Abstract
Background
We have previously shown that a 12-day treatment with cyclosporine A (CyA) facilitates induction of tolerance to class-I disparate kidneys, as demonstrated by acceptance of second, donor-matched kidneys without immunosuppression. In the present study, we have examined 1) the duration of tolerance in the absence of donor antigen and 2) the pathway of antigen recognition determining maintenance or loss of tolerance.
Methods
Seventeen miniature swine received class-I mismatched kidneys with 12 days of CyA, and received second donor-matched kidneys without immunosuppression at 0, 1, 3, or 4 months after nephrectomy of the primary graft. Five were sensitized 6 weeks after nephrectomy of the primary graft, three with donor-matched skin grafts, and two with donor class-I peptides to eliminate direct pathway involvement. In addition, two long-term tolerant animals received class-Ic peptides.
Results
Rejection of second grafts required at least a 3 month absence of donor antigen. Although donor-matched skin grafts in animals tolerant to kidneys induced antidonor cytotoxic T lymphocyte responses, second renal transplants revealed no evidence of sensitization. In contrast, immunization of recipients with donor class-I peptides after nephrectomy of the primary graft led to loss of tolerance at both T-cell and B-cell levels, as evidenced by rejection of the second graft in 5 days and development of antidonor immunoglobulin G. Peptide immunization of long-term tolerant in recipients bearing long-term renal grafts did not break tolerance.
Conclusions
These data indicate that the renal allograft is required for the indefinite maintenance of tolerance, that indirect antigen presentation is capable of breaking tolerance, and that in tolerant animals, direct antigen presentation may suppress rejection, allowing tolerance to persist.
Keywords: Tolerance, Kidney transplantation, Miniature swine, Indirect pathway
The induction of donor specific tolerance remains a major goal of clinical transplantation. Partially inbred Massachusetts General Hospital (MGH) miniature swine in which swine leukocyte antigens (SLA) have been well defined and utilized extensively as a preclinical model for tolerance induction (1, 2). We have previously reported that a short course of cyclosporine A (CyA) permits the uniform development of long-term, donor-specific tolerance to renal allografts across a two-haplotype class-I disparity in miniature swine aged 3 to 8 months (3). These recipients accepted donor matched kidneys with stable renal function without further immunosuppressionpression when the second grafts were transplanted on the same day as the primary graft nephrectomies. In contrast, primary kidney allografts transplanted without CyA were uniformly rejected within 2 weeks of transplantation (4). Since large animal studies are limited to the use of small numbers of experimental animals, the reproducibility of the “all or none” phenomenon of graft survival in these two protocols provides a unique opportunity to study both the induction and maintenance of tolerance in a model where differences from the previous results—even in a small number of animals—can provide significant information.
The mechanisms of tolerance can be elucidated in part by attempts to interfere with or break tolerance. Utilizing the class I disparate kidney model, we have demonstrated that a brief course of human recombinant interleukin (IL)-2 (hrIL2), administered 8, 9, and 10 days after renal transplantation, led uniformly to rejection in animals (5). This result is consistent with the hypothesis that a calcineurin-induced deficit in T-cell help permits the development of tolerance (3, 4). Similarly, manipulations of the host thymus including either thymectomy or thymic biopsies performed 3 weeks prior to transplantation have been found to interfere with tolerance induction (6), indicating that the thymus plays an important role in this process. In contrast, however, neither of these manipulations was effective in breaking tolerance if performed during the maintenance phase of tolerance (5, 7). In addition, our previous studies have shown that additional T-cell help by a skin graft bearing third party class-II antigens and donor-matched class-I antigens on the tolerant recipients did not break systemic tolerance to kidney allografts, despite being sufficient to induce strong antidonor cytotoxic T lymphocyte (CTLs) in vitro (3, 8). These results indicate that the tolerant state induced by this model is very robust and stable once tolerance is established.
The present study is directed toward elucidating the mechanism by which tolerance is maintained. In particular, we have investigated the importance of the persistence of donor alloantigen in the form of the vascularized renal allograft in maintaining donor-specific unresponsiveness. In addition, we have addressed the role of the indirect pathway in maintaining tolerance after an absence of donor alloantigen. For this purpose, we first determined the period of the absence of the donor antigen required for loss of tolerance by transplanting donor matched second kidneys at various times after the removal of the primary kidney graft. We then investigated whether sensitization by either donor matched skin grafts or donor class-I major histocompatibility complex (MHC) allopeptides during the absence of the donor kidney graft would accelerate the loss of tolerance. The data in the present study indicate that the persistence of the donor renal graft is essential for the indefinite maintenance of tolerance and that the pathway of antigen recognition after the antigen has been removed plays an important role in breaking tolerance.
MATERIALS AND METHODS
Animals
Animals were cared for according to the guidelines of the Massachusetts General Hospital Institutional Animal Care and Use Committee. The immunogenetic characteristics of this herd and of the intra-MHC recombinant haplotypes available have been described previously (9, 10).
Experimental Groups
Recipients were divided into two groups: a nonsensitized group (Group 1) and a sensitized group (Group 2; Table 1). Recipients in Group 1a (n=4) underwent immediate re-transplants after the removal of their primary renal grafts on day 90. Recipients in Groups 1b (n=2) and 1c (n=4) received second donor-matched kidneys one month (1b) or 3–4 months (1c) after the removal of the primary donor renal graft. Recipients received recipient-type kidney grafts on the day of graftectomy of the primary graft in groups 1b and 1c in order to maintain renal function in the recipients while the donor renal grafts were absent. With the exception of two animals (Group 2c), all recipients in Group 2 received donor-matched kidneys 3 months after removal of the primary renal grafts. These recipients (2a, b) were sensitized 6 weeks after the nephrectomy of the primary kidney (i.e., 6 weeks prior to the second donor kidney transplant) with donor-matched (SLAgg) skin grafts in Group 2a (n=3) and subcutaneous injection of donor MHC class-I (class-Ic) peptides in Group 2b (n=2; see below). Recipients in Group 2c (n=2) served as controls for Group 2b. These animals received class-Ic peptide immunizations without receiving a donor kidney nephrectomy.
