Summary
Neurogenesis continues throughout adulthood in the brains of many animals, including some insects[1–3], and relies on the presence of mitotic neural stem cells. However, in mammals, unlike the liver or skin, the regenerative capacity of the adult nervous system is extremely limited. Using the Drosophila brain as a model, we investigate the mechanisms that restrict adult neurogenesis, with the eventual goal of devising ways to replace neurons lost by injury or disease. We find that in Drosophila, adult neurogenesis is absent due to the elimination of neural stem cells (neuroblasts) during development by cell death. Prior to their elimination, neuroblasts undergo a developmentally regulated reduction in their growth and proliferation rates due to downregulation of insulin/PI3 kinase signaling, resulting in increased levels of nuclear Foxo. These small, growth-impaired neuroblasts are often eliminated by caspase-dependent cell death and not exclusively by terminal differentiation as has been previously proposed[4]. Eliminating Foxo, together with inhibition of pro-apoptotic proteins promotes long-term survival of neuroblasts and sustained neurogenesis in the adult mushroom body (mb), the center for learning and memory in Drosophila. Foxo likely activates an autophagic cell death response, since simultaneous inhibition of ATG1 (autophagy-specific gene 1) and apoptosis also promotes long-term mb neuroblast survival. mb neurons generated in the adult incorporate into the existing mb neuropil suggesting that both neuroblast identity and neuronal pathfinding cues are intact. Thus neurogenesis can be induced in adults by antagonizing pathways that normally function to eliminate neural stem cells during development.
Results and Discussion
The Drosophila brain provides an excellent model system in which to investigate how neurogenesis becomes progressively restricted because neurons are generated only during development and not in adulthood[5–7]. All neurons within the central brain are generated from the asymmetric cell divisions of neural stem cells known as neuroblasts[8–10]. Neuroblasts are large in size and mitotically active, likely dividing every one to two hours during later larval development[6, 11]. Because central brain neuroblasts divide only asymmetrically and never symmetrically, their numbers remain constant. Therefore they can be followed unambiguously over time using neuroblast-specific molecular markers[12]. Since neurogenesis at all stages of development is characterized by the presence of neuroblasts, we first examined whether adult Drosophila brains have neuroblasts. We detected no cells displaying the molecular signature of neuroblasts in young (3–5 days post-eclosion) adult brains i.e. expression of Deadpan (Dpn), a neuroblast transcription factor, Miranda (Mira), a cargo carrier of cell fate determinants and nuclear exclusion of Prospero (Pros) (n>10 adult brains). Consistent with previous studies[5–7], we also found no evidence for cell proliferation in these young adult brains using either MARCM[13], a genetic technique which positively marks cells only after cell division, or by administering BrdU, a thymidine analogue. Therefore adult neurogenesis is likely absent because no actively dividing neuroblasts are present in the adult Drosophila brain.
Reduction in neuroblast growth precedes neuroblast disappearance
To understand why adult neuroblasts are not present, we examined brains at earlier developmental stages when neuroblasts are still present. Using an antibody to Dpn and an E2F-responsive GFP reporter (pcna:eGFP)[14] (Figure1A,D), we identified 100 (mean, S.D.±2.73, n=8 brain lobes) large neuroblasts within the central brain of each brain lobe in mid L3 larvae (96 hours ALH, after larval hatching) during the height of neurogenesis (Figure1B,C). This is consistent with previous reports[6, 12]. Approximately 30 hours later, at five hours after pupal formation (APF), neuroblast number remained unchanged yet neuroblast cell size and mitotic activity were reduced (Figure1B,C), suggesting that neuroblast growth and division rates were changing. By 30 hours APF, only a few small neuroblasts remained, other than the mb neuroblasts (Figure1B and data not shown) (see below). This coincided with a complete shut down in cell proliferation as assessed by the lack of pcna:eGFP expressing cells (compare Figure1A to1D). Thus during early pupal stages, most central brain neuroblasts decrease their rates of growth and proliferation, raising the possibility that these changes trigger neuroblast disappearance and terminate neurogenesis.
Figure 1. Neuroblast growth becomes restricted during pupal development.
