Abstract
There are currently no consistent objective biochemical markers of alcohol abuse and alcoholism. Development of reliable diagnostic biomarkers that permit accurate assessment of alcohol intake and patterns of drinking is of prime importance to treatment and research fields. Diagnostic biomarker development in other diseases has demonstrated the utility of both open, systems biology, screening for biomarkers and more rational focused efforts on specific biomolecules or families of biomolecules. Long term alcohol consumption leads to altered inflammatory cell and adaptive immune responses with associated pathologies and increased incidence of infections. This has led researchers to focus attention on identifying cytokine biomarkers in models of alcohol abuse. Alcohol is known to alter cytokine levels in plasma and a variety of tissues including lung, liver, and very importantly brain. A number of cytokine biomarker candidates have been identified, including: TNF alpha, IL1-alpha, IL1-beta, IL6, IL8, IL12 and MCP-1. This is an emerging and potentially exciting avenue of research in that circulating cytokines may contribute to diagnostic biomarker panels and a combination of multiple biomarkers may significantly increase the sensitivity and specificity of the biochemical tests aiding reliable and accurate detection of excessive alcohol intake.
Keywords: alcoholism, alcohol abuse, biomarkers, cytokines, diagnostics
Introduction
Alcohol abuse and alcoholism remain serious societal problems in the US and the rest of the world. The lack of reliable diagnostic criteria and markers is a major obstacle to the diagnosis, treatment, and research of alcohol abuse and alcoholism. This review will address the definition of biomarkers, and the types of biomolecules used as biomarkers. The potential uses and previously proposed biomarkers in alcohol-related disorders are then discussed. Lastly, the potential use of cytokines as biomarkers of alcohol intake and immune dysfunction is considered.
Alcohol consumption levels directly relate to the behavioral aspects of alcoholism. Efficient and accurate clinical tests for alcohol consumption, typology, and alcohol-induced disorders are critically needed in alcoholism and alcohol abuse treatment and research. Although meticulous efforts have been made to construct interview formats such as Alcohol Use Disorders Identification Test Consumption (AUDIT-C) (Bradley et al., 2007;Bush et al., 1998), and CAGE (Ewing, 1984) that quantify alcohol intake, there are serious limitations associated with these approaches. This is particularly true in cases where individuals are forced to deny or minimize the magnitude of drinking behavior in order to mitigate personal, professional, or legal ramifications of alcohol abuse (Pernanen, 1974;Fuller et al., 1988). Thus, it is clear that a measurable clinical biomarker test that could provide an objective assessment of drinking intake/behavior would alleviate the uncertainties of currently available reporting methods. Furthermore, novel clinical diagnostics that identify alcohol-induced tissue damage could improve clinical care and have applications in alcohol abuse treatment.
What is a biomarker?
The discussion of biomarker diagnostics in alcohol abuse and alcoholism begins with a definition of what a biomarker is and the potential uses of biomarker diagnostics. The NIH Biomarkers Definitions Working Group has defined a biomarker strictly as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic intervention” (Biomarkers Definitions Working Group, 2001). The critical aspect of this definition is that a biomarker is indicative of a specifically-defined state. While the term biomarker is often misused in reference to any difference in biomolecule level means between two populations, this is a misapplication of the term because such a difference in mean levels may not be sufficiently robust to diagnose the disease in individual patients. We also suggest the use of the term biomarker to refer only to validated biomarkers while biomolecules that show promise as biomarkers but remain to be validated be referred to as biomarker targets.
Types of biomolecules used as biomarkers
The types of biomolecules that may serve as the best biomarkers of alcohol intake behavior and subsequent alcohol-induced tissue injury are not known. Macromolecules such as nucleic acids, proteins, or protein adducts could all be potential markers. Alternatively, small molecule or non-biochemical tests such as in vivo imaging could serve as a potential biomarker. In this regard, numerous studies have focused their attention on biomarkers that could be effectively used for alcohol abuse and alcoholism. Previous studies in this direction provided valuable examples of technologies that can be used in alcohol research based on the analysis of nucleic acids (Biermann et al., 2007;Walker and Grant, 2006), proteins (Freeman et al., 2006;Nomura et al., 2007), or small molecules (Bradford et al., 2008;De et al., 2007;Stephanson et al., 2007). Irrespective of the nature of the molecule or test, the best biomarker is the one that can effectively diagnose drinking behavior. Thus, while it may be appealing to think of a biomarker as a single molecule (e.g., an mRNA or a protein), increasing evidence suggests that a panel of molecules in combination may function as the best biomarkers with regard to sensitivity and specificity as shown in the case of lung cancer (Spira et al., 2007), Alzheimer’s disease (Ray et al., 2007) and amyotrophic lateral sclerosis (ALS) (Mitchell et al., 2009). Indeed, this approach has begun to be tested in alcohol diagnostics (Anton et al., 2002;Rinck et al., 2007).
Potential uses of biomarkers in alcohol-related disorders
With a biomarker defined as an indicator of a specific state, the next step for those in the alcohol research field is to define what state(s) is (are) being classified. One major goal of alcohol biomarker development is the generation of clinical diagnostics for identification of alcoholism. A biomarker of alcoholism is not strictly possible as DSM-IV criteria are based on the behavior of the patient and classify patients into alcohol abusing and alcohol dependent categories (Table 1). However, biomarkers may be used to define alcohol intake levels as an indicator of alcohol use and potentially abuse. Therefore, biomarkers may be used to identify patients with historically high alcohol intake levels who are likely to then be diagnosed as alcohol abusing or alcohol dependent by the standard DSM-IV psychological criteria. Another potential use of biomarkers is in identifying alcohol-induced tissue damage. Biomarkers developed for this purpose could be used as diagnostics of specific complications such as cardiovascular disease, liver disease, central and peripheral nervous system damage, and immune deficiency. Whatever the state intended to be indicated/diagnosed by a biomarker, metrics of a biomarker’s performance, such as sensitivity and specificity as a diagnostic are needed.
