Abstract
Copulatory behaviors in most rodents are highly sexually dimorphic, even when circulating hormones are equated between the sexes. Prairie voles (Microtus ochrogaster) are monomorphic in their display of some social behaviors, including partner preferences and parenting, but differences between the sexes in their masculine and feminine copulatory behavior potentials have not been studied in detail. Furthermore, the role of neonatal aromatization of testosterone to estradiol on the development of prairie vole sexual behavior potentials or their brain is unknown. To address these issues, prairie vole pups were injected daily for the first week after birth with 0.5 mg of the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) or oil. Masculine and feminine copulatory behaviors in response to testosterone or estradiol were later examined in both sexes. Males and females showed high mounting and thrusting in response to testosterone, but only males reliably showed ejaculatory behavior. Conversely, males never showed feminine copulatory behaviors in response to estradiol. Sex differences in these behaviors were not affected by neonatal ATD, but ATD-treated females received fewer mounts and thrusts than controls, possibly indicating reduced attractiveness to males. In other groups of subjects, neonatal ATD demasculinized males’ tyrosine hydroxylase expression in the anteroventral periventricular preoptic area, and estrogen receptor alpha expression in the medial preoptic area. Thus, although sexual behavior in both sexes of prairie voles is highly masculinized, aromatase during neonatal life is necessary only for females’ femininity. Furthermore, copulatory behavior potentials and brain development in male prairie voles are dissociable by their requirement for neonatal aromatase.
Keywords: aromatase, estradiol, Microtus, neonatal, sexual behavior, sexual differentiation, voles
Masculine and feminine copulatory behaviors are highly sexually dimorphic in many rodent species, even when levels of steroid hormones are equated in adult males and females (see Wallen and Baum, 2002). Indeed, treating male rats or mice with ovarian hormones rarely results in high levels of feminine sexual behaviors (Dominguez-Salazar et al., 2002; Edwards and Burge, 1971; Kudwa et al., 2005; Parsons et al., 1984; Whalen et al., 1986; Whalen and Olsen, 1981). Furthermore, although female rats treated with testosterone may mount other females, the frequency is often low compared to males, and intromissive and ejaculatory behaviors are rarely displayed in either rats or mice given testosterone (Clemens et al., 1978; Dominguez-Salazar et al., 2002; Edwards and Burge, 1971; Gladue and Clemens, 1980; Levine and Mullins, 1964; Whalen and Olsen, 1981).
Prairie voles (Microtus ochrogaster) are an interesting species to study sex differences in behavioral potentials because they are relatively monomorphic in some of their social behaviors, as both sexes form pairbonds after mating and display parental behaviors towards pups (Carter et al., 1995). Nonetheless, their copulatory behavior potentials have been suggested to be sexually dimorphic (Carter et al., 1987; Roberts et al., 1997; Smale et al., 1985). These studies report the percentage of subjects mounting or displaying lordosis, which gives valuable but somewhat incomplete information about their behavioral potentials. A detailed analysis of masculine and feminine sexual behavioral potentials in both sexes of prairie voles, and the behavior of the conspecifics they interact with, has not been performed. This is particularly important for understanding sexual differentiation of intromissive and ejaculatory behaviors, which in other rodents are more sexually differentiated than mounting (Clemens et al., 1978; Dominguez-Salazar et al., 2002; Edwards and Burge, 1971; Levine and Mullins, 1964; Whalen and Olsen, 1981).
Sexual differentiation of copulatory behaviors in many laboratory rodents requires exposure to gonadal hormones during perinatal life, with the aromatization of testosterone to estradiol and subsequent activation of neural estrogen receptors (ERs) often critical (e.g. De Vries and Simerly, 2002; Lonstein and Auger, 2008; McCarthy, 1994; Wallen and Baum, 2002). Masculinization of copulatory behavior in male rats is prevented by neonatal treatment with the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) or an ER antagonist (Bakker et al., 1993b; Booth, 1977; Brand et al., 1991; Houtsmuller et al., 1994; Swaab et al., 1995). These treatments also result in males with incompletely defeminized copulatory behaviors (Bakker et al., 1993a, 1993b, 1996; Booth, 1977; Brand et al., 1991; Fadem and Barfield, 1981; McEwen et al., 1977). Little is known about the role of aromatase in sexual differentiation of non-traditional laboratory rodents, but studies investigating sexual differentiation in prairie voles and other members of Microtus reveal some unexpected findings. For example, neonatal castration significantly feminizes male prairie voles’ parental responding and extra-hypothalamic arginine-vasopressin expression, but administration of testosterone for the week after surgery cannot maintain masculinity in neonatally castrated males (Lonstein et al., 2002, 2005). Perinatal testosterone also cannot masculinize parental behaviors or vasopressin expression in female prairie voles (Lonstein et al., 2002, 2005), while perinatal injections of estrogens does masculinize their brain (Lansing and Lonstein, 2006; Lonstein et al., 2005). Although this suggests that perinatal estrogens are important for some aspects of sexual differentiation in voles, copulatory behaviors in male gray-tailed voles (Microtus canicaudus) are not defeminized by perinatal aromatase inhibition (Petersen, 1986). Thus, the function of aromatization and ER activity in the sexual differentiation of prairie voles and other members of Microtus differs from that of some other laboratory rodents.
The goal of the present study was to provide the first detailed analysis of sexual behavior potentials in male and female prairie voles, and examine the effects of neonatal treatment with ATD on these potentials. We also determined the effects of neonatal ATD on sex differences in tyrosine hydroxylase (TH) expression in the anteroventral periventricular preoptic area (AVPV) and estrogen receptor alpha (ERα) expression in the medial preoptic area (mPOA), bed nucleus of the stria terminalis (BST), and medial amygdala (MeA). These systems have been reported to be sexually dimorphic in prairie voles (Cushing et al., 2004; Hnatczuk et al., 1994; Kramer et al., 2006; Lansing and Lonstein, 2006), are influenced by neonatal gonadal hormones (Cushing and Kramer, 2005; Lansing and Lonstein, 2006), and are associated with the display of copulatory behaviors in rodents (Clancy et al., 2000; Huddleston et al., 2006; Hull et al., 1999; Simerly and Swanson, 1987; Simerly et al., 1985; Wersinger et al., 1997).
Methods
Subjects
Prairie voles (Microtus ochrogaster) were raised in our colony at Michigan State University, which originated from voles captured in 1994 in Urbana, Illinois and later housed in colonies belonging to Dr. Betty McGuire (Smith College, Northampton, MA) and Dr. Zuoxin Wang (Florida State University, Tallahassee, FL). These voles were outbred at the University of Massachusetts in 2000 with voles of Illinois origin provided by Dr. C. Sue Carter (University of Illinois at Chicago), and were brought to Michigan State University in 2002. Animals were kept in clear plastic cages (48 × 28 × 16 cm) containing wood chips, wood shavings, and hay. They were provided ad libitum with water and a food mixture of cracked corn, whole oats, sunflower seeds, and rabbit chow (Teklad rodent diet #2031) in a 1:1:2:2 ratio. Our colony was maintained on a 14:10 light:dark cycle with an ambient temperature of approximately 21°C. All procedures were in accordance with the guidelines for animal use of the National Institutes of Health and the Institutional Animal Care and Use Committee at Michigan State University.
Neonatal injections
To obtain subjects for this study, unrelated adult male and female prairie voles were isolated for 4 days, after which time a female was placed in the home cage of an individual male. After 21 days, the pairs were checked daily at ~1400 hr for the presence of pups. On the day of birth and continuing for the next six days, pups were gently removed from the nest or the dam’s nipples and subcutaneously injected with 0.05 ml sesame oil (21 litters) or 0.5 mg of the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD; Steraloids, Newport, RI) dissolved in 0.05 ml sesame oil (18 litters), and returned to the nest. All pups in a litter received the same treatment. This dose of ATD was chosen because it was the same or similar to doses used in previous studies in voles (Lonstein and De Vries, 2000), and was the same dose previously used in neonatal rats to disrupt masculinization of their sexual behavior (Whalen et al., 1986). We also found that a higher dose (1 mg/pup/day) caused some pup mortality, while 0.5 mg did not seem to have any negative physical effects, as evidenced by body weights that were the same across treatment groups when subjects were gonadectomized during adulthood (oil-treated males: 42.6 ± 9.0 g, ATD-treated males: 39.2 ± 8.0 g, oil-treated females: 37.7 ± 9.8 g, ATD-treated females: 38.4 ± 11.1 g). Pups remained with their parents until weaning at 20 days of age. Litters were later separated into same-sex sibling groups at 45–55 days of age and used as subjects when 60 to 90 days old. Only one male and one female from each litter were typically used for each component of the experiment, but in a few cases two voles of the same sex from the same litter were used.