(A,D) Z projection of the brain (optic lobe, OL, and central brain, CB) and ventral nerve cord (VNC) from wild type (WT) animals (pcna:eGFP transgenic) at 15 (A) or 48 (D) hours after pupal formation (APF). Neuroblasts express Deadpan (Dpn, red) and the E2F reporter pcna:eGFP (GFP, green). (D) Only the four mushroom body (mb) neuroblasts (nbs) located on the dorsal CB surface persist late (arrowheads in A,D). Dotted box denotes area of CB magnified in larger inset with one of the four mb neuroblasts in small inset. Scale bar (A) equals 50µm. (B,E) Quantitation of CB, (including the mb neuroblasts) (B) and mb (E) neuroblast number and number of mitotic neuroblasts based on PH3 (phospho-histone H3) per brain lobe over time. n=number of brain lobes scored per time point. (C) Distribution of CB neuroblast cell size over time. The average diameter of all CB neuroblasts from three brain lobes (3 animals) was measured for each of 3 time points. (F) Quantitation of the average diameter (diam.) of mb neuroblasts over time. Number of mb neuroblasts measured for each time point shown as white number in black columns. *P-value <0.001. Columns represent mean, ±std.dev in this and all subsequent figures. ALH, after larval hatching.
Of all the neuroblasts in the central brain, only the mb neuroblasts remain midway through pupal development[6, 15], and are all located on the dorsal surface of the central brain, superficial to the mb calyx (Figure 1D,E). At 15 hours APF, mb neuroblasts can be positively identified among the remaining central brain neuroblasts (Figure1A), solely based on their size (average diameter=11.6 µm, S.D.±0.93, n=48). They remained large and mitotically active, similar to larval neuroblasts until 72 hours APF (Figure1B,C,E,F). After this, we observed a significant decrease in mb neuroblast cell size, followed 6 hours later by a strong reduction in their mitotic activity (Figure1E,F). By 96 hours APF, approximately ten hours before adult eclosion, essentially no mb neuroblasts were detected (Figure1E). Therefore all neuroblasts including the late persisting mb neuroblasts experience a reduction in growth prior to their disappearance, suggesting that a common lineage-independent mechanism links a decrease in growth with neuroblast disappearance.
Mushroom body neuroblasts are eliminated via Reaper-dependent cell death
A recent study[4] concluded that the most likely explanation for neuroblast disappearance within the central brain is terminal differentiation, which requires Pros to localize to the neuroblast nucleus after the final cell division. Therefore, we first examined mb neuroblasts for evidence of nuclear Pros at the time that best approximates when the final cell divisions of these neuroblasts occur (Figure1E). While nuclear localization of Pros could be transient and hence difficult to observe, we and others[15] found no evidence of Pros in the nuclei of mb neuroblasts at 84 or 90 hours APF (Figure S1C,D). This suggests that a mechanism other than terminal differentiation could account for mb neuroblast disappearance. Because neuroblasts located in the abdominal segments of the ventral nerve cord are eliminated by apoptosis[16, 17], we next tested whether central brain neuroblasts, including the mb neuroblasts also undergo cell death. Indeed, at 25 and 90 hours APF, when either central brain or mb neuroblast number was dramatically declining, we observed neuroblasts displaying characteristics of dying cells, i.e. no nuclear envelope, activated caspase, and fragmented DNA (Figure2A–C, FigureS1A,B). Taken together, these results suggest that at least some central brain neuroblasts including the mb neuroblasts are eliminated by apoptosis and not differentiation.
Figure 2. Reaper-family pro-apoptotic regulators and caspase activation are required for the proper timing of neuroblast cell death.
(A–C) Wild type (WT) mb neuroblasts undergo cell death (B,C). Developmental time and genotype are listed above panels and markers are listed within panels in this and all subsequent figures. (A–C) Top two rows, single channel images with colored overlay in bottom row, showing co-localization of cell death and neuroblast markers (B,C). Mb neuroblast (brackets) at early pupal stages (A), days prior to mb neuroblast cell death (B,C). DNA fragmentation (C, TUNEL) and nuclear envelope breakdown, as indicated by an absence of Lamin staining (B), are hallmarks of cell death (compare with Lamin staining of a mb neuroblast with an intact nuclear envelope, B, inset). (D–F, H–J) Z-projections of the dorsal, anterior CB surface. Aberrantly persisting neuroblasts are marked with white arrows and mb neuroblasts are marked with arrowheads. In rpr− and RHG miRNA adults, the aberrantly persisting neuroblasts are the mb neuroblasts. Only one of the 4 mb neuroblasts is shown in panel F. (H–J) The mb calyx is outlined by the white dash. (G,K) Quantitation of Dpn-expressing CB or mb neuroblast number per brain lobe over time. n=number of brain lobes scored at each time point. worniuGAL4 is used to induce UAS transgene expression (F,G,J,K). Scale bar (A,D) equals 10µm. see also FigureS1,S2.
