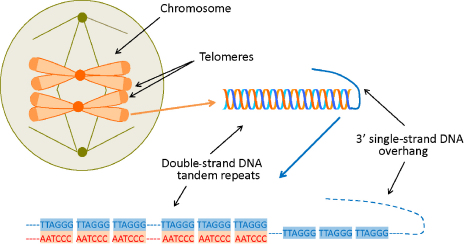
The Nobel Prize in Medicine in 2009 was awarded to Elizabeth Blackburn, Carol Greider and Jack Szostak for discovering the molecular structure of the far ends of chromosomes, called telomeres (Fig. 1), and how these protect chromosomes from degradation. Their discoveries shed light on a basic biological mechanism which stimulated research in a new exciting field aiming to explore the role of telomeres in normal ageing, cancer and age-related disease pathology.
Fig. 1.
Schematic presentation of telomeres.
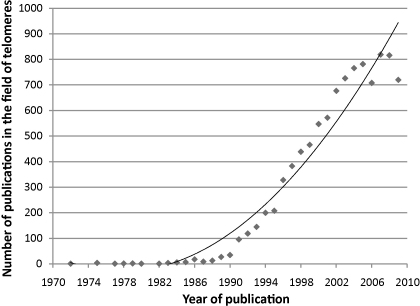
Elizabeth Blackburn first announced the identification of the repeated sequence of DNA in telomeres at a conference in 1980 and together with Jack Szostak in 1982 revealed that telomeres constitute a fundamental mechanism offering protection to chromosomes from degradation throughout different species [1]. In 1984 Carol Greider working with Elizabeth Blackburn discovered the enzyme which forms telomeric sequences [2,3]. This enzyme prevents telomere shortening with cell division, which otherwise takes place due to the incapability of DNA polymerase to fully copy the very end sequences of chromosomes during DNA replication, the so-called end-replication problem [4]. The impact of Blackburn's, Greider's and Szostak's work during the early 1980s is indicated by the increasing rate of publications in the field of telomeres thereafter (Fig. 2).
Fig. 2.
The rate of publications in the field of telomeres over the last 40 years, using data from the “Web of Science”.
We now know that telomeres’ biological function goes beyond the protection of chromosome ends from degradation or fusion, playing an important role in the cell's ageing process [5]. The length of telomeres serves as a mechanism of normal cell senescence [6]. In somatic cells, where the enzyme telomerase is not expressed, telomeres become shorter with each cell division, due to the end-replication problem. Once the length reduces below a critical value replicative senescence, also called the Hayflick limit, is induced [7]. The rate of telomere shortening in telomerase negative cells is not only dependent on the number of cell divisions, but also on DNA damage. The ends of telomeres constitute 3′ single-strand overhangs which are prone to single-strand breaks, particularly those caused by oxidative damage, due to their G-rich content. The accumulation of such breaks along the telomeres leads to additional loss during replication [8,9]. Therefore, the length of telomeres indicates the replicative capacity and cumulative genomic damage of somatic cells, reflecting in this way the tissue's “biological age”.
In recent years, the role of telomere length in the pathology of cardiovascular disease (CVD) and diabetes, where tissue ageing and senescence play major roles, has attracted a continuously growing research interest, and in the last two years alone six articles on telomere length have been published in Atherosclerosis. An article by Adaikalakoteswari et al. in the November 2007 issue associated shorter leukocyte telomere length (LTL) with impaired glucose tolerance, type 2 diabetes (T2D) and atherosclerotic plaques in T2D patients [10]. In June 2008, Satoh et al. showed that telomere length was shorter and telomerase activity lower in endothelial progenitor cells from patients with coronary heart disease (CHD) and even more reduced in CHD patients with metabolic syndrome. At the same time, oxidative DNA damage in these subjects displayed the opposite trend [11]. Following this, LTL was shown to negatively correlate with homocysteine levels by Richards et al. [12] and to positively correlate with HDL in the study of Chen et al. [13]. In a recent issue of Atherosclerosis, Olivieri et al. [14] showed that LTL is shorter in T2D patients compared to healthy subjects and even shorter in T2D patients with CHD. More recently, in this issue, our study [15] confirms the shorter LTL in T2D patients and also correlates LTL with plasma oxidative stress and variation in a gene regulating mitochondrial production of reactive oxygen species.
