (Online Figure V) and implied that sinus horn defects in Wt1- and Tbx18-mutants are of different etiology.
Loss of Raldh2 results in defects in sinus horn formation
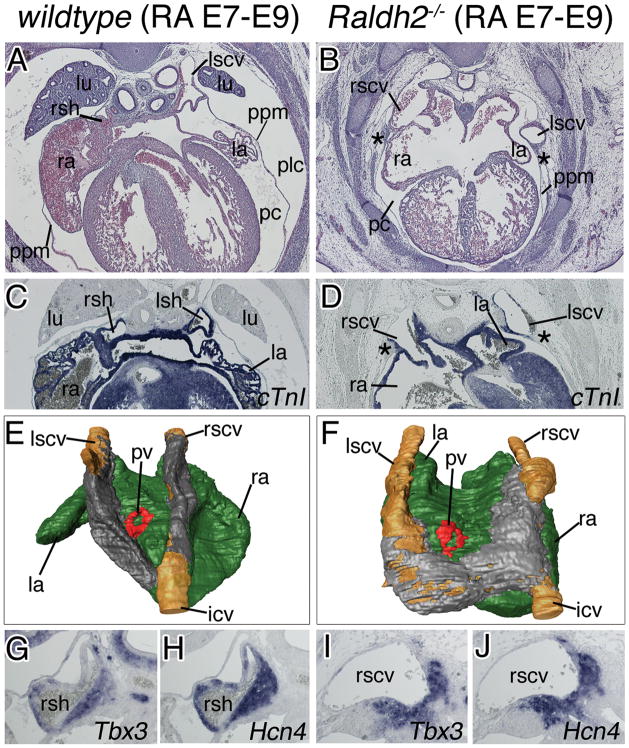
Loss of Raldh2 expression in Wt1 mutants suggested that Raldh2 is a downstream effector of Wt1 in sinus horn formation. We analyzed Raldh2-mutant mice11 at E14.5 (after sinus horn formation) that were rescued from early sinoatrial defects by RA food supply between E7.5 and E9.5 (Figure 7). Raldh2(RA E7-9)-deficient hearts featured thin ventricular walls and enlarged atria in accordance with the known role of RA in epicardial development and posteriorizing the chambers.8,16 Compared to wildtype embryos similarly supplied with exogenous RA, the Raldh2−/− cardinal veins were thinner, less myocardialized, and buried in an abnormal position within the lateralized PPMs (Figure 7A–F). Pleural and pericardial cavities communicated on the left side, and the pleural cavity was not expanded leaving the pericardium ventrally attached to the body wall with a mesh of persisting mesenchymal cells (Figure 7B,D). Analysis of Tbx3 and Hcn4 expression indicated presence of myocardium of the SAN, whose atrial border, however, appeared rather diffuse (Figure 7G–J). Expression of Wt1 and other markers for the lateral body wall mesenchyme was not changed in Raldh2−/−(RA E7-9) embryos at E12.5 (Online Figure VI). Similar to Wt1-deficient embryos and in contrast to the wildtype situation, apoptotic cell clusters were absent from the subcoelomic mesenchyme underlying the PPMs at E12.5 and E13.5 in Raldh2-deficient embryos supplied with RA from E7.5 to E9.5 (Online Figure VII). Thus, embryos with a lack of RA-signaling in the time-interval critical for sinus horn formation (E9.5 to E14.5) exhibit pericardial and cardinal vein defects highly reminiscent of those of Wt1−/− embryos, suggesting that Raldh2 and RA-signaling mediate Wt1 function in the subcoelomic mesenchyme of the lateral body wall.
Figure 7.
RA-signaling is required for cardinal vein formation after E9.5 in the mouse heart. Histological, molecular and morphological analyses of sinus horns (A–F) and the SAN (G–J) were carried out on transverse sections of the venous pole region of E14.5 wildtype (left column) and Raldh2-deficient embryos food supplied with RA between E7 and E9 (RA E7–E9) (right column). A–F, Histological stainings with haematoxylin and eosin (A,B), in situ hybridization analysis of cTnI expression (C,D), and 3-D reconstructions of serial sections stained for cTnI in a dorsal-posterior view. Atrial myocardium is shown in green, cardinal vein myocardium in grey, the lumen of the cardinal veins in brown, and the pulmonary vein myocardium in red (E,F). Asterisks (B,D) mark persisting subcoelomic mesenchyme. G–J, in situ hybridization analysis of sections through the base of the cardinal veins for expression of SAN marker genes with probes as indicated. Abbreviations are as in Figure 1 and 2.
