Summary
Spontaneous mutations altering mouse coat colors have been a classic resource for discovery of numerous molecular pathways. Although often overlooked, the mouse iris is also densely pigmented and easily observed, thus representing a similarly powerful opportunity for studying pigment cell biology. Here, we present an analysis of iris phenotypes among sixteen mouse strains with mutations influencing melanosomes. Many of these strains exhibit biologically and medically relevant phenotypes, including pigment dispersion, a common feature of several human ocular diseases. Pigment dispersion was identified in several strains with mutant alleles known to influence melanosomes, including beige, light, and vitiligo. Pigment dispersion was also detected in the recently arising spontaneous coat color variant, nm2798. We have identified the nm2798 mutation as a missense mutation in the Dct gene, an identical re-occurrence of the slaty light mutation. These results suggest that dysregulated events of melanosomes can be potent contributors to the pigment dispersion phenotype. Combined, these findings illustrate the utility of studying iris phenotypes as a means of discovering new pathways, and re-linking old ones, to processes of pigmented cells in health and disease.
Keywords: Tyrp1, Dct, eye, iris, pigment dispersion, glaucoma
Introduction
The iris is densely pigmented and readily observed; and a growing number of studies have made use of this as an opportunity for studying pigment cell biology. The iris contains three populations of melanin containing cells: iris stromal melanocytes, iris pigment epithelial cells, and antigen presenting cells of the iris stroma. Of these, melanocytes and iris pigment epithelial cells both synthesize melanin, whereas the antigen presenting cells become pigmented via phagocytosis. As with melanocytes contributing to the skin and hair (Lin and Fisher, 2007), pigment producing cells of the iris utilize many of the same well known pathways to synthesize melanin within melanosomes. Contrasting these similarities, different populations of pigment producing cells of the iris also exhibit many unique properties. For example, cultured murine iris melanocytes do not respond to α-melanocyte-stimulating hormone, which is potently mitogenic and melanogenic to melanocytes cultured from skin (Li et al., 2006). Similarly, pigment producing cells of the iris also exhibit many other distinct properties (Hong et al., 2006, Hu, 2005, Li et al., 2006, Liu et al., 2005, Smith-Thomas et al., 2001, Ward and Simon, 2007, Wistow et al., 2002). Each of these presumably reflects an adaptation or consequence of the genetic and environmental factors impacting different populations of pigment producing cells. Thus, studies of the iris afford an opportunity to uncover new molecular pathways driving these differences, and represent a powerful opportunity to gain new insight into the events shaping pigment cell biology.
A priori, there are several reasons that iris pigmentation might be expected to be controlled by processes that are particularly interesting. The importance of iris pigmentation extends well beyond its immediate cosmetic influence. In health, the iris and iridial melanin play key roles influencing vision (Iwata et al., 2000, Summers, 1996, Wong et al., 2005). In disease, the iris is often afflicted. Several diseases change the pigmentation of the iris or otherwise involve pigment of the iris, including forms of oculocutaneous albinism, Hermansky-Pudlak syndrome, Horner’s syndrome Waardenburg syndrome, Fuch’s heterochromic iridocyclitis, and pigment dispersion. Despite this importance, much remains unknown concerning the basic molecular and cellular processes influencing pigmented cells of the iris.
Our approach for studying iris biology has been to capitalize on a phenotype-driven approach in mice. As a primary screening tool, we have performed ophthalmic slit-lamp exams. Here, we report a survey of iris phenotypes among three groups of mice with mutations that influence melanosomes: 1) a diverse group of different substrains with pigment-related mutations all maintained on the C57BL/6J genetic background, 2) a group of mutant strains genetically associated with Tyrp1 pathways, and 3) the spontaneously occurring nm2798 strain of mice. Examined by an ophthalmic slit-lamp, many of these strains exhibit interesting phenotypes, including variable degrees of iris hypopigmentation, transillumination defects, and pigment dispersion. Combined, these results illustrate the diversity of iris phenotypes that occur among laboratory mice and establish a baseline for future experiments that will continue to make use of mouse iris phenotypes as an opportunity for studying the biology of pigment producing cells.
Results
Because mouse strains with coat color variations harbor mutations that influence melanosomes, these mutations may also affect melanosomes of the iris and give rise to abnormal ocular phenotypes. To test this, we utilized an ophthalmic slit-lamp to examine cohorts of mice for ocular phenotypes. Our screen utilized two types of slit-lamp illumination, broad beam and transilluminating illumination. With broad beam illumination, a wide beam of light is shone at an angle across the surface of the eye, highlighting surface morphology. With transilluminating illumination, a small beam of light is shone directly through the pupil and the eye is assessed for light reflected off inner surfaces of the back of the eye. In healthy irides, reflected light is blocked by the iris and the iris appears black. In thin, depigmented, or diseased irides, reflected light passes through areas of the iris and appears as areas of red (some light passing though) or white light (greater amounts of light passing through). Using these techniques, we performed an analysis comparing the iris of wild-type mice to several mutant strains.
Iris phenotypes of wild-type C57BL/6J mice
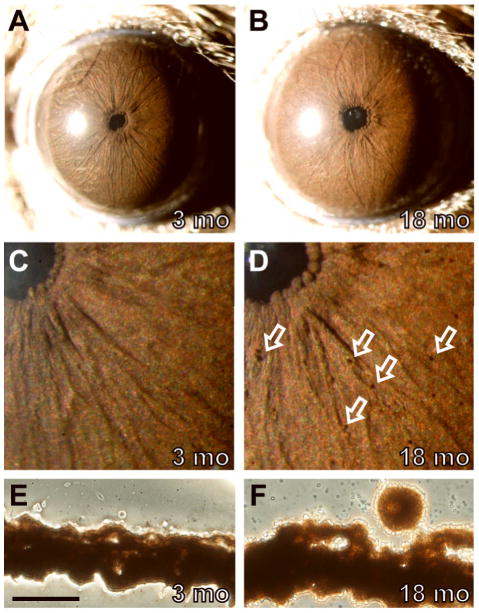
The iris of C57BL/6J mice is densely pigmented with a complex morphology (Figure 1). The iris appears uniformly deep sienna-brown in color with a smooth surface accentuated by a number of small vessels lying near the surface. The pupil is round, bordered by a beaded concentric ring of pupillary rough, and often contains a small notch inferiorly (Smith et al., 2000). As expected for densely pigmented intact irides, the iris appears solid black when viewed by transilluminating illumination. These features showed little visible variability between different individuals or sexes.
Figure 1.
Normal iris phenotypes of wild-type C57BL/6J mice. Comparisons of a representative 3-mo eye (left column) to an 18-mo eye (right column). (A) The normal C57BL/6J iris of young mice as viewed with broadbeam illumination originally imaged at 25X magnification. The iris is characterized by a sienna-brown color, a complex surface morphology with several small underlying vessels, and a circular pupil. The bright white circle to the left of the pupil is a reflection from flash photography. (B) With age, a number of clump cells are present on the surface of the iris and the iris becomes slightly more reddish in color. (C,D) At higher 40X magnification and less image reduction, clump cells are more readily visible, each casting a characteristic small crescent shadow (several are indicated by open white arrows, others in the field are unmarked). (E,F) Unstained cryosections of the same eyes shown above showing the presence of a melanin engulfed phagocytic clump cell in panel F on the surface of the iris stroma as a normal consequence of aging. These cells were not visible in 0/64 sections from the young eye in E and were present on the iris in 6/70 sections analyzed from the aged eye in F. Scale bar = 50 μm.
As C57BL/6J mice aged, two changes occurred (Figure 1B, D, F). First, iris color changed, gradually transitioning from a slightly darker hue in young mice toward a more reddish hue with age. Second, small amounts of dispersed pigment became visible. These typically appeared as rounded cells on the iris surface and likely correspond to the phagocytic clump cells described by Koganei (Wobmann and Fine, 1972). In mice 2–6 mo in age, dispersed pigment was rare (1 eye exhibited pigment on the cornea, 59 others were normal). In mice older than 6 mo, the presence of a small number of clump cells on the iris surface was nearly universal and tended to become more prominent with increasing age (n= 12 mice 6–9 mo, 15 mice 9–12 mo, and 15 mice 12–15 mo). Transillumination defects remained absent with age. Combined, these results indicate that small amounts of pigment dispersion are a normal feature of aging on the C57BL/6J genetic background, but C57BL/6J mice otherwise maintain an intact healthy iris into advanced age.
Iris phenotypes among a survey of 12 mouse substrains with coat color variations
We next analyzed a diverse group of 11 substrains with coat color variations or mutations that otherwise influence pigmentation (Figure 2, 3, 4, Summarized in Table 1), all with an identical genetic background (C57BL/6J). For simplicity, we will refer to each of these by their original allele names (see Materials and methods for full nomenclature). From observations of these strains, an interesting dichotomy became apparent; some alleles have apparently discordant effects on coat color and iris phenotypes, whereas others are quite similar.