Masculine Sexual Behavior Testing
Voles of both sexes were anesthetized with a cocktail of ketamine (62.5 mg/kg), xylazine (7.5 mg/kg), and acepromazine (0.8 mg/kg). Males were castrated via a midline incision in the scrotum, and females ovariectomized via a single midline ventral incision. Males and females tested for masculine sexual behavior were implanted subcutaneously with a 1.3-cm long Silastic capsule (1.57 mm inner diameter, 2.41 cm outer diameter, 3 mm effective release length) containing crystalline testosterone propionate (TP; Sigma, St. Louis, MO). A similarly sized capsule containing TP has previously been shown to produce physiological levels of testosterone in male prairie voles (Cushing et al., 2004). Two weeks after gonadectomy, males (9 oil-treated, 10 ATD-treated) and females (10 oil-treated, 8 ATD-treated) were tested with a randomly selected sexually receptive female prairie vole between 1200 and 1700 hr under dim ambient light (20 lux). Previously unmanipulated females from our colony were used as stimuli; they were ovariectomized, allowed to recover from surgery for 2 weeks, and injected subcutaneously with estradiol benzoate (10 µg in 50 µl sesame oil; Sigma, St. Louis, MO) 24 and 48 hours before testing to induce receptivity (Carter et al., 1987). Just before testing, receptivity of stimulus females was confirmed by placing them in a clean cage with a sexually experienced male from the colony for 5 min, and only females displaying lordosis were used for testing (~65% of primed females).
Subjects were placed in a clean cage containing bedding, food, and water, and were allowed to habituate to the cage and testing room for 15 min. The stimulus female was then placed in the cage and behavior recorded for 30 min using a Panasonic low-light-sensitive camera connected to a Panasonic VCR. If the subject did not mount the stimulus female in 30 min, the stimulus female was removed from the cage and a different receptive female was introduced. This was done because we believed it possible that subjects’ lack of response could be due to a particular stimulus female. If the subject failed to mount the second female within 15 min, however, the test was terminated and only the first test was scored. If the subject did mount the second female within 15 min (as occurred for one oil-treated male, one ATD-treated male, two oil-treated females, and three ATD-treated females), then the second test was continued until 30 min had elapsed, and only this test was scored. Therefore, all subjects had 30 minutes of testing scored. Sexual, affiliative, aggressive, and non-social behaviors (feeding, drinking, and exploring) displayed by subjects and stimulus females were scored using custom-made data acquisition software.
Feminine Sexual Behavior Testing
Males (7 oil-treated, 8 ATD-treated) and females (8 oil-treated, 8 ATD-treated) not previously tested for masculine sexual behaviors were gonadectomized as described above. Two weeks after gonadectomy, subjects were tested with a sexually experienced stud male for feminine sexual behaviors. Twenty four and 48 hours prior to the test, subjects were subcutaneously injected with estradiol benzoate (10 µg in 50 µl sesame oil; Sigma, St. Louis, MO). On the day of testing, subjects were placed into a clean cage containing bedding, food, and water, and were allowed to habituate to the cage and testing room for 15 min. A gonadally intact and sexually experienced stud male from our colony was then placed into the cage. The test continued for 30 min, and was recorded as detailed above. If the stud male failed to mount the subject during the test, the stud male was removed and a second stud male was placed in the cage. If the second stud male failed to mount within 15 min, the test was terminated and only the first test was scored. If the second stud male did mount the subject within 15 min, which only occurred for one oil-treated female, the test was continued until 30 min had elapsed, and only this second test was scored. Therefore, all subjects had 30 minutes of testing scored. Sexual, affiliative, aggressive, and non-social behaviors displayed by subjects and stud males were scored as detailed above. We did not measure lordosis intensity, as it is difficult to reliably measure in prairie voles, but there were no noticeable differences in intensity between subjects that displayed lordosis.
Brain Collection and Tissue Processing
Three weeks after gonadectomy, brains were harvested from virgin female voles treated for the first week of life with sesame oil, and from virgin males treated for the first week of life with oil or ATD as described above. These subjects were not treated with hormones or behaviorally tested during adulthood. Voles were gonadectomized before sacrifice because this is necessary to see sex differences in TH expression in the AVPV of prairie voles (Lansing and Lonstein, 2006), and because sex differences in ERα expression are still seen in laboratory rats and prairie voles after adult gonadectomy (Kuhnemann et al., 1995; Northcutt and Lonstein, unpublished data). Voles were overdosed with anesthetic and perfused through the heart with 100 ml of 0.9% saline followed by 100 ml of 4% paraformaldehyde in 0.1 M sodium phosphate buffer. Brains were then removed and postfixed overnight in 4% paraformaldehyde. After fixation, brains were placed in a 20% sucrose solution for at least two days, sectioned into 40 µm coronal sections on a freezing microtome, and stored in cryoprotectant. One series of every third section through the brain was processed immunocytochemically for tyrosine hydroxylase (TH), and a second series of sections was processed immunocytochemically for estrogen receptor alpha (ERα).
TH and ERα immunocytochemistry
The procedures followed for TH immunocytochemistry were identical to those previously described (Lansing and Lonstein, 2006). Brains from 9 oil-treated males, 11 ATD-treated males, and 13 oil-treated females were processed. Sections were rinsed three times in Tris-buffered saline (TBS; pH 7.6) between each step. First, sections were incubated in 0.1% sodium borohydride for 15 min, incubated in 1% hydrogen peroxide and 0.3% Triton X-100 in TBS for 10 min, blocked in 20% normal goat serum and 0.3% Triton X-100 in TBS for 30 min, and incubated in rabbit anti-TH primary antiserum (1:2000; AB152; Chemicon, Temecula, CA) in 2% normal goat serum and 0.3% Triton X-100 in TBS at room temperature for 18 hours. Sections were then incubated in a biotinylated goat anti-rabbit secondary antiserum (1:500; Vector Laboratories, Burlingame, CA) in 2% normal goat serum and 0.3% Triton X-100 in TBS for 1 hour, followed by a 1 hour incubation in avidin-biotin complex (Vectastain Elite, Vector Laboratories, Burlingame, CA). TH-containing cells were visualized with the chromagen Vector SG (Vector Laboratories, Burlingame, CA). Sections were mounted onto slides, dehydrated, and coverslipped. Immunocytochemical control procedures included omission of the primary or secondary antiserum, which abolished any specific labeling. Multiple immunocytochemical runs were performed, with a similar number of subjects from each group included in each run.
The ERα immunocytochemistry protocol was similar to that described by Cushing et al. (2004). Brains from 8 oil-treated males, 10 ATD-treated males, and 11 oil-treated females were processed in a single immunocytochemical run. Sections were rinsed in 0.05 M potassium phosphate buffered saline (KPBS; pH 7.6) between all steps. First, sections were incubated in 1% sodium borohydride in KPBS for 20 min, incubated in 0.5% hydrogen peroxide in KPBS for 10 min, and incubated in rabbit anti-ERα antiserum (1:15,000; C1355; Upstate, Lake Placid, NY) and 0.4% Triton X-100 in KPBS for 1 hour at room temperature and 40 hours at 4°C. The C1355 antibody binds both unbound and bound ERα (Greco et al., 2001). Then, sections were incubated in goat anti-rabbit antiserum (1:600; Vector Laboratories, Burlingame, CA) and 0.4% Triton X-100 in KPBS for 1 hour, followed by a 1 hour incubation in avidin-biotin complex (Vectastain Elite, Vector Laboratories, Burlingame, CA) and 0.4% Triton X-100 in KPBS. After rinses in KPBS, sections were rinsed in 0.175 M sodium acetate (pH 6.5), and ERα-immunoreactive cells were visualized with nickel-intensified diaminobenzidine (Sigma, St. Louis, MO) in sodium acetate. Sections were then mounted onto slides, dehydrated, and coverslipped. Immunocytochemical control procedures included omission of the primary or secondary antiserum, which abolished any specific labeling.