Discussion
Our study has established that the transcription factor Wt1 is required for the correct formation of the cardiac venous pole. We suggest that Wt1 functions primarily in the subcoelomic mesenchyme of the lateral body wall to mediate the release of the PPMs by apoptosis. This, in turn, mediates the correct re-positioning of the cardinal veins medially to the lungs and the dorsal mesocardium. Wt1 function in the subcoelomic mesenchyme may be mediated by RA-signaling. Tbx18 acts independently in the sinus horn mesenchyme to warrant its growth and myocardial differentiation.
Sinus horn defects are secondary to changes in PPM formation
Our analysis of Wt1−/− embryos revealed that sinus horn defects are tightly associated with pleuropericardial abnormalities suggesting that alterations in mesothelial development may accompany or cause cardinal vein malformations. These pericardial defects are similar to congenital defects in human that have been described as rare anomalies. They may or may not be symptomatic, with symptomatic patients often experiencing chest pain. In the more frequently occurring partial pericardial absence, surgical intervention may be required to prevent strangulation of the heart. The etiology of these human defects is not well understood, but descriptive studies of human embryos have pointed to an important role of the PPMs.17,18 The proximal aspects of the common cardinal veins (the ducts of Cuvier) are embedded in the PPMs. Thus, failure of one or both PPMs to close will result in lateralization of cardinal veins. At this point, it remains unclear why myocardialization of these lateralized cardinal veins fails, but physical separation from sources of differentiation signals (e.g. the sinoatrial region) may be involved. Different opinions for the causative factor for the non-closure of PPMs have been put forward.18 Perna (1909) proposed that the premature atrophy of the left duct of Cuvier results from insufficient blood supply to the PPMs.19 Alternatively, altered growth of the heart may inappropriately stretch and tear the PPMs.20 To our knowledge, normal and abnormal pericardial development has not been described in any detail in the mouse. Our phenotypic characterization of Wt1- and Raldh2-deficient embryos pinpoint to the decisive role of the subcoelomic mesenchyme of the lateral body wall in the etiology of pericardial defects. The persisting subcoelomic mesenchyme in these mutants tethers the PPMs to the lateral body wall, thus, preventing appropriate expansion and cardinal vein relocalization. It also hampers the inflation of the pleural cavity that may rely on a dorsal to ventral wave of PPM release by the swelling and subsequent disappearance of the underlying mesenchyme.
RA-signaling may mediate Wt1 function in the subcoelomic mesenchyme
RA has been identified as an important signaling factor in numerous developmental processes.21 RA also controls heart morphogenesis and differentiation,16 and acts as an epicardial factor for myocardial differentiation.22 Our analysis of the expression and function of the main RA biosynthetic enzyme Raldh2 expands the role of RA-signaling to pericardial development and sinus horn formation. Raldh2-mutant embryos rescued from early cardiac defects by RA food supply exhibited a spectrum of phenotypic changes including reduced PPMs, left sided pericardial-pleural communication, persistence of the body wall mesenchyme and lateralized cardinal veins that is compatible with a primary requirement of RA for pericardial development. Using a RA reporter mouse, we detected RA-signaling in the subcoelomic mesenchyme of the lateral body wall adjacent to the developing PPMs. RA-signaling was spatially dynamic since it shifted ventrally preceding PPM detachment. Localized apoptosis of the RA-signaling positive mesenchyme may mediate PPM release from the lateral body wall. This situation is somehow reminiscent of the role of RA-signaling in vertebrate limb development where it mediates apoptosis of the interdigital mesenchyme to release the digits.23 Noteworthy, administration of all-trans RA in patients with acute promyelocytic leukaemia can lead to RA syndrome that features pleural and pericardial effusion.24 Loss of the RA-metabolizing enzyme Cyp26a1 in zebrafish results in defects in common cardinal vein formation.25 Together, this suggests that RA is important for normal development and function of mesothelia and cardinal veins.