If this were the case, then central brain neuroblasts would persist in animals with reduced levels of the Reaper-family of pro-apoptotic regulators. We examined brains from animals transheterozygous for two overlapping deletions Df(3L)H99 and XR38 [17]. These animals, hereafter referred to as rpr mutants, were homozygous-null for reaper (rpr) and heterozygous for hid, grim, and sickle, all four being pro-apoptotic genes[18]. At 30 hours APF, rpr mutant brains had more neuroblasts than wild type controls (Figure2G). This was not due to the initial specification of extra neuroblasts or symmetric neuroblast cell divisions, since rpr mutant larval brains have a normal number of large, Dpn-expressing neuroblasts at earlier stages (data not shown). Central brain neuroblasts aberrantly persisted even at 48 hours APF (Figure2D,E,G), yet their numbers were considerably reduced. We also observed transient persistence of mb neuroblasts in brains of young adult rpr mutants (Figure2H,I,K, MovieS1,S2). This delay in neuroblast disappearance could be due to residual pro-apoptotic activity in rpr mutants. Therefore, we used MARCM[13] to generate neuroblasts that are homozygous null for reaper, hid and grim (H99). We observed Dpn-expressing cells in H99 mutant mb neuroblast clones only (FigureS1E), in brains of young (3–5 days post eclosion) adult animals (n>40 central brain neuroblast clones scored), suggesting that neuroblasts are still eliminated despite removal of additional pro-apoptotic genes. Unfortunately the low frequency of H99 mutant mb neuroblast clones precluded the use of this method for a detailed study of the fate of persisting adult mb neuroblasts at all stages. Therefore we engineered a transgene, UAS-RHG miRNA, which generates microRNAs that simultaneously inhibit reaper, hid and grim and targeted expression of this transgene to neuroblasts using worniuGAL4 (hereafter referred to as RHG miRNA) (FigureS2). In the brains of young RHG miRNA adults, the only neuroblasts observed were mb neuroblasts, whose persistence, again, was only transient (Figure2J,K). Similar results were observed following worniuGAL4 induced expression of baculovirus p35, an inhibitor of active caspases (Figure2F,G,K). Thus, Reaper-family proteins and caspase activation are necessary for the proper timing of neuroblast removal. In their absence, neuroblast elimination is only delayed, suggesting that an alternative caspase-independent pathway compensates to ensure neuroblast removal. Interestingly, neuroblasts persisted longer in rpr mutant adults than in those expressing RHG miRNA, which could be due to downregulation of worniuGAL4 (FigureS1F,G). This may indicate that neuroblasts rescued from an apoptotic fate have decreased transcription of neuroblast-specific genes, which may contribute to their disappearance via an alternative mechanism.
Cell death-inhibited mushroom body neuroblasts have impaired growth
Next, we examined the fate of the persisting mb neuroblasts in the brains of young (3–5 days post eclosion) rpr mutant adults. Persisting mb neuroblasts expressed both pcna:eGFP and Mira, showed an enrichment of membrane bound Scribble (Scrib), and excluded Pros from the nucleus, all indicators of proper neuroblast cell fate (FigureS3A,B,D). They also expressed the mb specific marker, Eyeless (Ey) and failed to express Elav or Repo, two markers characteristic of differentiated neuronal and glial cell fates respectively (FigureS3C,E). However, the persisting adult mb neuroblasts were only half the size of mb neuroblasts present during earlier stages of development (Figure4A) and they divided slowly (FigureS3F–H), generating very few new adult mb neurons (FigureS3I–L). Thus, growth of persisting mb neuroblasts may be impaired, which could contribute to neuroblast elimination via a caspase-independent pathway.