1. Telomere length in cardiovascular disease
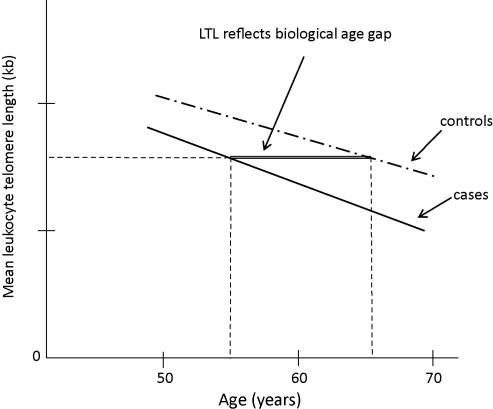
The association of telomere length with atherosclerosis and CVD has been supported by a large number of studies over the last few years [16–21]. Indicative of this effect is that CHD patients have mean LTL equivalent to that of 11 years older healthy subjects, as shown in the study of Brouilette et al. [19], which reflects the biological ageing of the vascular wall (Fig. 3).
Fig. 3.
Schematic presentation of LTL decrease with age in CHD cases (solid line) and controls (dashed line). The double line illustrates the biological age gap between cases and controls.
The evidence so far, suggests that in atherosclerosis telomere length probably contributes as a primary abnormality. In support of this are studies showing that family history of CHD is in part inherited through short LTL [22,23], most importantly the prospective studies associating baseline LTL with the risk to develop CVD [20,21,24], as well as the association of LTL with markers of subclinical CVD, such as intima-media thickness [10,21].
For pragmatic reasons, most of the studies have used LTL instead of vascular wall cell telomere length. However, Wilson et al. [25] have shown that LTL is a good predictor of vascular wall telomere length. Nonetheless, as LTL is only a surrogate of the telomere length in the cells contributing to atherosclerosis, it is likely that the real effect on CHD risk is greater than the observed when using LTL.
2. Telomere length in diabetes
It is now becoming apparent that type 2 diabetes (T2D) is also characterised by shorter telomeres [10,26,14,15]. It is not clear though, from the cross-sectional data available so far, whether the observed shorter telomeres in diabetes are a cause or consequence of the disease. Although the data are scarce, shorter telomeres have also been observed in type 1 diabetes patients [27]. The etiology of the disease in type 1 diabetes is in part different with that in type 2, although in both cases beta cell failure is the final trigger. Thus, one could speculate that critically short telomeres contribute to the onset of diabetes by eliciting senescent phenotypes in beta cells. However, in the case of type 1 diabetes again the data are cross-sectional, so the possibility that short telomeres are a result of the disease cannot be excluded. There is a need for a prospective study of the risk for diabetes in respect to telomere length in order to address this question.
Nevertheless, telomere length seems like a useful marker for T2D since it is associated with its progression. In the study of Adaikalakoteswari et al. telomeres were shorter in patients with only impaired glucose tolerance compared to controls and even shorter in T2D patients [10]. In addition, telomere shortening has been linked to diabetes complications, such as diabetic nephropathy [28], microalbuminuria [29] and epithelial cancers [30], while telomere shortening seems to be attenuated in patients with well-controlled diabetes [27].
3. Telomere length in co-existence of cardiovascular disease and type 2 diabetes
A very interesting finding, confirmed in independent studies, is that patients with diabetes or prediabetes exhibiting atherosclerotic manifestations have the shortest telomeres compared to patients with diabetes or CVD alone. Adaikalakoteswari et al. found that among T2D patients those with atherosclerotic plaques had the shorter telomeres [10]. The study of Olivieri et al. [14] showed that T2D patients with MI had shorter telomeres than T2D subjects free of MI and in our study [15], among the T2D subjects those with CHD had the shorter telomeres. Finally, Satoh et al. showed that CHD patients with metabolic syndrome had shorter telomeres than CHD patients without metabolic syndrome [11]. These observations suggest that substantially decreased telomere length, either caused by the common risk factors between CVD and diabetes and/or inherited short telomeres, possibly reflects greater tissue ageing and greater prevalence of senescent phenotypes in various tissues, including the vascular wall and pancreatic islets. Therefore, LTL might be a very useful marker of tissue ageing and progression of both CVD and diabetes.