Colocalization of Wt1 and Raldh2 expression in the lateral body wall mesenchyme, loss of Raldh2 expression in this domain in Wt1−/− embryos, and phenotypic similarities of the pericardial defects of either mutant strongly suggests that RA-signaling acts downstream of Wt1. We tried to rescue pericardial defects of Wt1-deficient mice by feeding pregnant mice with RA starting from E8.5. i.e. shortly before onset of sinus horn formation, until E14.5 when this process ends. A concentration of 0.25g RA per gram food, that was efficient to rescue sinoatrial defects of Raldh2-mutant mice, did not rescue sinus horn and PPM defects in Wt1−/−mice. However, cardinal vein defects were not rescued in the Raldh2−/− embryos supplied with RA until E14.5 either, suggesting that exogenous RA does reach its target tissue after E9.5 in the embryo (Online Figure VIII). Hence, it remains open whether Wt1 regulates additional genetic circuits that synergize with RA to mediate its downstream functions.
A link between Wt1 and Raldh family members has already been described in other developmental contexts. Raldh1 expression is down-regulated in the urogenital ridge of Wt1−/−embryos.26 Wt1 and Raldh2 are co-expressed in the epicardium and epicardially derived cells of the developing avian heart.27 A direct regulation of Raldh2 expression by Wt1 has also been proposed during liver development.28 Here, Wt1 and Raldh2 expression coincide in the coelomic lining, and in Wt1−/− mice Raldh2 expression is down-regulated in these mesothelial cells leading to defects in liver morphogenesis. Additionally, the left pleuroperitoneal membrane is not fully formed. The regulation of Raldh2 expression by Wt1 may therefore represent a general mechanism during the development of murine mesothelia.
Cellular contributions at the cardiac venous pole
We have recently shown that the myocardialized proximal aspects of the superior and inferior caval veins form only after heart looping by recruitment and subsequent myocardial differentiation of Tbx18-positive pericardial precursors.5 Wt1 expression in the subcoelomic mesenchyme directly abuts the Tbx18 expression domain at the venous pole, suggesting that Wt1-positive precursors feed into the pool of Tbx18-positive sinus horn cells. However, our lineage tracing experiments negate such a possibility, but instead stress that Tbx18 exclusively marks the sinus horn myocardial lineage throughout heart development.6 In Tbx18−/− mice the cardinal veins are positioned inside the PPMs, but appear less lateralized compared to Wt1-deficient embryos. Delay but not failure of myocardialization of proximal cardinal veins in Tbx18−/− embryos5 may relate to the different topological situation of the PPMs that are not tethered to the lateral body wall as in Wt1−/− embryos. Expression of Wt1 and Raldh2 is unchanged in Tbx18−/− mice, indicating that Wt1 and Tbx18 regulate different cellular and molecular programs during pericardial and caval vein development. Although the sinus horns fail to form in Wt1−/− embryos, the volume of the SAN is largely unchanged. This contrasts the complete lack of the large SAN head structure in Tbx18−/− embryos,6 and suggests that Wt1 controls the formation of myocardialized sinus horns but not of the region destined to form the SAN head that is regulated by Tbx18. Altered SAN morphology in Wt1−/−embryos, however, argues that positioning of the developing SAN next to the myocardialized sinus horns is critical for correct morphogenesis of this structure.
What is known?
The sinus horns, the myocardialized parts of the intrapericardial aspects of the common cardinal veins form late in cardiac development, from a pool of pericardial cells that is distinguished from precursors of the other cardiac components by the presence of Tbx18 and absence of Nkx2-5expression.
The sinus horns contribute to the mature systemic venous return system but also to the pace maker tissue of the heart, the sinoatrial node (SAN), which is a common focus of congenital malformations and atrial arrhythmias.
What new information does this article contribute?
The Wilms tumor 1 gene (Wt1) Wt1 is expressed in the subcoelomic mesenchyme of the lateral body wall surrounding the cardinal veins, but this Wt1-positive mesenchyme does not contribute cells to the sinus horn myocardium.