Figure 4. Long-term surviving mb neuroblasts proliferate and generate new adult mb neurons.
(A) Summary of mb neuroblast cell size over time. Genotypes color-coded at top for panels a–c. Each dash (A) or diamond (B,C) represents one neuroblast. (A) *P-value <0.003, **P-value <0.0003. (B,C) Number of BrdU progeny generated in 24 hours relative to mb neuroblast cell size. Foxo restricts mb neuroblast proliferation rate and cell size. Mb neuroblast cell size and proliferation rate correlate in 2-week-old RHG miRNA, foxo− adults (C) but not in 3–5 day adults (B). R2=coefficient of determination for a linear correlation. (D–H) Mb neuroblasts (white brackets) and progeny (numbered) following a 24 hour BrdU treatment. (H) Only 14 of 18 progeny are shown. (I,K) Month-old adult mb neuroblasts (I, bracket) generate new mb neurons, cell bodies (I, outline) and axons (K). Inset is double-labeled neuroblast, mCD8:GFP (green) and Dpn (red). genotype: worniuGAL4,UASmCD8:GFP,UAS-RHGmiRNA,foxo−/foxo− (K) Reconstruction of the mushroom body (red) with overlay of newborn adult neurons (mCD8:GFP, green). (J) Dendrites from new neurons project normally to the mb calyx (arrow, 2-week adult). (K,right) Axons project either normally through the mb pedunculus or mistarget (K,left). Single arrow marks premature axon bifurcation (K,left) and double arrow marks termination at the top of the α lobe. (L) model (see discussion). worniuGAL4 is used to induce UAS transgene expression (A–E,G–K). Scale bar (D,I–K) equals 10µm. see also FigureS4.
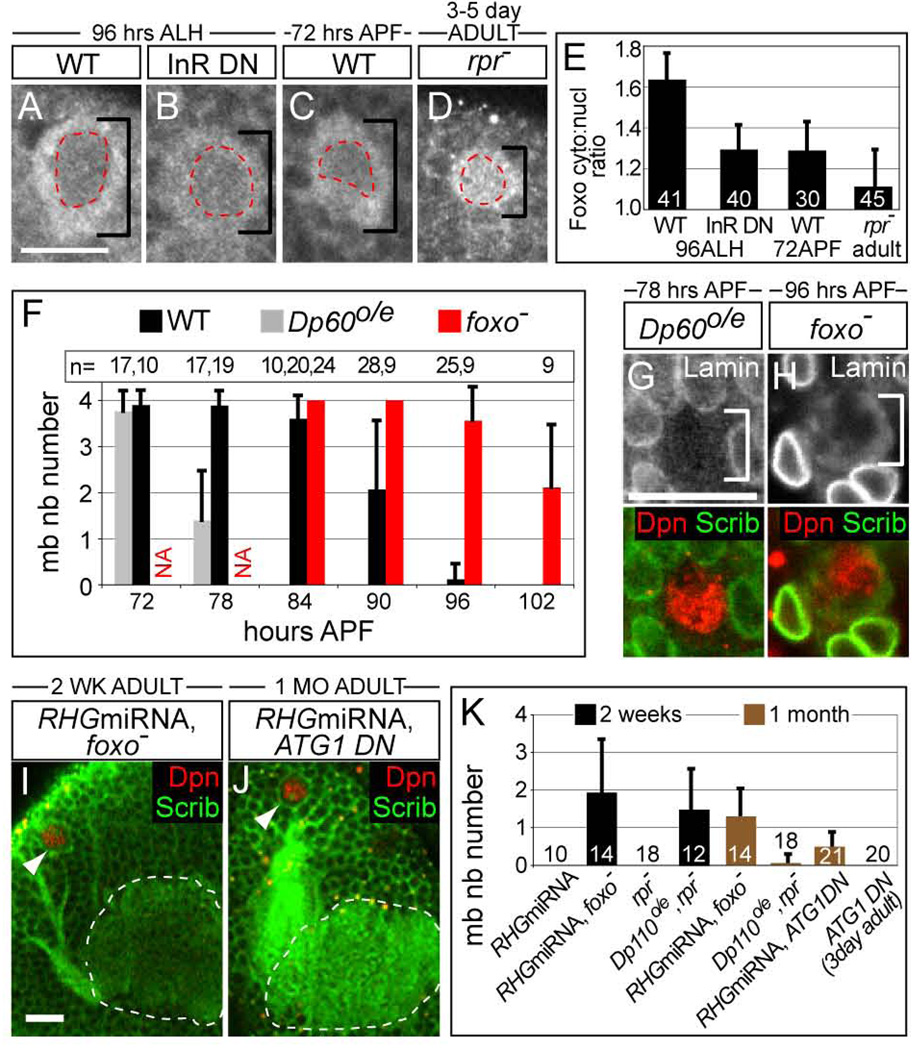
Consistent with this possibility, we observed that changes in the subcellular localization of the transcription factor Foxo correlate with changes in mb neuroblast cell size. Foxo was mostly cytoplasmic in wild type larval neuroblasts, when growth rates are high, consistent with elevated insulin/PI3 kinase signaling[19, 20] (Figure3A,E). However, more Foxo was observed in the nucleus in 72-hour-old pupal mb neuroblasts (Figure3C,E), when neuroblast size decreases (Figure1E,F), suggesting that insulin/PI3K signaling is reduced in these cells. This increase in nuclear Foxo was equivalent to that observed in larval neuroblasts that expressed a dominant negative Insulin receptor (InR DN) transgene (Figure3B,E). Levels of nuclear Foxo were even further elevated in the persisting mb neuroblasts observed in rpr mutant adults (Figure3D,E). Thus, the Reaper-independent pathway that eliminates neuroblasts may be activated by a reduction in insulin/PI3 kinase signaling.
Figure 3. Increased levels of nuclear Foxo restrict long-term survival of mb neuroblasts.