Table 1.
DSM-IV Criteria for Alcohol Abuse and Alcohol Dependence
Alcohol Abuse (1 or more criteria for over 1 year)
|
Alcohol Dependence (3 criteria for over 1 year)
|
A potential source of confusion in the field is that biomarkers of alcohol consumption may relate to, or even include, some of the same specific molecules as biomarkers of alcohol-induced tissue damage and vice versa. It is important to again remember that a biomarker is defined by its ability to classify a particular state. Biomarkers of high alcohol intake levels may originate with alcohol-induced tissue damage, but are optimized to detect a level of drinking, while a biomarker for tissue damage will be selected to most accurately diagnose a certain type of tissue damage. Therefore, biomarkers are developed to achieve different outcomes or measure different (and selective) states.
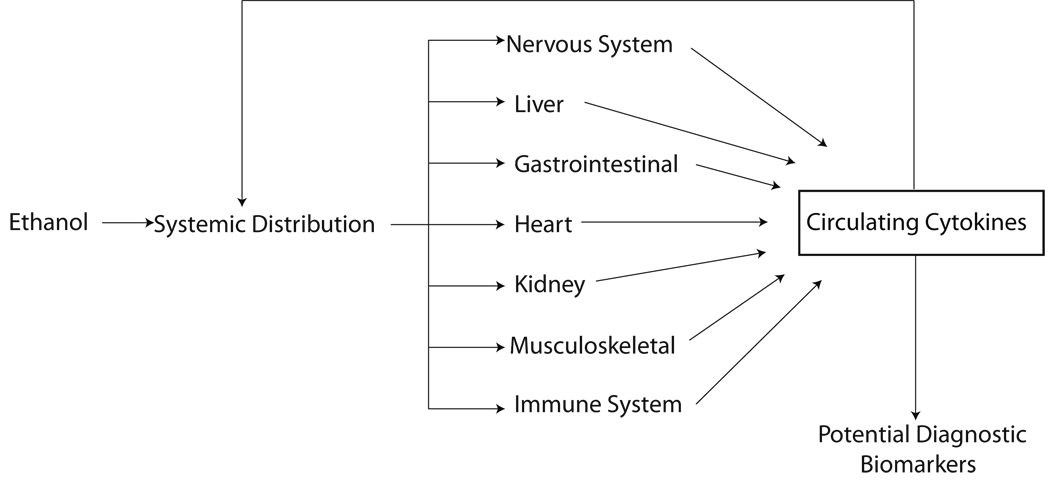
Successful development of reliable biochemical biomarkers that permit accurate assessment of alcohol intake, drinking patterns, or tissue damage would have obvious and widespread utility. One emerging and exciting avenue of research is the use of plasma or serum protein levels, particularly cytokines, as potential biomarkers of alcoholism and alcohol-induced tissue damage. As noted in Figure 1, the circulation represents a non-invasive window on drinking pathology. Alcohol consumption can lead to alterations in cytokine production and release from all compartments of the body. Growing evidence suggests that these circulating cytokines can directly affect behavior and mood. In the present context, however, they may also serve as potential biomarkers of drinking behavior and alcohol-induced tissue damage. We, herein, develop the hypothesis that circulating cytokines may contribute to diagnostic biomarker panels. The combination of multiple biomarkers may significantly increase the sensitivity and specificity of the biochemical tests aiding early and accurate detection.
Figure 1.
Alterations of circulating cytokines in response to ethanol originate in a variety of tissues/organs. With increasing amount and duration of ethanol administration, initial changes in cytokine levels may feedback on the body causing secondary changes. Either individually or as panels, circulating cytokines may serve as diagnostic markers of alcohol abuse and alcohol-induced tissue damage.
The desire for serum or plasma protein biomarkers of health and disease is not a novel concept and has been applied clinically for many years in ELISAs and other tests. Proteomics as a tool for biomarker discovery remains relatively new (Xiao et al., 2005) however biomarker discovery is a different question than the simple determination of a differentially expressed protein in the disease state versus the control state. A usable biomarker must be both sensitive (% of diseased subjects with marker) and specific (% of non-diseased subjects without the marker) in a population with normal prevalence. The majority of reports on proteomic discovery of serum biomarkers have been in the cancer field (Shau et al., 2003). We have chosen to classify protein biomarkers into three potential types: binary, single, and pattern. Binary biomarkers are the simplest and consist of a protein that is only present in either the diseased or control state; an on/off state with disease. Single biomarkers are single proteins that change in abundance with disease. A number of biomarkers such as this exist (e.g., prostate specific antigen, PSA). Pattern biomarkers, on the other hand, represent the use of multiple proteins for determination (Harasymiw and Bean, 2001;Javors and Johnson, 2003) and offer the promise of more rigorous detection.
Current biomarkers of alcoholism
The search for the development of diagnostic tools of alcohol abuse is not new. There has been significant research progress in this area to develop diagnostics of alcohol intake with a notable success being in directly measuring acute alcohol intake. A number of accurate methods for determining blood alcohol concentrations (BAC) through breath and blood now exist. This technology has developed to the point that small and inexpensive instruments are currently being used by thousands of law enforcement, medical, and security personnel.
