Abstract
Worldwide, contaminated drinking water poses a major health threat, particularly to child development. Diarrhoea represents a large part of the water-related disease burden and enteric infections have been linked to nutritional and growth shortfalls as well as long-term physical and cognitive impairment in children. Previous studies detailed the frequency of infection and the consequences for child health in a shanty town in north-east Brazil. To determine the frequency of contaminated water, we measured faecal contamination in primary drinking water samples from 231 randomly selected households. Risk for contamination was compared across source and storage types. Nearly a third of the study households (70/231: 30.3%) had contaminated drinking water; the source with the highest frequency of contamination was well water (23/24: 95.8%). For tap water, the type of storage had a significant effect on the susceptibility to contamination (χ2 = 12.090; p = 0.007). The observed pattern of contamination demonstrated the relative potential contributions of both source and storage. With evidence that supports the inclusion of source and storage in water quality surveys, this study, like others, suggests that contaminated drinking water in storage vessels may be an important factor for the documented diarrhoea disease burden in the Brazilian shanty town.
Keywords: Brazil, diarrhoea, favela, water contamination, water origin, water storage
INTRODUCTION
The quality of drinking water directly affects the well-being of individuals with cumulative effects at every social level. The World Health Organization attributed 4.0% of all deaths and 5.7% of the global disease burden to water-related illnesses, which stemmed from poor water quality, hygiene and sanitation (Pruss et al. 2002). These diseases disproportionately affect the developing world and young children. As opposed to the developed world, which has nearly complete coverage, less than half of sub-Saharan Africa has access to safe drinking water (United Nations 2005). As the combination of unsafe drinking water, absence of acquired immunity and lack of hygienic facilities increase the risk of infection, young children in developing regions are especially vulnerable. A review of studies between 1992 and 2000 found that, annually, 2.5 million deaths in children less than five years old are accounted for by diarrhoea (Kosek et al. 2003), the primary component of the water-related disease burden.
While mortality is an overwhelming impact of contaminated drinking water, early childhood diarrhoea has a myriad of debilitating effects that contribute to the disability-associated burden. Persistent as well as recurrent diarrhoea is associated with nutritional shortfalls in a cohort of children in north-east Brazil (Schorling & Guerrant 1990; Lima et al. 2000). This malnutrition represented an unhealthy feedback cycle: malnutrition predisposes children towards diarrhoeal illness and vice versa (Lima et al. 1992), with potential long-term consequences for physical development (Stephensen 1999). Consequently, in addition to a well-documented relationship between diarrhoea and immediate growth shortfalls (Martorell et al. 1975; Rowland & McCollum 1977; Black et al. 1984), Moore et al. (2001) and Guerrant et al. (1999) found that the frequency of early childhood diarrhoea impaired growth, physical fitness and even cognitive function 4.5 to 7 years later. In a follow-up study, several indicators of intelligence at 7–9 years of age were negatively correlated with diarrhoeal disease in early childhood (under two years old), confirming the initial findings (Niehaus et al. 2002). From a developmental perspective, the growth shortfalls from early childhood enteric infections associate with the even more troubling effect on cognitive development (Berkman et al. 2002).
Several waterborne pathogens have been implicated as causative agents of diarrhoea (Leclerc et al. 2002). For transmission, these organisms depend on a faecal-oral route through the environment. In addition, contaminated food, from either direct contamination or from contaminated water, also serves as an important vehicle of pathogen transmission in unhygienic conditions. Untreated sources are readily contaminated by faecal matter, resulting in a high background level of infectious diarrhoea in developing countries (5–12 episodes per child/year; Guerrant et al. 1990). In contrast, the thorough treatment of source water in developed countries corresponds with a much lower prevalence of diarrhoea (2 episodes per child/year). However, a survey found 34 major waterborne outbreaks in the United States from 1991 to 1992, reflecting the limitations of water treatment (Moore et al. 1993). For example, Cryptosporidium infected an estimated 403,000 people in Milwaukee, Wisconsin, as a result of inadequate filtration to remove chlorine-resistant oocysts (MacKenzie et al. 1994).