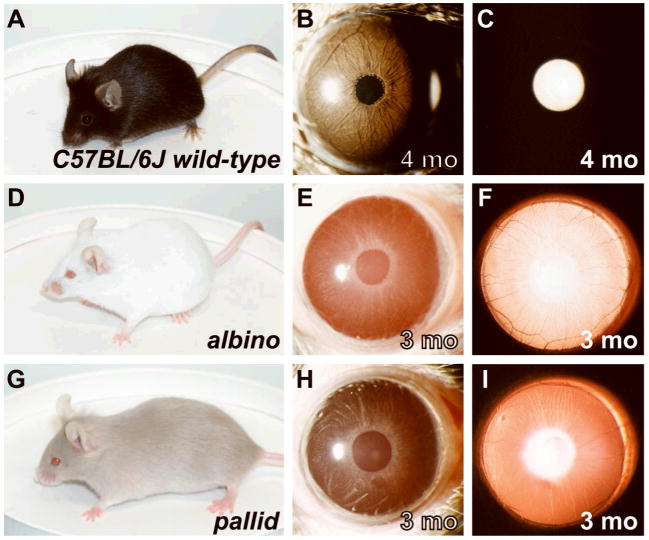
Figure 2.
Substrains exhibiting a correlation between coat and iris appearance. Coat color (left column), broadbeam illumination of iris (middle column), and transilluminating view of iris (right column). The bright white circle (to the left of the pupil with broadbeam illumination and central with transilluminating illumination) is a reflection from flash photography. (A–C) Wild-type C57BL/6J mice have darkly pigmented coats and darkly pigmented irides that are sienna-brown in color. Because the irides are darkly pigmented, the iris appears black with transilluminating illumination. (D–E) albino mice have coats and irides totally lacking melanin pigment. (G–I) pallid mice have severely diluted coats and irides. All mice were homozygous for the indicated mutations and were maintained on a C57BL/6J genetic background.
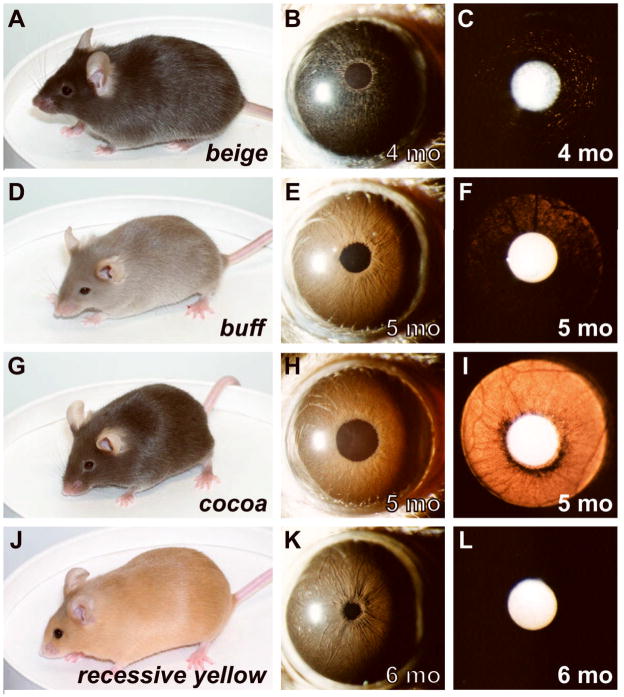
Figure 3.
Substrains exhibiting discordant coat color and iris appearances. Coat color (left column), broadbeam illumination of iris (middle column), and transilluminating view of iris (right column). (A–C) beige mice have slightly diluted coats, but irides that appear very dark. (D–F) buff mice have relatively severe dilution of coat color, but only a slight dilution of iris pigmentation evident by mild transillumination defects. The transillumination defects exhibited no discernable pattern, other than that the extent of the defects tended to increase peripherally. No influence of age was detectable. (G–I) cocoa mice have a modest dilution of coat color but strikingly hypopigmented irides (J–L) recessive yellow mice have yellow coats, but normally pigmented irides that are indistinguishable from those of normally pigmented C57BL/6J mice. All mice were homozygous for the indicated mutations and were maintained on a C57BL/6J genetic background.
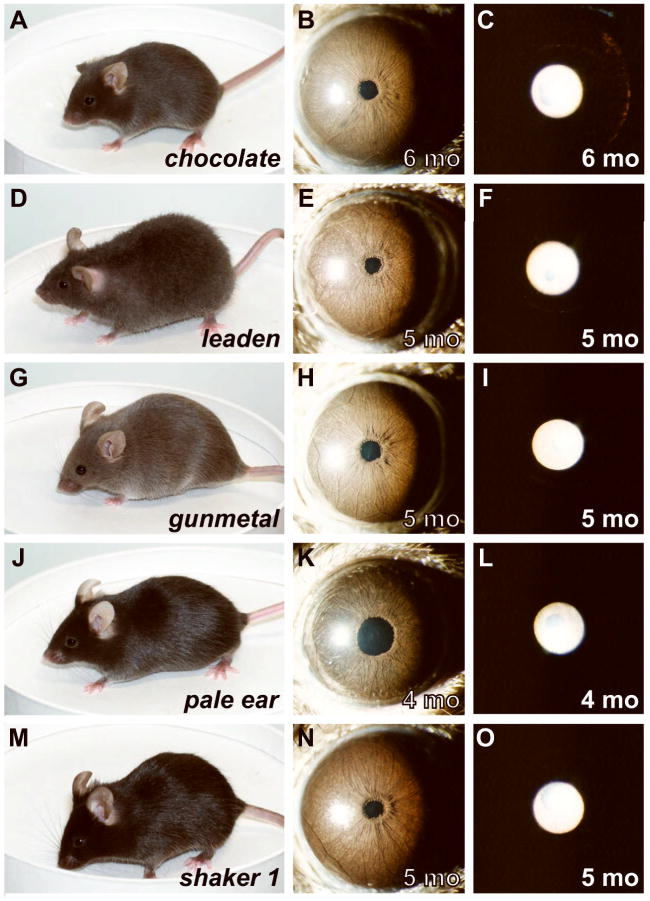
Figure 4.
Substrains with mild, or no, change in iris appearance. Coat color (left column), broadbeam illumination of iris (middle column), and transilluminating view of iris (right column). (A–C) chocolate mice have a modest alteration in coat color and mild transillumination defects that tended to increase peripherally. These transillumination defects did not worsen with increasing age among the mice examined. In contrast, other strains with similarly modest alterations in coat color maintain completely normal irides; including, (D–F) leaden mice have slightly diluted coats and normal appearing irides (the leaden substrain utilized here also carried the fuzzy allele, so it can additionally be concluded that fuzzy does not influence the iris), and (G–I) gunmetal mice have slightly diluted coats and normal appearing irides. Two substrains with mutations that do not influence adult coat color at all, but do influence melanosomes in other tissues were also examined; including, (J–L) As adults, pale ear mice have normal coat color but irides that appear slightly darker (note that the ears and tail of pale ear mice are also light colored), and (M–O) shaker 1 mice have normally colored coats and normal appearing irides. All mice were homozygous for the indicated mutations and were maintained on a C57BL/6J genetic background.
Table 1.
Summary of iris phenotypes survey
| Strain name | Symbol | Gene | Background | Total no mice | Oldest (months) | Comments |
|---|---|---|---|---|---|---|
| albino | TyrC–2J | Tyrosinase | C57BL/6J | 12 | 7 | Total albinism |
| beige | Lystbg-J | Lysosomal trafficking regulator | C57BL/6J | 13 | 10 | Very dark iris and pigment dispersion |
| buff | Vps33abf | Vacuolar protein sorting 33A (yeast) | C57BL/6J | 7 | 5 | Mild transillumination increasing peripherally |
| chocolate | Rab38cht | Rab38, member of RAS oncogene family | C57BL/6J | 11 | 12 | Mild transillumination increasing peripherally |
| cocoa | Hps3coa | Hermansky-Pudlak syndrome 3 homolog | C57BL/6J | 12 | 7 | Uniformly hypopigmented |
| gunmetal | Rabggtagm | Rab geranylgeranyl transferase, a subunit | C57BL/6J | 7 | 5 | Wild-type |
| leaden | Mlphln | Melanophilin | C57BL/6J | 12 | 8 | Wild-type |
| light | Tyrp1B-lt | Tyrosinase related protein 1 | LT/SvEiJ | 46 | 22 | Pigment dispersion |
| nm2798 | DctSlt-lt3J | DOPAchrome tautomerase | C57BL16J | 18 | 19 | Pigment dispersion |
| pale ear | Hps1ep | Hermansky-Pudlak syndrome 1 homolog | C57BL/6J | 9 | 6 | Slightly dark iris |
| pallid | Pldnpa | Pallidin | C57BL/6J | 9 | 6.5 | Near total albinism |
| recessive yellow | Mc1re-J | Melanocortin 1 receptor | C57BL/6J | 7 | 5.5 | Wild-type |
| shaker 1 | Myo7aShl–8j | Myosin VIIa | C57BL/6J | 9 | 7 | Wild-type |
| silver | SiSi | Silver | a Tyrp1b Sisi/J | 15 | 24 | Wild-type, does not alter Tyrp1b |
| vitiligo | Mitfml-vit | Microphthalmia-associated transcription factor | C57BL/6J | 12 | 21.5 | Pigment dispersion |
Some substrains exhibited a correlation between coat and iris appearance. For example, wild-type C57BL/6J mice have dark coats and dark irides (Figure 2A–C), albino mice completely lacking coat pigmentation also completely lacked iris pigment (Figure 2D–F), and pallid mice with severely diluted coat color exhibited severely diluted irides (Figure 2G–I). These results indicate that some alleles indeed have similar effects on pigmentation of the mouse iris and coat, likely because they represent key molecules that are absolutely required for melanin synthesis of any type.