The literature on potential plasma, blood, urine, and hair biomarkers has been thoroughly reviewed elsewhere (Conigrave et al., 2003;Conigrave et al., 2002;Das et al., 2008;Hannuksela et al., 2007;Helander, 2003). At present, the most common laboratory tests for alcohol intake include: gamma-glutamyltransferase (GGT) (Taracha et al., 2001), mean corpuscular volume (MCV) (Koch et al., 2004), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) (Niemela, 2007), sialylation of apolipoprotein J (SIJ) (Ghosh et al., 2001), carbohydrate-deficient transferrin (CDT) (Golka and Wiese, 2004;Hock et al., 2005), ethyl glucuronide (EtG) (Kissack et al., 2008), 5-hydroxytryptophol (5HTOL) (Helander and Eriksson, 2002), Phosphatidylethanol (PEth) (Comasco et al., 2009;Hansson et al., 1997), and homocysteine (Bleich and Hillemacher, 2009), are small molecule markers of alcohol intake. A detailed quantitative comparison of specificity, sensitivity and usefulness of these different biomarkers is beyond the scope of this review. However, in a broad sense, the utility of these biomarkers as diagnostic tools is greatly hampered due to variable results in different populations and low sensitivity and specificity. For example, clinical studies of CDT have found a range of sensitivities (77–100%) and specificities (10–85%) (Berner et al., 2006;Koch et al., 2004;Miller et al., 2006). These limitations may arise from several factors. 1) Most of these biomarkers have been proposed as unitary measures; however, combining multiple markers has been demonstrated to be more effective, suggesting that a panel of markers may result in better sensitivity and specificity (Anton et al., 2002;Korzec et al., 2005;Rinck et al., 2007;Korzec et al., 2009). 2) Many biomarkers relate to hepatic function, which is well known to be altered with alcohol abuse. Hepatic function is also impaired, however, in a number of other conditions that leads to false positives and reduced specificity. 3) Certain biomarkers may be detectable only during high alcohol intake or in conjugation with co-morbidities. In light of these limitations, together with other variations such as geographic and nutritional factors, there will be a higher level of false negative data and reduced sensitivity. While all of the biomarkers currently implicated have utility, they have not been universally accepted nor generally adopted in clinical practice. Thus, a need for more robust and dependable biomarkers of alcoholism is required and the current review examines one such group of potential biomarkers - the cytokines.
Cytokines as clinical biomarkers in other neurological disorders
In the last decade, a number of studies have examined the potential utility of plasma or CSF cytokines as disease diagnostics in neurological disorders as case studies of how alcohol research could potentially can benefit from cytokine profiling. An excellent review has previously summarized the potential use of cytokine biomarkers in various CNS disorders such as stroke, epilepsy, Parkinson’s disease (PD), Alzheimer’s disease (AD) and other neurological disorders (Lucas et al., 2006). Elevated levels of various cytokines in the course of CNS disorders have also been implicated from the point of view of potential therapeutic intervention (Perry, 2004). In epilepsy studies, an increase in serum cytokine levels has been documented, including alterations in TNF-alpha, IFN-gamma, IL-1beta, IL-2, IL-4, and IL-6 as compared to controls (Bauer et al., 2009). CSF analysis was also carried out indicating the presence of IL-6, IL-1, IL-2, IL-4, and IFN-gamma. Thus, increased post-ictal serum cytokine levels in patients with seizure disorders could be used as biomarkers for detection (Sinha et al., 2008), which is in agreement with earlier studies (Lucas et al., 2006).
In an effort to have molecular and biochemical markers of AD and to complement clinical approaches for early and accurate detection, levels of chemokines and cytokines in the serum and CSF of patients have been measured (Choi et al., 2008). The results indicate that MCP-1 was the only cytokine detectable in CSF, and that its levels did not differ between control and disease groups. The data indicate that immunological responses are not major contributors to the pathogenesis of AD and PD (Choi et al., 2008). However, in the case of PD, cytokines such as IL-1, TNF-alpha, IL-6 have been previously implicated although there is considerable controversy [as discussed by (Lucas et al., 2006)]. A landmark study identified a panel of plasma proteins, including a number of cytokines that serves as an accurate diagnostic of AD with ~90% accuracy (Ray et al., 2007). Recently, clinical studies using CSF from amyotrophic lateral sclerosis (ALS) patients have shown the existence of distinct biomarker profiles. Higher expression of IL-8 was found to be associated with a group of patients with lower levels of physical function. A CSF inflammatory profile analysis of cytokine and growth factors may distinguish patients with ALS from other neurologic disease controls, and has been suggested as a biomarker panel to aid in diagnosis (Mitchell et al., 2009).
These reports clearly document the potential use of cytokine markers in developing biomarker panel signatures of neurological disease. Inflammation is not only involved in acute CNS conditions such as stroke and traumatic injury, but also may be a key factor in chronic and neurodegenerative conditions such as AD, PD, and ALS. The availability and promise of effective treatments for neurodegenerative disorders is increasing the importance for early diagnosis through novel highly sensitive biomarkers such as cytokines. These results have led us to focus our attention towards identifying cytokine biomarkers in models of alcohol abuse. Taking these findings of cytokine profiling in these CNS diseases as a model, there is a strong rationale for examining cytokines as biomarkers of alcohol intake and alcohol induced tissue damage as well.
Consequences of alcoholism on the host immune system
Low levels of ethanol are commonly consumed as part of normal daily behavior. It is commonly accepted that moderate amounts of polyphenol rich beverages such as beer or wine may have beneficial health effects including on the immune system. On the other hand, high doses of alcohol consumption can directly suppress a wide range of immune responses, and alcohol abuse is associated with an increased incidence of a number of infectious diseases (Romeo et al., 2007).