Even with a clean source for drinking water, studies show a substantial amount of contamination at the household level. In a pilot survey of water quality in a Brazilian shanty town (the focus of the current study), where water was typically stored before consumption, faecal coliforms were absent from samples of the improved water source (tap water), compared with 58% of stored water samples (B. Beers, personal communication, July 2008). Similarly, although Oxfam had provided an improved source free of faecal contamination in an area of Sierra Leone, over 90% of stored household water samples contained thermotolerant coliforms (Clasen & Bastable 2003). In examining the causes of diarrhoea in young Rwandan children, authors of another study identified unclean tools for transport and storage as the means of water contamination (Gasana et al. 2002). Ultimately, in combination, the source and the household practice determine the levels of contamination and the drinker’s risk for diarrhoea.
The disproportionate burden of diarrhoea on children in the developing world motivated this investigation to focus on water contamination in a shanty town in north-eastern Brazil. Previous research determined that children less than three years old averaged 5.25 diarrhoea illnesses per year, and 8% of these infections were persistent (Lima et al. 2000), with consequent effects on malnutrition, growth and cognitive development (Lima et al. 1992; Guerrant et al. 1999; Moore et al. 2001; Niehaus et al. 2002). The high incidence of diarrhoeal illness may result from drinking water contamination as a potential source for faecal-oral transmission. This survey attempted to determine the relative contributions of source versus storage to household water contamination in Parque Universitario.
METHODS
From 19 May to 22 July 2005, research was conducted in the shanty town Parque Universitario in Fortaleza, Brazil. An earlier pilot study had shown a significant height-for-age deficit (averaging 2.4 cm) in children living in households with contaminated (vs. coliform-free) drinking water in this community (B. Beers, personal communication, July 2008). The community is situated in an area approximately 1.6 km by 1.6 km and divided into 22 blocks, housing 13,700 people (11% are less than five years old). After a census identifying households with at least one child under eight years old, approximately 30% of households were randomly selected for this study, yielding a potential sample size of 297 households and 491 children. An experienced team of community workers collected background household information as well as water and stool samples.
Participating households (n = 231) were invited to temporary stations within the community to record background and growth information for each child under eight years old. Sixty-six households did not participate for the following reasons: unoccupied (38), refused (9), no children (4), not found (2) and not at home (13). The mother received a stool collection cup labelled for each child; stool samples were refrigerated before collection the next day. After identifying the source and storage, a community worker collected 150 ml of the drinking water for the children in autoclaved plastic containers from the point of use. This report focuses on the analysis of those water samples.
Water was stored in a plastic bottle, a clay pot with a ceramic filter, a bottle and filtered clay pot, or a pot. A bottle, commonly a reused 2-l soda bottle, could be sealed with a screw-on cap. On the other hand, a filtered clay pot had a wide mouth, removable lid and a spigot that led to an infrequently cleaned and vulnerable filter. Rarely, water was transferred from the spigot to a bottle before consumption. The pots, without spigots, also had wide mouths for scooping water.
Two complementary methods for determining the concentration of faecal coliform colony forming units (cfu) were initiated within four hours of collection. For direct counts a sample of 100 µl was pipetted onto MacConkey agar (the agar was the best method available in Fortaleza as methyl-umbelliferryl β- glucuronidase (MUG) technologies were unavailable) and incubated for 24 hours to select for lactose and non-lactose fermenting gram-negative microorganisms. Filtering 100 ml, transferring the filter to MacConkey Agar, and incubating for 24 hours (37°C) attained a potentially more sensitive, indirect count. The 100 to 0.1 ml ratio of sample volumes implied a 1,000-fold higher sensitivity of the indirect versus direct methods. At the end of incubation, the number and type (lactose or non-lactose fermenting) of the colonies were recorded at the collaborating lab in Brazil.
Frequency and level of contamination of community water samples were summarized with descriptive statistics. Associations between categorical variables were assessed using the Pearson’s chi-square (χ2) test. One-way analysis of variance (ANOVA) tests were used to compare mean differences in level of contamination by origin and type of storage. SPSS© (Statistical Package for Social Surveys, Version 15.0) was used for all statistical analyses.