TABLE 1.
Experimental protocol
| Groups | Procedures |
|---|---|
| Group 1a | |
| Group 1b | |
| Group 1c | |
| Group 2a | |
| Group 2b | |
| Group 2c |
Kidney Transplantation and Retransplantation
The surgical procedures for primary and kidney retransplantation have been previously described in detail (3, 6).
Skin Graft
Split-thickness skin grafts (4×6 cm) were harvested from donors and placed on the dorsum of recipients.
Immunosuppression and Rejection Monitoring
CyA (Sandimmune) was provided by Novartis Pharmaceutical Corp. (Hanover, NJ) and administered as an intravenous suspension. CyA was administered daily at a dose of 10 to 13 mg/kg (adjusted to maintain a blood level of 400–800 ng/ml) for 12 days, starting on the day of the primary renal transplantation. Whole blood trough levels were determined by a monoclonal radioimmunoassay.
Histopathology and Immunohistochemistry
Renal open-wedge biopsies were performed on days 30 and 60 posttransplant or retransplant, during periods of renal failure, and at postmortem. Allograft rejection was scored by standard pathologic criteria according to the Cooperative Clinical Trials in Transplantation criteria (11). Immunohistochemical staining for anti-donor immunoglobulin (Ig) M and IgG deposition in renal allografts was examined by fluorescence microscopy using frozen sections stained with saturating concentrations of fluorescent isothiocyanate-labeled goat antiswine IgM or IgG (6).
Antibodies and Flow Cytometry
The presence of antidonor class-I IgM and IgG in the serum of experimental swine was detected by indirect flow cytometry. Fluorescence-activated cell sorting (FACS) was performed using a Becton Dickinson FACScan microfluorometer (Sunnyvale, CA).
Synthetic MHC Class-Ic Peptides
Most of the polymorphic sites of the two known class-I MHC loci in the pig (designated P1 and P14) are contained within the hypervariable regions of the α1 and α2 domains, as determined by comparison of the MHC class-Ic (donor type) and Id (recipient type) genetic sequences (12). Four MHC class-Ic peptides spanning the full length of the hypervariable regions of the P1 α1 helix were synthesized (13) and labeled as PC1-1 (amino acids [aa] 3–27), PC1-2 (aa 35–52), PC1-3 (aa 53–73), and PC1-4 (aa 71–90). Three MHC class-Ic peptides spanning the full length of the hypervariable regions of the P14 α1 helix were synthesized and labeled as PC14-1 (aa 3–27), PC14-2 (aa 45–59), and PC14-3 (aa 60–85). Peptide purity was >90% as verified by high-performance liquid chromatography and mass spectrometry.
Preparation of Peripheral Blood Lymphocytes (PBL)
For separation of PBLs, freshly heparinized whole blood was diluted 1:2 with Hank’s balanced salt solution (HBSS; GIBCO BRL, Gaithersburg, MD) and the mononuclear cells were obtained by gradient centrifugation using lymphocyte separation medium (Organon Teknika, Durham, NC) as previously described (6).
Cell-Mediated Lympholysis (CML) Assays
The procedure for CML assays has been described elsewhere (3, 6, 14).51Cr release was determined on a gamma counter. The results were expressed as:
Mixed Lymphocyte Reaction (MLR) Peptide Assays
Details of MLR peptide assays have been reported previously (13). To prevent contamination of responder cells with donor antigen-presenting cells (APCs), nylon-wool nonadherent (thereby APC depleted) PBLs were used as responders and naive nylon wool-adherent PBMCs that were pulsed in vitro with class-I peptides were used as APCs (15). Seven class-I peptides (see above) were used in these assays (12, 13).
RESULTS
Role of Persistence of Donor Alloantigen in Maintenance of Tolerance to Class-I Disparate Renal Allografts
Donor-matched kidney re-transplants performed immediately after the primary transplant nephrectomies were uniformly accepted without immunosuppression
We have previously reported that a 12-day course of CyA facilitated the induction of tolerance to class-I disparate renal allografts in juvenile (3–8 months of age) MGH miniature swine (3). All recipient animals accepted donor matched kidneys that were transplanted at least 3 months after the primary graft without immunosuppression. We first repeated this study and confirmed the historical findings of this established model.
Four recipients (animals 1, 2, 3, 4: group 1a) of class I–mismatched kidneys with a 12-day course of CyA received donor matched kidneys immediately after nephrectomy of the primary grafts. A minimal rise in creatinine was observed between days 10 and 20 after the second kidney transplant (Fig. 1A). A similar bump in creatinine levels after the second kidney transplant was reported in historical controls that were treated identically (16). Histology 30 and 60 days after the transplantation of the second donor matched kidney grafts showed only minimal cell infiltrates, and neither vasculitis nor glomerulitis was observed (Fig. 2A).
FIGURE 1.