Alcohol intake has been shown to play a pivotal role in alterations of innate immune responses. Individuals consuming alcohol have been reported to demonstrate a delayed and impaired hypersensitivity response (Tonnesen et al., 1992). The same observations of delayed response have also been made in a mouse model of chronic alcohol administration (Jayasinghe et al., 1992). It has also been reported that severe alcohol intake inhibits the monocyte-derived myeloid Dendritic Cell’s (DCs) capacity for inducing activation of T-cells in humans. Moreover, alcohol-treated DCs showed reduced IL-12, increased IL-10 production, and a decrease in expression of the co-stimulatory molecules CD80 and CD86 (Mandrekar et al., 2004;Szabo et al., 2004). Decreased production of IL-6, IL-12, IL-17A, IFN-gamma and increased levels of IL-13 cytokines as a result of ovalbumin stimulation in alcohol-consuming mice have also been observed (Heinz and Waltenbaugh, 2007). The data indicate that ethanol alters CD11c+/CD8(alpha)+ DC function, affecting the cytokines responsible for adaptive immune responses.
The effects of different levels of alcohol consumption as well as type of alcoholic beverage on the activity, number, distribution, balance, interaction and response of immunocompetent cells have been extensively reviewed (Diaz et al., 2002;Szabo, 1999). In total, alcohol consumption leads to altered inflammatory cell and adaptive immune responses and to increased incidence of infections and during infection, poor presentation of infectious agents and other organ-specific immune-mediated effects (Szabo and Mandrekar, 2009). Furthermore, this altered immune response may be observed through measurements of plasma/serum cytokine levels.
Cytokine association with alcohol induced pathologies
Cytokines are a class of multifunctional proteins that are implicated in cellular communication and activation. Cytokines are critical to the development and functioning of both innate and adaptive immune response, and not just limited to the immune system, but also involved in developmental processes. The cytokines could be of type Th1 (proinflammatory) or Th2 (anti-inflammatory) depending upon their role in the immune system. Cytokines impact tissues in a complex manner that regulates inflammation, cell death, cell proliferation, cell migration, and healing mechanisms. Alcohol is known to alter cytokine levels in a variety of tissues including plasma, lung, liver, brain (Crews et al., 2006a). These alterations could contribute to changes in the central nervous system leading to long-term changes in behavior and neurodegeneration (Figure 1). These studies suggest that measurement of cytokines could be a key course of action in understanding alcohol neural pathogenesis. As described below, much of the literature to date has focused on alcohol-induced liver disease (Table 2). These studies highlight the importance of examining cytokine changes across the time course of alcoholism to understand the initial changes directly resulting from alcohol use and the secondary pathology that occurs with late stage alcohol-induced tissue damage. The relationship between cytokines and the metabolic consequences of chronic alcoholism has been studied since the early 1990s. Circulating cytokines such as tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1) and IL-6 are found to be elevated in both chronic and acute alcohol-induced liver disease. These have been primarily correlated with the metabolic consequences and abnormalities of liver injury due to alcohol intake (Khoruts et al., 1991). The concentrations of all three cytokines have been correlated with biochemical parameters of liver injury, hepatic protein synthesis and serum IgG concentration.
Table 2.
Summary of potential cytokine changes implicated during human alcoholism
| Human alcoholism and associated pathology | Source | Cytokine | Reference |
|---|---|---|---|
| Actively drinking with no chronic liver disease |
Plasma | TNF-α↔ | Khoruts et el., 1991 |
| IL1↔ | |||
| IL6↔ | |||
| Non-drinking alcoholic with stable cirrhosis |
TNF-α↑ | ||
| IL1↑ | |||
| IL6↑ | |||
| Acute alcoholic hepatitis | TNF-α↑ | ||
| IL1↑ | |||
| IL6↑ | |||
| Alcoholic | Brain | MCP-1↑ | He and Crews, 2008 |
| Light/moderate/heavy drinkers with no liver disease |
Serum | TNF-α↔ | Gonzalez-Quintela et al., 2008 |
| Alcoholics with liver cirrhosis | TNF-α↑ | ||
| Alcoholic hepatitis 1990) |
Plasma | TNF-α↑ | Felver et al., 1990 |
| IL1-α↑ | |||
| IL1-β↔ | |||
| Alcoholic cirrhosis | TNF-α↔ | ||
| Alcoholics with no hepatitis | TNF-α↔ | ||
| Severe alcoholic hepatitis 1991) |
Plasma | IL6↑↑ | Sheron et al., 1991 |
| Alcoholic cirrhosis | IL6↑ | ||
| Alcoholic without liver disease/ inactive alcoholic cirrhosis/ non-alcoholic chronic liver disease/ chronic liver failure |
IL6↔ | ||
| Alcoholic hepatitis | Plasma | TNF-α↑↑ | Bird et al., 1990 |
| Inactive alcoholic cirrhosis | TNF-α↑ | ||
| Alcoholic without liver disease | TNF-α↔ | ||
| Alcoholic hepatitis | Serum | IL6↑ | Fujimoto et al., 2000 |
| IL8↑ | |||
| TNF-α(nd) | |||
| Alcoholic hepatitis and cirrhosis | IL6↑ | ||
| IL8↑ | |||
| TNF-α(nd) | |||
| Chronic alcoholic without liver disease | PB monocytes | IL1-β↑ | Laso et al., 2007 |
| IL6↑ | |||
| IL12↑ | |||
| TNF-α↑ | |||
| Alcoholic with Liver cirrhosis |
IL1-β↔ | ||
| IL6↔ | |||
| IL12↔ | |||
| TNF-α↔ | |||
| Chronic alcoholic with liver disease | IL1-β↔ | ||
| IL6↔ | |||
| IL12↔ | |||
| TNF-α↔ | |||
| No or mild liver hepatitis/ liver cirrhosis |
Plasma | TNF-α↔ | Le Moine et al., 1995 |
Note: nd, not detected, ↔ no change, ↑ slight increase, ↑↑ significant increase
Recently, the measurement of serum TNF-alpha levels has become routine in clinical practice and it has been shown that TNF-alpha contributes to alcohol-induced organ dysfunction, including alcoholic hepatitis. Serum levels of TNF-alpha levels have been demonstrated to be higher in hospitalized alcoholics than in the general population, regardless of alcohol consumption level (Gonzalez-Quintela et al., 2008). Furthermore the highest TNF-alpha levels, were correlated with liver dysfunction. It is important to note that light-to-moderate drinking had no significant effect on the levels of serum TNF-alpha levels (Gonzalez-Quintela et al., 2008).