However, coat color did not always predict iris appearance. This was strikingly observable in three different comparisons: 1) the slight dilution of coat color in beige mice is similar to many other substrains, but the iris appears very dark (Figure 3A–C). Eyes of beige mice also exhibited a unique pattern of transillumination defects and pronounced pigment dispersion. 2) The buff substrain exhibits a relatively severe dilution of coat color, but only a slight dilution of iris pigmentation (Figure 3D–F). Conversely, the cocoa substrain exhibits only a modest dilution of coat color, but pronounced iris transillumination defects (Figure 3G–I). 3) The recessive yellow substrain exhibits yellow coats, but irides of a color and morphology indistinguishable from wild-type C57BL/6J mice with black coats (Figure 3J–O). This result also offers further in vivo support for the prior finding that mouse iris melanocytes are insensitive to signalling pathways modulated by α-melanocyte-stimulating hormone (Li et al., 2006). Combined, these results identify alleles with distinguishable influence to the coat and iris, perhaps because they disrupt processes of differing importance to melanosomes in the unique context of each tissue.
Some substrains had irides with subtle, or no, changes in appearance. One substrain with modest alterations in coat color (chocolate) exhibited relatively mild transillumination defects (Figure 4A–C); two others with similarly modest alterations in coat color (leaden and gunmetal) maintained completely normal irides (Figure 4D–I). These observations correlate well with a recent report of ocular phenotypes observed in chocolate mice (Brooks et al., 2007). These results indicate that not every mutation influencing coat color will necessarily visibly influence the iris.
Two substrains with mutations that do not influence adult coat color at all (pale ear and shaker 1), but do influence melanosomes in other distinct populations of pigmented cells were also examined (Futter et al., 2004, Nguyen and Wei, 2007). Irides of pale ear mice exhibited a subtle phenotype characterized by a slight darkening across the surface of the iris stroma (Figure 4J–L). In contrast, eyes of shaker 1 mice maintained completely normal irides (Figure 4M–O). These results indicate that mutations influencing melanosomes, including mutations that do not influence coat color, can also give rise to abnormal iris phenotypes.
From these exams, an interesting observation regarding iris color became apparent. It has long been appreciated that mutations influencing melanin color of the coat also influences melanin color of the iris (Pierro, 1963). We were, therefore, surprised to find that the majority of substrains exhibited similar sienna-brown iris colors. Given the wide range of coat colors represented within the screen, this again emphasizes that there are differences in the pathways regulating pigmentation of the coat versus iris.
Iris phenotypes among mutant mouse strains genetically associated with Tyrp1
We have been particularly interested in identifying mouse strains exhibiting pigment dispersion (Anderson et al., 2006, Anderson et al., 2002, Anderson et al., 2001). Here, we use “pigment dispersion” to indicate the phenotype characterized by any aberrant deposition of pigment throughout the anterior chamber of the eye. The pigment may consist of dispersed melanin pigment itself or pigment that has become engulfed by other cells. In humans, pigment dispersion is a primary feature of pigment dispersion syndrome and can also occur in pseudoexfoliation syndrome, intraocular melanoma, and uveitis (Ball, 2004, Ritch et al., 1996, Shuba et al., 2007, Sowka, 2004). Because all of these diseases are of substantial medical importance, the identification of mouse strains exhibiting pigment dispersion could be of substantial benefit.
In addition to the identification of pigment dispersion in beige mice reported above, forms of pigment dispersion in mice have thus far only been observed in a small number of strains (Anderson et al., 2006, Anderson et al., 2002, Anderson et al., 2001, Boissy et al., 1987, Collier et al., 1984, John et al., 1998, Marneros and Olsen, 2003, Rodemer et al., 2003). Among these, the form of pigment dispersion occurring in DBA/2J mice is of particular relevance to pigment-related genes. DBA/2J mice develop a pigment dispersing iris disease as a consequence of mutations in two genes that both encode melanosomal proteins, Tyrp1b (brown) and GpnmbR150X (Anderson et al., 2002, John et al., 1998). Iris phenotypes of congenic strains containing either the Tyrp1b or GpnmbR150X mutations within the C57BL/6J genetic background have also recently been described in detail (Anderson et al., 2006). It is unknown whether pigment dispersion might also occur in strains with different alleles of these genes or in strains with other mutations in associated genetic pathways. While relatively little is currently known concerning the functions of Gpnmb in pigment producing cells of the mouse, Tyrp1 has been more extensively studied and multiple mouse strains relevant to Tyrp1 exist. Some of these, such as albino (genetically upstream of Tyrp1) and chocolate (targeting of TYRP1 to melanosomes), were already examined in the above experiments (Figures 2 and 4, respectively). In addition to those, we examined more strains genetically associated with Tyrp1; LT/SvEiJ mice carrying the Tyrp1B-lt mutation, vitiligo mice carrying the Mitfmi-vit mutation, and silver mice.
Inbred LT/SvEiJ mice are homozygous for the Tyrp1 allele, light, which acts dominantly to influence melanocyte survival and coat color (Johnson and Jackson, 1992, Mac, 1950). To test whether the previously described iris disease associated with Tyrp1 is specific to only the brown allele (Anderson et al., 2002, Chang et al., 1999, Libby et al., 2005), we analyzed the eyes of LT/SvEiJ mice (Figure 5A–C). Pigment dispersion was apparent in some eyes of young LT/SvEiJ mice (few specks of dispersed pigment within the pupil in 4/36 irides of 1–6 mo mice) and became increasingly common and severe with age (specks of dispersed pigment present in pupil of 8/18 irides of 6–12 mo mice, 13/24 irides of 12–18 mo mice). In advanced age, abundant dispersed pigment was present in almost all eyes (specks and accumulations of dispersed pigment present in pupil or on cornea in 10/12 irides of 21-mo mice; Figure 5B). In mice over 12 mo of age, mild small foci of iris transillumination defects became visible (data not shown). Several other non-age related ocular abnormalities occurred in light mice, including mild lens opacities (all eyes), lens-corneal adhesions (12/92 eyes), misshapen pupils (34/92 eyes), and dysmorphic pupillary rough (8/92 eyes). With respect to our current experiments, the most relevant of these findings pertains to pigment dispersion; iris disease associated with Tyrp1 mutation is not a specific consequence of the brown allele.
Figure 5.