4. Telomere length determinants
As to what determines telomere length, the data so far suggest a contribution of oxidative stress to the observed shorter LTLs in patients. Adaikalakoteswari et al. [10] found a negative correlation with a lipid peroxidation marker, Satoh et al. [11] with oxidative DNA damage and our study [15] showed a negative correlation with plasma oxidative stress and association with variation in a gene regulating mitochondrial reactive oxygen species production. The oxidative-induced telomere shortening has been established with in vitro experiments [8,9]. What is not clear yet is which factors, and to what extent contribute to the high levels of oxidative stress. The inverse correlation of LTL with variables reflecting the glycaemic state of patients in the study of Olivieri et al. [14] and the attenuation of telomere shortening in patients with good glycaemic control, as showed by Uziel et al. [27], suggest that hyperglycaemia might be driving the oxidative-induced telomere loss in diabetes. To this end the contribution of inflammation cannot be excluded, although many studies, including Oliveiri's et al. and ours, have failed to detect an association with inflammatory markers [14,15]. On the other hand, the rate of telomere shortening was associated with longitudinal cumulative HDL in the study of Chen et al. This suggests that LTL probably reflects the lifelong accumulating burden of increased oxidative stress and inflammation, whereas instant markers of these are not as representative [13]. What is determining the rate of oxidative stress-induced telomere shortening and if inflammation contributes to this effect needs to be further investigated with in vitro experiments. Worthy of remark is that in the study of Chen et al. [13] the rate of telomere shortening was dependent on the baseline telomere length, which is also supported by the findings of Nordfjall et al. [31]. Thus, it is possible that in longer telomeres, greater loss per cell division is more likely to occur. This, coupled with the high heritability in telomere length shown by twin studies [32,33], supports the hypothesis that telomere length is, to a large extent, genetically determined. It also supports the theory, that predisposition to CVD and/or diabetes might be expressed through inherited short telomeres.
5. Future work
Telomere length may prove to be very useful in the management and possibly the prediction of CVD and diabetes, representing the contribution of tissue ageing to their pathology. In order to explore this potential, further studies are needed to investigate in more depth what is the role of telomeres in the development of these diseases and whether it is important or not. Specifically, there is a need for prospective studies to establish whether telomere shortening is causative and examine the usefulness of LTL in predicting disease risk, especially for diabetes, since there is no previous record. In addition, it remains to be confirmed if LTL is a good surrogate measure of beta cells’ telomere length, as it has been shown for the vascular wall cells, before examining whether it is a useful marker in T2D. More importantly, there is a need to shed light on the basic biological functions of telomeres, like the mechanism involved in the trigger of cell senescence by telomere length or structure, how this is regulated and the possible interactions of telomeres with other chromosome regions.
Acknowledgments
We acknowledge the British Heart Foundation for funding Klelia D. Salpea (FS/06/053) and Steve E. Humphries (RG2005/014).
References
- 1.Szostak J.W., Blackburn E.H. Cloning yeast telomeres on linear plasmid vectors. Cell. 1982;29:245–255. doi: 10.1016/0092-8674(82)90109-x. [DOI] [PubMed] [Google Scholar]
- 2.Greider C.W., Blackburn E.H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 3.Greider C.W., Blackburn E.H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- 4.Olovnikov A.M. Principle of marginotomy in template synthesis of polynucleotides. Doklady Akademii nauk SSSR. 1971;201:1496–1499. [PubMed] [Google Scholar]
- 5.Blackburn E.H., Greider C.W., Szostak J.W. Telomeres and telomerase: the path from maize, Tetrahymena and yeast to human cancer and aging. Nature Medicine. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 6.Allsopp R.C., Harley C.B. Evidence for a critical telomere length in senescent human fibroblasts. Experimental Cell Research. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 7.Sozou P.D., Kirkwood T.B. A stochastic model of cell replicative senescence based on telomere shortening, oxidative stress, and somatic mutations in nuclear and mitochondrial DNA. Journal of Theoretical Biology. 2001;213:573–586. doi: 10.1006/jtbi.2001.2432. [DOI] [PubMed] [Google Scholar]
- 8.Petersen S, Saretzki G., von Zglinicki T. Preferential accumulation of single-stranded regions in telomeres of human fibroblasts. Experimental Cell Research. 1998;239:152–160. doi: 10.1006/excr.1997.3893. [DOI] [PubMed] [Google Scholar]
- 9.Serra V., Grune T., Sitte N., Saretzki G., von Zglinicki T. Telomere length as a marker of oxidative stress in primary human fibroblast cultures. Annals of the New York Academy of Sciences. 2000;908:327–330. doi: 10.1111/j.1749-6632.2000.tb06666.x. [DOI] [PubMed] [Google Scholar]
