Abstract
Chronic muscle pain is a major clinical problem that is often associated with fatigue. Conversely, chronic fatigue conditions are commonly associated with muscle pain. We tested the hypothesis that muscle fatigue enhances hyperalgesia associated with injection of acidic saline into muscle. We evaluated mechanical sensitivity of the paw (von Frey) in mice after 2 intramuscular injections of saline (20 µL; pH 4, pH 5, pH 6, pH 7.2) in a fatigue and a control group. To induce fatigue, mice were run for 2 h/day for 2 days prior to the first injection and 2 h/day for 2 days prior to the second injection. Muscle lactate, pCO2, pO2, creatinine kinase, phosphate, and histology were examined after the fatigue task and compared to a control group. Grip force was significantly decreased after 2 h of running indicating fatigue. The fatigue task did not induce muscle damage as there was no difference in muscle lactate, pCO2, pO2, creatinine kinase, phosphate, or histology. The fatigue task altered the dose-response relationship to intramuscular acidic saline injections. Mechanical hyperalgesia was observed in both fatigue and control groups after intramuscular injection of pH 4.0, but only the fatigue group after injection of pH 5. Neither the fatigue nor the control group developed hyperalgesia in response to intramuscular injection of pH 6 or pH 7.2. In conclusion, fatigue modified the susceptibility of mice to acid injection of pH 5.0 to result in mechanical hyperalgesia after 2 injections of pH 5.0. The fatigue task did not produce measurable changes in the muscle tissue suggesting a central mechanism mediating the enhancement of hyperalgesia.
Perspective
These data therefore show that muscle fatigue can enhance the likelihood that one develops pain to a mild insult. Clinically, this could relate to the development of pain from such conditions as repetitive strain injury, and may relate to the interrelationship between chronic pain and fatigue.
Keywords: Fatigue, exercise, acid, protons, low-pH saline
Pain arising from musculoskeletal disorders is a major clinical problem globally,58 and fibromyalgia affects 2% of population in the United States.57 As high as 76% of people with chronic widespread musculoskeletal pain conditions, such as fibromyalgia and arthritis, also report fatigue.26 Conversely, as high as 94% of people with chronic fatigue syndrome report musculoskeletal pain and approximately half of the population has chronic widespread musculoskeletal pain.56,60 Pain is increased during and after exercise in people with fibromyalgia and chronic fatigue syndrome. 20,48,54,55 Similarly, muscle fatigue is increased in people with chronic fatigue syndrome.3,10,23 This overlap between muscle fatigue and pain syndromes suggests an interaction between fatigue and pain such that fatigue may enhance pain.
To assess mechanisms of chronic widespread pain, we developed an animal model that mimics the clinical symptoms. Two injections of acidic saline (pH 4.0), 5 days apart, into 1 muscle results in bilateral long-lasting hyperalgesia of the paw,41 muscle,59 and the viscera28 without muscle tissue damage.41 The intramuscular pH decreases to a peak of pH 6.0 after intramuscular injection of pH 4.0 saline and is reversed 6 to 7 minutes after injection.41 After 2 unilateral injections mechanical hyperalgesia develops not only ipsilaterally but also contralaterally, and lasts for 4 weeks. Blockade of NMDA receptors in the spinal cord or local anesthetic delivered to the rostral ventromedial medulla reverses the hyperalgesia once developed.38,51 Further, removal of primary afferent input by local anesthetic in the muscle, or ipsilateral dorsal rhizotomy has no effect on the contralateral hyperalgesia.41 Thus, the hyperalgesia associated with this model is maintained by changes in the central nervous system, and once developed is independent of input from the periphery.38,39,41
Muscle fatigue is defined as a temporary loss of muscle force resulting from activity.50 It can occur as a result of concentric, eccentric, or isometric contractions.35 However, muscle damage and delayed onset muscle soreness occur predominately with eccentric contractions. Eccentric induced soreness is generally associated with inflammatory changes in exercised muscle that includes infiltration of leukocytes, increases in lactate, increases in phosphate, fatigue, and pain.2,8,11,30 In human subjects with experimental muscle pain, there is a decrease in muscle peak force and endurance in painful and in synergistic muscles.7 A low-intensity, 60-minute-duration tooth clenching task produces fatigue and pain during the task,49 suggesting fatigue produces pain. However, after the tooth clenching task, subjects report continue to report pain and headaches.52 Further injection of glutamate into the fatigued muscle enhanced pain sensation and headaches, particularly in female subjects.52 However, the role of fatigue as a precipitating factor in the development of clinical pain syndromes is unknown. We hypothesized that muscle fatigue would enhance the hyperalgesia associated with injection of acidic saline into muscle by inducing muscle damage and increasing metabolites.
Materials and Methods
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the guidelines of the National Institute of Health and the International Association for the Study of Pain policies on the use of laboratory animals.
Animals
Male mice (C57BL/6J, 19–22 g), kept at a 12-hour darklight cycle (7 am to 7 pm light; 7 pm to 7 am dark) were used for the experiments. Animals were brought to the behavioral testing room 2 hours before the testing to acclimatize them to the testing environment. Behavioral tests were performed between 10 am and 3 pm.
