Abstract
The primary aim of this project was to examine the role of alcohol use in smoking lapse behavior, as alcohol consumption is a known risk factor for poor smoking cessation outcomes. We have developed a novel human laboratory model to examine two primary aspects of alcohol-mediated tobacco relapse: 1) Does alcohol facilitate the initiation of the first cigarette? 2) Once the first cigarette is initiated, does alcohol facilitate subsequent smoking? Using a within-subject design, 16 daily smokers who were also heavy social drinkers received a priming drink (0.03 g/dl or taste masked placebo) and then had the option of initiating a tobacco self-administration session or delaying initiation by five minute increments for up to 50 minutes in exchange for monetary reinforcement. Subsequently, the tobacco self-administration session consisted of a one-hour period, in which subjects could choose to smoke their preferred brand of cigarettes using a smoking topography system or receive monetary reinforcement for cigarettes not smoked. Alcohol craving, tobacco craving, subjective reactivity to alcohol, and nicotine withdrawal were assessed as secondary outcomes. Results demonstrated that after consuming the alcohol beverage, subjects were less able to resist the first cigarette and initiated their smoking sessions sooner, and smoked more cigarettes compared to the placebo beverage. These findings have implications for smoking cessation in alcohol drinkers and model development to assess smoking lapse behavior.
Keywords: smoking, lapse behavior, alcohol, craving, human laboratory, monetary reinforcement
It is well established that alcohol consumption and tobacco use are highly correlated in both clinical and non-clinical samples. Among alcoholics, 80% to 95% are smokers (DiFranza & Guerrera, 1990; Hughes, 1993; Kalman et al., 2005; Patten et al., 1996; Sobell et al., 1990), as compared to 23% in the general population (CDC, 2003). Smokers have a four to ten-fold increased risk for developing alcohol use disorders (DiFranza & Guerrera, 1990; Grant et al., 2004; Hurt et al., 1994) and the severity of alcohol and tobacco dependence is positively correlated (Ellingstad et al., 1999; Gulliver et al., 1995). Smoking is also highly correlated with drinking in nonalcoholic individuals (Carmody et al., 1985; Istvan & Matarazza, 1984), particularly among those who are heavy drinkers. Both laboratory studies and naturalistic observations have demonstrated that alcohol consumption is strongly associated with increased rates of smoking (Glautier et al., 1996; Mitchell et al., 1995; Shiffman et al., 1994). Additionally, there is evidence for cross-substance craving from both clinical and non-clinical samples (Drobes et al., 2000; Tiffany, 1995). Given the high co-occurrence of alcohol and tobacco use, it is not surprising that alcohol has been identified as a risk factor for poor smoking cessation outcomes (Baer & Lichenstein, 1988; Shiffman 1986; Zimmerman et al., 1990). For example, Shiffman (1986) found that 20% of all relapse episodes in smokers involved alcohol consumption. In a real-time examination of first lapse episodes, 25% of drinkers identified alcohol as the primary trigger for smoking (Shiffman et al., 1997).
Given the significant impact of alcohol on the first instance of smoking during a cessation attempt (i.e., a lapse), we decided to focus our investigation on the effect of alcohol on smoking lapse behavior. Moreover, it is known that the first instance of smoking after the initiation of a quit attempt is one of the best predictors of relapse (Brandon et al., 1990; Garvey et al., 1992; Kenford et al., 1994; Marlatt et al., 1988; Nides et al., 1995; Norregaard et al., 1993). Lapses typically occur soon after quitting, and are usually defined as any smoking, even one puff (e.g., Brandon et al., 1990; Shiffman et al., 1996). In a sample of self-quitters, Garvey et al. (1992) found that 13% relapsed on the first day and that 49% had relapsed by the first week. Further, they demonstrated that in those who had any smoking in the post-cessation period, 95% returned to regular smoking. Conversely, Westman et al. (1997) found that smokers who maintained abstinence on the quit day were 10 times more likely to be abstinent in the long term.
It is clear that that the first occurrence of smoking during a cessation attempt is a critical transition and represents an important target for investigation. Human laboratory paradigms have been used to investigate various nicotine dependence phenomena such as nicotine discrimination (Perkins et al., 1997; Perkins et al., 1999), nicotine reinforcement (Perkins et al., 2001), deprivation effects (Hatsukami et al., 1984), self-administration behavior (Hatsukami et al., 1998; Perkins et al., 1997), and cue reactivity (Carter & Tiffany, 1999 for review). Researchers have also examined a human analogue of reinstatement as a model of smoking relapse, which involves a non-contingent cigarette exposure after a period of abstinence (Chornock et al., 1992; Juliano & Stitzer, 2003; King & Meyer, 2000). However, currently available models examining tobacco-related phenomena have not yet modeled the ability to resist the first cigarette, nor have they modeled the effect of alcohol on smoking lapse behavior.