The level of contamination was categorized as: none, low, moderate, high or very high (Table 1). The rationale for the categories was based both on the ability to reliably count to 100 cfu on a plate and on the principle that a single count of 30 cfu or less cannot accurately represent the sample. Selected samples were further analysed by immuno-magnetic bead-based detection of Cryptosporidium and a species-specific faecal marker, human immunoglobulin (shown to be specific and sensitive to a 10−10 dilution of stool), to determine the feasibility of these sensitive tests for household drinking water samples, and to reinforce the coliform counts (Sevilleja et al. 2007). For the faecal marker test, household water samples were selected randomly within contamination levels and weighted to the extremes: 6 without contamination, 1 moderately, 1 highly and 11 very highly contaminated samples. From the highest contamination group, seven samples were also tested for Cryptosporidium after Sevilleja et al. (2007). Two samples without contamination and two moderately contaminated samples were tested for Cryptosporidium but not for the faecal marker.
Table 1.
Criteria for level of contamination categories
| Level | Range (cfu 100 ml−1) |
Description | Indirect count: Cultured a filter of 100 ml (cfu 100 ml−1) |
Direct count: Cultured 0.1 ml (cfu 0.1 ml−1) |
|---|---|---|---|---|
| 0 | 0 | None | 0 | 0 |
| 1 | 1–30 | Low | 1–30 | 0 |
| 2 | 31–100 | Moderate | 31–100 | 0 |
| 3 | 101–105 | High | >100 | 1–100 |
| 4 | >105 | Very high | >100 | >100 |
RESULTS AND DISCUSSION
Residents of the shanty town utilized a variety of water practices (Table 2). Tap water is the primary source for the community (193/231: 83.5%), followed by well water (24/231: 10.4%). The uncommon sources, fountain and mineral, as well as the combinations of ‘tap and mineral’ and ‘tap and well’ accounted for the other 6%: 0.9 (2/231), 3.9 (9/231), 0.4 (1/231), and 0.9 percent (2/231), respectively. Bottles (113/231: 48.9%) and filtered clay pots (108/231: 46.8%) were major storage methods compared with ‘bottle and filtered clay pot’ (2/231: 0.9%) and non-filtered clay pots (8/231: 3.5%).
Table 2.
Origin and Water Source Usage Pattern
| Origin | Storage type | ||||
|---|---|---|---|---|---|
| Bottle | Filter | Filter and bottle | Pot | Total | |
| Fountain | 1 | 1 | 0 | 0 | 2 |
| Mineral | 7 | 1 | 1 | 0 | 9 |
| Tap and mineral | 1 | 0 | 0 | 0 | 1 |
| Tap | 86 | 99 | 1 | 7 | 193 |
| Well | 17 | 6 | 0 | 1 | 24 |
| Well and tap | 1 | 1 | 0 | 0 | 2 |
| Total | 113 | 108 | 2 | 8 | 231 |
The community water samples demonstrated faecal contamination in slightly under a third of household drinking water samples (70/231: 30.3%; Figure 1). In addition, of those that were contaminated, the substantial majority contained more than 100 cfu 100 ml−1 (50/70: 71.4%). Fourteen samples (14/231: 6.1%) contained more than 1,000 cfu per 100 ml.
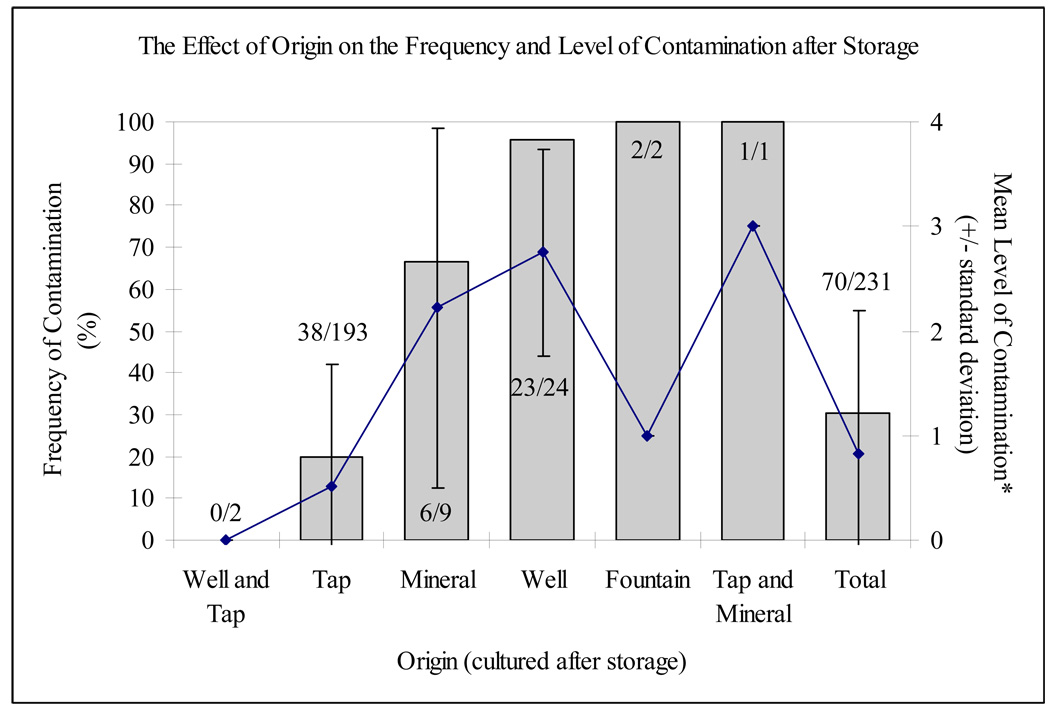
Figure 1.