Fatigue Task
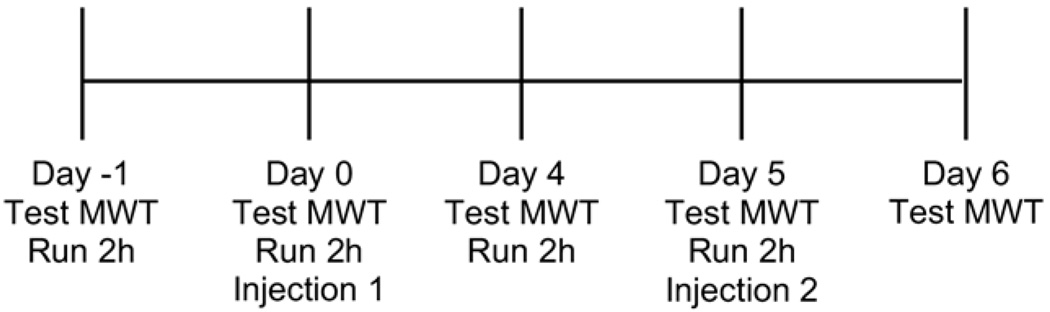
Before running, mice were acclimatized for 3 days in a mouse running wheel with a 10.3-cm diameter. The number of rotations was calculated with an external sensor and converted to kilometers, based on the size of the wheel. On the first training day, they were placed in the wheel for 10 minutes 3 times with 1-hour intervals between each session. On the second and third training day, mice were required to run for 10 minutes, in 3 separate sessions 1 hour apart. To ensure continuous running in the wheel, the top of the cage was tapped when the mice stopped running for greater than 5 seconds. For the running days, mice were run in the wheel for 2 hours and the total running distance was recorded. Mice ran 2 hours per day for 2 days immediately before injection 1, and for 2 hours per day for 2 days immediately before injection 2 (Fig 1). The 2-hour duration of running was chosen through preliminary experiments that showed fatigue in response to the running task. Two days of running before each injection were chosen since we hypothesized that the 2-hour running task would induce muscle damage and increase muscle metabolites; and we thus expected to show a greater effect with 2 days of running before each injection.
Figure 1.
Time line for the experiment is shown. The running task was performed on day -1, day 0, day 4, and day 5. Before the running task, the mechanical withdrawal threshold of the paw (MWT) was tested on each day and again 24 hours after the second intramuscular acid injection. Injection of acid was delivered immediately after the running tasks on day 0 and day 5.
Injection of Muscle
Immediately after the second day of running (day 0), mice were anesthetized briefly with halothane (2% to 4%) and 1 gastrocnemius muscle was injected with 20 µL of pH 4.0 (n = 7), pH 5.0 (n = 7), pH 6.0 (n = 7), or pH 7.2 (n = 7) sterile saline.41 The pH was adjusted with HCl to within 0.1 pH. Five days later, immediately after the fourth day of running (day 5), the animals were re-anesthetized with halothane (2% to 4%) and the same gas-trocnemius muscle was re-injected with 20 µL of the same sterile saline at the same pH as on day 0 (Fig 1). A control group that did not run received 2 injections of pH 4.0 (n = 7), pH 5.0 (n = 6), pH 6.0 (n = 6), or pH 7.2 (n = 6) on day 0 and day 5.
Mechanical Withdrawal Response of the Paw
Mice were tested for response to mechanical stimulation of the paw with von Frey filaments. The animals were placed in lucite cubicles on an elevated screen platform and allowed to acclimate for 45–60 minutes. Von Frey filaments of varying bending forces (1.5, 0.7, 0.3, 0.2, 0.08 mN) were applied 10 times to the each plantar surface of the hindpaw and the number of withdrawals was recorded.36 The mechanical withdrawal threshold was measured before the first run (day -1), before injection 1 (day 0), before the third run (day 4), before injection 2 (day 5), and 24 hours after the second injection (day 6) (Fig 1). In the current study, we interpreted an increase in the number of withdrawals as cutaneous hyperalgesia of the paw.
Measurement of Grip Force
In a separate group of animals, fatigue was confirmed by measurement of grip force immediately after running and comparing to the grip force before running for both the hind limbs and the forelimbs (n = 8). This was compared with a control group of mice that did not perform the running task (n = 8). Mice were trained 3 days before data collection to the grip force device. The mice were familiarized twice per day to the apparatus by performing the grip force task. Mice were pulled by the tail to the right to read grip force on the forelimb and then pulled to the left to read grip force on hind limb. Mice were pulled until a good grip was felt. Grip force was measured before and after every run: Day 0, day 1, day 4, day 5. Grip force was analyzed on both forelimb and hind limb and an average of 5 trials recorded. Each mouse had forelimb and hind limb measured before moving on to the next mouse. A decrease in grip force after running was interpreted as muscle fatigue.
Muscle Histology
To test for potential tissue damage, muscle histology was performed after the second and fourth day of 2 hours running (n = 4) and compared with a control group of mice that did not run (n = 4). Evans blue dye was used to assess for potential myocyte membrane damage. The gastrocnemius muscle was removed and frozen in isopentane cooled to −160°C. Muscles were step-sectioned at 100-µm intervals. Cross sections were cut on a cryostat at 7 to 10 µm, stained with hematoxylin and eosin, and examined by light microscopy.
Metabolic Changes in Muscle
Some animals (n = 8 fatigue and n = 8 control) were anesthetized with halothane (2% to 5%) and the gastrocnemius muscle was removed immediately after the second session of running on day 0. The muscles were analyzed for pco2 and po2 with an ABL5 radiometer (GMI Inc, Ramsey, MN) and for lactate with YSI 2300 STAT Plus (YSI Inc, Yellow Springs, OH). In a separate group of animals after the 4th day of running, (n = 4 fatigue, n = 4 control) mice were deeply anesthetized, guillotined, and the gastrocnemius muscle was removed and placed into cold buffer containing 1% Triton X, 10 mmol/L Tris pH 4, 20 mmol/L EDTA, 10 mmol/L EGTA, 150 mmol/L NaCl, leupeptin, pepstatin A, and PMSF. Samples were homogenized, centrifuged, and filtered through a 0.2-µm filter. CK-MB was measured with an ELISA kit obtained from Diagnostic Automotion, Inc (Calabasas, CA), and phosphate was measured using a kit obtained from Biomedical Research Service Center (Buffalo, NY). CK-MM was also measured by ELISA with a method developed in our laboratory. For CK-MM ELISA, the 1:200 primary antibody mouse anti-human CK-MM obtained from Scripts Laboratories (San Diego, CA) was loaded into PVC microtiter plate. The plate was covered and stored overnight at 4°C. The wells were washed twice with phosphate-buffered saline (PBS) and blocked with3%bovine serum albumin/PBS for 2 hours. The wells were washed twice with PBS. The wells were loaded with 50 mL of standard or sample. 50 mL of 1:250 biotinylated anti mouse IgG was added to each of the wells and incubated for 1 hour, then the plate was washed twice with PBS. 50 mL 1:500 streptavidin-horse-radish peroxidase was added to each well. The plates were then washed 3 times with PBS. 50 mL of TMB was added and the plate incubated at room temp for 15 minutes. The reaction was stopped with 50 mL of 0.1N sulfuric acid. The CK-MM and CK-MB plates were read at 490 nm and the phosphate plate was read at 650 nm on a spectromax (Molecular Devices, Sunnyvale, CA), all samples except blood gases and lactate were measured in duplicate.