For the current study, we decided to focus our model on early lapse behavior, and in particular, the ability to resist the first cigarette after the consumption of alcohol. We developed a paradigm designed to examine two central questions related to alcohol-mediated tobacco relapse; 1) Does alcohol facilitate the initiation of the first cigarette? 2) Once the first cigarette is initiated, does alcohol facilitate subsequent smoking? The proposed paradigm attempts to model the effects of a single alcoholic drink on resisting the first cigarette (a lapse) and on subsequent smoking behavior. Using a within-subject design, moderate to heavy drinking smokers who were minimally nicotine deprived received a priming drink (0.03 g/dl or taste masked placebo) and then had the option of initiating a tobacco self-administration session or delaying initiation by five minute increments for up to 50 minutes in exchange for monetary reinforcement. This portion of the experiment was intended to model the ability to resist smoking. Once subjects ‘gave in’ and decided to smoke (or alternatively, waited the full 50 minutes), the tobacco self-administration session consisted of a one-hour period, in which subjects could choose to smoke their preferred brand of cigarettes using a smoking topography system or receive monetary reinforcement for cigarettes not smoked. Money was provided as an alternative reinforcer in order to provide some incentive for not smoking and to enhance the likelihood that the effects of alcohol on the relative reinforcing value of tobacco would be detected (see Higgins, 1997; Rodefer et al., 1997). We predicted that alcohol would reduce the ability to resist the first cigarette and would increase subsequent smoking.
Additionally, we examined secondary outcomes of alcohol and tobacco craving as potential mechanisms of alcohol-mediated smoking lapse behavior. Given the high co-occurrence of drinking and smoking behavior, it follows that one substance can act as a conditioned cue for the other substance (see Tiffany, 1995 for review). For example, Drobes et al. (2000) demonstrated that alcohol cues increased craving for cigarettes and tobacco cues increased craving for alcohol in subjects who were dependent on both substances. Burton and Tiffany (1997) examined the effects of in-vivo and imaginal smoking cues under alcohol or placebo conditions in social drinkers. Overall, alcohol consumption was found to increase urges to smoke regardless of the cue condition. We predicted that alcohol and tobacco craving would be increased after consuming the alcohol beverage, relative to the placebo beverage. Furthermore, we examined whether subjective effects of alcohol and nicotine withdrawal varied as a function of alcohol consumption.
MATERIALS AND METHODS
Participants
Participants were eligible to enroll in this study if they were 21 to 55 years of age, smoked at least 15 cigarettes per day, drank alcohol at least 2 days per week, and drank at least 3 drinks per episode for men and at least 2 drinks per episode for women. Subjects were excluded from participation if they were alcohol dependent, using illicit drugs, currently seeking treatment for alcohol use or smoking behavior, presented with current severe psychiatric disorders, or had medical conditions that would contraindicate alcohol use or smoking behavior. A total of 16 subjects completed the study (6 females, 10 males). The average age was 27.75 (SD = 8.26), and participants were primarily Caucasian (81%; 19% African American), had at least some college education (81%; 19% high-school graduates), and were not married (88%). Participants smoked on average 20.07 (SD = 5.08) cigarettes per day, had baseline carbon monoxide readings of 21.00 ppm (SD = 7.13), baseline urine cotinine values of 1152.12 ng/ml (SD = 664.79), and average Fagerstrom Nicotine Dependence Scores (FTND; Heatherton et al., 1991) of 4.63 (SD = 1.75; range 1–10). Participants consumed an average of 23.77 (SD = 13.76) standard drinks per week, drank an average of 4.39 (SD = 1.65) times per week, had average Alcohol Use Disorders Identification Test (AUDIT; Babor et al., 1992) scores of 11.00 (SD = 3.31; scores of 8 or greater indicate problematic drinking), and 44% met DSM-IV criteria for current alcohol abuse.
Procedures
Intake Sessions
Written informed consent was obtained from all participants at the start of the intake session. The Structured Clinical Interview for DSM-IV (First et al., 1995) was used to exclude individuals who met diagnostic criteria for alcohol dependence or other Axis I disorders. The Timeline Followback (Sobell & Sobell, 1993) was used to assess past 30-day drinking and smoking behavior. A physical examination was conducted in addition to an EKG, urine toxicology, pregnancy tests for women, and basic blood chemistries including liver function tests.
Laboratory Sessions
Each subject completed two 6.5 hour laboratory sessions (alcohol vs. taste-masked placebo) which took place at the Yale General Clinical Research Center. The order of sessions was counterbalanced. For women, laboratory sessions were scheduled during the luteal phase of the menstrual cycle, to control for potential menstrual cycle effects (e.g., Perkins et al., 2000). The average time between laboratory sessions was 23.22 days (SD = 11.72) and this interval did not differ across gender. Subjects were paid $75 to complete each laboratory session and received a $30 bonus for completing the study.