Analysis of TH- and ERα-immunoreactive cells
Slides were first coded for analysis and all analyses were performed by one person (KVN). Cells in the anteroventral periventricular preoptic area (AVPV) and zona incerta (ZI) containing detectable TH-immunoreactivity were counted using a Nikon E400 microscope at 200X magnification with the aid of a reticle in one ocular lens. As previously described (Lansing and Lonstein, 2006), periventricular cells were counted from the first two sections where the anterior commissure decussates, which corresponds to plate 19 from Swanson’s rat brain atlas (Swanson, 1998). TH-immunoreactive cells from the first two rostral sections of the ZI (approximately plate 28 from Swanson’s rat brain atlas) were also analyzed and used as a control region because neither perinatal nor adult gonadal hormones influence the number of TH-immunoreactive cells in this region in prairie voles (Lansing and Lonstein, 2006).
We analyzed ERα immunoreactivity, using a densitometry method, from two sections of the anterior region of the medial preoptic area (mPOA; approximately plate 20 of Swanson’s rat brain atlas), from one section of the principal nucleus of the bed nucleus of the stria terminalis (BST; approximately plate 22 from Swanson’s atlas), and from four sections of the posterodorsal medial amygdala (MeA; Swanson’s plates 28–29) at 100X magnification on an Olympus BX60 microscope. NIH Scion Image was used to determine the optical density of the background staining, a threshold was set at two standard deviations above the mean background staining, and the number of pixels in each site with an optical density above this threshold was determined bilaterally for each section. We used this conservative densitometry method because we found a sex difference in ERα immunoreactivity using this method, but did not find any sex differences when we initially analyzed these subjects by quantifying the total number of cells containing any detectable ERα immunoreactivity (Northcutt and Lonstein, unpublished data). This indicated that the sexes did not differ in the number of cells containing any ERα immunoreactivity, but did differ in the amount of immunoreactivity per cell. Two subjects (an oil-treated male and an oil-treated female) were excluded from the analyses of ERα immunoreactivity in the MeA because tissue containing the MeA was lost during immunocytochemical processing.
Statistical Analysis
Non-responders were given scores of “zero” for behavioral frequencies, and excluded from measures of latency. If a second behavioral test was used for analysis, the latencies were calculated from the beginning of the second test. Behavioral data were analyzed with two-way ANOVAs (sex and neonatal treatment as factors) for each behavioral measure. The G-test of independence was used to compare proportions of subjects responding behaviorally. Males did not engage in many of the behaviors examined during the feminine sexual behavior tests, so the effects of ATD treatment on females were analyzed with unpaired t-tests. Finally, one-way ANOVAs were used to analyze immunocytochemical results. When ANOVAs revealed significant differences between groups, Fisher’s LSD post-hoc tests were used to compare individual groups. In all cases, statistical significance was indicated by p ≤ 0.05.
Results
Sex differences in masculine copulatory behaviors and effects of neonatal ATD
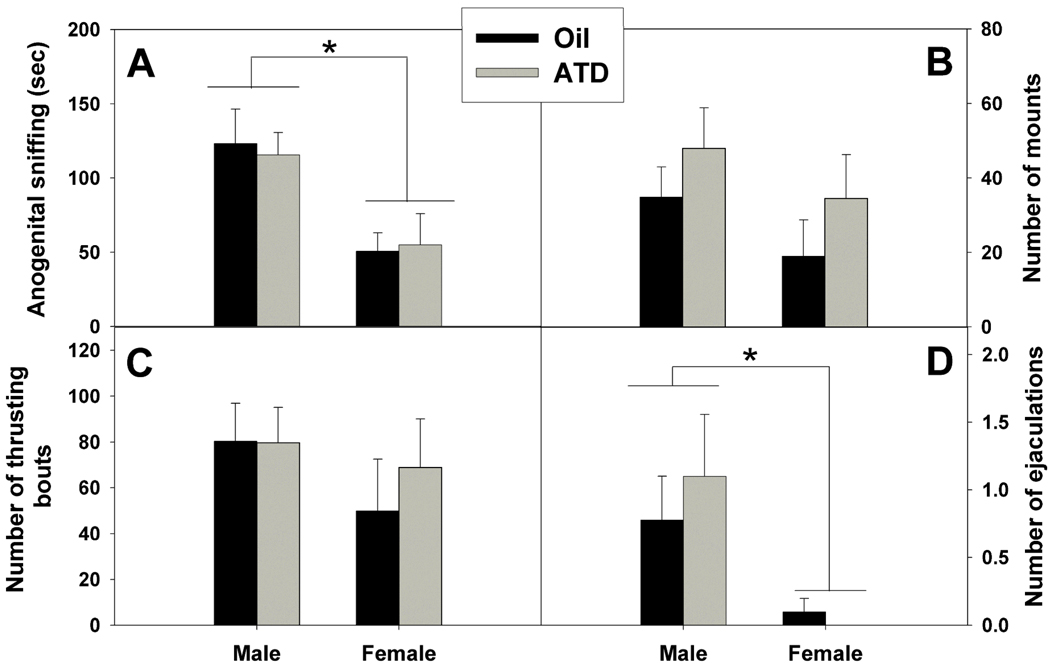
Both male and female prairie voles displayed very high levels of masculine copulatory behaviors, although several sex differences were observed (Table 1). Male subjects spent more time than female subjects sniffing the anogenital region of stimulus females (Fig. 1A; F(1,33) = 13.75, p < 0.001). The frequency of mounts and thrusting bouts did not differ between males and females (Fig. 1B and C; mounts: F(1,33) = 2.06, p = 0.16; thrusts: F(1,33) = 1.15, p = 0.29), but a greater proportion of males than females mounted and thrusted at least once (males vs. females, collapsed across neonatal treatment group; mounting: G = 6.28, df = 1, p = 0.012, thrusting: G = 9.89, df = 1, p = 0.002). Males also showed thrusting bouts of longer duration than did females (F(1,33) = 11.43, p = 0.002). Finally, males showed the stereotypical ejaculation posture more frequently than did females (Fig. 1D; F(1,33) = 8.74, p = 0.006), and a greater proportion of males displayed ejaculatory postures, given that only one female showed this behavior (males vs. females, collapsed across neonatal treatment group: G = 8.33, df = 1, p = 0.004).
Table 1.
Sexual and non-sexual behaviors displayed by male and female prairie voles treated for the first week after birth with oil or the aromatase inhibitor ATD, and tested as adults with sexually receptive females.
| Oil Male | ATD Male | Oil Female | ATD Female | |
|---|---|---|---|---|
| Latency (sec) | ||||
| Contact stimulus female |
10 ± 4 | 9 ± 4 | 11 ± 4 | 7 ± 3 |
| Mount | 249 ± 114 | 179 ± 70 | 181 ± 65 | 131 ± 31 |
| Thrust | 308 ± 127 | 195 ± 69 | 213 ± 87 | 148 ± 38 |
| Ejaculate | 580 ± 202 | 396 ± 107 | 845 ± 0 | - |
| Percent Responding | ||||
| Mounting | 100% (9/9)a | 100% (10/10)a | 80% (8/10)b | 75% (6/8)b |
| Thrusting | 100% (9/9)a | 100% (10/10)a | 60% (6/10)b | 75% (6/8)b |
| Ejaculating | 44% (4/9)a | 40% (4/10)a | 10% (1/10)b | 0% (0/10)b |
| Frequency | ||||
| Mounts | 35 ± 8 | 48 ± 11 | 24 ± 12 | 46 ± 12 |
| Thrusting bouts | 80 ± 16 | 80 ± 15 | 50 ± 23 | 60 ± 21 |
| Ejaculations | 0.8 ± 0.3a | 1.1 ± 0.5a | 0.1 ± 0.1b | 0 ± 0b |
| Duration (sec) | ||||
| Mounting | 223 ± 39 | 235 ± 34 | 214 ± 90 | 281 ± 62 |
| Thrusting | 69 ± 14 | 72 ± 16 | 38 ± 18 | 49 ± 14 |
| Average thrusting bout | 0.8 ± 0.06a | 0.8 ± 0.08a | 0.4 ± 0.1b | 0.6 ± 0.1b |
| Anogenital sniffing | 123 ± 23a | 116 ± 15a | 44 ± 8b | 73 ± 23b |
| Non-anogenital sniffing | 262 ± 30 | 257 ± 24 | 293 ± 52 | 334 ± 100 |
| Grooming female | 42 ± 13 | 37 ± 19 | 20 ± 6 | 22 ± 13 |
| Grooming self | 230 ± 60 | 271 ± 47 | 164 ± 37 | 162 ± 25 |
| Attacking female | 0.9 ± 0.4a | 0.8 ± 0.5a | 17.6 ± 8b | 5.8 ± 5b |
| General activity | 212 ± 71 | 346 ± 73 | 215 ± 40 | 247 ± 44 |
| Inactive with female | 57 ± 27 | 28 ± 9 | 40 ± 22 | 40 ± 20 |
| Inactive alone | 179 ± 79a | 68 ± 17a | 284 ± 78b | 293 ± 104b |
Values are expressed as Mean ± SEM. Different letter superscripts indicate significant group differences (p ≤ 0.05).