Statistical Analysis
A repeated-measures ANOVA tested for changes in the number of responses to repeated mechanical withdrawal threshold across time and between groups for each individual von Frey filaments. A repeated-measures ANOVA also tested for changes in grip force across time and between groups. A one-way ANOVA for group tested for differences between groups (fatigue vs control) for po2, pco2, lactate, phosphate, CK-MM, and CK-MB. Data were considered significant if P < .05. Data are represented as mean ± SEM.
Results
Induction of Fatigue
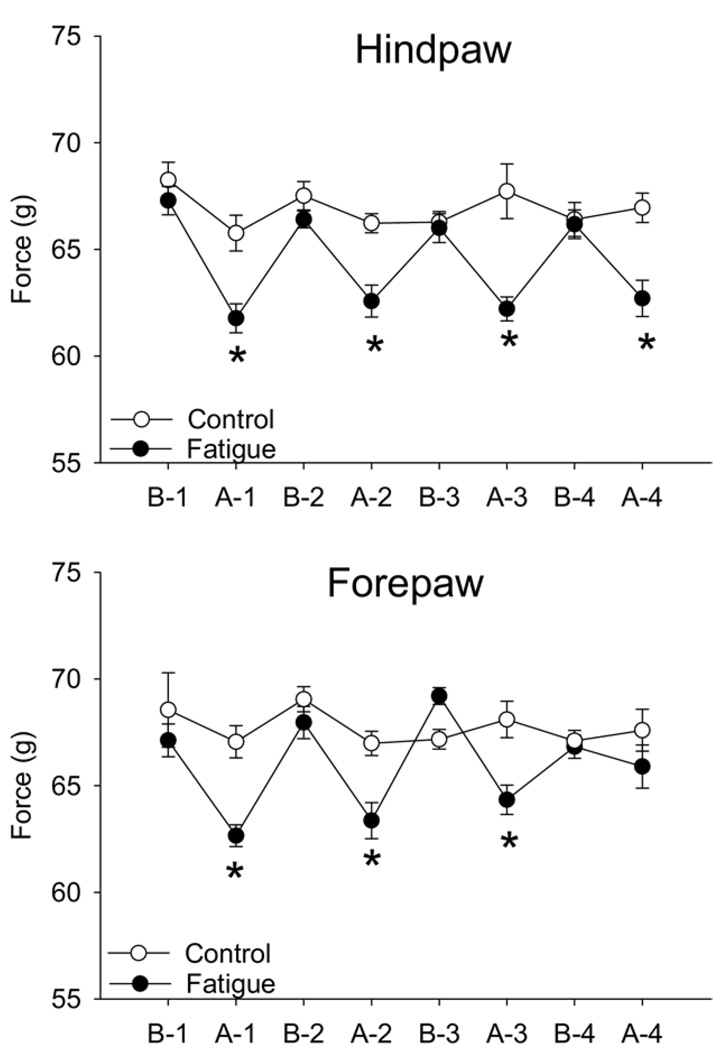
Running distance averaged 2.16 ± 0.52 km/2 h for all groups and the distance was similar at each time and between groups. In the running group, the grip force was significantly less than the baseline grip force immediately after the running task and from the control non-running group for both the forelimbs and the hind limbs (Fig 2). For the forepaws, the grip force after the 4th run was increased in 4 of 8 animals and decreased in another 4 of 8 animals, which resulted in a net effect of no change.
Figure 2.
Line graphs show the withdrawal force in grams before (B) and after (A) each 2-hour running session (1, 2, 3, 4) for both the hindpaws and the forepaws. There was a significant decrease in force immediately after each run (*P < .05) in the running group (fatigue; closed symbols) compared with the group that did not run (control; open symbols). Data are mean SEM.