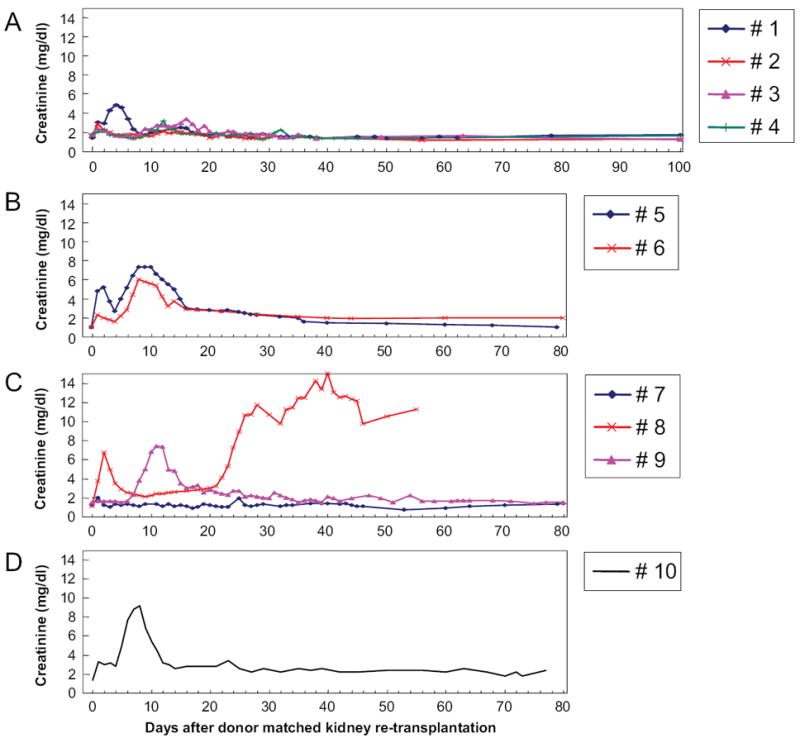
Clinical course of recipients undergoing second donor-matched kidney transplantation (A) immediately (group 1a), (B) 1 month (group 1b), (C) 3 months (group 1c), and (D) 4 months after removal of the primary renal graft.
FIGURE 2.
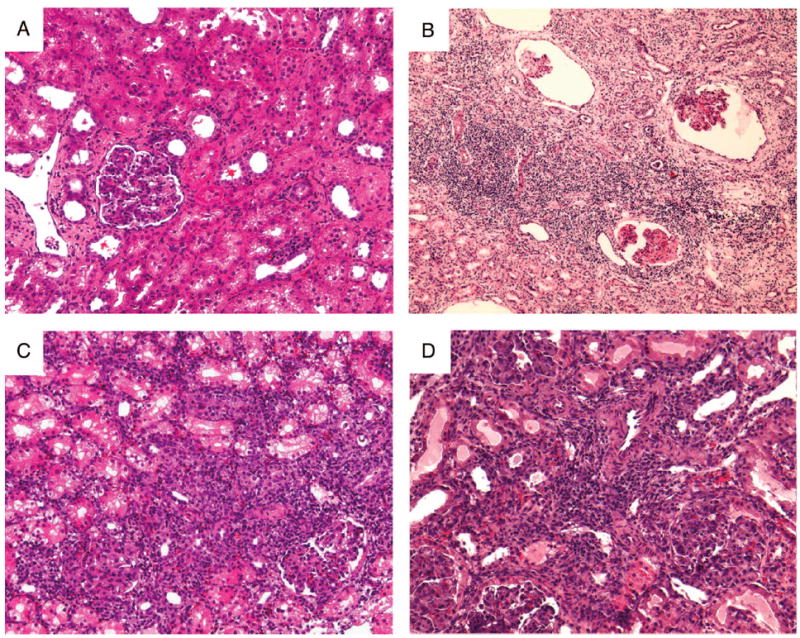
Histological examination of second donor-matched renal allografts in group 1 animals. (A) Representative hematoxylin and eosin stained samples on day 60 obtained from animal 3 (group 1a). (B) Biopsy specimen on day 55 taken from animal 8 (group 1c) demonstrated chronic glomerular changes with a diffuse interstitial mononuclear cell infiltrate and fibrosis. (C) Biopsy specimen on day 9 taken from animal 9 (group 1c) with a diffuse interstitial mononuclear cell infiltrate. (D) Biopsy specimen on day 7 taken from animal 10 with a diffuse interstitial mononuclear cell infiltrate.
A 3-month interval between removal of the primary kidney graft and the re-transplantation of a donor-matched graft led to the rejection of the replacement graft by one of three animals
We next varied the time between the removal of the primary graft and the retransplantation of a donor-matched graft to determine the period of absence of donor kidney antigen necessary for the complete loss of tolerance in this model. For this purpose, we first retransplanted two animals without immunosuppression one month after the removal of the primary kidney graft (group 1b). Both recipients demonstrated a period of transient renal dysfunction during the second postretransplantation week. However, renal function improved spontaneously within one week. Both animals maintained stable renal function thereafter and went on to maintain their grafts long-term (Fig. 1B).
We next extended the duration of donor antigen absence to 3 months. Three animals (animals 7, 8, and 9) were retransplanted with a donor-matched kidney three months after the removal of the primary kidney allograft (group 1c). These three animals showed varied clinical courses (Fig. 1C). Animal 7 showed only minimal renal dysfunction immediately postoperatively (as determined by creatinine levels) and has since maintained normal renal function for more than 300 days. Animal 8, on the other hand, showed a transient episode of renal failure immediately postoperatively that resolved spontaneously by the second week. However, this animal’s renal function deteriorated rapidly from the third postoperative week, and the graft was eventually rejected 55 days after re-transplantation (Fig. 2B). The third animal (animal 9) also had severe rejection crisis from day 7 (Figs. 1C and 2C). However, the rejection crisis spontaneously resolved from the third postoperative week and the animal maintained stable renal function thereafter.