In a recent study, alcoholic patients were examined for the production of cytokines by peripheral blood (PB) monocytes and this was related to the amount of alcohol intake, as well as liver disease (Laso et al., 2007). As shown in Table 2, a significant increase in spontaneous production of IL1-beta, IL6, IL12, and TNF-alpha was observed in chronic alcoholics without liver disease compared with those who are alcoholic with liver cirrhosis. On the other hand, chronic alcoholics with liver disease still consuming alcohol showed abnormally low production of IL1-beta and TNF-alpha (Laso et al., 2007).
In addition to liver injury, there is a growing understanding of the role of cytokines in alcohol-induced neurodegeneration. In a rat model, alcohol-induced neurodegeneration is shown to be due to neuronal death during intoxication and is related to increased oxidative stress and proinflammatory proteins that are neurotoxic (Crews and Nixon, 2009). Degeneration is associated with increased NF-kappaB proinflammatory transcription and decreased CREB transcription. However, abstinence after binge ethanol intoxication results in neurogenesis and regeneration of brain structure and function (Crews and Nixon, 2009). Studies using animal models have documented the effects of alcohol on acute adolescent neurogenesis by alcohol (Crews et al., 2006b). Studies on human postmortem brains from alcoholics have shown increased concentrations of monocyte chemoattractant protein 1 (MCP-1) and microglial activities that may have contributed to the alcohol-induced pathogenesis (He and Crews, 2008).
The interactions between altered levels of cytokines in alcoholics and infectious agents provide mechanistic insight into alcohol pathobiology. (Molina et al., 2006)have studied the effect of chronic alcoholism on nutritional, metabolic and immune alterations during the initial 10-month asymptomatic phase of SIV infection in nonhuman primate rhesus macaques. Chronic alcohol/SIV(+) animals showed a higher viral load at 3 months post-SIV infection, as well as muscle TNF-alpha mRNA expression was markedly increased (Molina et al., 2006).
Interestingly, there are also reported associations between cytokine gene polymorphisms and predisposition to alcoholism. Marcos and colleagues reported that an IL-10 gene polymorphism is associated with alcoholism and not with alcoholic liver disease as a result of alcoholism (Marcos et al., 2008). This study in Spanish subjects demonstrated that the frequency of allele A carriers (CA and AA genotypes) was significantly higher in patients with alcohol dependence and alcohol abuse than in healthy controls. The mechanism by which cytokine polymorphisms such as these contribute to alcoholic predisposition remain to be understood but further emphasize the importance of examining cytokine functions in alcohol abuse and alcoholism.
These examples from the alcohol research field are exciting first steps in understanding the relationship of alcohol intake and alcohol-induced tissue damage to levels of circulating cytokines. Much more detailed analysis of many more cytokines is needed to understand the global pattern of cytokine levels in the plasma. While the focus of this review is on the use of cytokines in diagnostic biomarkers, these findings will also add mechanistic insight into the effects of alcohol on a variety of organ systems.
With the promise of cytokines as diagnostic markers there is also the potential that cytokines alone will not provide a sufficiently sensitive and specific test. Addition of other circulating proteins, such as growth factors, to a biomarker panel may reach the required accuracy. Interactions of alcohol with levels of growth factor during pregnancy highlight the potential roles of circulating factors in neurodevelopment. Studies have examined peripheral hepatocyte growth factor (HGF), epidermal growth factor (EGF) and placenta growth factor (PlGF) in pregnant women consuming alcohol (Vuorela et al., 2002). The data indicate that alcohol abuse during pregnancy has a profound effect on circulating EGF and PlGF but not HGF concentrations. However, the data showed no associations between clinical outcome such as birth weight, Apgar score and placental weight and maternal circulating cytokines analyzed during pregnancy. Interestingly, although there were clear differences in the cytokine concentrations between alcohol abusing and alcohol abstinent women, the changes were speculated to be due to altered expression, tissue release or breakdown of these cytokines and thus could not be correlated. Thus, the changes occurring during pregnancy could be primarily due to alcohol or could be part of a cascade of secondary events leading to alcohol-induced placental pathophysiology (Vuorela et al., 2002).
Conclusions
From the above discussion, it is clear that cytokines such as TNF alpha, IL1-alpha, IL1-beta, IL6, IL8, IL12 and MCP-1, can be regulated by alcohol and alcohol-induced tissue damage. As it has become increasingly clear that, in chronic alcoholic patients, the levels of circulating inflammatory cytokines produced by monocytes and macrophages is distorted. Further studies will be needed to determine the course of these changes over the pathological sequelae of alcoholism. The literature available on this aspect is constantly being updated, and a consensus on the pattern of cytokine changes remains to be reached. The complex interactions of these cytokines, such as release of IL-6 in response to TNF alpha remain to be understood (Sheron et al., 1991). Similarly, the role of other factors in cytokine release associated with alcoholism, such as nutrition, age, sex, and method of analysis and co-morbid drug use remains to be examined. However, given the existing literature, there is great promise in using circulating cytokine levels as diagnostics of alcohol abuse and alcoholism. Cytokines, in combination with other circulating proteins, such as the growth factors may provide highly accurate diagnostics of alcohol intake and alcohol-induced tissue damage. These biomarkers could also be measured using existing technology, speeding the translation of new diagnostics. Ultimately, the success or failure of biomarkers of alcohol intake and alcohol-induced tissue damage will depend on the ability of biomarkers to improve patient treatment in a cost effective manner (Kapoor et al., 2009).