Figure 1.
The A) duration of sniffing of the anogenital region of the stimulus female, B) number of mounts, C) number of thrusting bouts, and D) number of ejaculations (all Mean ± SEM) subjects displayed in the masculine sexual behavior test. * = Significant sex difference (p ≤ 0.05).
Sex differences were also found in two non-copulatory behaviors. Male subjects spent less time than female subjects attacking the stimulus female (F(1,33) = 5.38, p = 0.03), and spent less time alone and inactive (F(1,33) = 5.11, p = 0.03). The duration of other behaviors scored, including general activity, inactivity while in contact with the stimulus female, grooming, and non-anogenital sniffing did not differ between males and females (Table 1).
Unexpectedly, there were no significant effects of neonatal ATD treatment on any behavior displayed by either sex during the masculine sexual behavior tests (Table 1).
Sex differences in feminine copulatory behaviors and effects of ATD
Male subjects, both oil and ATD-treated, were never mounted by stud males. Conversely, 15 out of 16 female subjects were mounted (Table 2; males vs. females, collapsed across neonatal treatment: G = 35.46, df = 1, p < 0.001). Consistent with this, male subjects spent considerably more time attacking the stud male than did female subjects (F(1,27) = 17.04, p < 0.001). Male subjects were also attacked by the stud male more often than were females (F(1,27) = 4.40, p = 0.05), but this sex difference was qualified by a significant interaction (F(1,27) = 4.32, p = 0.05), with ATD-treated males attacked for as little time as females. While subjects did not differ in the amount of time they spent investigating the anogenital region of the stud male (F(1,27) = 1.02, p = 0.3), stud males spent more time investigating the anogenital region of females than males (F(1,27) = 15.69, p < 0.001). Finally, male subjects spent less time grooming themselves and more time alone and inactive than did females (grooming: F(1,27) = 18.12, p < 0.001; inactive: F(1,27) = 15.63, p < 0.001).
Table 2.
Sexual and non-sexual behaviors displayed by male and female prairie voles treated for the first week after birth with oil or the aromatase inhibitor ATD, and tested as adults with sexually experienced stud males.
| Oil Male | ATD Male | Oil Female | ATD Female | |
|---|---|---|---|---|
| Latency (sec) | ||||
| Contact stud male | 22 ± 8 | 46 ± 15 | 16 ± 6 | 22 ± 9 |
| Receive anogenital sniffs | 39 ± 10 | 63 ± 16 | 55 ± 22 | 83 ± 22 |
| Receive mount | - | - | 73 ± 7a | 628 ± 205b |
| Lordose | - | - | 74 ± 7a | 629 ± 205b |
| Receive thrust | - | - | 93 ± 13a | 540 ± 208b |
| Receive ejaculation | - | - | 380 ± 77 | 330 ± 112 |
| Percentage of subjects | ||||
| Receiving mounts | 0% (0/7)a | 0% (0/8)a | 88% (7/8)b | 100% (8/8)b |
| Receiving thrusts | - | - | 88% (7/8) | 88% (7/8) |
| Receiving ejaculations | - | - | 63% (5/8) | 25% (2/8) |
| Frequency | ||||
| Mounts received | 0a | 0a | 48 ± 15b | 18 ± 6b |
| Lordoses | - | - | 47 ± 15 | 18 ± 6 |
| Lordosis Quotient | - | - | 0.97 ± 0.01a | 1.00 ± 0b |
| Thrusting bouts received | - | - | 127 ± 32a | 44 ± 14b |
| Ejaculations received | - | - | 1.6 ± 0.5 | 0.5 ± 0.3 |
| Duration (sec) | ||||
| Mounting received | - | - | 413 ± 98a | 138 ± 41b |
| Lordosis | - | - | 413 ± 101a | 142 ± 41b |
| Thrusting received | - | - | 118 ± 33a | 35 ± 11b |
| Anogenital sniffing received from stud male |
6 ± 2a | 18 ± 6a | 40 ± 12b | 54 ± 14b |
| Anogenital sniffing of stud male |
8.2 ± 4 | 14 ± 3 | 13 ± 5 | 22 ± 12 |
| Non-anogenital sniffing of stud male |
264 ± 65 | 304 ± 84 | 182 ± 47 | 249 ± 42 |
| Grooming stud male | 8 ± 3 | 18 ± 12 | 21 ± 19 | 45 ± 37 |
| Grooming self | 88 ± 14a | 150 ± 85a | 366 ± 42b | 334 ± 42b |
| Attacking stud male | 71 ± 18a | 67 ± 21a | 6.7 ± 3b | 17 ± 5b |
| Attacking received | 9.3 ± 3a | 2.0 ± 1b | 2.4 ± 1b | 2.0 ± 0.5b |
| General activity | 175 ± 29 | 131 ± 15 | 114 ± 38 | 167 ± 46 |
| Inactive with stud male | 53 ± 42 | 100 ± 56 | 43 ± 12 | 39 ± 16 |
| Inactive alone | 649 ± 167a | 449 ± 120a | 150 ± 40b | 180 ± 36b |
Values are expressed as Mean ± SEM. Different letter superscripts indicate significant group differences (p ≤ 0.05).
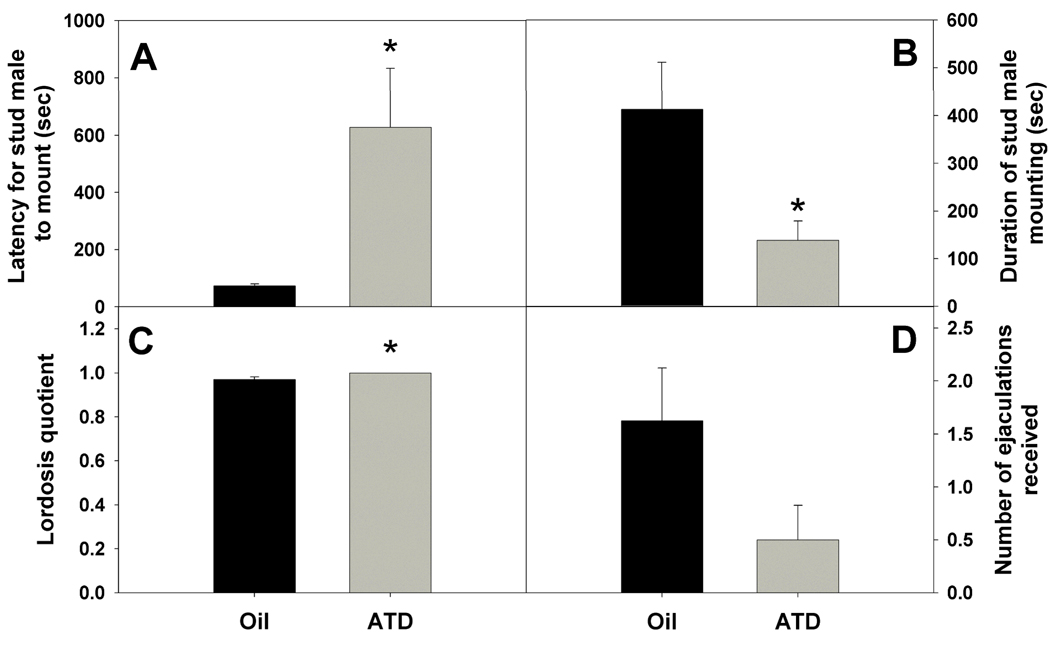
Other than the duration of time being attacked noted above, there was no effect of neonatal ATD on males’ behavior. However, neonatal ATD did affect sexual behavior of females. Stud males mounted ATD-treated females with a longer latency after the beginning of testing than they mounted oil-treated females (Fig. 2A; t(13) = 2.52, p = 0.03). Stud males also spent less time mounting and thrusting with ATD-treated females than they did with oil-treated females (Fig. 2B; mounting: t(14) = 2.58; p = 0.02; thrusting: t(14) = 2.36, p = 0.03). Nonetheless, both groups of females were highly receptive, and ATD-treated females had slightly but significantly higher lordosis quotients than did oil-treated females (Fig. 2C; t(13) = 2.74, p = 0.02). Finally, there was a trend for ATD-treated females to receive fewer ejaculations from stud males than did oil-treated females (Fig. 2D; t(14) = 1.89, p = 0.08).