Cutaneous Hyperalgesia
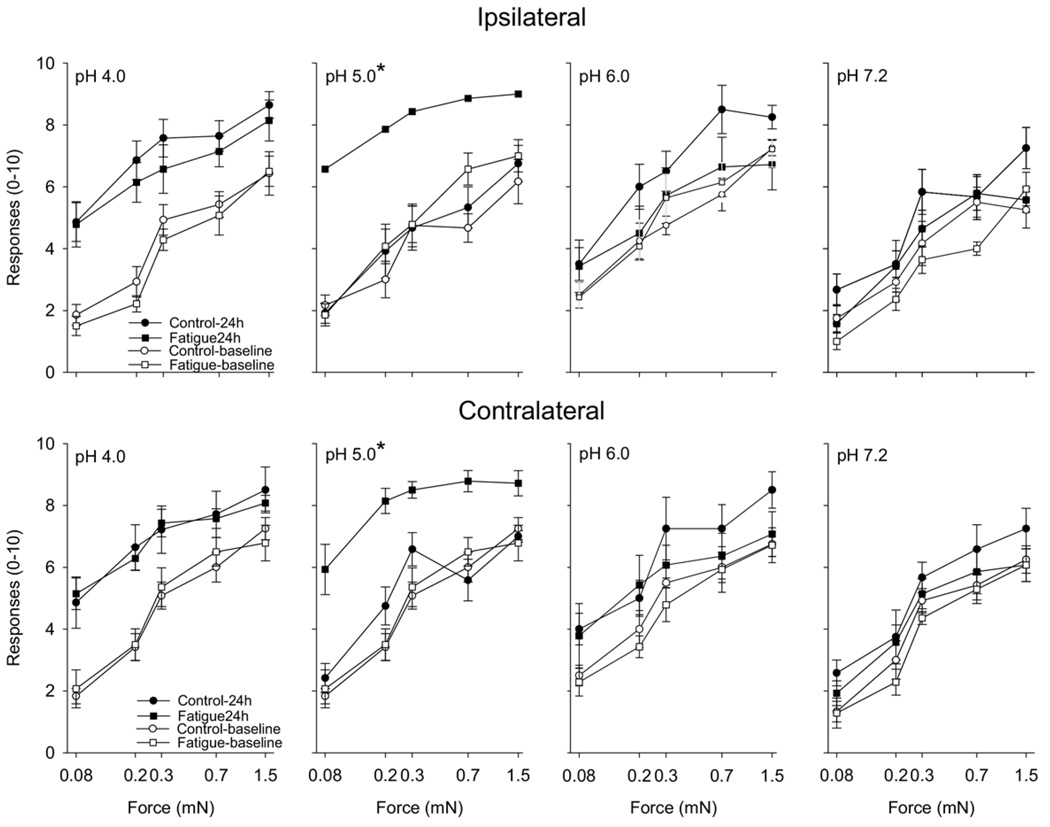
For the mice injected intramuscularly with pH 4.0 saline, both the fatigued and the control groups showed a bilateral increase in the number of responses to mechanical stimuli 24 hours after injection (Fig 3). The pH 5 group responded differently between groups; there was a significant increase in the number of responses to mechanical stimuli 24 hours bilaterally after the second injection of acidic saline for mice that were fatigued when compared with control animals (Fig 3). This increase in mechanical sensitivity after injection of pH 5.0 into fatigued mice was of the same magnitude observed after injection of pH 4.0 (Fig 3). On the other hand, injections of pH 6 and pH 7.2 did not produce significant change in the number of responses to mechanical stimuli in the both groups when compared with baseline values. There were no changes at other time points tested for either the ipsilateral or the contralateral hindpaw (data not shown).
Figure 3.
Line graphs show the number of withdrawals (of 10) to von Frey filaments (0.08, 0.2, 0.4, 0.7, 1.5 mN) for the ipsilateral (top panels) and contralateral (bottom panels) paws from animals that ran (fatigue; squares) and control animals that did not run (control; circles). Each panel shows data for rats that received 2 intramuscular injections of different pH (4.0, 5.0, 6.0, 7.2). Open symbols represent data before the first intramuscular injection of saline and the closed symbols represent data 24 hours after the second injection of saline. Significant difference between the fatigue and control groups was observed 24 hours after the second intramuscular injection of pH 5.0 saline (*P < .05). Data are mean ± SEM.