One additional animal (animal 10) received a second kidney 4 months after the removal of the primary graft. Like two of the three recipients that received a donor-matched graft after a three month period of donor antigen absence, this animal experienced severe renal dysfunction during the first and second postoperative weeks (Fig. 1D). A kidney biopsy revealed diffuse mononuclear cell infiltrates and acute glomerulitis on day 7 (Fig. 2D), similar to that seen in animal 9 at the same time point (Fig. 2C). However, this episode must be termed a rejection crisis because renal function spontaneously improved and remained normal thereafter (creatinine <2.0 mg/dl >90 days). Antidonor CTL responses were slightly increased after graftectomy; however, it became donor unresponsive by 30 days after second kidney transplantation (Fig. 3A). Neither IgM nor IgG developed during the absence of donor antigen (Fig. 3B).
FIGURE 3.
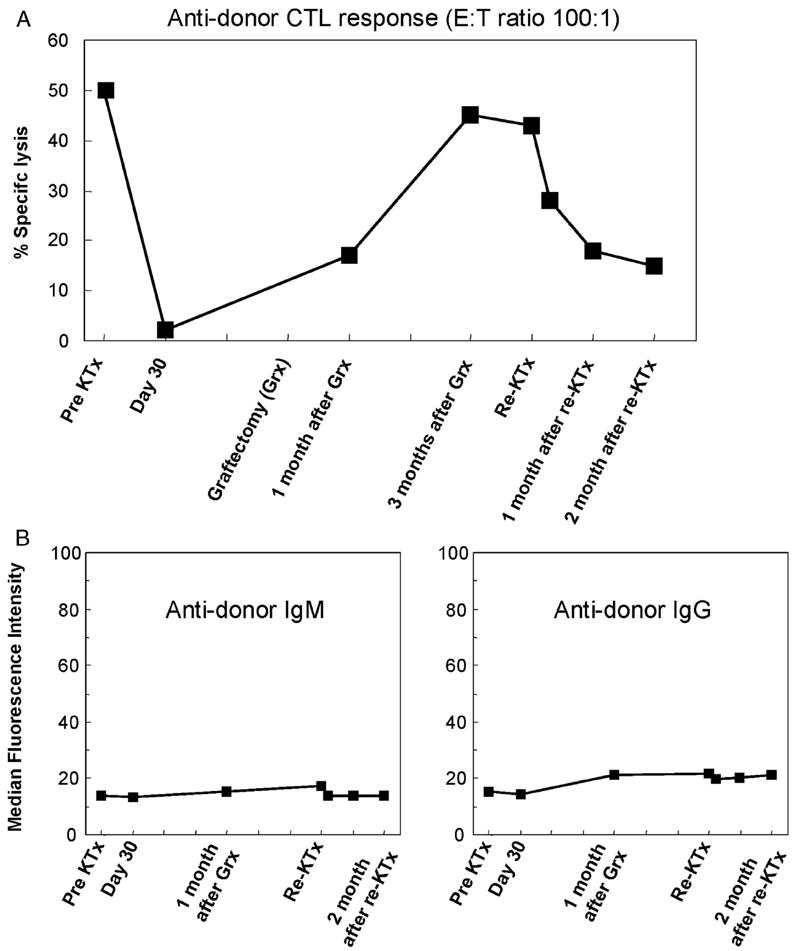
(A) Antidonor CTL responses of animal 10 before and after the primary kidney graftectomy. (B) Flow cytometric analysis of antidonor IgM and IgG in the serum of animal 10.
Attempts to Break Tolerance by Stimulation With Donor Class-I During the Absence of Donor Antigen
Donor-type class-I peptides administered during the absence of donor antigens broke tolerance, while donor-matched (SLAgg) skin grafts did not
Results in group 1 demonstrated that the persistence of donor kidneys appeared to be essential for the maintenance of stable tolerance. Despite several previous attempts to break tolerance in this model, rejection of a second, donor-matched kidney was not seen except after a 3-month absence of donor antigen (5, 7, 8). Although additional experiments would be required in order to establish firmly the duration of antigen absence that would result in uniform graft loss, we chose instead to study whether the addition of T-cell help during the first 3 months in the absence of donor antigen would facilitate the complete loss of tolerance. To test the effect of stimulation of antidonor class-I responses in the absence of donor antigen, we examined the response to immunization either with donor type skin (class-I mismatched to recipient) or with donor type class I peptide.
Effect of SLAgg Skin Grafts on Tolerance
We have previously reported that donor matched (SLAgg) skin grafts placed on long-term tolerant SLAdd swine bearing SLAgg kidneys did not restore in vitro antidonor responses or lead to loss of the kidney allografts (3). In the present study, three recipients of kidney grafts received an SLAgg skin graft 6 weeks after removal of tolerated kidney grafts (group 2a: animals 11, 12, and 13). These SLAgg skin grafts were rejected at 14, 15, and 16 days after skin placement, which was somewhat earlier than the grafts of the historical controls bearing kidney grafts (25.6 ± 4.8 days) (3). In addition, the rejection of SLAgg skin grafts led to the restoration of strong anti-donor CTL responses (Fig. 4A). One (animal 11) of the three animals developed antidonor class I IgM, but none developed antidonor IgG (Fig. 4B).
FIGURE 4.