(A–D) Subcellular localization of Foxo in neuroblasts. All neuroblasts imaged under same settings for direct comparison. Neuroblasts are marked with black brackets and their nuclei are outlined in red. (E) Quantitation of the ratio of cytoplasmic to nuclear Foxo protein, based on fluorescence pixel intensities. White number in black columns equals number of neuroblasts scored for each genotype/time. (F) Quantitation of mb neuroblast number over time. (mean, std deviation). Dp60 overexpression (o/e) inhibits PI3K signaling leading to premature mb neuroblast cell death (F,G), while foxo mutant mb neuroblasts delay death (F,H). n equals number of brain lobes scored for each genotype at each time point (F). (G,H) Dying mb neuroblasts lack a nuclear envelope. Compare Lamin staining with inset in Figure2B. Top panels are single image and bottom row is overlay. (I,J) Z-projections of the dorsal brain surface showing persisting adult mb neuroblasts (arrowheads) near the mb calyx (white outline). (K) Quantitation of mb neuroblast number over time. Numbers in columns equals number of brain lobes scored for each genotype/time. worniuGAL4 is used to induce UAS transgene expression (B,E,F,G,I–K). Scale bar (A,G,I) equals 10µm. see also FigureS3.
Next we asked whether insulin/PI3 kinase activity regulates mb neuroblast cell death. We overexpressed Dp60 in mb neuroblasts to inhibit PI3 kinase and observed that mb neuroblasts undergo premature cell death (Figure3F,G). Conversely mb neuroblasts persist slightly longer in foxo mutant mb neuroblasts as well as in mb neuroblasts that express a constitutively active insulin receptor transgene, yet are still eliminated via cell death (Figure3F,H, and data not shown). Therefore levels of insulin/PI3 kinase signaling regulate timing of mb neuroblast caspase-induced cell death.
Foxo restricts growth and survival of apoptosis-inhibited mushroom body neuroblasts
To examine the consequences of maintaining insulin/PI3 kinase signaling in mb neuroblasts with decreased function of Reaper-family proteins, we either removed foxo function[19] or overexpressed Dp110, the catalytic subunit of PI3 kinase[21] (Dp110o/e). We observed Dpn-expressing mb neuroblasts in 2-week-old and even in 1-month-old RHG miRNA, foxo mutant adults, as well as in Dp110o/e, rpr mutant adults (Figure3I,K,4I, FigureS4A). In marked contrast, no neuroblasts were observed at these later time points in RHG miRNA expressing adults or those mutant only for either rpr or foxo (Fig3H, FigureS5A,C). Thus activation of the insulin/PI3 kinase pathway overcomes the caspase-independent mechanism that eliminates mb neuroblasts in rpr mutants and promotes long-term survival of mb neuroblasts in adult brains.