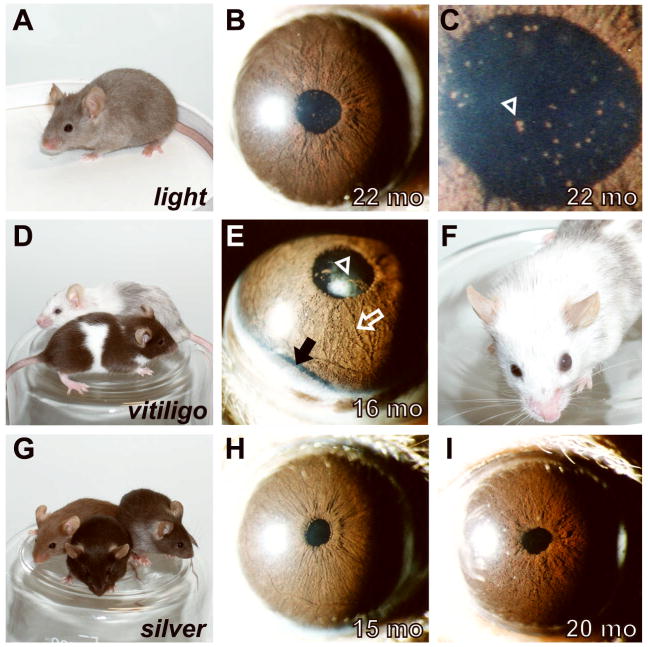
Pigment dispersion is a common, but not universal, feature of mouse strains with mutations related to Tyrp1. Comparisons of coat color (left column) and ocular phenotypes (right columns). (A) The light allele in LT/SvEiJ mice results in the production of hair that is pigmented at the tip, but very lightly or not pigmented along the hair shaft. As a consequence, coat color is rapidly lightened as hair lengthens. (B) Pigment dispersion was commonly present in the eyes of aged light mice. (C) Higher magnification image of same pupil shown in panel B. Several clumps of dispersed pigment are clearly visible in the pupil (one prominent example indicated with arrowhead, several other unmarked also visible). (D) The vitiligo allele results in a coat that is initially lighter than normal, with extensive white spotting (front mouse). With increasing age, the coat becomes progressively whiter due to increasing numbers of white hairs with each molt (back mouse). (E) Pigment dispersion was striking in eyes of aged vitiligo mice, as shown here in the pupil (arrowhead), across the surface of the iris (open white arrow), and in a pronounced pool accumulated inferiorly (dark band marked by solid black arrow). (F) Eyes of several vitiligo mice became severely enlarged, as sometimes occurs in mice with glaucoma. (G) The silver allele results in a mix of normal and hypopigmented hairs, which together cause a characteristic silvering of the coat. Heterozygosity for Tyrp1b enhances the silver coat color phenotype as shown here among three young silver homozygotes with differing Tyrp1 genotypes (Tyrp1+/+ front, Tyrp1b/b left, and Tyrp1b/+ right). (H) Mice heterozygous for Tyrp1b and homozygous for Sisi maintain healthy irides lacking pigment dispersion. (I) Mice homozygous for both Tyrp1b and Sisi exhibit a characteristic Tyrp1 mutant iris phenotype characterized by iris atrophy and pigment dispersion. Neither the onset nor severity of these Tyrp1b mediated phenotypes were influenced by Si. Note, because of the unique photographic flash settings used to capture the pigment accumulation near the highly reflective sclera in panel E, the apparent color of this iris is not directly comparable to the images in other panels.
The Mitf gene encodes a transcription factor previously demonstrated to regulate expression of several genes important to ocular development and function, including Tyrp1 (Smith et al., 1998, Murisier and Beermann, 2006, Fang et al., 2002). Whereas some mutant alleles of Mitf in mice result in the presence of micro-ophthalmic or albino eyes (Steingrimsson et al., 2004), the vitiligo allele allows grossly normal ocular development and pigmentation to initially occur and is an interesting candidate for exhibiting pigment dispersion. In order to test this possibility, we performed slit-lamp exams on the vitiligo strain of mice (Figure 5D–F). Dispersed pigment became evident by 7–8 mo (single specks of dispersed pigment present in pupil of 2/18 eyes). Past 12 mo of age, dispersed pigment was readily apparent across the iris surface, in the pupil, and on the cornea of all mice (Figure 5E). The distinctly rounded appearance of the dispersed pigment suggests that it likely correlates to pigment engulfed macrophages previously observed in histologic sections of vitiligo eyes (Boissy et al., 1987). Interestingly, several eyes and anterior chambers with pigment dispersion also became enlarged (6/30 eyes of 15–21 mo mice), suggesting that the accumulation of pigment within these eyes may be raising intraocular pressure (Figure 5F). No transillumination defects were observed. The occurrence of pigment dispersion in these mice indicates that at least some genes in genetic pathways influencing Tyrp1 can also contribute to the pigment dispersion phenotype.
Interestingly, the iris phenotype observed in vitiligo mice was more severe than previously observed Tyrp1 mutant phenotypes (Figure 5; and (Anderson et al., 2006, Anderson et al., 2002). This observation suggests that in addition to regulating Tyrp1 expression, MITF may transcriptionally regulate additional target genes impacting iris integrity. A key candidate for such a factor is Gpnmb. In mice, mutation of Gpnmb causes significant pigment dispersion (Anderson et al., 2006, Anderson et al., 2002) and experiments in quail have functionally associated an ortholog of Gpnmb, QNR-71, as a likely target of MITF (Aksan and Goding, 1998, Turque et al., 1996). As a step toward determining whether MITF regulates Gpnmb in mice, we examined the immediate flanking region of the Gpnmb transcription start site for potential MITF binding M-box sequences (Aksan and Goding, 1998). A consensus M-box sequence (5′-TCATGTG-3′) was identified at −34 bp relative to the Gpnmb transcription start site (M-box starting at NCBIM37:6:48986590; based on the transcription start site indicated by Gpnmb mRNA RefSeq NM_053110). A similar M-box related sequence is also located at −96 bp of the mouse gene and both elements appear present at similar positions 5′ of the human GPNMB gene. This observation supports the hypothesis that Gpnmb may be a downstream target of MITF. Further experiments will be required to test this directly.
The Si gene encodes a close homolog of Gpnmb (Shikano et al., 2001, Weterman et al., 1995). Interestingly, both Gpnmb and Si interact genetically with the Tyrp1b mutation (Anderson et al., 2002, Theos et al., 2005). Our initial studies involved silver mice with a mixed genetic background in which the Tyrp1b allele was also segregating (Figure 5G–I). In mice homozygous for Sisi and heterozygous for Tyrp1b, pigment dispersion was not detected through advanced age (14 mice aged 10–22 mo examined; Figure 5H). In mice homozygous for both Sisi and Tyrp1b (17 mice aged 10–31 mo), eyes developed iris atrophy typical for mice carrying the Tyrp1b mutation (Anderson et al., 2006, Anderson et al., 2001, Chang et al., 1999). The silver allele had no influence on the age of onset nor severity of these phenotypes and no other ocular phenotypes were present (Figure 5I). Similar results were obtained with mice from the silver stock cryopreserved by The Jackson Laboratory (STOCK a Tyrp1b Sisi/J, data not shown). These results indicate that despite the evidence linking Si to the pigment dispersion related Tyrp1 and Gpnmb genes, the silver allele does not influence pigment dispersion.
Combined, these results indicate that mutations influencing Tyrp1 are a common, but not universal, cause of iris disease. As explained below, a central relevance of this genetic pathway to pigment dispersion was further supported by our analysis of a new spontaneous coat color mutant strain exhibiting pigment dispersion.
Iris phenotypes in the nm2798 strain of mice
The nm2798 mutation is a spontaneously occurring coat color variant isolated at The Jackson Laboratory causing a semi-dominant affect on coat color whereby a normally black coat appears light grey in homozygotes and dark grey in heterozygotes (Figure 6A). Because of our interest in relationships between coat color and iris phenotypes, we screened this strain for potential iris phenotypes (n= 5 mice at 1 mo, 12 mice at 4–6 mo, 2 mice at 8 mo). Irides of nm2798 homozygotes had a healthy appearance through 5 mo (Figure 6B). After 8 mo, all nm2798 homozygotes showed signs of iris pigment dispersion. In follow-up analysis of aged mice (6 homozygous mice aged 14–19 mo), all eyes exhibited pigment dispersion across the iris surface and mild transillumination defects (Figure 6C, D). Similarly aged cohorts of nm2798 heterozygotes maintained healthy irides lacking pigment dispersion and transillumination defects.
Figure 6. Phenotypes and sequence analysis of the spontaneously occurring nm2798 mutation.