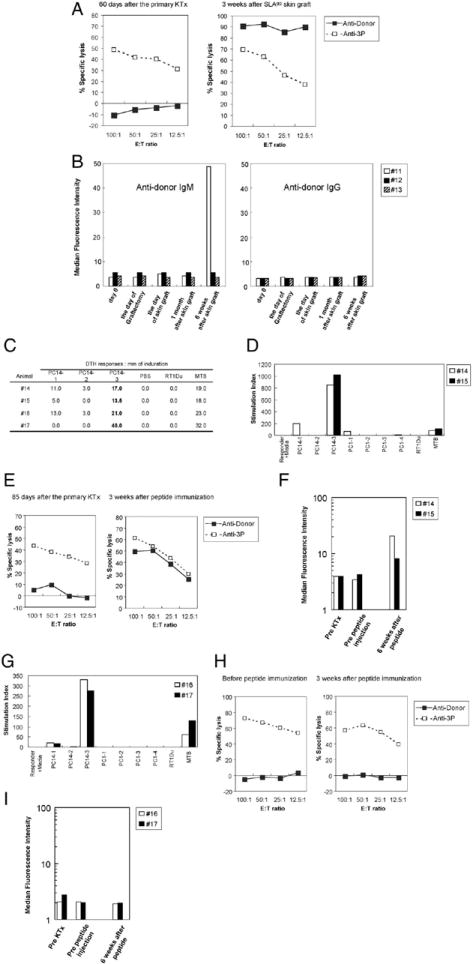
(A) Representative CTL responses for group 2a animals on day 60 after the primary kidney transplantation and 21 days after SLAgg skin graft. Recipient responses to donor stimulation (solid lines and boxes) and third-party stimulation (dotted lines and striped boxes). (B) Flow cytometric analysis of antidonor IgM and IgG in the serum of recipients in group 2a. Only animal 11 developed antidonor IgM by 6 weeks after SLAgg skin graft placement, but none developed antidonor IgG. (C) DTH responses in group 2b and 2c animals. All animals immunized with class-Ic peptides developed a strong reactivity to PC14-3 in DTH. Measurements represent the average of two independent readings. (D) MLR peptide assays conducted on group 2b animals. These assays were performed 3 weeks after immunization with donor peptides. (E) Representative CTL responses for group 2b animals on day 85 after the primary kidney transplantation (left) and 3 weeks after immunization with donor peptides (right). Recipient responses to donor stimulation (solid lines) and third-party stimulation (dotted lines). (F) Flow cytometric analysis of antidonor IgG in the serum of recipients in group 2b. Both recipients developed anti-donor IgG by 6 weeks after immunization. (G) Both animals in group 2c developed a strong reactivity to PC14-3 in MLR peptide assays. These results were similar to those seen in group 2b animals. (H) Representative CTL responses for group 2c animals before immunization (left) and 3 weeks after immunization with donor peptides (right). Recipient responses to donor stimulation (solid lines) and third-party stimulation (dotted lines). (i) Flow cytometric analysis of anti donor IgG in the serum of recipients in group 2c. No antidonor class I IgG developed after peptide immunization in both animals.
Despite sensitization at the T-cell level that was measured in vitro, none of these recipients showed accelerated rejection of a second SLAgg kidney graft transplanted 6 weeks later. Animal 11 developed antidonor IgM after skin graft placement and experienced an acute rejection crisis during the first postoperative week after the donor matched kidney retransplantation that resolved spontaneously. Animal 12 showed stable renal function until day 30 after retransplantation and then experienced delayed rejection. The third animal (animal 13) maintained stable renal function after retransplantation (Fig. 5A). Taken together, these clinical courses are similar to those seen in recipients of 3-month delayed second kidney grafts without skin grafts.
FIGURE 5.
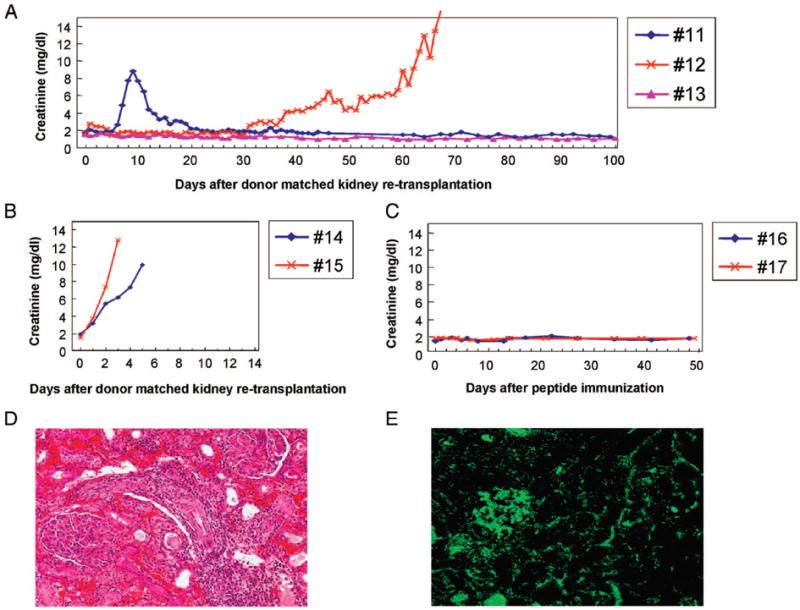
Clinical course of recipients undergoing donor-matched kidney retransplantation 6 weeks after sensitization with (A) SLAgg skin grafts (group 2a) and (B) class-Ic peptides (group 2b). (C) Clinical course of long-term tolerant recipients bearing tolerated class-I disparate renal allografts after peptide immunization (group 2c). Representative histological examination and immunofluorescence examination of second donor-matched renal allografts in group 2b (D and E). (D) Biopsy specimen of animal 14 on the day of rejection showing a diffuse and extensive mononuclear cell infiltrate as well as neutrophil infiltrate and interstitial hemorrhages. (E) Antidonor IgG deposits were detected in the glomeruli and peritubular capillaries in animal 14 on the day of rejection.