Baseline Assessment Period
Laboratory sessions started at 11:30 am. Subjects were asked to not consume alcohol on the day of the session but were free to smoke as they normally would up until 11:30 am. Baseline assessments of breath CO (MCO2 Monitor, MicroDirect, Auburn, ME), breath alcohol (Alco-Sensor III, Intoximeter, St. Louis, MI), plasma nicotine levels, urine drug screen, urine pregnancy screen, height, and weight were obtained. All breath alcohol, pregnancy, and drug screens were required to be negative. Lunch was provided at 12:30 pm to standardize the time and amount of last food administration. At 1:00 pm, participants smoked a single cigarette with the smoking topography equipment (CRESS, Plowshare Technologies, Baltimore, MD) to familiarize themselves with the equipment and to standardize the time of last cigarette exposure. For the next 3 hours, subjects were not able to smoke but were able to watch TV and read during this period. These activities ceased at 4:00 pm. A 3-hour window of nicotine deprivation was selected to increase desire to smoke, but without resulting in a ceiling effect in craving scores prior to alcohol being consumed.
Priming Dose
The priming drink was administered at 4:00 pm. The alcohol priming drink consisted of 1 part 80 proof liquor of the subject’s choosing to 3 parts mixer chosen from a selection of equicaloric, noncaffeinated, non-carbonated drinks. The amount of alcohol was designed to raise blood alcohol levels to 0.03 g/dl and was based on a formula that takes into account the gender, age and weight of each subject (Watson, 1989). This priming dose of alcohol is equivalent to a single standard alcoholic drink, and has been successfully used in a model of alcohol drinking to prime further drinking behavior (see O’Malley et al., 2002). The placebo beverage used the same mixer and total mls as the alcohol beverage with 1% liquor (per total mls) floated on the top of the drink as a taste mask (see King et al., 2002). Both the subject and the experimenter were blind as to the beverage condition. Subjects were instructed to consume the beverage within 5 minutes.
Delay Period
At 4:05pm participants were presented with a tray containing 8 ‘half-cigarettes’ of their preferred brand, a lighter, and an ashtray. Each half cigarette consisted of the filter plus 1 inch of the tobacco portion (depending on the brand, the tobacco portion of a cigarette typically ranges from 2 to 3 inches). Half cigarettes, rather than full cigarettes, were provided to increase the number of choice options available. Participants were instructed that they could commence smoking at any point over the next 50 minutes. However, for each 5-minute block of time that they delayed or ‘resisted’ smoking they would earn $1, for a maximum of $10 over the next 50 minutes. A 50 minute period was initially chosen as this timeframe is sufficient to observe the ascending and descending limb of beverage designed to raise BALs to 0.03 g/dl (see O’Malley et al., 2002). Assessment of breath alcohol was completed every 10 minutes during the delay period. Assessment of tobacco craving, alcohol craving, subjective effects of alcohol, and nicotine withdrawal occurred at the start of the delay period and at the end of the delay period, prior to smoking. We recorded the time (in minutes) that subjects announced that they wanted to smoke (range 0–50 minutes).
Smoking Self-Administration Period
The ad-lib smoking session was 60 minutes in length and started once participants decided to end the delay period (or delayed for the full 50 minutes). Participants were instructed to ‘smoke as little or as much as you wish’ using the smoking topography equipment. Participants were further instructed that for each ½ cigarette they lit, it would cost them $1 of their $8 ‘smoking tab’. Assessments (BAL, tobacco craving, alcohol craving, subjective effects of alcohol) were completed at +30 minutes and +60 minutes during the ad-lib period. Nicotine withdrawal was assessed at the +60 timepoint. Money earned for delaying smoking and any unused portion of the ‘smoking tab’ was paid to the subjects at the end of each laboratory session.
Measures
The Alcohol Urge Questionnaire (AUQ; Bohn et al., 1995) was used to assess alcohol craving. This self-report measure consists of 8-items designed to assess an individual’s desire to drink alcohol right now (Visual Analogue Scale [VAS], range 1–100). Tobacco craving was assessed with the Tiffany Questionnaire of Smoking Urges- Brief (QSU-Brief; Cox et al., 2001), which consists of 10 items to evaluate urges to smoke in response to positive (Factor 1) or negative (Factor 2) reinforcement (VAS scale, range 1–100). We also assessed alcohol and tobacco craving with the Yale Craving Scale (YCS). This developmental measure uses single items to assess alcohol and tobacco craving (range 1–100). Based on psychophysical methods, the scale uses magnitude estimation procedures to assess craving (Bartoshuk, 2002). The Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993) is a 14-item self- report, unipolar adjective rating scale used to measure the stimulant and sedative effects of alcohol (VAS scale, range 1–100). The Alcohol Effects Scale (AES) assessed subjective alcohol effects with 5 items (high, like, rush, feel-good, intoxicated). Participants indicated on a visual analogue scale (range 1–100), how much of an alcohol effect they were experiencing. This scale was adopted from others found in the literature (Schuckit, 1984). DSM-IV symptoms of nicotine withdrawal were assessed with the 8-item Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986). Instructions were worded to assess current symptoms of withdrawal (range 0–32). Serum nicotine was measured by reversed-phase HPLC with UV detection, modified from the literature (Hariharan et al., 1988) to include a micro acid back extraction clean up step which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4ng/ml.