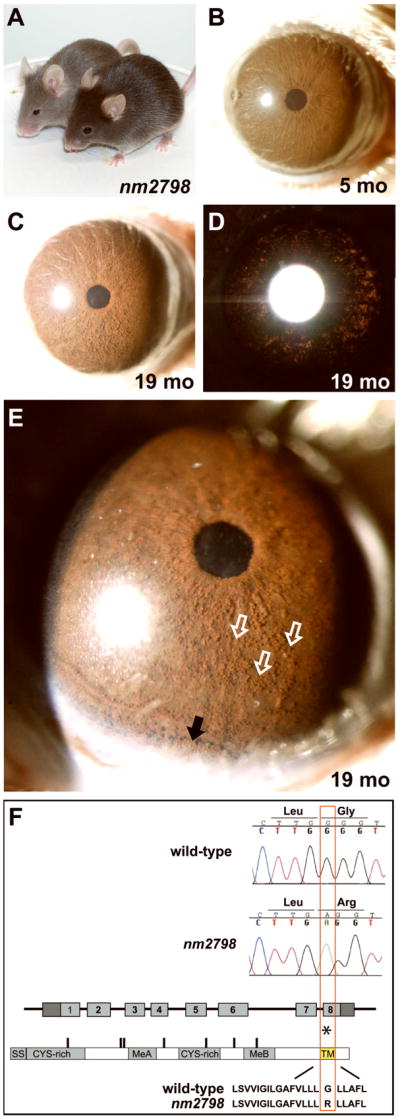
(A) The nm2798 mutation acts semi-dominantly to influence coat color, as shown here in a comparison of a heterozygote (front) and homozygote (back). (B) Healthy iris of a young nm2798 homozygote. (C) Aged nm2798 homozygote showing dispersed pigment across the surface of the iris and (D) mild transillumination defects spread across the iris. (E) Higher magnification view with a narrower slit of illumination showing the same iris as in panels C,D. The discretely rounded appearance of the dispersed pigment along the iris surface (three examples marked with open white arrows, several others in field unmarked) indicates that it is likely present within phagocytic clump cells. Pigment is also accumulating inferiorly (solid black arrow). (F) Sequence comparison of wild-type (B6) versus mutant (nm2798) alleles. A single base pair substitution within exon 8 of the Dct gene results in a mis-sense mutation changing a glycine to arginine (asterisk). The base pair and amino acid changes are both identical to previously described slaty light mutation. The change occurs within the predicted transmembrane spanning domain (TM). Other motifs of the DCT protein include a signal sequence (SS); cysteine rich domains (CYS-rich); and metal-binding motifs (MeA, MeB).
Genetic crosses between nm2798 and CAST/EiJ mice were utilized to map the nm2798 mutation to chromosome 14, closely linked to D14Mit42 (data not shown). Because the Dct gene is located near this position and the nm2798 coat color resembles slaty variants, a candidate gene approach was utilized to analyze Dct for possible mutations. A single G to A base change resulting in an amino acid substitution within the predicted transmembrane encoding portion of the DCT protein was identified (Figure 6F). All mice exhibiting the homozygous coat color phenotype were homozygous for the change, and all mice exhibiting the heterozygous coat color phenotype were heterozygous for the change (n= 6 mice of each genotype). No other changes were detected, indicating that the G to A change is highly likely to be the disease causing mutation.
Dct (also referred to as Tyrp2) encodes a transmembrane melanosomal protein sharing significant homology with TYRP1 (Budd and Jackson, 1995). DCT influences melanin synthesis and melanosome morphology (Hirobe and Abe, 2007, Matsunaga and Riley, 2002, Solano et al., 2000a, Winder et al., 1994). Interestingly, the exact mutation identified in nm2798 has been previously identified as the cause of the slaty light phenotype in a completely distinct strain of mice (precisely the same nucleotide change resulting in precisely the same amino acid substitution) (Budd and Jackson, 1995). Accordingly, we propose to hereafter refer to this allele as slaty light 3J. This mutation represents a slightly unusual case whereby the exact same genetic mutation has spontaneously arisen multiple times. Apparently, this amino acid position plays a critical role in enzyme function, one that that is perhaps uniquely disrupted by this substitution.
Discussion
Mice with coat color variations have had a significant influence on the discovery of genes influencing pigmentation (Bennett and Lamoreux, 2003), and on the history of mouse genetics itself (Paigen, 2003). Experiments with these strains have uncovered a wide array of important genetic pathways. In considering strategies for continuing to learn about these important pigment-related pathways, several recent studies have chosen to take advantage of approaches utilizing the iris. Here, we have used an ophthalmic slit-lamp to test the hypothesis that mutations influencing melanosomes will often give rise to abnormal iris phenotypes. The results confirm that strains with coat color variations indeed manifest several interesting iris phenotypes, many of which were previously unrecognized. Of the phenotypes observed, we have particularly focused on two classes: 1) mutants with discordant coat color and iris appearances, and 2) mutants exhibiting pigment dispersion. These efforts also drew us to analyzing the nm2798 mutant, which is caused by an identical re-occurrence of the slaty light mutation. Each of these findings has important implications.
Strains with discordant coat color and iris appearances
Some mutations have significantly different influence on appearance of the iris in comparison to the coat. Although there were correlations between coat color and iris appearance in some strains, there were many more in which coat color did not predict iris appearance. Only the albino and pallid strains exhibited coat color and iris phenotypes that were largely similar, in these cases causing severe hypopigmentation in both tissues. This result likely indicates that the proteins responsible for both phenotypes are similarly required within both tissues (tyrosinase as a rate-limiting enzyme required for melanin synthesis and pallidin as a member of the BLOC-1 complex) (Falcon-Perez et al., 2002, Murisier and Beermann, 2006). Undoubtedly, many other gene functions are also likely to be required in both tissues as well.
More surprisingly, many strains exhibited significant incongruities between coat and iris phenotypes. One biological factor likely contributing to these differences is embryologic. Whereas melanocytes influencing coat color are derived soley from neural crest, the iris has a diversity of pigmented cell types. The mammalian iris contains two populations of melanin producing cells, neural crest derived melanocytes of the iris stroma and neuroepithelial derived cells of the iris pigment epithelium. The iris also contains bone marrow derived antigen presenting cells typically located within the stroma that become pigmented as a secondary consequence of phagocytosis (Fine and Yanoff, 1979, Rodrigues et al., 1982). Thus, genes influencing neural crest derived melanocytes might well be expected to influence both the coat and iris. For other genes, the differing embryonic origins of the iris pigment epithelium and phagocytic cells could give rise to differing phenotypes between the coat and iris.
There are also several differences in the physiology of the iris and coat which may be regulated in part by this class of genes. For example, while melanin contributing to coat color is constantly being transferred into growing hair and ultimately shed (Slominski et al., 2005), iris melanosomes are normally maintained by the cells that generate them. Our demonstration of low level pigment dispersion in aged mice, and the prior description of low levels of ongoing melanin synthesis in the adult mouse iris (Lindquist et al., 1998), both suggest that there is some level of melanin turn-over in the iris, though likely at a level far less than which occurs in the coat. This fundamental difference in melanin maintenance would certainly lead to many proteins involving melanosome transfer that may influence the coat but not the iris. Conversely, the iris may well require additional compensatory pathways for dealing with aged melanin that would primarily influence only the iris. Defining the precise mechanisms contributing to these tissue-specificities, and how the alleles identified here influence them, will be an interesting area for additional experimentation.
Although our current experiments included a significant number of strains, they account for only a small fraction of all genes encoding melanosomal proteins and there remain many interesting genes to assess. Among these, the Oca2 (pink-eyed dilution) and Herc2 genes, orthologs of which are thought to influence human iris color (Kayser et al., 2008, Sturm et al., 2008), would be of particular interest. Eyes of pink-eyed dilution mice have been described as resembling albinos, though small amounts of melanin are found in the iris (Silvers, 1979). To our knowledge, eyes of Herc2 mutant mice have never been examined. In the future, it would be worthwhile to perform slit-lamp exams of these strains, and many others, to compare their phenotypes to the baselines established here.
Defining the class of mouse mutants exhibiting pigment dispersion
Pigment dispersion is a phenotype characterized by aberrant deposition of pigment throughout the anterior chamber of the eye. Because of its importance to human health (Ball, 2004, Ritch et al., 1996, Shuba et al., 2007, Sowka, 2004), we have attempted to expand known genetic pathways of pigment dispersion by identifying mouse strains with this phenotype. Among the coat color variant strains surveyed here, pigment dispersion was identified in four additional strains: beige, light, vitiligo, and nm2798. It is also significant that small amounts of dispersed pigment were observed in aged C57BL/6J mice. These results indicate that the iris pigment dispersion phenotype can be initiated by multiple, though not all, pigment-related genes and alleles, and is also influenced by age.
Previous experiments in DBA/2J mice initially suggested that one class of mutations causing pigment dispersion likely involved genes encoding melanosomal proteins (Anderson et al., 2002, Chang et al., 1999). Our current results add an additional insight; many alleles causing pigment dispersion can be specifically associated with toxic intermediates of melanin synthesis. A key function of melanosomes is to sequester the potentially cytotoxic intermediates produced during melanin production (Pawelek and Lerner, 1978, Smit et al., 2000). Interestingly, both the Tyrp1 and Dct alleles have previously been suggested to influence the toxicity of these intermediates (Chang et al., 1999, Costin et al., 2005, Johnson and Jackson, 1992, Urabe et al., 1994). Because Mitf likely regulates transcription of Tyrp1, and perhaps Dct (Murisier and Beermann, 2006) and Gpnmb (Aksan and Goding, 1998, Turque et al., 1996) as well, the pigment dispersion phenotype of vitiligo mice may also be linked to intermediates of melanin synthesis via this regulation. No link between beige and intermediates of melanin synthesis has previously been suggested, though one may well exist. Interestingly, beige melanosomes are substantially enlarged (Shiflett et al., 2002). Though speculative, the enlarged size of beige melanosomes could well influence the toxicity of intermediates of melanin synthesis. If the toxicity of melanin intermediates is moderated by protective molecules of the melanosomal membrane (such as TYRP1, GPNMB, or DCT), the altered surface:volume ratio of the enlarged beige mutant melanosomes could also influence toxicity because the relative ratio of these protective molecules at the melanosomal membrane to toxic intermediates would be distorted. In sum, it increasingly appears that a recurrent molecular theme among most, if not all, mutations causing pigment dispersion that we have identified is that this class of iris mutations share links to the toxic intermediates of melanin synthesis.