Local Muscle Histology and Metabolism
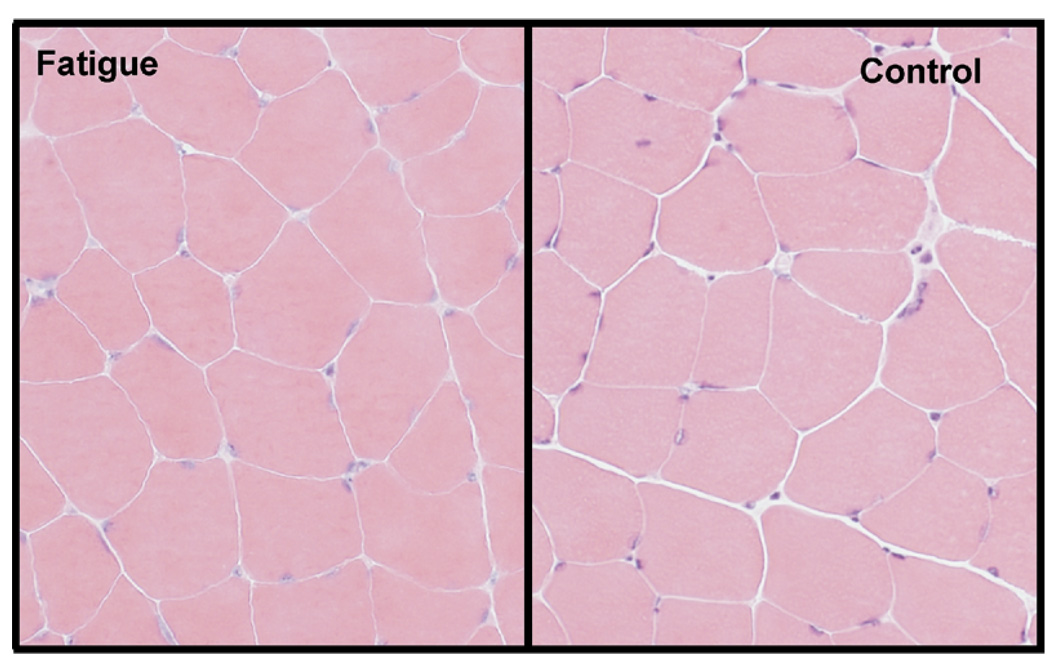
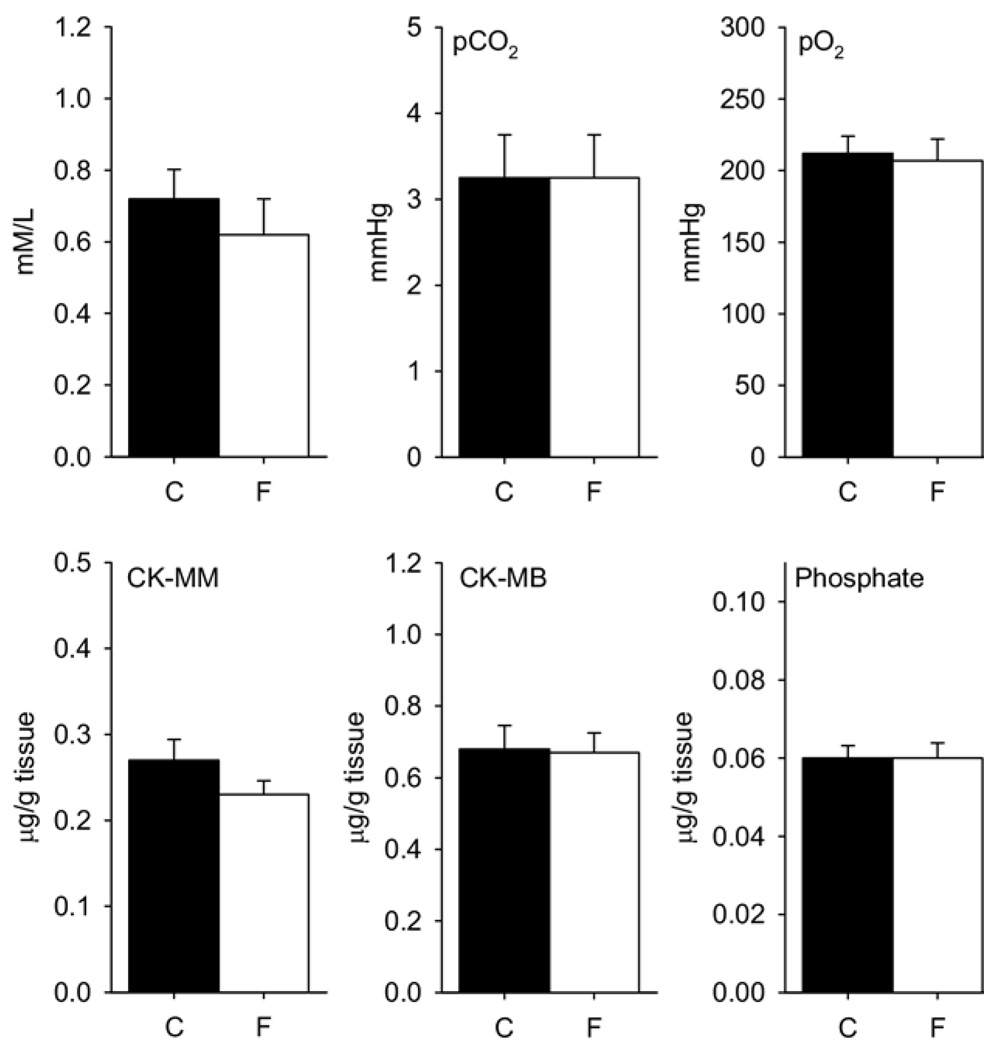
No signs of muscle damage were observed in the fatigue group. No visual histological changes were observed in the muscle after 2 days of 2-hour running (Fig 4), or after 4 days of 2-hour running (data not shown). Similarly, there was no difference in muscle lactate, pco2, and po2 after 2 days of 2-hour running; or for phosphate and creatinine kinase (CK-MB or CK-MM) after 4 days of 2-hour running when comparing the fatigue group with the nonfatigue group (Fig 5).
Figure 4.
Representative sections from muscle from an animal that was fatigued compared with a nonfatigued animal. There was no visible tissue damage in the fatigue group (fatigue; n = 4) when compared with control group (control; n = 4).
Figure 5.
Bar graphs depict muscle concentrations of lactate, pCO2, pO2, CK-MM, CK-MB, and phosphate for the control (C) and the fatigue (F) groups. There was no significant difference in any measure. Data are mean ± SEM.
Discussion
The current study shows there was a similar increase in sensitivity to mechanical stimuli after 2 injections of pH 4.0 in animals with muscle fatigue and in control mice that were not fatigued. In control mice, 2 intramuscular injections of pH 5.0, 6.0, or 7.2 had no effect on the sensitivity to mechanical stimuli. However, in animals with muscle fatigue, 2 injections of pH 5.0 increased the mechanical sensitivity of the paw similar to that observed after 2 injections of pH 4.0. These data suggest that the fatigue task primed the nervous system to enhance nociceptive responses to acidic pH that were previously subthreshold.