Frequency and level of contamination by origin (these averages were calculated using the assigned values of 0, 1, 2, 3 and 4 to none, low, moderate, high and very high levels of contamination, respectively; see Table 1)
The origin of the stored water had a significant effect on the frequency of faecal contamination (x2 = 72.50; p < 0.001). Over 65% of water samples with well (23/24: 95.8%) or only mineral (6/9: 66.7%) water as a source were contaminated. While contamination of the untreated well water was anticipated, mineral water was popularly considered a cleaner alternative to tap water and thus was purchased at a higher price. Still, the contamination of nearly 20% of samples, after collection of clean tap water (B. Beers, personal communication, July 2009), indicates the vulnerability of water quality to problematic household water storage practices.
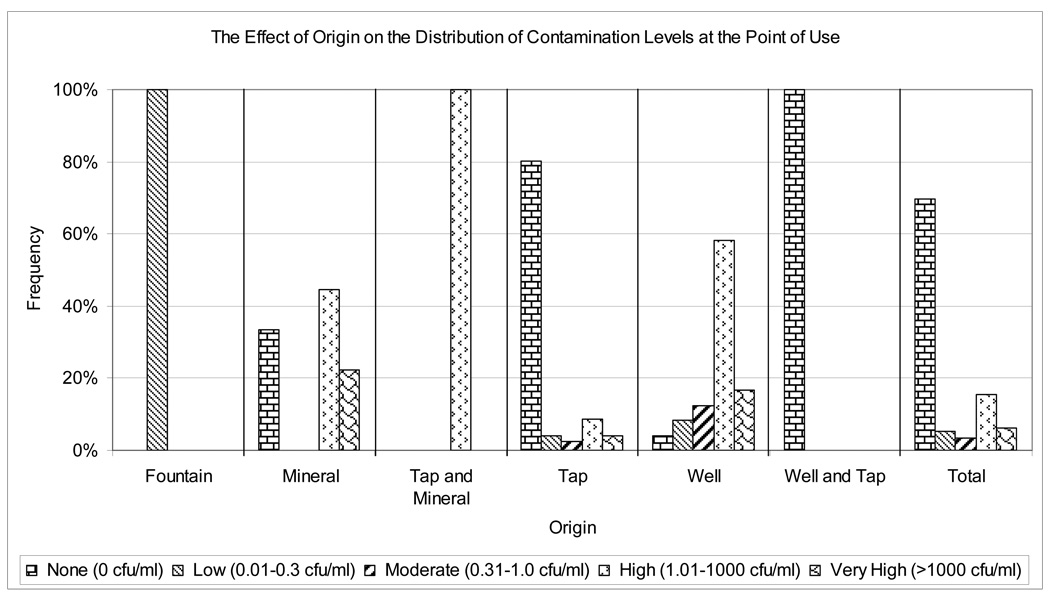
Drinking water origin also determined the level of contamination (F = 19.39; p < 0.001; Figure 1 and Figure 2). The few contaminated tap water samples (38/193: 19.7%) showed a wide range of levels of contamination: low (21.1%: 8/38), moderate (13.2%: 5/38), high (44.7%: 17/38) and very high (21.1%: 8/38). Of the 23 contaminated well water samples, 18 (78.3%) had higher levels of contamination (>100 cfu 100 ml−1).
Figure 2.