Statistical Analysis
Paired t-tests were used to examine the primary outcomes of the length of the delay period and number of ½ cigarettes smoked during the ad-lib period, across alcohol and taste-masked placebo beverages. To examine secondary outcomes during the delay period, multivariate analyses of variance were used examine AUQ, QSU-B, YCS, AES, BAES, and MNWS scales within beverage condition (alcohol vs. taste-masked placebo) and within time (start of delay period vs. end of delay period). We also conducted exploratory analyses to identify potential correlates of the length of the delay period and number of cigarettes smoked. Using Pearson correlation coefficients, we examined associations of demographic (age), smoking (FTND, cigarettes per day, cotinine values) and drinking variables (drinks per episode, frequency per week, AUDIT scores) with the difference score on the length of delay across the beverage conditions (placebo minus alcohol session).
Smoking topography data were cleaned with a utility program (PuffCleanUp, Plowshare Technologies), which combines or deletes data (i.e., false puffs), which can arise from movement artifacts. We used the recommended parameters for data cleaning. To examine mean smoking topography measures per ½ cigarette over the 60 minute ad-lib period, we conducted paired t-tests examining puff number, puff volume (ml), puff duration (s), inter-puff interval (s), and peak puff velocity (ml/s) within beverage conditions (alcohol vs. taste-masked placebo) 1,2. To examine secondary outcomes during the ad-lib period, multivariate analyses of variance were used examine AUQ, QSU-B, YCS, AES, BAES, and MNWS scales within beverage condition (alcohol vs. taste-masked placebo) and within time (start of ad-lib, +30 minutes, +60 minutes).
We also repeated the above analysis with gender as a between subject factor. We found no significant effects of gender on our primary or secondary outcomes. Additionally, there were no substantive effects of beverage order on our primary or secondary outcomes.
RESULTS
Baseline nicotine exposure
Across the two laboratory sessions, subjects had equivalent baseline nicotine plasma levels (p = .51; alcohol session mean = 14.37 ng/ml, SD = 7.20; placebo session mean = 15.55 ng/ml, SD = 6.81).
Delay Period
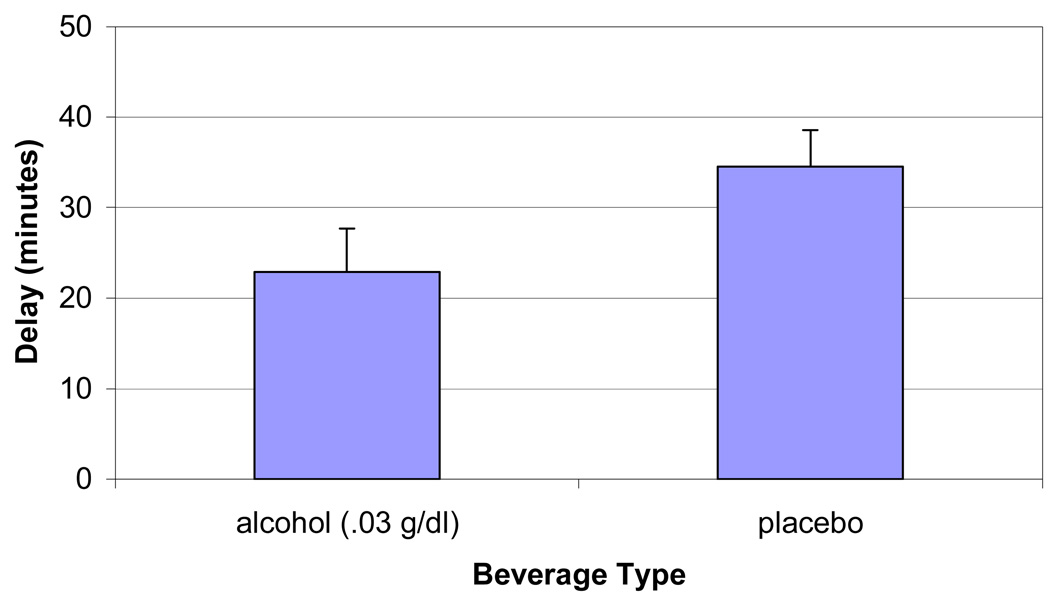
Subjects were less able to resist smoking and terminated the delay period sooner after consuming the alcohol beverage, compared to the placebo beverage [t (15) = 2.88, p<.01]. Multivariate analyses of subjective ratings during the delay period demonstrated significant effects of beverage by time for alcohol craving, cigarette craving, and subjective alcohol effects. After consuming the alcohol beverage YCS – Cigarette scores (p = 0.02), and AES scores (p = 0.02) significantly increased from the start to the end of the delay period, compared to the placebo beverage. AUQ scores demonstrated a similar effect, although it was not statistically significant (p = 0.08). Other measures (QSU-B, YCS – Alcohol, MNWS scores) demonstrated significant effects of time but not beverage condition, increasing from the start to the end of the delay period (p’s < .05). When assessing potential correlates of delay period behavior, there was a significant correlation between delay difference scores (length of placebo delay minus alcohol delay) and FTND scores (r = .55, p < .03), indicating that greater nicotine dependence was associated with less ability to delay after consuming the alcohol beverage, compared to the placebo beverage.