Figure 2.
The A) latency for stud males to mount, B) duration of stud male mounting, C) lordosis quotients, and D) number of stud male ejaculations received (all Mean ± SEM) for female subjects in the feminine sexual behavior test. * = Significant effect of neonatal ATD treatment (p ≤ 0.05). ATD also tended to reduce the number of ejaculations that females received (D; p = 0.08).
Sex differences and ATD effects on TH-expressing cells
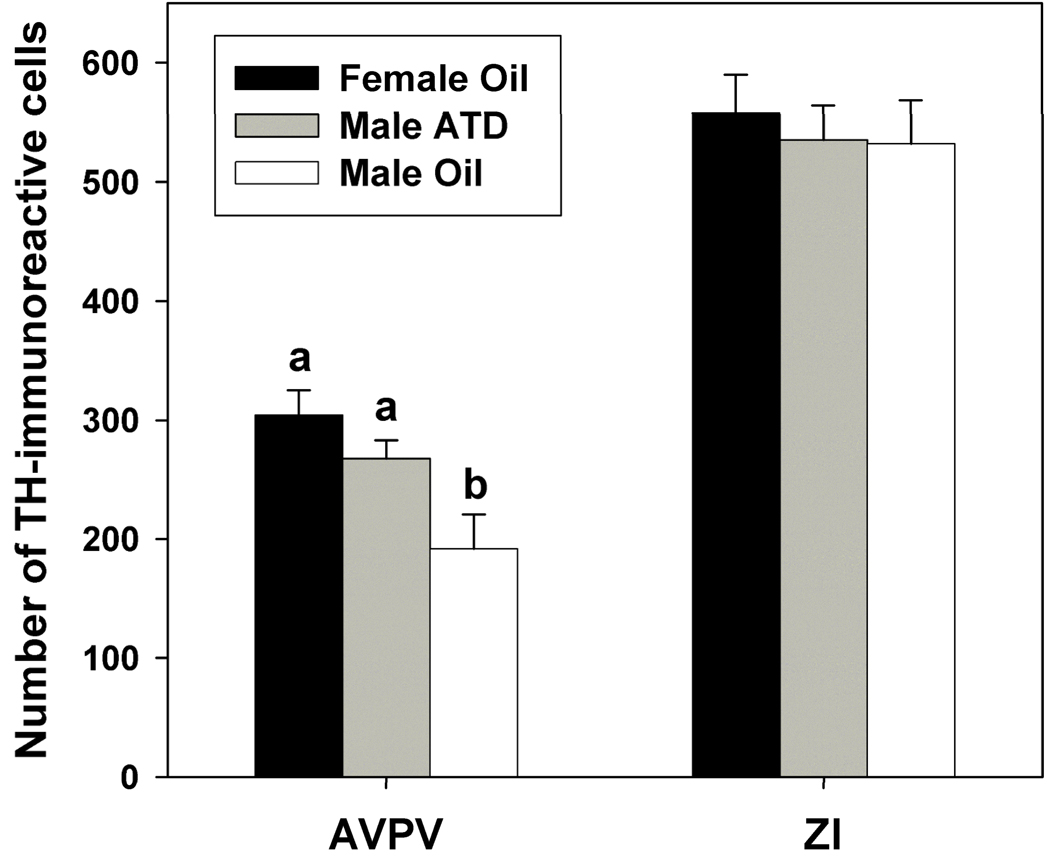
The number of cells in the AVPV containing TH immunoreactivity significant differed between oil-treated females, ATD-treated males, and oil-treated males (Fig. 3 and Fig. 4; F(2,28) = 6.48, p = 0.005). Post-hoc analysis revealed a significant sex difference (p = 0.001), such that females had more TH-immunoreactive cells in the AVPV than oil-treated males, as previously reported (Lansing and Lonstein, 2006). This sex difference was eliminated by neonatal ATD, which increased the number of cells containing TH immunoreactivity in males to the point where they did not significantly differ from females (p = 0.21), but had more TH-immunoreactive cells than oil-treated males (p = 0.025).
Figure 3.
The number of TH-immunoreactive cells (Mean ± SEM) in the AVPV and ZI of female prairie voles treated neonatally with oil, and males treated neonatally with oil or ATD. Different letters above bars indicate significant group differences (p ≤ 0.05).
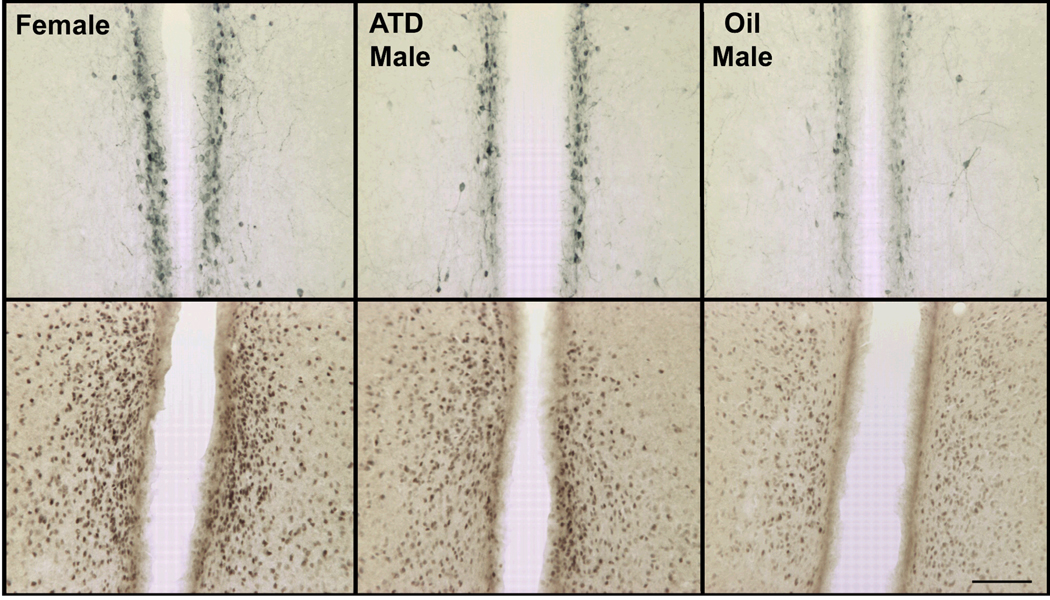
Figure 4.
Photomicrographs of TH-immunoreactive cells in the AVPV (top row) and ERα-immunoreactive cells in the mPOA (bottom row) of representative female prairie voles treated neonatally with oil, and males treated neonatally with ATD or oil. Scale bar = 50 µm.
We did not find a sex difference or effect of neonatal ATD treatment on the number of TH-immunoreactive cells in the zona incerta (overall ANOVA: F(2,28) = 0.67, p = 0.52), indicating that sex differences and ATD effects are not found in every TH-expressing population of cells in the forebrain.