Distribution of levels of contamination by origin
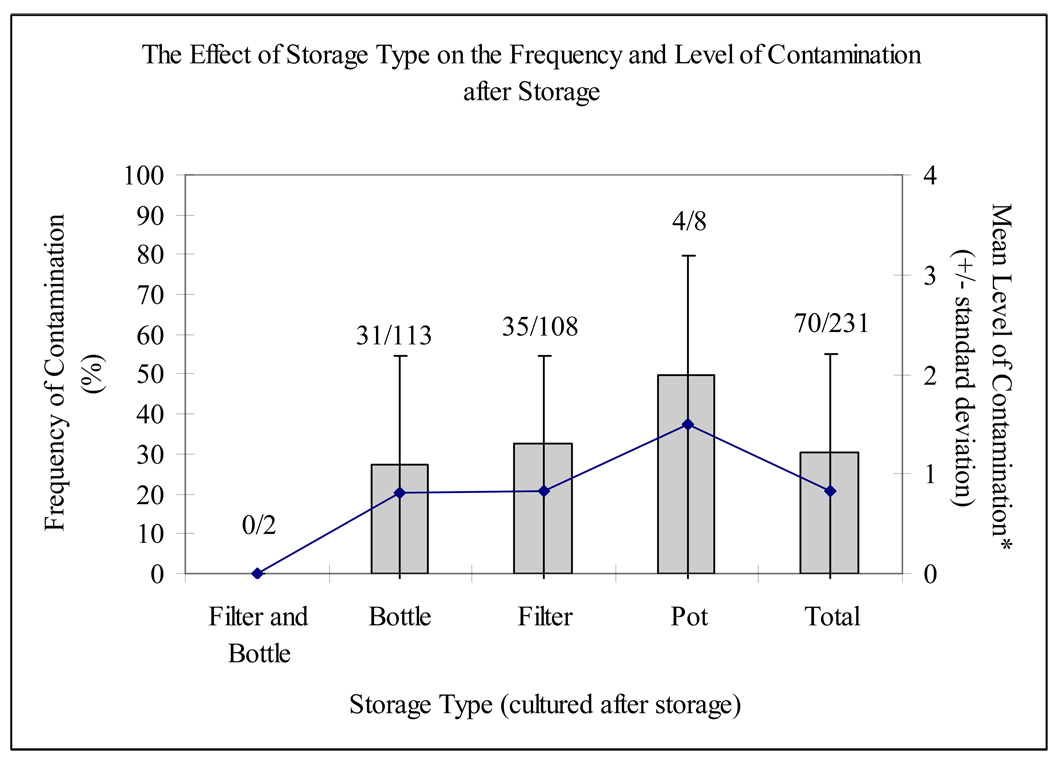
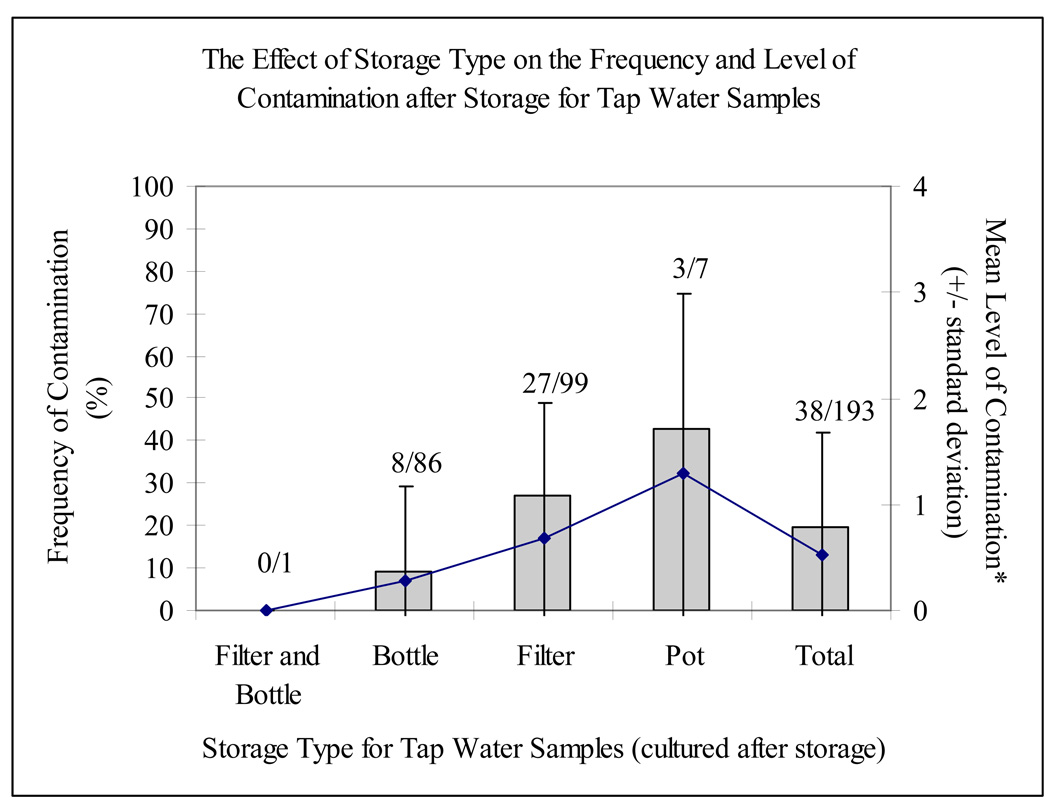
In comparison with the significant contribution of origin to the risk for and level of contamination, the type of storage was not a major determinant of either without controlling for origin (x2 = 0.39 for risk and F = 1.93 for level, p > 0.05; Figure 3). However, selecting for stored tap water samples (n = 193), a substantial relationship between faecal contamination and storage emerged (x2 = 12.09; p = 0.007; Figure 4). For tap water, samples were three times more likely to be contaminated during storage in a filtered clay pot than in a bottle (27.3 versus 9.3%). Similarly, three out of seven tap water samples stored in a pot (42.9%) tested positive. There was no statistical effect of storage type on categorical level of contamination for any source.
Figure 3.
Frequency and level of contamination by storage type (these averages were calculated using the assigned values of 0, 1, 2, 3 and 4 to none, low, moderate, high and very high levels of contamination, respectively; see Table 1)
Figure 4.
Frequency and level of contamination by storage type of tap water (these averages were calculated using the assigned values of 0, 1, 2, 3 and 4 to none, low, moderate, high and very high levels of contamination, respectively; see Table 1)
Of the 11 samples tested using the method of Sevilleja and colleagues (2007), seven of the very highly contaminated samples (>105 cfu 100 ml−1) also contained the human specific faecal marker (total Ig), implicating a human source of contamination. While all of the samples without contamination (0/6), as well as the sole highly contaminated sample (101–105 cfu 100 ml−1), were negative for the indicator, the moderately contaminated sample (31–100 cfu 100 ml−1) was positive. In the diagnostic test for Cryptosporidium, only one sample, which also yielded a very high coliform count, was positive at a level of 5–6 parasites per ml. Interestingly, with a negative result for the human faecal marker, this sample may have had a non-human origin (e.g. cattle) for both the faecal coliforms and Cryptosporidium. Furthermore, while the small number of samples limit an analysis of source and storage effects, this case was the only sample tested for the human faecal marker and Cryptosporidium stored in a non-filtered clay pot.