Does insulin/PI3 kinase activation suppress autophagy in persisting mb neuroblasts? Autophagic cell death due to downregulation of PI3 kinase signaling and apoptosis are both required for proper elimination of the Drosophila larval salivary gland[22]. To determine whether elimination of Foxo promotes long-term survival of RHG miRNA mb neuroblasts via inhibition of autophagy, we expressed a dominant-negative version of the autophagy-promoting protein ATG1[23] in RHG miRNA mb neuroblasts. Again, we observed Dpn-expressing mb neuroblasts in one month old ATG1 DN, RHG miRNA animals, but not in ATG1 DN neuroblast expressing animals alone. Similar to foxo mutant animals, ATG1 DN mb neuroblasts also have a slight delay in their disappearance. Therefore downregulation of insulin/PI3 kinase may serve as a potent back-up mechanism to ensure mb neuroblast elimination via activation of an autophagic cell death response.
Next we assayed the behavior of these long-term persisting adult mb neuroblasts. We observed that some, but not all, 2-week-old RHG miRNA, foxo mutant mb neuroblasts were large in size and generated many new progeny compared to young (3–5 days post eclosion) neuroblasts that either expressed RHG miRNA or were mutant for rpr (Figure4A–C,D,F,H, FigureS4F,H). Similarly, some young (3–5 day post eclosion) RHG miRNA, foxo mb neuroblasts were also larger in size and generated many more progeny (Figure4A,B,E), suggesting that increased levels of nuclear Foxo also restrict proliferation and growth in mb neuroblasts inhibited for Reaper family proteins. Interestingly, we observed a strong correlation between mb neuroblast cell size and progeny number in 2-week-old RHG miRNA, foxo mutant adults, but not in younger mutant adults (Figure4B,C, FigureS6A). Thus, activation of the growth-promoting insulin/PI3 kinase pathway sustains not only long-term survival of adult mb neuroblasts but also increases their proliferation and growth rate. Furthermore, Foxo inactivation may indirectly allow long-term persisting adult mb neuroblasts to acquire growth properties common to neuroblasts during development.
Long-term surviving adult mb neuroblasts generate new mb neurons
Lastly, we analyzed the morphology of the newborn cells by visualizing mCD8:GFP, which perdures in daughter cells following neuroblast cell divisions (Figure4I,K, FigureS3B). Dendrites appeared to project normally into the mb calyx (Figure4J, arrow), while the trajectory of axons was more variable (Figure4K). We observed bundles of axons that projected normally through the mb pedunculus (Figure4K, right) and some that mistargeted, bifurcating prematurely and projecting anterior of the pedunculus (Figure4K,left). Axon pathfinding defects could arise from limited guidance cues in the fully-developed adult brain. Alternatively, some of these supernumerary neurons may have an inappropriately specified identity. We observed no defects in the mushroom body structure of RHG miRNA or foxo mutant adults. Thus, at least a subset of supernumerary mb neurons generated from long-term surviving mb neuroblasts appear to incorporate into the existing mature adult mushroom body structure.
Conclusions
Our findings demonstrate that two pathways act in concert to eliminate mb neuroblasts and terminate neurogenesis (Figure4L). Downregulation of insulin/PI3 kinase signaling occurs first and may activate both autophagy and a program of caspase-dependent cell death. In the absence of one of these cell death pathways, mb neuroblasts persist, but only transiently. Thus a failsafe mechanism likely exits to ensure mb neuroblast elimination, similar to salivary gland cells[22].