Fatigue is defined as a temporary loss in force as a result of recent muscle contraction.50 The current study shows an approximately 10% decrease in force after 2 hours of wheel running and is thus defined as a fatigue task. Since fatigue recovers quickly this loss of force probably is a conservative estimate. There are numerous mechanisms of fatigue that include changes in the central nervous system, changes in the microenvironment of the muscle (peripheral fatigue), or changes in excitation-contraction coupling (peripheral fatigue).1,2,50
Peripheral Mechanisms for Enhanced Nociception After Fatigue
One peripheral mechanism by which an enhanced response to acidic pH could occur is through metabolic changes in the muscle as a result of fatigue. Peripherally, fatiguing exercise can result in changes in the muscle tissue that includes increases in lactate, phosphate and creatinine kinase, decreases in pH, and infiltration of neutrophils.2,8,11,30 Changes in these substances could directly activate nociceptors through acid sensing ion channels and increased release of cytokines and prostaglandins.5,22,27,31,42,43,47 Alternatively, there could be changes in the buffering capacity of the muscle to maintain pH such that a pH of 5 is buffered slower than under nonfatigue conditions. 13,33 However, the current study was unable to detect changes in lactate, phosphate, creatinine kinase, or to detect infiltration of neutrophils in the muscle, suggesting that metabolic changes in the muscle associated with damage are unlikely to contribute to the enhanced nociceptive response to pH. Changes in blood lactate clearly occur during a fatiguing exercise bout,12,16,34,37 and these lactate changes can last up to 15 minutes after a high-intensity exercise bout.16,17,37 In the current study, the animals spontaneously ran in the running wheel and thus were not likely exercising at a strong intensity typically observed in studies that measure lactate. It is further possible that increases in lactate occurred during the 2-hour fatigue task, even though we were unable to measure the changes after the fatigue task. Regardless, we measured metabolic changes in the muscle at the same time as we would have given the intramuscular injection of saline. Thus, if metabolic changes occurred they were resolved quickly and were not present at the time of injection. It should be also pointed out that there are minimal changes in the periphery after injection of acidic saline. The intramuscular pH after injection of pH 4 decreases to an average of pH 6.5 and after injection of pH 5 to an average of pH 6.9. There is also no change in muscle after injection of pH 4 examined histologically. Therefore, a peripheral mechanism is unlikely since the induction and maintenance of the hyperalgesia associated with this model involves changes in the central nervous system38,39,41,51 (outlined below) and we were unable to detect long-lasting changes in muscle metabolism or muscle damage after the fatigue task.
Central Mechanisms for Enhanced Nociception After Fatigue
An alternate mechanism could be that the enhanced response to pH after fatigue is mediated by changes in the central nervous system. Indeed, in this model of muscle-induced hyperalgesia there is a long-lasting increased mechanical sensitivity that is maintained by changes in the central nervous system.38–41,44,51 The contralateral hyperalgesia that develops after the second intramuscular acid injection is independent of input from the periphery41 and prevented or reversed by blockade of receptors in the spinal cord.38 There is also an enhanced release of the excitatory neurotransmitter glutamate in the spinal cord in response to the second injection of acidic saline.39 Recently, we showed that anesthetic blockade of supraspinal sites in the medulla 1) prevent the development of the hyperalgesia that occurs in response to the second injection of acidic saline and 2) reverse the mechanical hyperalgesia once developed.51 These sites in the rostral ventromedial medulla integrate both sensory and motor systems and thus changes in these sites could enhance nociceptive responses after a fatigue task. Serotonergic neurons in the nucleus raphe obscurus and pallidus in the medulla are implicated in fatigue since there is a strong correlation between neuronal activity and motor activity.19 Both the nucleus raphe obscurus and pallidus, in addition to the nucleus raphe magnus, have also been shown to facilitate nociception61 specifically in this muscle-induced model of hyperalgesia.51
Exercise Training and Analgesia
Chronic running could also result in decreased endogenous inhibition. Allowing rats free access to a running wheel for 3 weeks resulted in lower tail flick latencies compared with nonrunning rats.9,21,24,25,45,46 The active rats showed a lower analgesic response to opioid analgesics administered peripherally, systemically, or into the periaqueductal gray in the midbrain.24,25 These data suggest that running wheel activity increases release of endogenous opioid peptides. With repeated running and continued opioid release tolerance occurs at opioid receptors and thus there is less endogenous inhibition. Noxious stimulation clearly increases activity in the descending inhibitory systems and removal of this inhibition enhances nociception. 53 In the current study, however, baseline withdrawal thresholds were similar between groups suggesting that opioid tolerance did not occur with 4 days of running.
On the other hand, low intensity, short duration treadmill exercise (15–30 min/d for 5 days) reduces hyperalgesia in rats after repeated intramuscular injections of pH 4.0 saline.4 Similarly, in rats with spinal cord injury, low-intensity treadmill exercise (20–25 min/d for 7 weeks) reduces the enhanced sensitivity to mechanical stimuli. Naloxone prevents the analgesic effects of low intensity exercise suggesting opioid mechanisms are responsible for the reduction in hyperalgesia.4 However, decreases in brain-derived neurotrophic factor (BDNF) mRNA in the spinal cord and muscle that normally occur after spinal cord injury are normalized by exercise, and neurotrophin-3mRNAincreases in the soleus after exercise training.18 Similarly, mice that overexpress neurotrophin-3 in muscle have decreased mechanical hyperalgesia to acidic saline15 and BDNF in the periphery or spinal cord reduces hyperalgesia.32 Together these studies suggest there may be both peripheral and central mechanisms underlying the responses to exercise, fatigue and enhanced nociception.
Clinical Implications
Clinically, fatigue is common in many musculoskeletal conditions; people with chronic fatigue have significant musculoskeletal pain. There is a clear association between the severity of musculoskeletal pain and disability as well as between the severity of fatigue and disability in people with chronic fatigue syndrome.29 This fatigue is described by subjects as a reduced amount of energy needed to perform normal activities of daily living. However, in people with musculoskeletal pain or chronic fatigue syndrome there is decreased muscle force compared with normal subjects,3,10,23 and there is commonly pain on or after exercise.20,48,54,55 Clearly, exercise is an effective treatment for people with musculoskeletal pain conditions such as fibromyalgia and chronic fatigue syndrome6,14 decreasing pain, fatigue, and/or improving function. This exercise, however, must be carefully monitored to avoid worsening of symptoms. In people with fibromyalgia and chronic fatigue syndrome ‘pacing’ is a commonly utilized strategy that balances the amount of physical activity with rest to achieve optimal physical function without exacerbation of symptoms. This type of strategy is utilized not only for developing an appropriate exercise program but also for all activities of daily living. Importantly, pacing suggests that there is a dose of exercise which is analgesic and one in which you can exacerbate the pain. It is therefore imperative to find a level of exercise that does not produce excessive fatigue and enhance or promote pain.