CONCLUSIONS
For the Brazilian shanty town under surveillance, these findings suggest a high prevalence of household level faecal contamination and a corresponding vulnerability to infection by diarrhoea-causing pathogens. Consequently, this research provides insight into a likely contributor (along with food contamination and direct person-to-person spread) to the documented frequency of diarrhoea (Lima et al. 2000) and resultant burden on childhood development (Lima et al. 1992; Guerrant et al. 1999; Niehaus et al. 2002). More generally, it illustrates the water quality disparities among communities in the developing and developed world. In contrast to the faecal contamination of over a quarter of household water samples found in this study, the US Environmental Protection Agency has established that no more than 5% of 100-ml water samples from a particular source can test positive for faecal colony forming units (USEPA 1989). As a result of the substantially lower water quality, the developing world, as exemplified in this community, shares a disproportionate burden of water related-disease (Pruss et al. 2002). A decrease in this water-related risk for early childhood infections would be likely to correspond to gains in physical as well as cognitive development (Guerrant et al. 1999; Niehaus et al. 2002).
In order to better manage the water-related disease burden, the observed effect of source on faecal contamination necessitates improvements to drinking water at the origin. For example, according to this study, a shift away from well to tap water would result in a three-quarters reduction in contamination at the household level. Continued reliance on well water, in contrast, compounds source and storage contamination via high environmental exposure and lack of chlorine/presence of organic matter.