The reduction in growth that precedes neuroblast apoptosis appears to be developmentally regulated since it occurs at an earlier time in central brain neuroblasts than in mushroom body neuroblasts. This may be due to either local differences in microenvironments or differences in the ability of neuroblasts to respond to circulating insulin-like peptides[24]. Moreover, the extended survival of mb neuroblasts under these conditions, but not other central brain neuroblasts, suggests that additional mechanisms such as terminal differentiation still function to ensure elimination of most neuroblasts[4, 25]. Indeed, during mammalian development, neural progenitors are eliminated via cell death and by terminal differentiation. The relative importance of death and differentiation for neuroblast elimination may be lineage dependent. Finally because cricket adult mb neuroblasts proliferate in response to insulin in explant cultures[26], a common mechanism may regulate adult neurogenesis among insects and possibly in more distantly related metazoans. Our findings may represent an important first step towards devising ways to manipulate the regenerative capacity of adult brains in diverse species and provide insight into how aberrantly persisting neural stem cells behave in vivo.
Experimental Procedures
We used the following fly stocks: w;FRT80B,Df(3L)H99, XR38, foxo25, UAS-GFP,tub-GAL4;FRT80B,tub-GAL80, worniu-GAL4[12], UAS-p35, UAS-flp, hsp70flp; act5c-FRT-stop-FRT-tau:lacZ, tub-GAL80[ts], UAS-mCD8:GFP, UAS-Dp60, UAS-Dp110, UAS-InRDN, UAS-ATG1DN (UAS-ATG1K38Q), pcnaeGFP, and OregonR. We replaced eGFP with GAL4 in the pRD119 transformation vector[14] to generate pcnaGAL4. We used the miRNA mir6.1 method[27] to generate UAS-RHG miRNA. rpr, hid, grim sequences (FigureS2) were subcloned into one of three stem-loop units and ligated into pUAST or pGMR. Transgenic animals were generated by BestGene (Chino Hills, CA).
Neuroblast clones were induced by shifting freshly eclosed adults (pcnaGAL4, UASflp, tubGAL80[ts],act5c-FRT-stop-FRT-tauLacZ;Df(3L)H99/Df(3L)XR38) to 30°C or to 37°C for 15 mins (hsflp;act>stop>tauLacZ;H99/XR28). MARCM animals were shifted to 37°C for 1 hour at white pupal stages. Adults were fed BrdU (.5mg/ml) mixed with yeast paste and food dye for 24 or 48 hrs.
Adult and pupal brains were fixed for 25 mins. in 4% paraformaldeyde/PEM buffer (Pipes, EGTA, MgCl2) and processed using standard methods[12]. White pupae were picked (t=0 hours) and aged accordingly. We used Lamin (DHSB, #ADL67.10) and other antibodies previously described[12, 20]. For BrdU labeling, brains were incubated in 2N HCl for 30 min. before processing. Cell death was assayed using a TUNEL kit (Roche). Labeling reaction proceeded for 1 hour at 37°C. All images collected on a confocal microscope and images processed with ImageJ, Photoshop, and Illustrator software.
Brains were imaged from the ventral to dorsal surface. When neuroblasts were too small to be distinguished by size, we counted only isolated Dpn-expressing cells. The small Dpn-expressing cells generated from intermediate progenitors that reside mostly in clusters on the dorsal brain surface were not counted. Average neuroblast diameter was determined by measuring the length of two perpendicular lines each passing through neuroblast center. Average pixel intensity was determined for three separate equal sized regions in the cytoplasm and in the nucleus, and then averaged. Student's two-tailed t-test was performed for statistical significance.