Sex differences and ATD effects on ERα -expressing cells
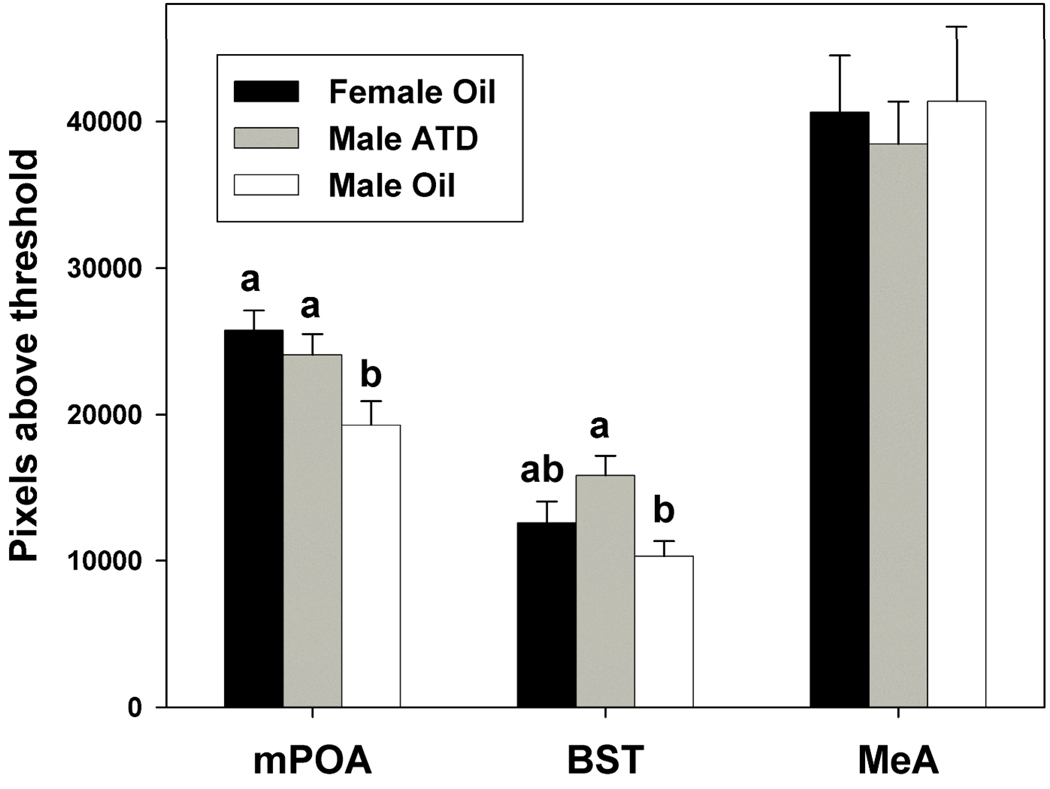
Groups differed in ERα immunoreactivity in the mPOA (Fig. 4 and Fig. 5; F(2,26) = 4.88, p = 0.02) and BST (Fig. 5; F(2,26) = 3.98, p = 0.03), but not the MeA (F(2,24) = 0.15, p = 0.86). In the mPOA, oil-treated males had less ERα immunoreactivity than females (p = 0.005), and this sex difference was eliminated by treating males with ATD (ATD-treated males compared to females: p = 0.411; ATD-treated males compared to oil-treated males: p = 0.034). There was no significant sex difference in the BST (p = 0.25), but ATD-treated males did have significantly more ERα immunoreactivity than oil-treated males (p = 0.01) and did not differ from females (p = 0.09).
Figure 5.
Optical density of ERα-immunoreactivity (Mean ± SEM) two standard deviations above background staining in the mPOA, BST, and MeA of gonadectomized adult prairie voles treated neonatally with oil (males and females) or ATD (males). Different letters above bars indicate significant group differences (p ≤ 0.05).
Discussion
Sex differences in masculine copulatory behaviors
We found few sex differences in masculine sexual behaviors after males and females were gonadectomized and given the same TP treatment in adulthood. These results are consistent with data from other rodents where females remain at least partially masculinized and respond as such when given testosterone in adulthood (Edwards and Burge, 1971; Phoenix et al., 1959; Roberts et al., 1997; Whalen and Olsen, 1981). However, female prairie voles seem particularly masculinized. Female rats and guinea pigs mount receptive female conspecifics after gonadectomy and testosterone treatment in adulthood, but often not as avidly as do males (Dominguez-Salazar et al., 2002; Gladue and Clemens, 1980; Phoenix et al., 1959). Female mice, on the other hand, mount receptive females quite vigorously, but do not readily show intromission-like behaviors (Edwards and Burge, 1971; Wersinger et al., 1997). In contrast to these other rodents, our data demonstrate that many female prairie voles mount and thrust at frequencies equal to males. It might be that many female prairie voles remain highly masculinized because they are perinatally exposed to greater levels of gonadal or other hormones. We know that testosterone is higher on the first few days after birth in male prairie vole pups compared to female prairie vole pups (Lansing, French, and Lonstein, unpublished data), and variability in these levels may contribute to later variability in females’ propensity to display masculine sexual behaviors.
In many rodent species, although not all, aromatization of testosterone to estradiol during neonatal life is at least partly responsible for masculinizing copulatory behaviors in males (Bakker et al., 1993b; Brand et al., 1991; Houtsmuller et al., 1994; Swaab et al., 1995). In a previous study of the effects of neonatal hormones on sexual behaviors in prairie voles, Roberts et al. (1997) found that neonatal castration did not reduce the percentage of males that later mount receptive females. Details of males’ sexual and non-sexual behaviors were not provided, and we believed it possible that altering aromatase activity could affect the details of males’ later copulatory behaviors. Nonetheless, there were no significant effects of ATD on any masculine copulatory behavior measured. One could suggest that this is because our daily ATD injections may not have reliably inhibited aromatase. We have found that implanting silastic capsules of ATD is unfeasible in prairie voles, as dams remove the implants or kill implanted pups. This dose of ATD did significantly affect other aspects of behavior and in the brain, though, suggesting that our treatment was sufficient to produce a biological effect. It is of note that ATD is a steroidal aromatase inhibitor that has been reported to be anti-androgenic at very high doses (Kaplan and McGinnis, 1989). Because even at high doses ATD is a relatively weak competitor for androgen receptors in androgen-sensitive tissues (Summerfield et al., 1995), and had no effects on masculine copulatory behaviors in our study, we do not believe that this is of particular concern for interpreting our behavioral results.
We predicted that the period for masculinization of copulation in prairie voles would extend to neonatal life as it does in rats, given their similar gestation period (around 22 days). Instead, it may be possible that this process occurs prenatally in prairie voles. Perinatal gonadal hormone levels in prairie vole fetuses are unknown, but a prenatal surge in testosterone may masculinize sexual behavior in males. It is also possible that non-aromatized metabolites of testosterone contribute to masculinization in prairie voles, as they do in ferrets and monkeys (reviewed in Wallen and Baum, 2002). Lastly, the process of masculinization in prairie voles may be more similar to mice than it is to rats, given that mice do not seem to require either aromatase or androgen receptors for their behavioral masculinization (Bakker et al., 2004; Bodo and Rissman, 2007; Sato et al., 2004, but see Olsen, 1992).
Defeminization and feminization of prairie vole copulatory behavior
Many studies of the effects of neonatal ATD on sexual behaviors demonstrate that aromatase inhibition greatly increases later receptivity and proceptivity in male rats, and also prevents defeminization of female rat pups given testosterone (Bakker et al., 1993a, 1993b, 1996; Brand et al., 1991; Fadem and Barfield, 1981; McEwen et al., 1977). Furthermore, neonatal activation of ERs, specifically ERβ, has been shown to be responsible for defeminization in mice (Kudwa et al., 2005). However, our negative results and those of Peterson (1986) indicate that neonatal aromatization may not be necessary for defeminization of sexual behaviors in any Microtus species. Again, it is possible that defeminization occurs prenatally. If this is the case, prenatal testosterone may act on androgen receptors to cause defeminization. Gladue and Clemens (1978, 1982) reported that prenatal administration of androgen receptor antagonists increases lordosis quotients in male and female rats, suggesting a role for non-aromatized androgens in defeminization in rats. However, sexual receptivity is readily defeminized in female prairie voles by neonatal, but not prenatal, testosterone (Roberts et al., 1997; Smale et al., 1985). It may be that male and female prairie voles are sensitive to the defeminizing effects of gonadal hormones at different time points during development.