Even with a complete transition to an improved source, however, the tap water data suggests that problematic storage would still yield a high contamination rate (~20%). The introduction of faecal matter to stored drinking water may result from several activities: scooping with contaminated hands in the container, manually clearing blocked filters and inadvertently contaminating vessels during cleaning. Independent of the specific route for transmission, vulnerable storage methods limit the potential gains from a protected water supply. This finding corroborates reports of contamination during storage in Bolivia (Quick et al. 1999), Peru (Oswald et al. 2007), Sierra Leone (Clasen & Bastable 2003) and Rwanda (Gasana et al. 2002). A comprehensive approach to improvements in household drinking water must focus on improving both water source and storage.
Future research should elaborate the pathways for faecal contamination at the source and during storage. For example, a detailed account of household water practice would differentiate between direct hand contact and unhygienic objects as the means for contamination. Moreover, various water improvement methods should be considered in the socioeconomic context of the community. Mintz et al. (1995) and Quick et al. (2002) obtained impressive results with small-necked containers that prevent children from putting their hands directly in the water. The feasibility of diagnostic tests for human-specific faecal contamination opens new possibilities for the assessment of the frequency and roles of human versus animal faecal contamination, which is likely to be relevant to the transmission of predominantly human pathogens, such as Cryptosporidium hominis and most enterotoxigenic and probable enteroaggregative E. coli, not to mention such viral pathogens as noroviruses, rotaviruses and hepatitis A viruses. Ultimately, future work must continue to investigate the underlying causes of and potential solutions to the extensive diarrhoea burden in impoverished communities around the world.
ACKNOWLEDGEMENTS
The authors are grateful for the field support from the community workers in Brazil and the financial support from NIAID, NIH, ICIDR Grant 5U01-AI026512, and ICIDR Biodefense Supplement.
REFERENCES
- Berkman D, Lescano A, Gilman R, Lopez S, Black M. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet. 2002;359:564–571. doi: 10.1016/S0140-6736(02)07744-9. [DOI] [PubMed] [Google Scholar]
- Black R, Brown K, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73(6):799–805. [PubMed] [Google Scholar]
- Clasen T, Bastable A. Faecal contamination of drinking water during collection and household storage: the need to extend protection to the point of use. J. Wat. Health. 2003;1(3):109–115. [PubMed] [Google Scholar]
- Gasana J, Morin J, Ndikuyeze A, Kamoso P. Impact of water supply and sanitation on diarrheal morbidity among young children in the socioeconomic and cultural context of Rwanda (Africa) Environ. Res. 2002;90:76–88. doi: 10.1006/enrs.2002.4394. [DOI] [PubMed] [Google Scholar]
- Guerrant R, Hughes J, Lima N, Crane J. Diarrhea in developed and developing countries: magnitude, special settings, and etiologies. Rev. Infect. Dis. 1990;1 supplement:41–50. doi: 10.1093/clinids/12.Supplement_1.S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant D, Moore S, Lima A, Patrick P, Schorling J, Guerrant R. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function 4 to 7 years later in a poor urban community in northeast Brazil. Am. J. Trop. Med. Hyg. 1999;61(5):707–713. doi: 10.4269/ajtmh.1999.61.707. [DOI] [PubMed] [Google Scholar]
- Kosek M, Bern C, Guerrant R. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull. World Health Organ. 2003;81(3):197–204. [PMC free article] [PubMed] [Google Scholar]
- Leclerc H, Schwartzbrod L, Dei-Cas E. Microbial agents associated with waterborne diseases. Crit. Rev. Microbiol. 2002;28(4):371–409. doi: 10.1080/1040-840291046768. [DOI] [PubMed] [Google Scholar]