Effect of Class-Ic Peptides on Tolerance
The response to peptide immunization was very different. We have previously demonstrated that cell–cell contact is required for the donor-specific suppressive effects of regulatory T-cells (Tregs) from tolerant animals on naive antidonor reactions (14, 17, 18). Thus, we hypothesized that one of the reasons SLAgg skin grafts did not accelerate the breaking of tolerance could be that the SLAgg skin graft both induced alloreactive T-cells and expanded the Treg population in the recipient animal. To eliminate the direct pathway of antigen presentation from these experiments, we immunized tolerant animals with donor MHC class-I (class-Ic) peptides instead of SLAgg skin grafts. Two animals (group 2b: animals 14 and 15) were immunized subcutaneously with a mixture of three allogenic MHC class-Ic PC14 peptides (PC14-1, PC14-2, and PC14-3: 500 μg of each peptide in complete Freund’s adjuvant) 6 weeks after the removal of the primary kidney graft.
Delayed type hypersensitivity (DTH) responses to class-Ic peptides were analyzed in both recipients immunized with the class-Ic PC14 peptides 14 days after immunization. PC14-3 elicited the strongest positive DTH response in the immunized animals. Both immunized pigs showed brisk DTH responses to the M. tuberculosis H37 RA positive control and negative responses to the phosphate-buffered saline control. These results confirmed the presence of indirect alloantigen presentation in vivo and validated the immunogenicity of specific class-I MHC peptides. To assess the in vitro reactivity of recipient PBLs to individual class-Ic peptides, MLR peptide assays were performed with PBLs from group 2b animals after immunization in the absence of donor kidney antigens. There was no T-cell proliferative response to any of the class-Ic peptides before immunization. After immunization, T-cell responses to PC14-3 developed, which was consistent with the positive DTH responses observed 14 days after immunization (Fig. 4C and D). Interestingly, class-Ic peptide immunization induced not only the generation of antidonor responses in CML assays but also the production of antidonor class-I MHC IgG antibody in FACS analysis by 6 weeks after immunization but before second donor-matched kidney transplantation (Fig. 4E and F).
Both recipients immunized with class-Ic peptides promptly rejected the subsequently transplanted donor matched kidney grafts on days 3 and 5, respectively (Fig. 5B). Histologic examination revealed that both animals rejected their grafts by severe accelerated cellular and humoral rejection. Both grafts had evidence of a diffuse and extensive mononuclear cell infiltrate as well as neutrophil infiltrate and interstitial hemorrhages (Fig. 5D). Frozen sections of renal biopsy specimens from second donor-matched kidneys were examined by immunohistochemistry. Biopsy specimens analyzed 1 hr after revascularization of the retransplants and on the day of rejection showed both antidonor IgM and IgG depositions (Fig. 5E).
As controls, two long-term tolerant recipients bearing tolerated class-I disparate kidney allografts (group 2c: animals 16, 17) were immunized with class-Ic peptides. These recipients were followed for 50 days after class-Ic peptides immunization, a time by which both animals in group 2b had rejected their second donor matched kidney grafts completely. The two control animals developed a strong reactivity to PC14-3 in both DTH and MLR peptide assays (Fig. 4G). The production of anti PC14-3 IgM and IgG by enzyme-linked immunosorbent assay was detected by 14 days after peptide injection in both animals, and IgG levels remained stable thereafter. However, CML responses maintained specific unresponsiveness to donor (Fig. 4H) and no antidonor class-I MHC IgM or IgG antibodies developed (Fig. 4I), as assessed by FACS after immunization in both animals. Renal graft function remained stable throughout the experimental period (Fig. 5C).
DISCUSSION
We have studied mechanisms of tolerance to renal allografts with a short course of CyA in MHC inbred miniature swine extensively (3, 5, 6, 8, 19-21). Long-term tolerance to class-I disparate renal allografts in miniature swine is uniformly induced by a 12-day course of CyA (3). This tolerance persists when the graft is immediately replaced with a new donor-matched kidney without immunosuppression, and the tolerated kidney maintains its function even when anti-donor CTL is induced by a class-II mismatched, class-I donor-matched skin graft (8, 19). We have also reported that 1) the thymus plays an important role in the induction of tolerance, but not in the maintenance of tolerance (6, 7); and that 2) regulatory cells develop after renal transplantation (14, 17, 18). These results suggest that a balance between Tregs and alloreactive T-cells after transplantation plays an essential role in the induction and maintenance of tolerance in this model. Therefore, in this study we aimed to address 1) the length of time tolerance is maintained in the absence of donor antigen and 2) the role of the indirect pathway in the maintenance or loss of tolerance in the absence of donor antigen. The present data demonstrates that 1) a donor organ is required for indefinite maintenance of tolerance in this model, although tolerance persists for up to 3 months in the absence of donor antigen; and 2) when donor kidney antigen is absent, immunization with class-I donor peptides, but not with class-I donor matched skin grafts breaks systemic tolerance at both T-cell and B-cell levels. To our knowledge, this is the first report demonstrating the breaking of tolerance in large animal models. Although statistical comparisons are limited by the small animal numbers, the consistency of observed data across experimental conditions assessed by different in vivo and vitro assays supports this interpretation.