Wt1-expression in the subcoelomic mesenchyme is required for sinus horn formation, release and growth of the pleuropericardial membranes (PPMs), and expansion of the pleural and pericardial cavities.
Retinoic acid signaling may mediate Wt1 function in the subcoelomic mesenchyme for PPM release, as well as relocalization and myocardialization of cardinal veins.
Novelty and significance
The cardiac venous pole is a common focus of congenital malformations and atrial arrhythmias, yet little is known about the cellular and molecular mechanisms that regulate its development. Here, we have identified expression of the Wilms tumor 1 gene (Wt1) and downstream retinoic acid signaling in the subcoelomic mesenchyme of the lateral body wall as a crucial requirement for the development of the myocardial components of the venous pole and of the pericardium. Our study uncovers the critical role of the subcoelomic mesenchyme in this program. Unexpectedly, this tissue does not represent a cellular source for sinus horn myocardium but controls sinus horn formation indirectly. We suggest that relocalization and myocardialization of cardinal veins depends on the correct temporal release of the pleuropericardial membranes in which the cardinal veins are embedded from the underlying body wall mesenchyme. Hydration and apoptotic removal of the mesenchyme may be cellular mediators in this process. Our results provide novel insight into the genetic and cellular pathways regulating the posterior extension of the mammalian heart, and the formation of its coelomic lining. Our study suggests that congenital malformations of the cardiac venous pole and pericardial defects present together and share a common etiology.
Supplementary Material
Acknowledgments
We thank Gregg Duester and Alexandre T. Soufan for technical help, John Burch and J. Rossant for mice.
Sources of Funding
This work was supported by grants from the Netherlands Organization for Scientific Research (Vidi grant 864.05.006 to V.M.C.), individual grants from DFG (La 1381/3-1 to 3) and the Wilhelm Sander-Stiftung (to E.L.), the National Institute of Health (R01 HL070733 to K.N.); the European Community’s Sixth Framework Programme contract (‘HeartRepair’ LSHM-CT-2005-018630 to V.M.C., M.J.B.vd and A.K.) and the German Research Foundation (DFG) for the Cluster of Excellence REBIRTH (From Regenerative Biology to Reconstructive Therapy) and for the Clinical Research Group KFO136 at Hannover Medical School (A.K.).
Non-standard abbreviations and acronyms
- 3D
three dimensional
- DAPI
4′,6-Diamidino-2-phenylindol
- E
embryonic day
- PPM
pleuropericardial membrane
- SAN
sinoatrial node
- RA
retinoic acid
- RARE
retinoic acid response element
Footnotes
Disclosures
None.
References
- 1.Anderson RH, Brown NA, Moorman AF. Development and structures of the venous pole of the heart. Dev Dyn. 2006;235:2–9. doi: 10.1002/dvdy.20578. [DOI] [PubMed] [Google Scholar]
- 2.Stieber J, Hofmann F, Ludwig A. Pacemaker channels and sinus node arrhythmia. Trends Cardiovasc Med. 2004;14:23–28. doi: 10.1016/j.tcm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Moorman AF, Christoffels VM, Anderson RH. Anatomic substrates for cardiac conduction. Heart Rhythm. 2005;2:875–886. doi: 10.1016/j.hrthm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Rochais F, Mesbah K, Kelly RG. Signaling pathways controlling second heart field development. Circ Res. 2009;104:933–942. doi: 10.1161/CIRCRESAHA.109.194464. [DOI] [PubMed] [Google Scholar]
- 5.Christoffels VM, Mommersteeg MT, Trowe MO, Prall OW, de Gier-de Vries C, Soufan AT, Bussen M, Schuster-Gossler K, Harvey RP, Moorman AF, Kispert A. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ Res. 2006;98:1555–1563. doi: 10.1161/01.RES.0000227571.84189.65. [DOI] [PubMed] [Google Scholar]
- 6.Wiese C, Grieskamp T, Airik R, Mommersteeg MT, Gardiwal A, de Gier-de Vries C, Schuster-Gossler K, Moorman AF, Kispert A, Christoffels VM. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ Res. 2009;104:388–397. doi: 10.1161/CIRCRESAHA.108.187062. [DOI] [PubMed] [Google Scholar]