- Lima A, Fang G, Schorling J, De Albuquerque L, Mcauliffe J, Mota S, Leite R, Guerrant R. Persistent diarrhea in northeast Brazil: etiologies and interactions with malnutrition. Acta Paediactr. 1992;81(381):39–44. doi: 10.1111/j.1651-2227.1992.tb12370.x. [DOI] [PubMed] [Google Scholar]
- Lima A, Moore S, Barboza M, Jr, Soares A, Schleupner M, Newman R, Sears C, Nataro J, Fedorko D, Wuhib T, Schorling J, Guerrant R. Persistent diarrhea signals a critical period of increased diarrhea burdens and nutritional shortfalls: a prospective cohort study among children in northeastern Brazil. J. Infect. Dis. 2000;181:1643–1651. doi: 10.1086/315423. [DOI] [PubMed] [Google Scholar]
- MacKenzie W, Hoxie N, Proctor M, Gradus M, Blair K, Peterson D, Kazimierczak J, Addiss D, Fox K, Rose J, Davis J. A massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. New Engl. J. Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- Martorell R, Yarbrough C, Lechtig A, Habicht J, Klein R. Diarrheal diseases and growth retardation in preschool Guatemalan children. Am. J. Phys. Anthropol. 1975;43(3):341–346. doi: 10.1002/ajpa.1330430307. [DOI] [PubMed] [Google Scholar]
- Mintz E, Reiff F, Tauxe R. Safe water treatment and storage in the home. A practical new strategy to prevent waterborne disease. J. Am. Med. Assoc. 1995;273(12):948–953. [PubMed] [Google Scholar]
- Moore A, Herwaldt B, Craun G, Calderon R, Highsmith A, Juranek D. Surveillance for waterborne disease outbreaks: United States, 1991–1992. MMWR CDC Surveillance Summaries. 1993;42(5):1–22. [PubMed] [Google Scholar]
- Moore S, Lima A, Conaway M, Schorling J, Soares A, Guerrant R. Early childhood diarrhea and helminthiases associate with long-term linear growth faltering. Int. J. Epidemiol. 2001;30:1457–1464. doi: 10.1093/ije/30.6.1457. [DOI] [PubMed] [Google Scholar]
- Niehaus M, Moore S, Patrick P, Derr L, Lorntz B, Lima A, Guerrant R. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian Shantytown. Am. J. Trop. Med. Hyg. 2002;66(5):590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- Oswald W, Lescano A, Bern C, Calderon M, Cabrera L, Gilman R. Fecal contamination of drinking water within peri-urban households, Lima, Peru. Am. J. Trop. Med. Hyg. 2007;77(4):699–704. [PubMed] [Google Scholar]
- Pruss A, Kay D, Fewtrell L, Bartram J. Estimating the burden of disease from water, sanitation, and hygiene at a global level. Environ. Health Perspect. 2002;110(5):537–542. doi: 10.1289/ehp.110-1240845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick R, Venczel L, Mintz E, Soleto L, Aparicio J, Gironaz M, Hutwagner L, Greene K, Bopp C, Maloney K, Chavez D, Sobsey M, Tauxe R. Diarrhoea prevention in Bolivia through point-of-use water treatment and safe storage: a promising new strategy. Epidemiol. Infect. 1999;122:83–90. doi: 10.1017/s0950268898001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick R, Kimura A, Thevos A, Tembo M, Shamputa I, Hutwagner L, Mintz E. Diarrhea prevention through household-level water disinfection and safe storage in Zambia. Am. J. Trop. Med. Hyg. 2002;66(5):584–589. doi: 10.4269/ajtmh.2002.66.584. [DOI] [PubMed] [Google Scholar]
- Rowland M, McCollum J. Malnutrition and gastroenteritis in Gambia. Trans. Roy. Soc. Trop. Med. Hyg. 1977;71(3):199–203. doi: 10.1016/0035-9203(77)90006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorling J, Guerrant R. Diarrhoea and catch-up growth. Lancet. 1990;335:599–600. doi: 10.1016/0140-6736(90)90378-i. [DOI] [PubMed] [Google Scholar]
- Sevilleja J, Copeland C, Guerrant R. Detection of a highly sensitive human fecal biomarker (10−10) in less than or equal to 10 ml contaminated drinking water using immunomagnetic separation. Am. J. Trop. Med. Hyg. Abstract Book: 56th ASTMH Annual Meeting. 2007;77(5):216. [Google Scholar]
- Stephensen C. Burden of infection on growth failure. J. Nutr. 1999;129(28):534S–538S. doi: 10.1093/jn/129.2.534S. [DOI] [PubMed] [Google Scholar]
- United Nations. Millennium Development Project Report 2005. 2005
- USEPA (US Environmental Protection Agency) Drinking water; national primary drinking water regulations; total coliforms (including fecal coliforms and E. coli); final rule. Federal Register. 1989;54:27544–27568. [Google Scholar]