Pigment dispersion is a feature of particular importance to the eye disease, pigment dispersion syndrome and it’s potentially blinding sequelae, pigmentary glaucoma (Ritch, 1996). In this disorder, iridial pigment accumulates in ocular drainage structures and is accompanied by decreased aqueous humor outflow, increased intraocular pressure, and glaucoma. Despite knowledge that heredity strongly influences pigment dispersion syndrome, the causative genetic pathway in humans remain unknown. The TYRP1 and GPNMB genes have previously been analyzed as candidates contributing to human pigmentary glaucoma (Anderson et al., 2002, Lynch et al., 2002). Although no overt mutations in these genes have yet been identified among the limited number of human patients analyzed in these studies, these genes and the additional pigment dispersion causing genes identified here remain interesting candidates for potentially contributing to pigment dispersion syndrome and other diseases of the human iris.
Among the strains exhibiting pigment dispersion, the nm2798 strain is particularly interesting. Because of the toxicity of the melanin intermediate dopachrome (the substrate for DCT), Dct mutations would be expected to increase the possibility for melanosomal mediated toxic events involving this intermediate (Costin et al., 2005, Urabe et al., 1994). It is also noteworthy that the mutation underlying this spontaneous mutant has arisen before (Budd and Jackson, 1995). The G to A base change occurring here in nm2798, and also previously described in slaty light mice, substitutes the arginine for glycine at position 486, an amino acid within the predicted transmembrane region of the DCT protein. This presence of arginine has previously been suggested to decrease the hydrophobicity of the transmembrane domain, likely causing the transmembrane protein of DCT to slide by approximately four amino acids toward the N-terminus (Budd and Jackson, 1995, Costin et al., 2005). How this change results in a loss of DCT function remains largely unknown. The spontaneous occurrence of this precise mutation twice in distinct colonies of mice is certainly unusual, and might be explained in several ways. In one scenario, this base pair may exist in a hyper-mutable state that renders this substitution as a relatively likely event. In another, this change may be one of the only changes capable of causing a semi-dominant Dct allele, and a large selective pressure for observing it has been applied as diligent animal caretakers observe colonies of mice taking note of spontaneous mutations altering coat color. In either case, the spontaneous re-occurrence of this mutation has again brought attention to this domain of the DCT protein and allowed us to establish a new link between Dct and pigment dispersion.
Conclusion
Among mouse strains with coat color variations, mutations influencing melanosomes often induce biologically and medically relevant iris phenotypes, including pigment dispersion. Considering the different mutations that cause pigment dispersion in mice, a molecular theme is emerging that genes in this class are often linked to the toxic intermediates of melanin synthesis. These findings suggest new candidates for contributing to human diseases involving pigment dispersion and establish a baseline for future experiments that will continue to capitalize on approaches utilizing the iris as a means of studying pathways influencing pigment cell biology.
Materials and methods
Animal husbandry
All mice were obtained from The Jackson Laboratory, Bar Harbor, Maine. Mice were maintained on a 4% fat NIH 31 diet provided ad libitum, and the water was acidified to pH 2.8–3.2. Mice were housed in cages containing white pine bedding and covered with polyester filters. The environment was kept at 21°C with a 14-h light:10-h dark cycle. All animals were treated according to the guidelines of the Association for Research in Vision and Ophthalmology for use of animals in research. All experimental protocols were approved by the Animal Care and Use Committee of The Jackson Laboratory or The University of Iowa.
Slit-lamp examination
The primary slit-lamp screen shown in Figures 2–4 and the survey of strains associated with Tyrp1 shown in Figure 5 were performed with a Haag-Streit slit-lamp bio-microscope. Eyes were photographed with a 40X objective lens and 35mm camera utilizing identical camera settings. Because slight variations in film development may cause small fluctuations in the color of these images, assessments of iris color were primarily made in side-by-side comparisons of mice at the slit-lamp. Photographs of the C57BL/6J and nm2798 strain shown in Figures 1 and 6 were performed with a Topcon SL-D7 slit-lamp. These eyes were photographed with a 25X objective and Nikon D100 digital camera utilizing identical camera settings.
Analysis of cryosections
To compare the appearance of pigmented cell populations in the anterior chamber with respect to age, enucleated eyes were embedded in Optimal Cutting Temperature embedding medium (Tissue-Tek O.C.T. Compound, Sakura Finetek U.S.A., Inc., Torrance, CA), 10-micrometersections cut, and sections transferred to glass slides (CryoJane, Instrumedics, Inc., St. Louis, MO). Sections were imaged with an Olympus BX52 upright microscope using a 40X objective and bright field illumination. Images were captured with an Olympus DP70 digital camera and treated in an identical fashion during software processing.
Genetic and molecular analysis of nm2798
The nm2798 (slaty light 3J) mutation arose in the B6.V-Lepob/J strain and was initially maintained by breeding to wild-type C57BL/6J mice. The coat color mutation segregated independently of Lepob, allowing the stock of C57BL/6J mice carrying only nm2798 utilized here to be generated. To determine the chromosomal location of the nm2798 mutation, a backcross between B6-nm2798 and CAST/EiJ mice was performed. Individual genomic DNA samples were prepared from tail samples of N2 mice. Following previously published reaction parameters (Chang et al., 2006), a genome scan of microsatellite DNA markers was initially performed on pooled DNA from 21 mice with the lightest coat colors. After detection of linkage on chromosome 14, the microsatellite markers D14Mit42, D14Mit105, D14Mit35, D14Mit145, and D14Mit69 were scored in individual DNA samples. To test the Dct gene as a candidate, 3 overlapping fragments were amplified from iris cDNA and analyzed with automated fluorescence tag sequencing.
Genetic nomenclature and stocks
The official full names of strains, Jackson Laboratory stock numbers, and alleles utilized in this study, include: C57BL/6J (stock 000664); albino, B6(Cg)-Tyrc-2J/J (stock 000058); beige, C57BL/6J-Lystbg-J/J (stock 000629); buff, C57BL/6J-Vps33abf/J (stock 000520); chocolate, C57BL/6J-Rab38cht/J (000976); cocoa, B6; B10-Hps3coa/J (stock 001025); gunmetal, C57BL/6J-Rabggtagm/J (stock 000110); leaden, B6.Cg-fz H54 Mlphln/+ H54 +/J (stock 000112); light, LT/SvEiJ (stock 006252), carries Tyrp1B-lt; nm2798/slaty light 3J, B6(Cg)-Dctslt-lt3J/J; pale ear, B6.C3Fe-Hps1ep/J (stock 000525); pallid, B6.Cg-Pldnpa/J (stock 000024); recessive yellow, B6.C-H2-Ab1bm12/KhEg-Mc1re-J/J (stock 003625); shaker 1, B6.Cg-Myo7ash1-8J/J (003184); vitiligo, C57BL/6J-Mitfmi-vit/J (stock 002134).
Two separate colonies of silver mice were utilized. Because of its availability, our initial studies utilized a silver stock that was maintained on a mixed genetic background. This colony was derived from crosses of the original silver stock (STOCK a Tyrp1b Sisi/J) bred to C57BR/cdJ mice for one generation, maintained as brother-sister matings for approximately eight generations, then later crossed to C57BL/6J for one generation. The images of silver coat colors in Figure 5G represent mice from an intercross of these mice. Si genotype was confirmed by amplifying a 258 bp region flanking the silver mutation (forward primer 5′-GATTAGCGTAGGCAGGTTGC-3′ and reverse primer 5′-GACTGCAGAAAACACAGGCA-3′) and assaying for the absence or presence of an AluI restriction enzyme site created by the silver mutation (Solano et al., 2000b). Tyrp1b genotype was confirmed utilizing flanking polymorphic markers D4Mit327 and D4Mit178. Initial ocular exams (as shown in Figure 5H, I) were performed with progeny of a backcross of these mice in which all progeny were obligate Sisi homozygotes and either heterozygous (yielding silver coats) or homozygous (yielding brown coats) for the Tyrp1b mutation. Later, mice from the cryopreserved silver strain, STOCK a Tyrp1b Sisi/J (stock 000064), were also examined. In these mice, all mice were obligate Sisi homozygotes and Tyrp1b was segregating.