Supplementary Material
Acknowledgements
We thank Eric Baehrecke, Chris Doe, Bob Duriano, Marc Freeman, Kristin White, Bruce Edgar, Robert Tjian, the Bloomington stock center, the Developmental studies hybridoma bank, and the Drosophila Genomics Resource Center, for fly stocks, antibodies, and/or constructs. We thank Karsten Siller, Nipam Patel, David Bilder, Abby Gerhold, Chris Doe, David Weisblat and Jason Boone for discussions and/or reading of the manuscript. SES is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-1960–07) and IKH is funded by the NIH (RO1 GM61672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dufour MC, Gadenne C. Adult neurogenesis in a moth brain. J Comp Neurol. 2006;495:635–643. doi: 10.1002/cne.20909. [DOI] [PubMed] [Google Scholar]
- 2.Zhao X, Coptis V, Farris SM. Metamorphosis and adult development of the mushroom bodies of the red flour beetle, Tribolium castaneum. Dev Neurobiol. 2008;68:1487–1502. doi: 10.1002/dneu.20669. [DOI] [PubMed] [Google Scholar]
- 3.Cayre M, Scotto-Lomassese S, Malaterre J, Strambi C, Strambi A. Understanding the regulation and function of adult neurogenesis: contribution from an insect model, the house cricket. Chem Senses. 2007;32:385–395. doi: 10.1093/chemse/bjm010. [DOI] [PubMed] [Google Scholar]
- 4.Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 5.von Trotha JW, Egger B, Brand AH. Cell proliferation in the Drosophila adult brain revealed by clonal analysis and bromodeoxyuridine labelling. Neural Dev. 2009;4:9. doi: 10.1186/1749-8104-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito K, Hotta Y. Proliferation pattern of postembryonic neuroblasts in the brain of Drosophila melanogaster. Dev Biol. 1992;149:134–148. doi: 10.1016/0012-1606(92)90270-q. [DOI] [PubMed] [Google Scholar]
- 7.Kato K, Awasaki T, Ito K. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development. 2009;136:51–59. doi: 10.1242/dev.023366. [DOI] [PubMed] [Google Scholar]
- 8.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- 10.Zhong W, Chia W. Neurogenesis and asymmetric cell division. Curr Opin Neurobiol. 2008;18:4–11. doi: 10.1016/j.conb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila melanogaster. Dev Biol. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 12.Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- 13.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 14.Thacker SA, Bonnette PC, Duronio RJ. The contribution of E2F-regulated transcription to Drosophila PCNA gene function. Curr Biol. 2003;13:53–58. doi: 10.1016/s0960-9822(02)01400-8. [DOI] [PubMed] [Google Scholar]
- 15.Kurusu M, Maruyama Y, Adachi Y, Okabe M, Suzuki E, Furukubo-Tokunaga K. A conserved nuclear receptor, Tailless, is required for efficient proliferation and prolonged maintenance of mushroom body progenitors in the Drosophila brain. Dev Biol. 2009;326:224–236. doi: 10.1016/j.ydbio.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Bello BC, Hirth F, Gould AP. A pulse of the Drosophila Hox protein Abdominal-A schedules the end of neural proliferation via neuroblast apoptosis. Neuron. 2003;37:209–219. doi: 10.1016/s0896-6273(02)01181-9. [DOI] [PubMed] [Google Scholar]
- 17.Peterson C, Carney GE, Taylor BJ, White K. reaper is required for neuroblast apoptosis during Drosophila development. Development. 2002;129:1467–1476. doi: 10.1242/dev.129.6.1467. [DOI] [PubMed] [Google Scholar]
- 18.Hay BA, Guo M. Caspase-dependent cell death in Drosophila. Annu Rev Cell Dev Biol. 2006;22:623–650. doi: 10.1146/annurev.cellbio.21.012804.093845. [DOI] [PubMed] [Google Scholar]
- 19.Junger MA, Rintelen F, Stocker H, Wasserman JD, Vegh M, Radimerski T, Greenberg ME, Hafen E. The Drosophila forkhead transcription factor FOXO mediates the reduction in cell number associated with reduced insulin signaling. J Biol. 2003;2:20. doi: 10.1186/1475-4924-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puig O, Marr MT, Ruhf ML, Tjian R. Control of cell number by Drosophila FOXO: downstream and feedback regulation of the insulin receptor pathway. Genes Dev. 2003;17:2006–2020. doi: 10.1101/gad.1098703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996;15:6584–6594. [PMC free article] [PubMed] [Google Scholar]
- 22.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott RC, Juhasz G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 25.Fichelson P, Moch C, Ivanovitch K, Martin C, Sidor CM, Lepesant JA, Bellaiche Y, Huynh JR. Live-imaging of single stem cells within their niche reveals that a U3snoRNP component segregates asymmetrically and is required for self-renewal in Drosophila. Nat Cell Biol. 2009;11:685–693. doi: 10.1038/ncb1874. [DOI] [PubMed] [Google Scholar]
- 26.Malaterre J, Strambi C, Aouane A, Strambi A, Rougon G, Cayre M. Effect of hormones and growth factors on the proliferation of adult cricket neural progenitor cells in vitro. J Neurobiol. 2003;56:387–397. doi: 10.1002/neu.10244. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila. Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.