Acknowledgments
This work was supported by NIH grant R01AA016613 (KEV). The authors thank Mr. Steven Swavely for his editorial contribution.
Sources of Support: This work was supported by NIH grant R01AA016613 (KEV)
Footnotes
Disclaimers: None
References
- Anton RF, Lieber C, Tabakoff B. Carbohydrate-deficient transferrin and gamma-glutamyltransferase for the detection and monitoring of alcohol use: results from a multisite study. Alcohol Clin Exp Res. 2002;26:1215–1222. doi: 10.1097/01.ALC.0000023986.42254.F5. [DOI] [PubMed] [Google Scholar]
- Bauer S, Cepok S, Todorova-Rudolph A, Nowak M, Koller M, Lorenz R, Oertel WH, Rosenow F, Hemmer B, Hamer HM. Etiology and site of temporal lobe epilepsy influence postictal cytokine release. Epilepsy Res. 2009;86:82–88. doi: 10.1016/j.eplepsyres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Berner MM, Bentele M, Kriston L, Manz C, Clement HW, Harter M, Mundle G. DOVER and QUVER-new marker combinations to detect and monitor at-risk drinking. Alcohol Clin Exp Res. 2006;30:1372–1380. doi: 10.1111/j.1530-0277.2006.00163.x. [DOI] [PubMed] [Google Scholar]
- Biermann T, Bonsch D, Reulbach U, Kornhuber J, Bleich S. Dopamine and N-methyl-D-aspartate receptor expression in peripheral blood of patients undergoing alcohol withdrawal. J Neural Transm. 2007;114:1081–1084. doi: 10.1007/s00702-007-0661-4. [DOI] [PubMed] [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–920. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- Bleich S, Hillemacher T. Homocysteine, alcoholism and its molecular networks. Pharmacopsychiatry. 2009;42 Suppl 1:S102–S109. doi: 10.1055/s-0029-1214396. [DOI] [PubMed] [Google Scholar]
- Bradford BU, O'Connell TM, Han J, Kosyk O, Shymonyak S, Ross PK, Winnike J, Kono H, Rusyn I. Metabolomic profiling of a modified alcohol liquid diet model for liver injury in the mouse uncovers new markers of disease. Toxicol Appl Pharmacol. 2008;232:236–243. doi: 10.1016/j.taap.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Choi C, Jeong JH, Jang JS, Choi K, Lee J, Kwon J, Choi KG, Lee JS, Kang SW. Multiplex analysis of cytokines in the serum and cerebrospinal fluid of patients with Alzheimer's disease by color-coded bead technology. J Clin Neurol. 2008;4:84–88. doi: 10.3988/jcn.2008.4.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Nordquist N, Leppert J, Oreland L, Kronstrand R, Alling C, Nilsson KW. Adolescent alcohol consumption: Biomarkers PEth and FAEE in relation to interview and questionnaire data. J Stud Alcohol Drugs. 2009;70:797–804. doi: 10.15288/jsad.2009.70.797. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Davies P, Haber P, Whitfield JB. Traditional markers of excessive alcohol use. Addiction. 2003;98 Suppl 2:31–43. doi: 10.1046/j.1359-6357.2003.00581.x. [DOI] [PubMed] [Google Scholar]
- Conigrave KM, Degenhardt LJ, Whitfield JB, Saunders JB, Helander A, Tabakoff B. CDT, GGT, and AST as markers of alcohol use: the WHO/ISBRA collaborative project. Alcohol Clin Exp Res. 2002;26:332–339. [PubMed] [Google Scholar]
- Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006a;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Dhanya L, Vasudevan DM. Biomarkers of alcoholism: an updated review. Scand J Clin Lab Invest. 2008;68:81–92. doi: 10.1080/00365510701532662. [DOI] [PubMed] [Google Scholar]
- De GN, Donadio G, Chiarotti M. The reliability of fatty acid ethyl esters (FAEE) as biological markers for the diagnosis of alcohol abuse. J Anal Toxicol. 2007;31:93–97. doi: 10.1093/jat/31.2.93. [DOI] [PubMed] [Google Scholar]
- Diaz LE, Montero A, Gonzalez-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56 Suppl 3:S50–S53. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- Ewing JA. Detecting alcoholism. The CAGE questionnaire. JAMA. 1984;252:1905–1907. doi: 10.1001/jama.252.14.1905. [DOI] [PubMed] [Google Scholar]
- Felver ME, Mezey E, McGuire M, Mitchell MC, Herlong HF, Veech GA, Veech RL. Plasma tumor necrosis factor alpha predicts decreased long-term survival in severe alcoholic hepatitis. Alcohol Clin Exp Res. 1990;14:255–259. doi: 10.1111/j.1530-0277.1990.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Gooch RS, Lull ME, Worst TJ, Walker SJ, Xu AS, Green H, Pierre PJ, Grant KA, Vrana KE. Apo-aii is an elevated biomarker of chronic non-human primate ethanol self-administration. Alcohol Alcohol. 2006;41:300–305. doi: 10.1093/alcalc/agl021. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Uemura M, Nakatani Y, Tsujita S, Hoppo K, Tamagawa T, Kitano H, Kikukawa M, Ann T, Ishii Y, Kojima H, Sakurai S, Tanaka R, Namisaki T, Noguchi R, Higashino T, Kikuchi E, Nishimura K, Takaya A, Fukui H. Plasma endotoxin and serum cytokine levels in patients with alcoholic hepatitis: relation to severity of liver disturbance. Alcohol Clin Exp Res. 2000;24:48S–54S. [PubMed] [Google Scholar]
- Fuller RK, Lee KK, Gordis E. Validity of self-report in alcoholism research: results of a Veterans Administration Cooperative Study. Alcohol Clin Exp Res. 1988;12:201–205. doi: 10.1111/j.1530-0277.1988.tb00180.x. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Hale EA, Lakshman MR. Plasma sialic-acid index of apolipoprotein J (SIJ): a new alcohol intake marker. Alcohol. 2001;25:173–179. doi: 10.1016/s0741-8329(01)00187-2. [DOI] [PubMed] [Google Scholar]