Ad-lib Smoking
Subjects smoked more ½ cigarettes after consuming the alcohol beverage (mean = 3.06, SE = .51) compared to the placebo beverage [mean = 2.19, SE = .37; t (15) = 2.21, p < .05]. Paired comparisons examining mean measures of smoking topography (number of puffs, puff volume, puff duration, inter-puff interval, peak puff velocity) per ½ cigarette did not reveal any significant differences across the beverage conditions. We examined difference scores in length of delay as a potential covariate for the smoking topography measures. We found that for the alcohol condition, length of delay was significant correlated with amount smoked (r = −.59, p < .02), but not for the placebo condition. There was no effect of the delay difference scores on measures of smoking topography.
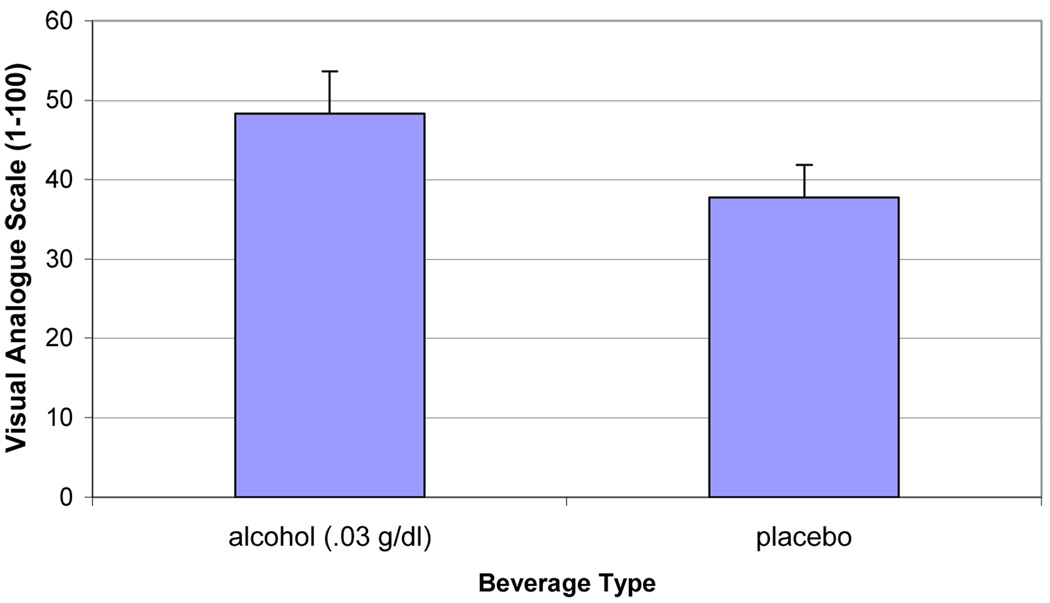
Multivariate analyses of subjective ratings during the ad-lib period demonstrated significant effects of beverage by time for alcohol craving [AUQ, F (1,15) = 5.10, p < .04; YCS – Alcohol, F (1,15) = 5.74, p < .04]. Within-subject contrasts revealed that AUQ scores were significantly elevated for the alcohol (mean = 34.79, SE = 6.28; p<.05), compared to the placebo condition (mean = 24.38, SE = 4.65), collapsing across the 0-minute and the 30-minute timepoints. YCS – Alcohol scores significantly decreased (p<.05) from the 30-minute to the 60-minute timepoints, for the alcohol condition (means = 17.0 vs 12.5, respectively), compared to the placebo condition (means = 11.69 vs. 12.69, respectively). There was a main effect of beverage condition for QSU-B: Factor 1 scores [F (1,15) = 4.77, p < .05]. During the alcohol beverage condition, subjects reported that they expected greater positive reinforcement from smoking than during the placebo beverage condition. There was also a main effect of beverage condition for MNWS scores [F (1,15) = 5.00, p< .05]. During the alcohol beverage condition, subjects reported greater nicotine withdrawal (mean = 5.64, SE = .153), compared to the placebo beverage condition (mean = 4.10, SE = .104). We then examined the MNWS without the craving item and found that there were no significant differences between the alcohol and placebo conditions. Other measures of tobacco craving (QSU-B: Factor 2, YCS – Cigarettes), subjective effects of alcohol (BAES – Stimulation, BAES – Sedation, Alcohol Effects Scale), and nicotine withdrawal (MNWS) all significantly decreased (Time; all p’s < .05) over the ad-lib period. There were no significant baseline correlates of differences in the amount smoked across alcohol and placebo sessions.