Acknowledgments
We thank Richard Samples, an employee of The Jackson Laboratory, who first recognized the nm2798 mutation and whose diligent observation made this work possible; and Robert F. Mullins and Edward Heffron for helpful discussions. This work was supported in part by National Eye Institute Grant F32EY07015 and a grant from The Glaucoma Foundation to MGA; National Eye Institute Grant EY11721 to SWMJ; National Eye Institute Grant EY07758 to BC; and National Cancer Institute Grant CA34196 to The Jackson Laboratory. SWMJ is an Investigator of The Howard Hughes Medical Institute.
References
- Aksan I, Goding CR. Targeting the microphthalmia basic helix-loop-helix-leucine zipper transcription factor to a subset of E-box elements in vitro and in vivo. Mol Cell Biol. 1998;18:6930–8. doi: 10.1128/mcb.18.12.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Libby RT, Mao M, Cosma IM, Wilson LA, Smith RS, John SW. Genetic context determines susceptibility to intraocular pressure elevation in a mouse pigmentary glaucoma. BMC Biol. 2006;4:20. doi: 10.1186/1741-7007-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SW. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–5. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Smith RS, Savinova OV, Hawes NL, Chang B, Zabaleta A, Wilpan R, Heckenlively JR, Davisson M, John SW. Genetic modification of glaucoma associated phenotypes between AKXD-28/Ty and DBA/2J mice. BMC Genet. 2001;2:1. doi: 10.1186/1471-2156-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball SF. Pigmentary Glaucoma. In: Yanoff M, Duker JS, editors. Ophthalmology. 2. St Louis: Mosby; 2004. [Google Scholar]
- Bennett DC, Lamoreux ML. The color loci of mice--a genetic century. Pigment Cell Res. 2003;16:333–44. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- Boissy RE, Moellmann GE, Lerner AB. Morphology of melanocytes in hair bulbs and eyes of vitiligo mice. Am J Pathol. 1987;127:380–8. [PMC free article] [PubMed] [Google Scholar]
- Brooks BP, Larson DM, Chan CC, Kjellstrom S, Smith RS, Crawford MA, Lamoreux L, Huizing M, Hess R, Jiao X, et al. Analysis of ocular hypopigmentation in Rab38cht/cht mice. Invest Ophthalmol Vis Sci. 2007;48:3905–13. doi: 10.1167/iovs.06-l464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budd PS, Jackson IJ. Structure of the mouse tyrosinase-related protein-2/dopachrome tautomerase (Tyrp2/Dct) gene and sequence of two novel slaty alleles. Genomics. 1995;29:35–43. doi: 10.1006/geno.1995.1212. [DOI] [PubMed] [Google Scholar]
- Chang B, Dacey MS, Hawes NL, Hitchcock PF, Milam AH, Atmaca-Sonmez P, Nusinowitz S, Heckenlively JR. Cone photoreceptor function loss-3, a novel mouse model of a chromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47:5017–21. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, Roderick TH, Heckenlively JR, Davisson MT, John SW. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–9. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Collier LL, Prieur DJ, King EJ. Ocular melanin pigmentation anomalies in cats, cattle, mink, and mice with Chediak-Higashi syndrome: histologic observations. Curr Eye Res. 1984;3:1241–51. doi: 10.3109/02713688409000828. [DOI] [PubMed] [Google Scholar]
- Costin GE, Valencia JC, Wakamatsu K, Ito S, Solano F, Milac AL, Vieira WD, Yamaguchi Y, Rouzaud F, Petrescu AJ, et al. Mutations in dopachrome tautomerase (Dct) affect eumelanin/pheomelanin synthesis, but do not affect intracellular trafficking of the mutant protein. Biochem J. 2005;391:249–59. doi: 10.1042/BJ20042070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcon-Perez JM, Starcevic M, Gautam R, Dell’angelica EC. BLOC-1, a novel complex containing the pallidin and muted proteins involved in the biogenesis of melanosomes and platelet-dense granules. J Biol Chem. 2002;277:28191–9. doi: 10.1074/jbc.M204011200. [DOI] [PubMed] [Google Scholar]
- Fang D, Tsuji Y, Setaluri V. Selective down-regulation of tyrosinase family gene TYRP1 by inhibition of the activity of melanocyte transcription factor, MITF. Nucleic Acids Res. 2002;30:3096–106. doi: 10.1093/nar/gkf424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine BS, Yanoff M. Ocular Histology. 2. Hagerstown: Harper & Row, Publishers, Inc; 1979. The Uveal Tract. [Google Scholar]
- Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell. 2004;15:2264–75. doi: 10.1091/mbc.E03-10-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirobe T, Abe H. Changes of melanosome morphology associated with the differentiation of epidermal melanocytes in slaty mice. Anat Rec (Hoboken) 2007;290:981–93. doi: 10.1002/ar.20547. [DOI] [PubMed] [Google Scholar]
- Hong L, Simon JD, Sarna T. Melanin structure and the potential functions of uveal melanosomes. Pigment Cell Res. 2006;19:465–6. doi: 10.1111/j.1600-0749.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Hu DN. Photobiology of ocular melanocytes and melanoma. Photochem Photobiol. 2005;81:506–9. doi: 10.1562/2004-08-24-IR-289. [DOI] [PubMed] [Google Scholar]
- Iwata F, Reed GF, Caruso RC, Kuehl EM, Gahl WA, Kaiser-Kupfer MI. Correlation of visual acuity and ocular pigmentation with the 16-bp duplication in the HPS-1 gene of Hermansky-Pudlak syndrome, a form of albinism. Ophthalmology. 2000;107:783–9. doi: 10.1016/s0161-6420(99)00150-5. [DOI] [PubMed] [Google Scholar]
- John SW, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci. 1998;39:951–62. [PubMed] [Google Scholar]
- Johnson R, Jackson IJ. Light is a dominant mouse mutation resulting in premature cell death. Nat Genet. 1992;1:226–9. doi: 10.1038/ng0692-226. [DOI] [PubMed] [Google Scholar]
- Kayser M, Liu F, Janssens AC, Rivadeneira F, Lao O, Van Duijn K, Vermeulen M, Arp P, Jhamai MM, Van Ijcken WF, et al. Three genome-wide association studies and a linkage analysis identify HERC2 as a human iris color gene. Am J Hum Genet. 2008;82:411–23. doi: 10.1016/j.ajhg.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Hu DN, Zhao H, Mccormick SA, Nordlund JJ, Boissy RE. Uveal melanocytes do not respond to or express receptors for alpha-melanocyte-stimulating hormone. Invest Ophthalmol Vis Sci. 2006;47:4507–12. doi: 10.1167/iovs.06-0391. [DOI] [PubMed] [Google Scholar]
- Libby RT, Anderson MG, Pang IH, Robinson ZH, Savinova OV, Cosma IM, Snow A, Wilson LA, Smith RS, Clark AF, et al. Inherited glaucoma in DBA/2J mice: pertinent disease features for studying the neurodegeneration. Vis Neurosci. 2005;22:637–48. doi: 10.1017/S0952523805225130. [DOI] [PubMed] [Google Scholar]
- Lin JY, Fisher DE. Melanocyte biology and skin pigmentation. Nature. 2007;445:843–50. doi: 10.1038/nature05660. [DOI] [PubMed] [Google Scholar]
- Lindquist NG, Larsson BS, Stjernschantz J, Sjoquist B. Age-related melanogenesis in the eye of mice, studied by microautoradiography of 3H-methimazole, a specific marker of melanin synthesis. Exp Eye Res. 1998;67:259–64. doi: 10.1006/exer.1998.0513. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hong L, Wakamatsu K, Ito S, Adhyaru BB, Cheng CY, Bowers CR, Simon JD. Comparisons of the structural and chemical properties of melanosomes isolated from retinal pigment epithelium, iris and choroid of newborn and mature bovine eyes. Photochem Photobiol. 2005;81:510–6. doi: 10.1562/2004-10-19-RA-345.1. [DOI] [PubMed] [Google Scholar]
- Lynch S, Yanagi G, Delbono E, Wiggs JL. DNA sequence variants in the tyrosinase-related protein 1 (TYRP1) gene are not associated with human pigmentary glaucoma. Mol Vis. 2002;8:127–9. [PubMed] [Google Scholar]
- Mac DE. “Light”--a new mouse color. J Hered. 1950;41:35–6. doi: 10.1093/oxfordjournals.jhered.a106079. [DOI] [PubMed] [Google Scholar]