Summary
In summary, the current study shows that muscle fatigue enhances the probability of the development of mechanical hyperalgesia in mice in response to acidic saline without histological change. This increased mechanical hyperalgesia occurs in mice intramuscularly injected with pH 5 saline, a pH that does not produce hyperalgesia in nonfatigued mice. The fatigue task did not produce measurable changes in the muscle tissue suggesting muscle damage did not occur not contribute to the hyperalgesia observed with pH 5. Thus, fatigue could play a critical role in the development of chronic musculoskeletal pain.
Acknowledgments
We thank J. Danielson, Dane Pratt, Steve Westra, and Lynn Burnes for excellent technical assistance.
Supported by the National Institutes of Health grants K02-AR02201, R01-AR052316, U54-NS05362, and R01-AR053509.
References
- 1.Allen DG, Lannergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol. 1995;80:497–527. doi: 10.1113/expphysiol.1995.sp003864. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- 3.Bazelmans E, Bleijenberg G, Voeten MJ, van der Meer JW, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychosom Res. 2005;59:201–208. doi: 10.1016/j.jpsychores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Bement MK, Sluka KA. Low-intensity exercise reverses chronic muscle pain in the rat in a naloxone-dependent manner. Arch Phys Med Rehabil. 2005;86:1736–1740. doi: 10.1016/j.apmr.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 5.Benson CJ, Xie J, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Busch A, Schachter CL, Peloso PM, Bombardier C. Exercise for treating fibromyalgia syndrome. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD003786. CD003786. [DOI] [PubMed] [Google Scholar]
- 7.Ciubotariu A, Rendt-Nielsen L, Graven-Nielsen T. The influence of muscle pain and fatigue on the activity of synergistic muscles of the leg. Eur J Appl Physiol. 2004;91:604–614. doi: 10.1007/s00421-003-1026-9. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson PM, Sayers SP. Etiology of exercise-induced muscle damage. Can J Appl Physiol. 1999;24:234–248. doi: 10.1139/h99-020. [DOI] [PubMed] [Google Scholar]
- 9.D’Anci KE, Gerstein AV, Kanarek RB. Long-term voluntary access to running wheels decreases kappa-opioid antinociception. Pharmacol Biochem Behav. 2000;66:343–346. doi: 10.1016/s0091-3057(00)00245-8. [DOI] [PubMed] [Google Scholar]
- 10.De BP, Roeykens J, Reynders M, McGregor N, De MK. Exercise capacity in chronic fatigue syndrome. Arch Intern Med. 2000;160:3270–3277. doi: 10.1001/archinte.160.21.3270. [DOI] [PubMed] [Google Scholar]
- 11.Debold EP, Dave H, Fitts RH. Fiber type and temperature dependence of inorganic phosphate: Implications for fatigue. Am J Physiol Cell Physiol. 2004;287:C673–C681. doi: 10.1152/ajpcell.00044.2004. [DOI] [PubMed] [Google Scholar]
- 12.Durand RJ, Castracane VD, Hollander DB, Tryniecki JL, Bamman MM, O’Neal S, Hebert EP, Kraemer RR. Hormonal responses from concentric and eccentric muscle contractions. Med Sci Sports Exerc. 2003;35:937–943. doi: 10.1249/01.MSS.0000069522.38141.0B. [DOI] [PubMed] [Google Scholar]
- 13.Edge J, Bishop D, Goodman C. The effects of training intensity on muscle buffer capacity in females. Eur J Appl Physiol. 2006;96:97–105. doi: 10.1007/s00421-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 14.Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2004 doi: 10.1002/14651858.CD003200.pub2. CD003200. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi R, Ryals JM, Wright DE. Neurotrophin-3 reverses chronic mechanical hyperalgesia induced by intramuscular acid injection. J Neurosci. 2004;24:9405–9413. doi: 10.1523/JNEUROSCI.0899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollander DB, Durand RJ, Trynicki JL, Larock D, Castracane VD, Hebert EP, Kraemer RR. RPE, pain, and physiological adjustment to concentric and eccentric contractions. Med Sci Sports Exerc. 2003;35:1017–1025. doi: 10.1249/01.MSS.0000069749.13258.4E. [DOI] [PubMed] [Google Scholar]
- 17.Horstmann T, Mayer F, Maschmann J, Niess A, Roecker K, Dickhuth HH. Metabolic reaction after concentric and eccentric endurance-exercise of the knee and ankle. Med Sci Sports Exerc. 2001;33:791–795. doi: 10.1097/00005768-200105000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Hutchinson KJ, Gomez-Pinilla F, Crowe MJ, Ying Z, Basso DM. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs BL, Martin-Cora FJ, Fornal CA. Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev. 2002;40:45–52. doi: 10.1016/s0165-0173(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 20.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings and pressure pain thresholds in healthy individuals and patients with fibromyalgia. Eur J Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Kanarek RB, Gerstein AV, Wildman RP, Mathes WF, D’Anci KE. Chronic running-wheel activity decreases sensitivity to morphine-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1998;61:19–27. doi: 10.1016/s0091-3057(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Yang TM, Liedtke W, Simon SA. Chronic IL-1beta signaling potentiates voltage-dependent sodium currents in trigeminal nociceptive neurons. J Neurophysiol. 2006;95:1478–1490. doi: 10.1152/jn.00509.2005. [DOI] [PubMed] [Google Scholar]
- 23.Maquet D, Croisier JL, Renard C, Crielaard JM. Muscle performance in patients with fibromyalgia. Joint Bone Spine. 2002;69:293–299. doi: 10.1016/s1297-319x(02)00373-1. [DOI] [PubMed] [Google Scholar]
- 24.Mathes WF, Kanarek RB. Wheel running attenuates the antinociceptive properties of morphine and its metabolite, morphine-6-glucuronide, in rats. Physiol Behav. 2001;74:245–251. doi: 10.1016/s0031-9384(01)00577-7. [DOI] [PubMed] [Google Scholar]