Blood Alcohol Levels
During the alcohol session, subjects had mean BALs of 0.0229 g/dl (SD = .021) when they terminated their delay period. As BALs were assessed every 10 minutes during the delay period, we examined whether levels were still ascending at the point of termination, or whether levels had peaked and then had started to descend. We determined that the majority of subjects (62.5%) terminated the delay period during the ascending limb. BALs then decreased over the ad-lib period [+30 timepoint = .008, SD = .004; +60 timepoint = .003, SD = .002].
DISCUSSION
Using a novel human laboratory paradigm, the current study demonstrated that alcohol consumption undermined the ability to resist smoking, in a sample of heavy social drinking smokers. This result is consistent with clinical findings demonstrating that alcohol use is associated with tobacco relapse episodes (Baer & Lichenstein, 1988; Shiffman 1986; Zimmerman et al., 1990). After consuming the equivalent of a single alcoholic drink, subjects were most likely to terminate the delay period during the ascending limb of the BAC. Others have demonstrated that alcohol associated increases in smoking behavior may be related to ascending limb effects (Mitchell et al., 1995). King and Epstein (2005) found that desire to smoke in nicotine-deprived light smokers was dose-dependently increased during the ascending limb of the BAC.
The current study also demonstrated that once subjects decided to ‘give in’ and smoke, they smoked more cigarettes if they had consumed alcohol, supporting other human laboratory findings that alcohol increases smoking behavior (Griffiths et al., 1976; Mintz et al., 1985; Mitchell et al., 1995). In contrast to our results, Rose et al. (2004) did not demonstrate any effect of alcohol on the number of cigarettes smoked. It is possible that the inclusion of a ‘smoking tab’ in the current study helped to elucidate the effect of alcohol on tobacco use by providing an incentive to resist smoking. With regard to smoking topography, we did not demonstrate any overall differences across beverage conditions.
We demonstrated some evidence that craving for both alcohol and tobacco increased from the start to the end of the delay period, during the alcohol beverage condition relative to the placebo beverage condition. Although there was some inconsistency in findings across the measures of craving, it supports the inclusion of multiple craving measures as suggested by Sayette et al. (2001). Our findings are consistent with others demonstrating cross-substance craving (Drobes et al., 2000; Kouri et al., 2004; Rose et al., 2004; Sayette et al., 2005) and findings that cue-reactivity is predictive of relapse behavior (e.g., Niaura et al., 1989). Additionally during the alcohol beverage condition, measures of alcohol craving and desire to smoke for positive reinforcement remained elevated during the ad-lib smoking period, despite increased cigarette use. We found that symptoms of nicotine withdrawal during the ad-lib period were greater after the consumption of the alcohol beverage, compared to the placebo beverage. However, this effect appeared to be primarily mediated by the item assessing tobacco craving. King and Epstein (2005) found that desire to smoke for positive reinforcement was elevated during the descending limb, but in nicotine deprived smokers. Alcohol and tobacco are thought to potentiate each other’s reinforcing effects (Rose et al., 2004; Shiffman & Balabanis, 1995). Our results suggest that concurrent alcohol and tobacco use may further serve to increase desire for continued alcohol and tobacco use in heavy social drinkers.
When examining potential correlates of the ability to resist smoking, we found that severity of nicotine dependence was significantly associated with delay period behavior. Those who had greater nicotine dependence scores (as assessed by the FTND) were less able to resist smoking after consuming alcohol. It is known that the severity of alcohol and tobacco dependence is positively correlated (Ellingstad et al., 1999; Gulliver et al., 1995). It is possible that those with greater nicotine dependence were more reactive to tobacco following alcohol consumption. Studies of light, social drinkers have demonstrated either minimal (Henningfield et al., 1984) or modest increases in smoking behavior following alcohol consumption (Mello et al., 1987), suggesting that the influence of drinking on smoking behavior may be more pronounced in heavier drinkers. In addition to our stated focus on understanding lapse behavior in abstaining smokers, this study may also have relevance for understanding the influence of alcohol on ongoing smoking behavior in those not attempting cessation.