- Marneros AG, Olsen BR. Age-dependent iris abnormalities in collagen XVIII/endostatin deficient mice with similarities to human pigment dispersion syndrome. Invest Ophthalmol Vis Sci. 2003;44:2367–72. doi: 10.1167/iovs.02-1180. [DOI] [PubMed] [Google Scholar]
- Matsunaga J, Riley PA. Catecholamine Research: From Molecular Insights to Clinical Medicine. New York: Kluwer Academic/Plenum Publishing; 2002. Biosynthesis of neuromelanin and melanin: the potential involvement of macrophage migration inhibitory factor and DOPAchrome tautermerase as rescue enzymes. [Google Scholar]
- Murisier F, Beermann F. Genetics of pigment cells: lessons from the tyrosinase gene family. Histol Histopathol. 2006;21:567–78. doi: 10.14670/HH-21.567. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J Invest Dermatol. 2007;127:421–8. doi: 10.1038/sj.jid.5700566. [DOI] [PubMed] [Google Scholar]
- Paigen K. One hundred years of mouse genetics: an intellectual history. I. The classical period (1902–1980) Genetics. 2003;163:1–7. doi: 10.1093/genetics/163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelek JM, Lerner AB. 5,6-Dihydroxyindole is a melanin precursor showing potent cytotoxicity. Nature. 1978;276:626–8. doi: 10.1038/276627a0. [DOI] [PubMed] [Google Scholar]
- Pierro LJ. Effects of the Light Mutation of Mouse Coat Color on Eye Pigmentation. J Exp Zool. 1963;153:81–7. doi: 10.1002/jez.1401530109. [DOI] [PubMed] [Google Scholar]
- Ritch R. A unification hypothesis of pigment dispersion syndrome. Trans Am Ophthalmol Soc. 1996;94:381–405. discussion 405–9. [PMC free article] [PubMed] [Google Scholar]
- Ritch R, Shields MB, Krupin T. The Glaucomas. St. Louis: Mosby; 1996. [Google Scholar]
- Rodemer C, Thai TP, Brugger B, Gorgas K, Just W. Targeted disruption of ether lipid synthesis in mice. Adv Exp Med Biol. 2003;544:355–68. doi: 10.1007/978-1-4419-9072-3_46. [DOI] [PubMed] [Google Scholar]
- Rodrigues MM, Hackett J, Donohoo P. Iris. In: Jakobiec FA, editor. Ocular Anatomy, Embryology, and Teratology. Philadelphia: Harper & Row, Publishers, Inc; 1982. [Google Scholar]
- Shiflett SL, Kaplan J, Ward DM. Chediak-Higashi Syndrome: a rare disorder of lysosomes and lysosome related organelles. Pigment Cell Res. 2002;15:251–7. doi: 10.1034/j.1600-0749.2002.02038.x. [DOI] [PubMed] [Google Scholar]
- Shikano S, Bonkobara M, Zukas PK, Ariizumi K. Molecular cloning of a dendritic cell-associated transmembrane protein, DC-HIL, that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 2001;276:8125–34. doi: 10.1074/jbc.M008539200. [DOI] [PubMed] [Google Scholar]
- Shuba L, Nicolela MT, Rafuse PE. Correlation of capsular pseudoexfoliation material and iridocorneal angle pigment with the severity of pseudoexfoliation glaucoma. J Glaucoma. 2007;16:94–7. doi: 10.1097/01.ijg.0000212283.70422.b0. [DOI] [PubMed] [Google Scholar]
- Silvers WK. The Coat Colors of Mice. New York: Springer-Verlag; 1979. Chapter 4: Dilue and Leaden, the p-locus, Ruby-Eye, and Ruby-Eye-2. [Google Scholar]
- Slominski A, Wortsman J, Plonka PM, Schallreuter KU, Paus R, Tobin DJ. Hair follicle pigmentation. J Invest Dermatol. 2005;124:13–21. doi: 10.1111/j.0022-202X.2004.23528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit NPM, Pavel S, Riley PA. Mechanisms of control of the cytotoxicity of orthoquinone intermediates of melanogenesis. In: Creveling CR, editor. Role of catechol quinone species in cellular toxicity. Johnson City, TN: F. P. Graham Publishing; 2000. [Google Scholar]
- Smith-Thomas LC, Moustafa M, Dawson RA, Wagner M, Balafa C, Haycock JW, Krauss AH, Woodward DF, Macneil S. Cellular and hormonal regulation of pigmentation in human ocular melanocytes. Pigment Cell Res. 2001;14:298–309. doi: 10.1034/j.1600-0749.2001.140411.x. [DOI] [PubMed] [Google Scholar]
- Smith RS, Zabaleta A, Kume T, Savinova OV, Kidson SH, Martin JE, Nishimura DY, Alward WL, Hogan BL, John SW. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000;9:1021–32. doi: 10.1093/hmg/9.7.1021. [DOI] [PubMed] [Google Scholar]
- Smith SB, Zhou BK, Orlow SJ. Expression of tyrosinase and the tyrosinase related proteins in the Mitfvit (vitiligo) mouse eye: implications for the function of the microphthalmia transcription factor. Exp Eye Res. 1998;66:403–10. doi: 10.1006/exer.1997.0443. [DOI] [PubMed] [Google Scholar]
- Solano F, Hearing VJ, Garcia-Borron JC. Neurotoxicity due to o-quinones: neuromelanin formation and possible mechanisms for o-quinone detoxification. Neurotox Res. 2000a;1:153–69. doi: 10.1007/BF03033287. [DOI] [PubMed] [Google Scholar]
- Solano F, Martinez-Esparza M, Jimenez-Cervantes C, Hill SP, Lozano JA, Garcia-Borron JC. New insights on the structure of the mouse silver locus and on the function of the silver protein. Pigment Cell Res. 2000b;13(Suppl 8):118–24. doi: 10.1111/j.0893-5785.2000.130821.x. [DOI] [PubMed] [Google Scholar]
- Sowka J. Pigment dispersion syndrome and pigmentary glaucoma. Optometry. 2004;75:115–22. doi: 10.1016/s1529-1839(04)70023-8. [DOI] [PubMed] [Google Scholar]
- Steingrimsson E, Copeland NG, Jenkins NA. Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet. 2004;38:365–411. doi: 10.1146/annurev.genet.38.072902.092717. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Duffy DL, Zhao ZZ, Leite FP, Stark MS, Hayward NK, Martin NG, Montgomery GW. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–31. doi: 10.1016/j.ajhg.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CG. Vision in albinism. Trans Am Ophthalmol Soc. 1996;94:1095–155. [PMC free article] [PubMed] [Google Scholar]
- Theos AC, Truschel ST, Raposo G, Marks MS. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 2005;18:322–36. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turque N, Denhez F, Martin P, Planque N, Bailly M, Begue A, Stehelin D, Saule S. Characterization of a new melanocyte-specific gene (QNR-71) expressed in v-myc-transformed quail neuroretina. Embo J. 1996;15:3338–50. [PMC free article] [PubMed] [Google Scholar]
- Urabe K, Aroca P, Tsukamoto K, Mascagna D, Palumbo A, Prota G, Hearing VJ. The inherent cytotoxicity of melanin precursors: a revision. Biochim Biophys Acta. 1994;1221:272–8. doi: 10.1016/0167-4889(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Ward WC, Simon JD. The differing embryonic origins of retinal and uveal (iris/ciliary body and choroid) melanosomes are mirrored by their phospholipid composition. Pigment Cell Res. 2007;20:61–9. doi: 10.1111/j.1600-0749.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- Weterman MA, Ajubi N, Van Dinter IM, Degen WG, Van Muijen GN, Ruitter DJ, Bloemers HP. nmb, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Int J Cancer. 1995;60:73–81. doi: 10.1002/ijc.2910600111. [DOI] [PubMed] [Google Scholar]
- Winder A, Kobayashi T, Tsukamoto K, Urabe K, Aroca P, Kameyama K, Hearing VJ. The tyrosinase gene family--interactions of melanogenic proteins to regulate melanogenesis. Cell Mol Biol Res. 1994;40:613–26. [PubMed] [Google Scholar]
- Wistow G, Bernstein SL, Ray S, Wyatt MK, Behal A, Touchman JW, Bouffard G, Smith D, Peterson K. Expressed sequence tag analysis of adult human iris for the NEIBank Project: steroid-response factors and similarities with retinal pigment epithelium. Mol Vis. 2002;8:185–95. [PubMed] [Google Scholar]
- Wobmann PR, Fine BS. The clump cells of Koganei. A light and electron microscopic study. Am J Ophthalmol. 1972;73:90–101. doi: 10.1016/0002-9394(72)90311-x. [DOI] [PubMed] [Google Scholar]
- Wong VW, Lam PT, Lai TY, Lam DS. Black diaphragm aniridia intraocular lens for aniridia and albinism. Graefes Arch Clin Exp Ophthalmol. 2005;243:501–4. doi: 10.1007/s00417-004-1058-9. [DOI] [PubMed] [Google Scholar]