An unexpected finding was that ATD seemed to reduce females’ attractiveness to males, suggesting that feminization requires neonatal exposure to estrogens. These results are consistent with the suggestion that perinatal exposure to estrogens is necessary for female-typical brains and reproductive behaviors (Bakker et al., 2002; Fitch and Denenberg, 1998; Gerall et al., 1973). It is possible that neonatal ATD altered females’ behavior to make them less attractive to males, but similar to Gray and Dewsbury (1973), we did not detect any proceptive behaviors that could have been affected. An alternate explanation is that ATD-treated females rejected the stud male more often than did oil-treated females. This was not the case, as attacks toward the male (the primary method of rejection that female prairie voles use) did not differ between ATD-treated and control females. Perhaps subtle changes in the behavior of ATD-treated females, or their sensory emissions, are responsible for decreased interest by males. It would be interesting to investigate whether males spend less time investigating soiled bedding of ATD-treated females compared to that of oil-treated females, which would indicate that the sensory emissions of ATD-treated females are less attractive. It would also be valuable to know whether ATD affects neural system that could decrease females’ attractiveness. Unfortunately, ATD’s effects on female sexual behavior were unexpected, so we did not collect these brains to analyze TH and ERα expression. We hope to investigate these possibilities in future studies.
Role of aromatase in TH and ERα expression
Neonatal ATD did not greatly affect sexual behavior potentials, but did affect sex differences in neural TH and ERα expression. ATD increased the number of TH-immunoreactive neurons in males such that a sex difference no longer existed. These data are similar to those from other rodents, which suggest that neonatal ER activation is necessary for sex differences in periventricular TH expression (Bodo et al., 2006; Simerly et al., 1997). Furthermore, they complement previous data from our lab showing that neonatal estradiol masculinizes TH expression in female prairie voles (Lansing and Lonstein, 2006). The sex difference in ERα expression in the mPOA was also eliminated by treating males neonatally with ATD. This seemingly conflicts with a report that neonatal castration does not significantly increase ERα immunoreactivity in the prairie vole mPOA (Cushing and Kramer, 2005), and could indicate that the source of relevant testosterone is not from the testes. Our results are consistent with data from rats demonstrating that sex differences in ERα expression in the mPOA can be eliminated by neonatal castration (DonCarlos et al., 1995; Kuhnemann et al., 1995; Orikasa et al., 1996). Interestingly, sexual differentiation of this system may proceed via a different mechanism in mice, as aromatase knock-out males have fewer ERα-immunoreactive cells in the mPOA than wild-type males (Kudwa et al., 2007).
Given previous reports (Cushing et al., 2004; Hnatczuk et al., 1994; Kramer et al., 2006; Yamamoto et al., 2006), we were surprised to find no sex difference in ERα immunoreactivity in the BST or MeA. Notable differences between our study and those reporting sex differences in these two regions are that we examined gonadectomized voles, while others measured ERα expression in gonadally intact voles, and we measured the optical density of ERα immunoreactivity, while others quantified the number of cells containing ERα immunoreactivity. These methodological differences do not explain the discrepancies, because we recently found no difference in ERα immunoreactivity in the mPOA, BST or MeA between gonadally intact voles and those gonadectomized during adulthood, and failed to find a sex difference in any of these sites when we quantified the number of ERα-immunoreactive cells (Northcutt and Lonstein, unpublished data). There is no sex difference in ERα expression in the mPOA, BST, or MeA of prairie voles obtained from Kansas (Cushing et al., 2004; Kramer et al., 2006), so there may be variability in prairie voles from the same, as well as from different, geographic origins.
Conclusions
Our results indicate that prairie voles are sexually dimorphic in their copulatory behavior potentials, although most females retain the capacity to display masculine behaviors. Although previous studies suggested that perhaps aromatase was unnecessary for sexual differentiation of neural and behavioral systems in Microtus (Lonstein et al., 2002, 2005; Petersen, 1986), our current results are evidence that aromatization of testosterone to estradiol is, indeed, necessary for their normal development. Even so, there were notable differences in the effects of neonatal aromatase inhibition on sexual behaviors when compared to the effects seen in other rodents. These results further highlight the diversity of mechanisms involved in sexual differentiation of the mammalian brain and behavior.
Acknowledgements
We would like to thank Dr. Juli Wade and Mr. Rayson Figueira for assistance with this work, and Dr. Bruce Cushing for his assistance with the immunocytochemical protocol for ERα. This research was funded by National Science Foundation grant #0515070 to JSL and a Graduate Research Fellowship from the National Science Foundation to KVN.
References
- Bakker J, Brand T, van Ophemert J, Slob AK. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav Neurosci. 1993a;107(3):480–487. doi: 10.1037//0735-7044.107.3.480. [DOI] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22(20):9104–9112. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46(1):1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Bakker J, van Ophemert J, Slob AK. Organization of partner preference and sexual behavior and its nocturnal rhythmicity in male rats. Behav Neurosci. 1993b;107(6):1049–1058. doi: 10.1037//0735-7044.107.6.1049. [DOI] [PubMed] [Google Scholar]
- Bakker J, Van Ophemert J, Slob AK. Sexual differentiation of odor and partner preference in the rat. Physiol Behav. 1996;60(2):489–494. doi: 10.1016/s0031-9384(96)80023-0. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147(1):415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25(7):2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Booth JE. Sexual behavior of male rats injected with anti-estrogen mer-25 during infancy. Physiol Behav. 1977;19:35–39. doi: 10.1016/0031-9384(77)90155-x. [DOI] [PubMed] [Google Scholar]
- Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm Behav. 1991;25(3):323–341. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19(2):303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Auksi T, Casten L. Estrogen and the induction of lordosis in female and male prairie voles (Microtus ochrogaster) Horm Behav. 1987;21(1):65–73. doi: 10.1016/0018-506x(87)90031-6. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Zumpe D, Michael RP. Estrogen in the medial preoptic area of male rats facilitates copulatory behavior. Horm Behav. 2000;38(2):86–93. doi: 10.1006/hbeh.2000.1602. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10(1):40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Kramer KM. Microtines: a model system for studying the evolution and regulation of social monogamy. Acta Theriol Sin. 2005;25:182–199. [Google Scholar]
- Cushing BS, Razzoli M, Murphy AZ, Epperson PM, Le WW, Hoffman GE. Intraspecific variation in estrogen receptor alpha and the expression of male sociosexual behavior in two populations of prairie voles. Brain Res. 2004;1016(2):247–254. doi: 10.1016/j.brainres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Simerly RB. Anatomy, development, and function of the sexually dimorphic neural circuits in the mammalian brain. In: Pfaff DW, editor. Hormones, Brain and behavior. NY: Academic Press; 2002. pp. 137–192. [Google Scholar]
- Dominguez-Salazar E, Portillo W, Baum MJ, Bakker J, Paredes RG. Effect of prenatal androgen receptor antagonist or aromatase inhibitor on sexual behavior, partner preference and neuronal Fos responses to estrous female odors in the rat accessory olfactory system. Physiol Behav. 2002;75(3):337–346. doi: 10.1016/s0031-9384(01)00674-6. [DOI] [PubMed] [Google Scholar]
- DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM. Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Dev Brain Res. 1995;84(2):253–260. doi: 10.1016/0165-3806(94)00179-4. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- Fadem BH, Barfield RJ. Neonatal hormonal influences on the development of proceptive and receptive feminine sexual behavior in rats. Horm Behav. 1981;15(3):282–288. doi: 10.1016/0018-506x(81)90017-9. [DOI] [PubMed] [Google Scholar]