- 10.Adaikalakoteswari A., Balasubramanyam M., Ravikumar R., Deepa R., Mohan V. Association of telomere shortening with impaired glucose tolerance and diabetic macroangiopathy. Atherosclerosis. 2007;195:83–89. doi: 10.1016/j.atherosclerosis.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M., Ishikawa Y., Takahashi Y., Itoh T., Minami Y., Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2008;198:347–353. doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 12.Richards J.B., Valdes A.M., Gardner J.P. Homocysteine levels and leukocyte telomere length. Atherosclerosis. 2008;200:271–277. doi: 10.1016/j.atherosclerosis.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Chen W., Gardner J.P., Kimura M. Leukocyte telomere length is associated with HDL cholesterol levels: The Bogalusa heart study. Atherosclerosis. 2009;205:620–625. doi: 10.1016/j.atherosclerosis.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 14.Olivieri F., Lorenzi M., Antonicelli R. Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis. 2009;206:588–593. doi: 10.1016/j.atherosclerosis.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Salpea K.D., Talmud P.J., Cooper J.A. Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samani N.J., Boultby R., Butler R., Thompson J.R., Goodall A.H. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 17.Minamino T., Miyauchi H., Yoshida T., Ishida Y., Yoshida H., Komuro I. Endothelial cell senescence in human atherosclerosis: role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–1544. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 18.Matthews C., Gorenne I., Scott S. Vascular smooth muscle cells undergo telomere-based senescence in human atherosclerosis: effects of telomerase and oxidative stress. Circulation Research. 2006;99:156–164. doi: 10.1161/01.RES.0000233315.38086.bc. [DOI] [PubMed] [Google Scholar]
- 19.Brouilette S., Singh R.K., Thompson J.R., Goodall A.H., Samani N.J. White cell telomere length and risk of premature myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:842–846. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 20.Brouilette S.W., Moore J.S., McMahon A.D. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case–control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 21.Fitzpatrick A.L, Kronmal R.A., Gardner J.P. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 22.Salpea K.D., Nicaud V., Tiret L., Talmud P.J., Humphries S.E. The association of telomere length with paternal history of premature myocardial infarction in the European Atherosclerosis Research Study II. Journal of Molecular Medicine (Berlin, Germany) 2008;86:815–824. doi: 10.1007/s00109-008-0347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouilette S.W., Whittaker A., Stevens S.E., van der Harst P., Goodall A.H., Samani N.J. Telomere length is shorter in healthy offspring of subjects with coronary artery disease: support for the telomere hypothesis. Heart. 2008;94:422–425. doi: 10.1136/hrt.2007.139675. [DOI] [PubMed] [Google Scholar]
- 24.Zee R.Y., Michaud S.E., Ridker P.M. Mean telomere length and risk of incident venous thromboembolism: a prospective, nested case–control approach. Clinica Chimica Acta: International Journal of Clinical Chemistry. 2009;406:148–150. doi: 10.1016/j.cca.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson W.R, Herbert K.E., Mistry Y. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. European Heart Journal. 2008;29:2689–2694. doi: 10.1093/eurheartj/ehn386. [DOI] [PubMed] [Google Scholar]
- 26.Sampson M.J., Winterbone M.S., Hughes J.C., Dozio N., Hughes D.A. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 27.Uziel O., Singer J.A., Danicek V. Telomere dynamics in arteries and mononuclear cells of diabetic patients: effect of diabetes and of glycemic control. Experimental Gerontology. 2007;42:971–978. doi: 10.1016/j.exger.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Verzola D., Gandolfo M.T., Gaetani G. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. American Journal of Physiology. 2008;295:F1563–1573. doi: 10.1152/ajprenal.90302.2008. [DOI] [PubMed] [Google Scholar]
- 29.Tentolouris N., Nzietchueng R., Cattan V. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:2909–2915. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- 30.Sampson M.J., Hughes D.A. Chromosomal telomere attrition as a mechanism for the increased risk of epithelial cancers and senescent phenotypes in type 2 diabetes. Diabetologia. 2006;49:1726–1731. doi: 10.1007/s00125-006-0322-4. [DOI] [PubMed] [Google Scholar]
- 31.Nordfjall K., Svenson U., Norrback K.F., Adolfsson R., Lenner P., Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genetics. 2009;5:e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slagboom P.E., Droog S., Boomsma D.I. Genetic determination of telomere size in humans: a twin study of three age groups. American Journal of Human Genetics. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- 33.Graakjaer J., Pascoe L., Der-Sarkissian H. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3:97–102. doi: 10.1111/j.1474-9728.2004.00093.x. [DOI] [PubMed] [Google Scholar]