- Golka K, Wiese A. Carbohydrate-deficient transferrin (CDT)--a biomarker for long-term alcohol consumption. J Toxicol Environ Health B Crit Rev. 2004;7:319–337. doi: 10.1080/10937400490432400. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Quintela A, Campos J, Loidi L, Quinteiro C, Perez LF, Gude F. Serum TNF-alpha levels in relation to alcohol consumption and common TNF gene polymorphisms. Alcohol. 2008;42:513–518. doi: 10.1016/j.alcohol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- Hannuksela ML, Liisanantti MK, Nissinen AE, Savolainen MJ. Biochemical markers of alcoholism. Clin Chem Lab Med. 2007;45:953–961. doi: 10.1515/CCLM.2007.190. [DOI] [PubMed] [Google Scholar]
- Hansson P, Caron M, Johnson G, Gustavsson L, Alling C. Blood phosphatidylethanol as a marker of alcohol abuse: levels in alcoholic males during withdrawal. Alcohol Clin Exp Res. 1997;21:108–110. [PubMed] [Google Scholar]
- Harasymiw J, Bean P. The combined use of the early detection of alcohol consumption (EDAC) test and carbohydrate-deficient transferrin to identify heavy drinking behaviour in males. Alcohol Alcohol. 2001;36:349–353. doi: 10.1093/alcalc/36.4.349. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol. 2008;210:349–358. doi: 10.1016/j.expneurol.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz R, Waltenbaugh C. Ethanol consumption modifies dendritic cell antigen presentation in mice. Alcohol Clin Exp Res. 2007;31:1759–1771. doi: 10.1111/j.1530-0277.2007.00479.x. [DOI] [PubMed] [Google Scholar]
- Helander A. Biological markers in alcoholism. J Neural Transm. 2003 Suppl:15–32. doi: 10.1007/978-3-7091-0541-2_2. [DOI] [PubMed] [Google Scholar]
- Helander A, Eriksson CJ. Laboratory tests for acute alcohol consumption: results of the WHO/ISBRA Study on State and Trait Markers of Alcohol Use and Dependence. Alcohol Clin Exp Res. 2002;26:1070–1077. [PubMed] [Google Scholar]
- Hock B, Schwarz M, Domke I, Grunert VP, Wuertemberger M, Schiemann U, Horster S, Limmer C, Stecker G, Soyka M. Validity of carbohydrate-deficient transferrin (%CDT), gamma-glutamyltransferase (gamma-GT) and mean corpuscular erythrocyte volume (MCV) as biomarkers for chronic alcohol abuse: a study in patients with alcohol dependence and liver disorders of non-alcoholic and alcoholic origin. Addiction. 2005;100:1477–1486. doi: 10.1111/j.1360-0443.2005.01216.x. [DOI] [PubMed] [Google Scholar]
- Javors MA, Johnson BA. Current status of carbohydrate deficient transferrin, total serum sialic acid, sialic acid index of apolipoprotein J and serum beta-hexosaminidase as markers for alcohol consumption. Addiction. 2003;98 Suppl 2:45–50. doi: 10.1046/j.1359-6357.2003.00582.x. [DOI] [PubMed] [Google Scholar]
- Jayasinghe R, Gianutsos G, Hubbard AK. Ethanol-induced suppression of cell-mediated immunity in the mouse. Alcohol Clin Exp Res. 1992;16:331–335. doi: 10.1111/j.1530-0277.1992.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Kraemer KL, Smith KJ, Roberts MS, Saitz R. Cost-effectiveness of screening for unhealthy alcohol use with %carbohydrate deficient transferrin: results from a literature-based decision analytic computer model. Alcohol Clin Exp Res. 2009;33:1440–1449. doi: 10.1111/j.1530-0277.2009.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- Kissack JC, Bishop J, Roper AL. Ethylglucuronide as a biomarker for ethanol detection. Pharmacotherapy. 2008;28:769–781. doi: 10.1592/phco.28.6.769. [DOI] [PubMed] [Google Scholar]
- Koch H, Meerkerk GJ, Zaat JO, Ham MF, Scholten RJ, Assendelft WJ. Accuracy of carbohydrate-deficient transferrin in the detection of excessive alcohol consumption: a systematic review. Alcohol Alcohol. 2004;39:75–85. doi: 10.1093/alcalc/agh031. [DOI] [PubMed] [Google Scholar]
- Korzec A, de BC, van LM. The Bayesian Alcoholism Test had better diagnostic properties for confirming diagnosis of hazardous and harmful alcohol use. J Clin Epidemiol. 2005;58:1024–1032. doi: 10.1016/j.jclinepi.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Korzec S, Korzec A, Conigrave K, Gisolf J, Tabakoff B. Validation of the Bayesian Alcoholism Test compared to single biomarkers in detecting harmful drinking. Alcohol Alcohol. 2009;44:398–402. doi: 10.1093/alcalc/agp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laso FJ, Vaquero JM, Almeida J, Marcos M, Orfao A. Production of inflammatory cytokines by peripheral blood monocytes in chronic alcoholism: relationship with ethanol intake and liver disease. Cytometry B Clin Cytom. 2007;72:408–415. doi: 10.1002/cyto.b.20169. [DOI] [PubMed] [Google Scholar]
- Le Moine O, Soupison T, Sogni P, Marchant A, Moreau R, Hadengue A, Goldman M, Deviere J, Lebrec D. Plasma endotoxin and tumor necrosis factor-alpha in the hyperkinetic state of cirrhosis. J Hepatol. 1995;23:391–395. doi: 10.1016/0168-8278(95)80196-0. [DOI] [PubMed] [Google Scholar]