This study had several limitations. First, it should be noted that the sample size was modest and only generalizeable to the population of heavy social drinkers. However, our sample size was comparable to other laboratory studies modeling initial smoking or drinking lapse episodes (King & Meyer, 2000; O’Malley et al., 2002). Second, it is possible that expectancy effects for alcohol influenced smoking behavior in the laboratory paradigm. Although this study was conducted using a double blind procedure for alcohol content, a no-alcohol beverage control should be included in future research to control for possible expectancy effects. Third, subjects were not treatment seeking which has been a criticism of laboratory studies examining various smoking cessation medication effects (see Perkins et al. 2006). However, money was provided as an alternative reinforcer in order to provide some incentive for not smoking and to enhance the likelihood that the effects of alcohol on the relative reinforcing value of tobacco would be detected (see Higgins, 1997; Rodefer et al., 1997). Perkins et al. (2006) and others (Stitzer et al., 1986; Gilbert et al., 1999) acknowledge that motivation to abstain can be temporarily raised through the use of monetary reinforcement.
For the current study, we developed a paradigm to evaluate two primary aspects of alcohol-mediated smoking lapse behavior; the ability to resist the first cigarette and subsequent smoking. Prior investigations that modeled smoking relapse involved a planned cigarette exposure after a period of abstinence (Chornock et al., 1992; Juliano & Stitzer, 2003; King & Meyer, 2000), and none had yet modeled the ability to resist the first cigarette. Ability to resist the first cigarette represents an important transition point in a quit attempt as the majority of abstinent smokers (up to 95%) who experience a lapse return to baseline smoking levels (Brandon et al., 1990; Garvey et al., 1992; Kenford et al., 1994). Our smoking lapse model can be conceptualized as an extension of cue reactivity paradigms. Similar to cue reactivity paradigms, participants were exposed to various primes (i.e., alcohol, cigarette availability) that are associated with relapse behavior. Unlike cue reactivity paradigms, which typically assess craving as the primary outcome, the primary outcomes were the length of the delay period (i.e., ability to resist smoking), and secondarily, the number of cigarettes smoked during the ad-lib period. We are currently adapting this basic model to examine the effect of other primes (e.g., nicotine deprivation, negative affect) and combinations thereof, on smoking lapse behavior. Additionally, we plan to vary the dose or duration of the primes (e.g., length of nicotine deprivation, alcohol dose), as well as the nature of the study samples to examine smoker characteristics that may influence lapse behavior (e.g., light vs. heavy smokers, presence vs. absence of various clinical syndromes). We also plan to investigate whether this laboratory model is predictive of actual lapse episodes, similar to studies examining the predictive validity of cue reactivity (e.g., Niaura et al., 1989). Finally, these models could be used to screen promising pharmacotherapies for smoking cessation. The proposed model has the potential of facilitating translational work in medication development by providing an intermediary step between preclinical studies and clinical trials. Perkins et al. (2006) advocates for the use of laboratory based paradigms as a means to efficiently evaluate new therapeutics.
Given the substantial health risks associated with concurrent alcohol and tobacco use (Rosengren et al., 1988), addressing smoking cessation in those experiencing alcohol problems is an important area for research. Although some studies have found no differences in the ability to quit smoking on the basis of alcohol problems (e.g., Hayford et al., 1999; Sobell et al., 1995), the majority of studies demonstrate that individuals with current or past history of alcohol problems are more nicotine dependent and are less likely to be successful at quitting smoking, compared to those without alcohol problems (e.g., Daeppen et al., 2000; Di Franza & Guerrera, 1990). One possible reason for this population’s reduced ability to quit smoking is that alcohol use promotes smoking relapse (Baer & Lichenstein, 1988; Shiffman 1986; Shiffman et al., 1996; Zimmerman et al., 1990). In our laboratory based model of smoking lapse behavior, we demonstrated that heavy social drinkers found it harder to resist smoking and smoked more cigarettes after consuming alcohol. Alcohol-mediated lapse behavior was most likely to occur during the ascending limb of the BAC and was associated with concomitant increases in both alcohol and tobacco craving. Further, alcohol consumption was associated with persistent elevations in alcohol craving and cravings to smoke for positive reinforcement even after smoking was initiated. These results suggest a number of mechanisms by which alcohol consumption promotes smoking cessation failure in heavy drinkers. The data support recent recommendations that smokers avoid alcohol consumption during the initial stages of a quit attempt (Fiore et al., 2000). Whether heavier drinkers will be able to comply with this advice remains to be determined. Pharmacotherapy that reduces alcohol reactivity and consumption should be investigated as a strategy to support smoking cessation efforts in heavy drinkers.
Figure 1.
Mean length of delay (minutes) to start smoking after consuming alcohol (0.03 g/dl) or placebo beverages.
Figure 2.
Mean Questionnaire of Smoking Urges-Brief Factor 1 scores (Positive Reinforcement) collapsed across the 60 minute ad-lib smoking period after consuming alcohol (0.03 g/dl) or placebo beverages.
Table 1.