Persistence of donor antigen via the presence of donor solid organs appears to be essential for the maintenance of peripheral transplant tolerance in rodent models (22, 23). Tolerance to murine allogeneic heart grafts induced by anti-CD4 monoclonal antibodies and donor specific transfusion (DST) was lost when second donor-matched hearts were transplanted 200 days after the removal of the primary hearts (22). Moreover, studies have shown that regulatory CD4+ T-cells require the continuous presence of donor antigens to survive in allograft tolerance models (23) (24). Data from this study of tolerance induction in miniature swine supports the results of these studies in rodent models (22, 23). However, while rodents maintained second grafts indefinitely after a 130-day period of absence of the donor organ, swine appeared to lose tolerance starting at about 3 months after the donor kidney was removed (22). Because one additional month (i.e., 4 months) of absence of donor antigen did not induce full rejection in our model, we attempted to examine the mechanisms of tolerance and breaking tolerance within this marginal period by determining whether the loss of tolerance could be accelerated by the addition of T-cell help via the direct and/or indirect pathway. Since we are not able to deplete APCs in the recipient to eliminate the direct pathway in our miniature swine model, we chose to isolate the indirect pathway by injecting donor class-I peptides, compared to skin grafts which would stimulate both the direct and indirect pathways.
CTL responses indicate a return of alloreactivity over time (Fig. 3) that may be due to a shift in the balance between tolerogenic Tregs and alloreactive T-cells accounting for the loss of tolerance after removal of the donor kidney graft. One reason for this observation would be that the relative numbers or potency of Tregs versus alloreactive T-cells could change in the absence of donor antigen. Unfortunately, at the time these studies were performed, no FoxP3 antibodies were available that reacted with swine lymphocytes, so we were unable to use this marker to determine if the change was due to an increase in alloreactivity or a decrease in regulatory potential in the animals. However, previous studies have demonstrated that class-I donor matched, but class-II third-party disparate skin grafts can induce antidonor CTL in tolerant animals, suggesting that alloreactive progenitors were not fully deleted during tolerance induction (8). These T-cells, as well as T-cells newly developed from the thymus after the removal of donor antigen, could overwhelm the effects of Tregs when the second graft is placed. We placed donor matched skin or immunized with donor type class-I peptide 6 weeks after the removal of the first kidney graft in an attempt to intentionally shift this balance between Tregs and allogeneic T-cells. As described in the Results section, strong CTL responses, but no antibody responses, against donor antigen were induced by a donor skin graft; however, the skin grafts did not affect the clinical course of recipients after transplantation of second kidney grafts. In contrast, both CTL and antidonor IgG were induced by donor peptide and resulted in the accelerated rejection of the second graft. Immunization with donor peptide did not induce antidonor antibodies in recipients bearing donor kidneys. We are currently investigating the role of recent thymic emigrants after removal of donor antigen in the breaking of tolerance by manipulating the host thymus.
Induced antialloantigen IgG after immunization with donor class-I peptide in the present study suggest that 1) alloreactive CD4 T-cells were not fully deleted in this model; 2) these alloreactive T-cells were likely suppressed by Tregs when the donor kidney graft was present; and 3) with the decrease in number or activity of Tregs after the removal of the primary kidney graft, these alloreactive memory CD4+ T-cells, boosted by donor-type MHC class-I peptide, provided T-cell help to B-cells through the indirect pathway, triggering the B-cells to produce antidonor MHC class-I IgG via a cognate interaction (25).
There is an alternative mechanism that could explain the ability of peptide exposure to induce antibody production only when the donor kidney graft is absent. When a donor kidney graft is present, circulating host APCs may pick up donor antigens in the graft, migrate to draining lymph nodes, and present those antigens in a way that favors the preferential expansion of Tregs relative to alloreactive T-cells. However, when donor kidney antigen is absent, the presence of donor MHC class-I peptide in the systemic circulation may cause the antigen to be picked up by multiple subtypes of host APCs that may present this antigen in a manner that favors activation or expansion of alloreactive T-cells relative to Tregs.
Donor-type MHC class-I peptide immunization induced antialloantibody in this model while donor-matched skin grafting did not. One plausible reason to explain this difference is that the skin graft would allow for both direct and indirect presentation of class-I antigen despite the fact that MHC class-II of APC in the skin graft is matched to the recipient’s. In addition to inducing alloreactive T-cell production, the direct pathway by the APC in the skin graft may expand Tregs by cell–cell contact at the site of the skin graft—a mechanism that has been supported by previous rodent studies. In a model of heart allograft tolerance induced by a deoxyspergualine analogue in rats, tolerance and expansion of CD4+CD25+ Tregs were dependent on donor-passenger leukocytes, suggesting that the direct presentation of donor antigens is required to expand Tregs in the thymus and in the periphery (26). Alternatively, tolerant recipients challenged with donor-antigen as skin grafts could have distinct clinical courses from those challenged with donor-antigen as peptides because an SLAgg skin graft provides both SLA class-Ic peptides and other peptides. While SLA class-Ic peptides alone might stimulate only alloreactive CD4 helper T-cells, other peptides might stimulate Tregs.
Acknowledgments
We thank Novartis Pharmaceutical Corp. (Hanover, NJ) for generously providing Cyclosporine A. Drs. Akihiko Okuyama and Parsia Vagefi for helpful review of this manuscript.
This work was supported by grants from the National Institutes of Health (R37 AI31046-16) and the Japan Cardiovascular Research Foundation (no. 050).