- Fitch RH, Denenberg VH. A role for ovarian hormones in sexual differentiation of the brain. Behav Brain Sci. 1998;21(3):311–327. doi: 10.1017/s0140525x98001216. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Dunlap JL, Hendricks SE. Effect of ovarian secretions on female behavioral potentiality in the rat. J Comp Physiol Psychol. 1973;82(3):449–465. doi: 10.1037/h0034113. [DOI] [PubMed] [Google Scholar]
- Gladue BA, Clemens LG. Androgenic influences on feminine sexual behavior in male and female rats: defeminization blocked by prenatal antiandrogen treatment. Endocrinology. 1978;103(5):1702–1709. doi: 10.1210/endo-103-5-1702. [DOI] [PubMed] [Google Scholar]
- Gladue BA, Clemens LG. Flutamide inhibits testosterone-induced masculine sexual behavior in male and female rats. Endocrinology. 1980;106(6):1917–1922. doi: 10.1210/endo-106-6-1917. [DOI] [PubMed] [Google Scholar]
- Gladue BA, Clemens LG. Development of feminine sexual behavior in the rat: androgenic and temporal influences. Physiol Behav. 1982;29(2):263–267. doi: 10.1016/0031-9384(82)90013-0. [DOI] [PubMed] [Google Scholar]
- Gray GD, Dewsbury DA. A quantitative description of copulatory behavior in prairie voles (Microtus ochrogaster) Brain Behav Evol. 1973;8(6):426–452. [PubMed] [Google Scholar]
- Greco B, Allegretto EA, Tetel MJ, Blaustein JD. Coexpression of ER beta with ER alpha and progestin receptor proteins in the female rat forebrain: effects of estradiol treatment. Endocrinology. 2001;142(12):5172–5181. doi: 10.1210/endo.142.12.8560. [DOI] [PubMed] [Google Scholar]
- Hnatczuk OC, Lisciotto CA, DonCarlos LL, Carter CS, Morrell JI. Estrogen receptor immunoreactivity in specific brain areas of the prairie vole (Microtus ochrogaster) is altered by sexual receptivity and genetic sex. J Neuroendocrinol. 1994;6(1):89–100. doi: 10.1111/j.1365-2826.1994.tb00558.x. [DOI] [PubMed] [Google Scholar]
- Houtsmuller EJ, Brand T, de Jonge FH, Joosten RN, van de Poll NE, Slob AK. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol Behav. 1994;56(3):535–541. doi: 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Huddleston GG, Paisley JC, Clancy AN. Effects of estrogen in the male rat medial amygdala: infusion of an aromatase inhibitor lowers mating and bovine serum albumin-conjugated estradiol implants do not promote mating. Neuroendocrinology. 2006;83(2):106–116. doi: 10.1159/000094400. [DOI] [PubMed] [Google Scholar]
- Hull EM, Lorrain DS, Du J, Matuszewich L, Lumley LA, Putnam SK, Moses J. Hormone-neurotransmitter interactions in the control of sexual behavior. Behav Brain Res. 1999;105(1):105–116. doi: 10.1016/s0166-4328(99)00086-8. [DOI] [PubMed] [Google Scholar]
- Kaplan ME, McGinnis MY. Effects of ATD on male sexual behavior and androgen receptor binding: a reexamination of the aromatization hypothesis. Horm Behav. 1989;23(1):10–26. doi: 10.1016/0018-506x(89)90071-8. [DOI] [PubMed] [Google Scholar]
- Kramer KM, Carr MS, Schmidt JV, Cushing BS. Parental regulation of central patterns of estrogen receptor alpha. Neuroscience. 2006;142(1):165–173. doi: 10.1016/j.neuroscience.2006.05.069. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Bodo C, Gustafsson JA, Rissman EF. A previously uncharacterized role for estrogen receptor beta: defeminization of male brain and behavior. Proc Natl Acad Sci U S A. 2005;102(12):4608–4612. doi: 10.1073/pnas.0500752102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Effects of organisational oestradiol on adult immunoreactive oestrogen receptors (alpha and beta) in the male mouse brain. J Neuroendocrinol. 2007;19(10):767–772. doi: 10.1111/j.1365-2826.2007.01585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnemann S, Brown TJ, Hochberg RB, MacLusky NJ. Sexual differentiation of estrogen receptor concentrations in the rat brain: effects of neonatal testosterone exposure. Brain Res. 1995;691(1–2):229–234. doi: 10.1016/0006-8993(95)00640-c. [DOI] [PubMed] [Google Scholar]
- Lansing SW, Lonstein JS. Tyrosine hydroxylase-synthesizing cells in the hypothalamus of prairie voles (Microtus ochrogaster): sex differences in the anteroventral periventricular preoptic area and effects of adult gonadectomy or neonatal gonadal hormones. J Neurobiol. 2006;66(3):197–204. doi: 10.1002/neu.20212. [DOI] [PubMed] [Google Scholar]
- Levine S, Mullins R., Jr Estrogen Administered Neonatally Affects Adult Sexual Behavior in Male and Female Rats. Science. 1964;144:185–187. doi: 10.1126/science.144.3615.185. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Auger AP. Perinatal gonadal hormone influences on neurobehavioral development. In: Blumberg M, Freeman J, Robinson S, editors. Handbook of Behavioral and Comparative Neuroscience. Oxford University Press; 2008. [Google Scholar]
- Lonstein JS, De Vries GJ. Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster) Behav Brain Res. 2000;114(1–2):79–87. doi: 10.1016/s0166-4328(00)00192-3. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Parental responsiveness is feminized after neonatal castration in virgin male prairie voles, but is not masculinized by perinatal testosterone in virgin females. Horm Behav. 2002;41(1):80–87. doi: 10.1006/hbeh.2001.1740. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Rood BD, De Vries GJ. Unexpected effects of perinatal gonadal hormone manipulations on sexual differentiation of the extrahypothalamic arginine-vasopressin system in prairie voles. Endocrinology. 2005;146(3):1559–1567. doi: 10.1210/en.2004-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology. 1994;19(5–7):415–427. doi: 10.1016/0306-4530(94)90029-9. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9(3):249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Olsen K. Genetic influences on sexual behavior differentiation. In: Gerall AA, Moltz H, Ward IL, editors. Handbook of Behavioral Neurobiology, Sexual Differentiation. Vol. 11. New York: Plenum Press; 1992. pp. 1–40. [Google Scholar]
- Orikasa C, Mizuno K, Sakuma Y, Hayashi S. Exogenous estrogen acts differently on production of estrogen receptor in the preoptic area and the mediobasal hypothalamic nuclei in the newborn rat. Neurosci Res. 1996;25(3):247–254. doi: 10.1016/0168-0102(96)01050-4. [DOI] [PubMed] [Google Scholar]
- Parsons B, Rainbow TC, McEwen BS. Organizational effects of testosterone via aromatization on feminine reproductive behavior and neural progestin receptors in rat brain. Endocrinology. 1984;115(4):1412–1417. doi: 10.1210/endo-115-4-1412. [DOI] [PubMed] [Google Scholar]
- Petersen SL. Perinatal androgen manipulations do not affect feminine behavioral potentials in voles. Physiol Behav. 1986;36(3):527–531. doi: 10.1016/0031-9384(86)90326-4. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Zullo AS, Carter CS. Sexual differentiation in prairie voles: the effects of corticosterone and testosterone. Physiol Behav. 1997;62(6):1379–1383. doi: 10.1016/s0031-9384(97)00365-x. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101(6):1673–1678. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res. 1987;400(1):11–34. doi: 10.1016/0006-8993(87)90649-4. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985;330(1):55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS. Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci U S A. 1997;94(25):14077–14082. doi: 10.1073/pnas.94.25.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Nelson RJ, Zucker I. Neuroendocrine responsiveness to oestradiol and male urine in neonatally androgenized prairie voles (Microtus ochrogaster) J Reprod Fertil. 1985;74(2):491–496. doi: 10.1530/jrf.0.0740491. [DOI] [PubMed] [Google Scholar]
- Summerfield AE, Diaz Cruz PJ, Dolenga MP, Smith HE, Strader CD, Toney JH. Tissue-specific pharmacology of testosterone and 5 alpha-dihydrotestosterone analogues: characterization of a novel canine liver androgen-binding protein. Mol Pharmacol. 1995;47(5):1080–1088. [PubMed] [Google Scholar]
- Swaab DF, Slob AK, Houtsmuller EJ, Brand T, Zhou JN. Increased number of vasopressin neurons in the suprachiasmatic nucleus (SCN) of 'bisexual' adult male rats following perinatal treatment with the aromatase blocker ATD. Dev Brain Res. 1995;85(2):273–279. doi: 10.1016/0165-3806(94)00218-o. [DOI] [PubMed] [Google Scholar]
- Swanson L. Brain Maps: Structure of the Rat Brain. 2nd ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: Comparative aspects of steroid hormone action. In: Pfaff DW, editor. Hormones, Brain and behavior. NY: Academic Press; 2002. pp. 385–423. [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32(3):176–183. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Gladue BA, Olsen KL. Lordotic behavior in male rats: genetic and hormonal regulation of sexual differentiation. Horm Behav. 1986;20(1):73–82. doi: 10.1016/0018-506x(86)90030-9. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Olsen KL. Role of aromatization in sexual differentiation: effects of prenatal ATD treatment and neonatal castration. Horm Behav. 1981;15(2):107–122. doi: 10.1016/0018-506x(81)90022-2. [DOI] [PubMed] [Google Scholar]