- 7.Lee SB, Haber DA. Wilms tumor and the WT1 gene. Exp Cell Res. 2001;264:74–99. doi: 10.1006/excr.2000.5131. [DOI] [PubMed] [Google Scholar]
- 8.Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms’ tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998;79:169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Estrada OM, Lettice LA, Essafi A, Guadix JA, Slight J, Velecela V, Hall E, Reichmann J, Devenney PS, Hohenstein P, Hosen N, Hill RE, Munoz-Chapuli R, Hastie ND. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat Gen. 2010;42:89–93. doi: 10.1038/ng.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- 11.Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Gen. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- 12.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Gen. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 13.Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res. 2001;29:E39. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossant J, Zirngibl R, Cado D, Shago M, Giguere V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- 15.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci USA. 2000;97:7963–7968. doi: 10.1073/pnas.130192197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–1031. doi: 10.1242/dev.128.7.1019. [DOI] [PubMed] [Google Scholar]
- 17.Abbas AE, Appleton CP, Liu PT, Sweeney JP. Congenital absence of the pericardium: case presentation and review of literature. Int J Cardiol. 2005;98:21–25. doi: 10.1016/j.ijcard.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Faridah Y, Julsrud PR. Congenital absence of pericardium revisited. Int J Cardiovasc Imaging. 2002;18:67–73. doi: 10.1023/a:1014350814067. [DOI] [PubMed] [Google Scholar]
- 19.Perna G. Sopra un arresto di sviluppo della sierosa pericardica nell’ uomo. Anat Anz. 1909;35:323. [Google Scholar]
- 20.Kaneko Y, Okabe H, Nagata N. Complete left pericardial defect with dual passage of the phrenic nerve: a challenge to the widely accepted embryogenic theory. Pediatr Cardiol. 1998;19:414–417. doi: 10.1007/s002469900338. [DOI] [PubMed] [Google Scholar]
- 21.Niederreither K, Dolle P. Retinoic acid in development: towards an integrated view. Nat Rev Genet. 2008;9:541–553. doi: 10.1038/nrg2340. [DOI] [PubMed] [Google Scholar]
- 22.Stuckmann I, Evans S, Lassar AB. Erythropoietin and retinoic acid, secreted from the epicardium, are required for cardiac myocyte proliferation. Dev Biol. 2003;255:334–349. doi: 10.1016/s0012-1606(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 23.Zuzarte-Luis V, Hurle JM. Programmed cell death in the embryonic vertebrate limb. Sem Cell Dev Biol. 2005;16:261–269. doi: 10.1016/j.semcdb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Leelasiri A, Numbenjapol T, Prayoonwiwat W, Mongkolsritrakul W, Srisawat C. Successful treatment of retinoic acid syndrome with dexamethasone: a case report. J Med Assoc Thai. 2005;88 (Suppl 3):S302–310. [PubMed] [Google Scholar]
- 25.Patatanian E, Thompson DF. Retinoic acid syndrome: a review. J Clin Pharm Ther. 2008;33:331–338. doi: 10.1111/j.1365-2710.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 26.Klattig J, Sierig R, Kruspe D, Makki MS, Englert C. WT1-mediated gene regulation in early urogenital ridge development. Sex Dev. 2007;1:238–254. doi: 10.1159/000104774. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Pomares JM, Phelps A, Sedmerova M, Carmona R, Gonzalez-Iriarte M, Munoz-Chapuli R, Wessels A. Experimental studies on the spatiotemporal expression of WT1 and RALDH2 in the embryonic avian heart: a model for the regulation of myocardial and valvuloseptal development by epicardially derived cells (EPDCs) Dev Biol. 2002;247:307–326. doi: 10.1006/dbio.2002.0706. [DOI] [PubMed] [Google Scholar]
- 28.Ijpenberg A, Perez-Pomares JM, Guadix JA, Carmona R, Portillo-Sanchez V, Macias D, Hohenstein P, Miles CM, Hastie ND, Munoz-Chapuli R. Wt1 and retinoic acid signaling are essential for stellate cell development and liver morphogenesis. Dev Biol. 2007;312:157–170. doi: 10.1016/j.ydbio.2007.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.