- 25.Mathes WF, Kanarek RB. Chronic running wheel activity attenuates the antinociceptive actions of morphine and morphine-6-glucouronide administration into the periaqueductal gray in rats. Pharmacol Biochem Behav. 2006;83:578–584. doi: 10.1016/j.pbb.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Meeus M, Nijs J, Meirleir KD. Chronic musculoskeletal pain in patients with the chronic fatigue syndrome: A systematic review. Eur J Pain. 2006 Dec 7; doi: 10.1016/j.ejpain.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E2. Brain Res. 1981;225:95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- 28.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Nijs J, De MK, Wolfs S, Duquet W. Disability evaluation in chronic fatigue syndrome: associations between exercise capacity and activity limitations/participation restrictions. Clin Rehabil. 2004;18:139–148. doi: 10.1191/0269215504cr708oa. [DOI] [PubMed] [Google Scholar]
- 30.Ogilvie RW, Armstrong RB, Baird KE, Bottoms CL. Lesions in the rat soleus muscle following eccentrically biased exercise. Am J Anat. 1988;182:335–346. doi: 10.1002/aja.1001820405. [DOI] [PubMed] [Google Scholar]
- 31.Ozaktay AC, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Dorsal root sensitivity to interleukin-1 beta, interleukin-6 and tumor necrosis factor in rats. Eur Spine J. 2002;11:467–475. doi: 10.1007/s00586-002-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezet S, McMahon SB. Neurotrophins: Mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 33.Pilegaard H, Asp S. Effect of prior eccentric contractions on lactate/H+ transport in rat skeletal muscle. Am J Physiol. 1998;274:E554–E559. doi: 10.1152/ajpendo.1998.274.3.E554. [DOI] [PubMed] [Google Scholar]
- 34.Racette R, Peronnet F, Massicotte D, Lavoie C. Metabolic response to prolonged cycling with (13)C-glucose ingestion following downhill running. Eur J Appl Physiol. 2005;93:598–605. doi: 10.1007/s00421-004-1240-0. [DOI] [PubMed] [Google Scholar]
- 35.Rijkelijkhuizen JM, de Ruiter CJ, Huijing PA, de HA. Low-frequency fatigue is fibre type related and most pronounced after eccentric activity in rat medial gastrocnemius muscle. Pflugers Arch. 2003;447:239–246. doi: 10.1007/s00424-003-1172-2. [DOI] [PubMed] [Google Scholar]
- 36.Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- 37.Sayers SP, Clarkson P, Patel JJ. Metabolic response to light exercise after exercise-induced rhabdomyolysis. Eur J Appl Physiol. 2002;86:280–282. doi: 10.1007/s00421-001-0540-x. [DOI] [PubMed] [Google Scholar]
- 38.Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- 39.Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 40.Sluka KA, Audette KM. Activation of protein kinase C in the spinal cord produces mechanical hyperalgesia by activating glutamate receptors, but does not mediate chronic muscle-induced hyperalgesia. Mol Pain. 2006;2:13. doi: 10.1186/1744-8069-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 43.Sluka KA, Price MP, Wemmie JA, Welsh MJ. ASIC3, but not ASIC1, channels are involved in the development of chronic muscle pain. Proc 10th World Congress Pain. 2003 [Google Scholar]
- 44.Sluka KA, Rohlwing JJ, Bussey RA, Eikenberry SA, Wilken JM. Chronic muscle pain induced by repeated acid injection is reversed by spinally administered μ−, and δ−, but not κ−, opioid receptor agonists. J Pharmacol Exp Ther. 2002;302:1146–1150. doi: 10.1124/jpet.102.033167. [DOI] [PubMed] [Google Scholar]
- 45.Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav. 2004;77:49–57. doi: 10.1016/j.pbb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 46.Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–434. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- 47.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 48.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 49.Svensson P, Burgaard A, Schlosser S. Fatigue and pain in human jaw muscles during a sustained, low-intensity clenching task. Arch Oral Biol. 2001;46:773–777. doi: 10.1016/s0003-9969(01)00028-0. [DOI] [PubMed] [Google Scholar]
- 50.Taylor JL, Todd G, Gandevia SC. Evidence for a supraspinal contribution to human muscle fatigue. Clin Exp Pharmacol Physiol. 2006;33:400–405. doi: 10.1111/j.1440-1681.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 51.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate bilateral hyperalgesia after muscle insult. Soc Neurosci Abstr. 2006;347:3. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torisu T, Wang K, Svensson P, De Laat A, Fujii H, Rendt-Nielsen L. Effects of muscle fatigue induced by low-level clenching on experimental muscle pain and resting jaw muscle activity: Gender differences. Exp Brain Res. 2006;174:566–574. doi: 10.1007/s00221-006-0497-4. [DOI] [PubMed] [Google Scholar]
- 53.Vanegas H, Schaible HG. Descending control of persistent pain: Inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Vierck CJ, Jr, Staud R, Price DD, Cannon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 55.Whiteside A, Hansen S, Chaudhuri A. Exercise lowers pain threshold in chronic fatigue syndrome. Pain. 2004;109:497–499. doi: 10.1016/j.pain.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–1417. [PubMed] [Google Scholar]
- 57.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 58.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 59.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Zautra AJ, Fasman R, Parish BP, Davis MC. Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain. 2006;128:128–135. doi: 10.1016/j.pain.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Zhuo M, Gebhart GF. Biphasic modulation of spinal nociceptive transmission from the medullary raphe nuclei in the rat. J Neurophysiol. 1997;78:746–758. doi: 10.1152/jn.1997.78.2.746. [DOI] [PubMed] [Google Scholar]