References
- 1.Sachs DH. MHC homozygous miniature swine. In: Swindle MM, Moody DC, Phillips LD, editors. Swine as Models in Biomedical Research. Ames, IA: Iowa State University Press; 1992. p. 3. [Google Scholar]
- 2.Mezrich JD, Haller GW, Arn JS, et al. Histocompatible miniature swine: An inbred large-animal model. Transplantation. 2003;75:904. doi: 10.1097/01.TP.0000054839.43852.BF. [DOI] [PubMed] [Google Scholar]
- 3.Rosengard BR, Ojikutu CA, Guzzetta PC, et al. Induction of specific tolerance to class I disparate renal allografts in miniature swine with cyclosporine. Transplantation. 1992;54:490. doi: 10.1097/00007890-199209000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Gianello PR, Sachs DH. Effect of major histocompatibility complex matching on the development of tolerance to primarily vascularized renal allografts: A study in miniature swine. Hum Immunol. 1996;50:1. doi: 10.1016/0198-8859(96)00059-6. [DOI] [PubMed] [Google Scholar]
- 5.Gianello PR, Blancho G, Fishbein JF, et al. Mechanism of cyclosporin-induced tolerance to primarily vascularized allografts in miniature swine. Effect of administration of exogenous IL-2. J Immunol. 1994;153:4788. [PubMed] [Google Scholar]
- 6.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186:497. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagefi PA, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature Swine: IV. The thymus is required during the induction phase, but not the maintenance phase, of renal allograft tolerance. Transplantation. 2004;77:979. doi: 10.1097/01.tp.0000116416.10799.c6. [DOI] [PubMed] [Google Scholar]
- 8.Gianello PR, Fishbein JF, Rosengard BR, et al. Tolerance to class I disparate renal allografts in miniature swine: Maintenance of tolerance despite induction of specific antidonor CTL responses. Transplantation. 1995;59:772. doi: 10.1097/00007890-199503150-00023. [DOI] [PubMed] [Google Scholar]
- 9.Sachs DH, Leight G, Cone J, et al. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Pennington LR, Lunney JK, Sachs DH. Transplantation in miniature swine. VIII. Recombination within the major histocompatibility complex of miniature swine. Transplantation. 1981;31:66. [PubMed] [Google Scholar]
- 11.Colvin RB. The renal allograft biopsy. Kidney Int. 1996;50:1069. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan JA, Oettinger HF, Sachs DH, Edge AS. Analysis of polymorphism in porcine MHC class I genes: Alterations in signals recognized by human cytotoxic lymphocytes. J Immunol. 1997;159:2318. [PubMed] [Google Scholar]
- 13.Lee RS, Yamada K, Houser SL, et al. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98:3276. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ierino FL, Yamada K, Hatch T, et al. Peripheral tolerance to class I mismatched renal allografts in miniature swine: Donor antigen-activated peripheral blood lymphocytes from tolerant swine inhibit antidonor CTL reactivity. J Immunol. 1999;162:550. [PubMed] [Google Scholar]
- 15.Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T-cell response. Evidence for allelic specificity of MLR and for both direct and indirect pathways of recognition. J Immunol. 1995;155:5249. [PubMed] [Google Scholar]
- 16.Gianello PR, Lorf T, Yamada K, et al. Induction of tolerance to renal allografts across single-haplotype MHC disparities in miniature swine. Transplantation. 1995;59:884. [PubMed] [Google Scholar]
- 17.Ierino FL, Yamada K, Hatch T, Sachs DH. Preliminary in vitro evidence for regulatory cells in a miniature swine renal allograft model. Transplant Proc. 1997;29:1165. doi: 10.1016/s0041-1345(96)00515-5. [DOI] [PubMed] [Google Scholar]
- 18.Mezrich JD, Kesselheim JA, Johnston DR, et al. The role of regulatory cells in miniature swine rendered tolerant to cardiac allografts by donor kidney cotransplantation. Am J Transplant. 2003;3:1107. doi: 10.1046/j.1600-6143.2003.00202.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosengard BR, Kortz EO, Ojikutu CA, et al. The failure of skin grafting to break tolerance to class I disparate renal allografts in miniature swine despite inducing marked anti-donor cellular immunity. Transplantation. 1991;52:1044. doi: 10.1097/00007890-199112000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine: II. Effect of steroids and age on the induction of tolerance to class I mismatched renal allografts. Transplantation. 1999;67:458. doi: 10.1097/00007890-199902150-00020. [DOI] [PubMed] [Google Scholar]
- 21.Yamada K, Ierino FL, Gianello PR, et al. Role of the thymus in transplantation tolerance in miniature swine. III. Surgical manipulation of the thymus interferes with stable induction of tolerance to class I-mismatched renal allografts. Transplantation. 1999;67:1112. doi: 10.1097/00007890-199904270-00005. [DOI] [PubMed] [Google Scholar]
- 22.Hamano K, Rawsthorne MA, Bushell AR, et al. Evidence that the continued presence of the organ graft and not peripheral donor microchimerism is essential for maintenance of tolerance to alloantigen in vivo in anti-CD4 treated recipients. Transplantation. 1996;62:856. doi: 10.1097/00007890-199609270-00026. [DOI] [PubMed] [Google Scholar]
- 23.Chen ZK, Cobbold SP, Waldmann H, Metcalfe S. Amplification of natural regulatory immune mechanisms for transplantation tolerance. Transplantation. 1996;62:1200. doi: 10.1097/00007890-199611150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Scully R, Qin S, Cobbold S, Waldmann H. Mechanisms in CD4 antibody-mediated transplantation tolerance: Kinetics of induction, antigen dependency and role of regulatory T cells. Eur J Immunol. 1994;24:2383. doi: 10.1002/eji.1830241019. [DOI] [PubMed] [Google Scholar]
- 25.Steele DJ, Laufer TM, Smiley ST, et al. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699. doi: 10.1084/jem.183.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiffoleau E, Beriou G, Dutartre P, et al. Role for thymic and splenic regulatory CD4+ T cells induced by donor dendritic cells in allograft tolerance by LF15–0195 treatment. J Immunol. 2002;168:5058. doi: 10.4049/jimmunol.168.10.5058. [DOI] [PubMed] [Google Scholar]