- Lucas SM, Rothwell NJ, Gibson RM. The role of inflammation in CNS injury and disease. Br J Pharmacol. 2006;147 Suppl 1:S232–S240. doi: 10.1038/sj.bjp.0706400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Marcos M, Pastor I, Gonzalez-Sarmiento R, Laso FJ. Interleukin-10 gene polymorphism is associated with alcoholism but not with alcoholic liver disease. Alcohol Alcohol. 2008;43:523–528. doi: 10.1093/alcalc/agn026. [DOI] [PubMed] [Google Scholar]
- Miller PM, Spies C, Neumann T, Javors MA, Hoyumpa AM, Roache J, Webb A, Kashi M, Sharkey FE, Anton RF, Egan BM, Basile J, Nguyen S, Fleming MF, Dillie KS. Alcohol biomarker screening in medical and surgical settings. Alcohol Clin Exp Res. 2006;30:185–193. doi: 10.1111/j.1530-0277.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Freeman WM, Randazzo WT, Stephens HE, Beard JL, Simmons Z, Connor JR. A CSF biomarker panel for identification of patients with amyotrophic lateral sclerosis. Neurology. 2009;72:14–19. doi: 10.1212/01.wnl.0000333251.36681.a5. [DOI] [PubMed] [Google Scholar]
- Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30:2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Niemela O. Biomarkers in alcoholism. Clin Chim Acta. 2007;377:39–49. doi: 10.1016/j.cca.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Nomura F, Tomonaga T, Sogawa K, Wu D, Ohashi T. Application of proteomic technologies to discover and identify biomarkers for excessive alcohol consumption: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;855:35–41. doi: 10.1016/j.jchromb.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Pernanen K. Validity of survey data on alcohol use. In: Gibbons, et al., editors. Research Advances in Alcohol and Drug Problems. New York: 1974. pp. 355–374. [Google Scholar]
- Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ray S, et al. Classification and prediction of clinical Alzheimer's diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Rinck D, Frieling H, Freitag A, Hillemacher T, Bayerlein K, Kornhuber J, Bleich S. Combinations of carbohydrate-deficient transferrin, mean corpuscular erythrocyte volume, gamma-glutamyltransferase, homocysteine and folate increase the significance of biological markers in alcohol dependent patients. Drug Alcohol Depend. 2007;89:60–65. doi: 10.1016/j.drugalcdep.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Romeo J, Warnberg J, Nova E, Diaz LE, Gomez-Martinez S, Marcos A. Moderate alcohol consumption and the immune system: a review. Br J Nutr. 2007;98 Suppl 1:S111–S115. doi: 10.1017/S0007114507838049. [DOI] [PubMed] [Google Scholar]
- Shau H, Chandler GS, Whitelegge JP, Gornbein JA, Faull KF, Chang HR. Proteomic profiling of cancer biomarkers. Brief Funct Genomic Proteomic. 2003;2:147–158. doi: 10.1093/bfgp/2.2.147. [DOI] [PubMed] [Google Scholar]
- Sheron N, Bird G, Goka J, Alexander G, Williams R. Elevated plasma interleukin-6 and increased severity and mortality in alcoholic hepatitis. Clin Exp Immunol. 1991;84:449–453. [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Patil SA, Jayalekshmy V, Satishchandra P. Do cytokines have any role in epilepsy? Epilepsy Res. 2008;82:171–176. doi: 10.1016/j.eplepsyres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Spira A, Beane JE, Shah V, Steiling K, Liu G, Schembri F, Gilman S, Dumas YM, Calner P, Sebastiani P, Sridhar S, Beamis J, Lamb C, Anderson T, Gerry N, Keane J, Lenburg ME, Brody JS. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13:361–366. doi: 10.1038/nm1556. [DOI] [PubMed] [Google Scholar]
- Stephanson N, Helander A, Beck O. Alcohol biomarker analysis: simultaneous determination of 5-hydroxytryptophol glucuronide and 5-hydroxyindoleacetic acid by direct injection of urine using ultra-performance liquid chromatography-tandem mass spectrometry. J Mass Spectrom. 2007;42:940–949. doi: 10.1002/jms.1231. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33:220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taracha E, Habrat B, Wozniak P, Walkowiak J, Szukalski B. The activity of beta-hexosaminidase (uHex) and gamma-glutamyl-transferase (uGGT) in urine as non-invasive markers of chronic alcohol abuse: I. Alcohol-dependent subjects. World J Biol Psychiatry. 2001;2:184–189. doi: 10.3109/15622970109026807. [DOI] [PubMed] [Google Scholar]
- Tonnesen H, Kaiser AH, Nielsen BB, Pedersen AE. Reversibility of alcohol-induced immune depression. Br J Addict. 1992;87:1025–1028. doi: 10.1111/j.1360-0443.1992.tb03119.x. [DOI] [PubMed] [Google Scholar]
- Vuorela P, Sarkola T, Alfthan H, Halmesmaki E. Hepatocyte growth factor, epidermal growth factor, and placenta growth factor concentrations in peripheral blood of pregnant women with alcohol abuse. Alcohol Clin Exp Res. 2002;26:682–687. [PubMed] [Google Scholar]
- Walker SJ, Grant KA. Peripheral blood alpha-synuclein mRNA levels are elevated in cynomolgus monkeys that chronically self-administer ethanol. Alcohol. 2006;38:1–4. doi: 10.1016/j.alcohol.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Prieto D, Conrads TP, Veenstra TD, Issaq HJ. Proteomic patterns: their potential for disease diagnosis. Mol Cell Endocrinol. 2005;230:95–106. doi: 10.1016/j.mce.2004.10.010. [DOI] [PubMed] [Google Scholar]