Mean (± SE) and analyses for subjective ratings for the delay period across alcohol and placebo beverage conditions.
| Alcohol Beverage Mean (±SE) |
Placebo Beverage Mean (±SE) |
Significant Effect |
F value (1,15) |
P value |
|||
|---|---|---|---|---|---|---|---|
| Variable | Delay Start |
Delay End |
Delay Start |
Delay End |
|||
| AUQ | 29.39 (4.92) | 39.48 (7.05) | 30.66 (5.95) | 26.71 (5.48) | beverage x time | 3.52 | 0.08 |
| QSU-B Factor 1 | 50.67 (6.34) | 67.45 (6.59) | 53.10 (5.04) | 63.93 (7.34) | time | 6.79 | 0.02 |
| QSU-B Factor 2 | 26.77 (7.08) | 31.48 (7.28) | 25.65 (6.17) | 36.52 (8.32) | time | 6.11 | 0.03 |
| YCS - Alcohol | 13.19 (3.48) | 23.38 (6.28) | 13.19 (3.36) | 16.06 (3.49) | time | 8.49 | 0.01 |
| YCS – Cigarettes | 27.94 (4.40) | 41.75 (5.44) | 26.44 (2.89) | 31.69 (3.45) | beverage x time | 6.88 | 0.02 |
| BAES - Stimulation | 1.74 (0.46) | 2.40 (0.63) | 2.08 (0.48) | 2.13 (0.52) | ns | ||
| BAES - Sedation | 2.14 (0.66) | 1.90 (0.62) | 1.84 (0.59) | 1.50 (0.53) | ns | ||
| AES | 9.93 (2.32) | 19.50 (3.56) | 11.01 (2.46) | 13.90 (2.51) | beverage x time | 6.90 | 0.02 |
| MNWS | 5.00 (0.71) | 6.62 (1.29) | 4.74 (0.93) | 5.50 (0.98) | time | 6.29 | 0.02 |
AUQ Alcohol Urge Questionnaire (1–100); QSU-B Factor 1 Questionnaire of Smoking Urges Brief– Positive Reinforcement (1–100); QSU-B Factor 2 Questionnaire of Smoking Urges Brief- Negative Reinforcement (1–100); BAES Biphasic Alcohol Effects Scale (1–100); AES Alcohol Effects Scale (1–100); MNWS Minnesota Nicotine Withdrawal Scale (0–32).
Table 2.
Mean smoking topography measures per ½ cigarette (± SE) over the 60 minute ad-lib smoking period across alcohol and placebo beverage conditions.
| Per ½ Cigarette Average | ||
|---|---|---|
| Variable | Alcohol Beverage Mean (±SE) |
Placebo Beverage Mean (±SE) |
| Puff # | 7.37 (.66) | 7.45 (.66) |
| Puff volume (ml) | 38.85 (2.98) | 39.11 (2.71) |
| Duration of puff (s) | 1.29 (.08) | 1.26 (.09) |
| Inter-puff interval (s) | 22.59 (2.99) | 24.41 (4.46) |
| Peak Puff velocity (ml/s) | 45.22 (3.99) | 45.63 (3.55) |
Acknowledgements
We would like to thank Dr. Peter Jatlow, PI of the Laboratory Core (P50AA015632) for analyzing the nicotine plasma samples.
Funding: P50DA13334, P50AA015632, R03AA013622, M01RR000125
Footnotes
There were 13 subjects available for these analyses. Two subjects did not smoke for at least one of their topography session, and there was equipment failure for a third subject.
For one subject there was disagreement between the recorded number of cigarettes smoked and the number recorded by the smoking topography equipment. As there was no equipment failure, we elected to use the data generated by the topography equipment.
Contributor Information
Sherry A. McKee, Department of Psychiatry, Yale University School of Medicine
Suchitra Krishnan-Sarin, Department of Psychiatry, Yale University School of Medicine
Julia Shi, Department of Internal Medicine, Yale University School of Medicine.
Tricia Mase, Department of Psychiatry, Yale University School of Medicine
Stephanie S O’Malley, Department of Psychiatry, Yale University School of Medicine
References
- Babor TF, de la Fuente JR, Saunders J, Grant M. AUDIT: The alcohol use disorders identification test: Guidelines for use in primary health care. Genva, Switzerland: World Health Organization; 1992. [Google Scholar]
- Baer JS, Lichenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. Journal of Consulting and Clinical Psychology. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM. Comparing sensory experiences across individuals: Recent psychophysical advances illuminate genetic variation in taste perception. Chemical Senses. 2002;25:447–460. doi: 10.1093/chemse/25.4.447. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Burton SM, Tiffany ST. The effect of alcohol consumption on craving to smoke. Addiction. 1997;92:15–26. [PubMed] [Google Scholar]
- Center for Disease Control CDC. Cigarette smoking among adults – United States, 2001. MMWR. 2003;52:#40. [PubMed] [Google Scholar]
- Carmody TP, Brishchetter CS, Matarazzo JD. Co-occurent use of cigarettes, alcohol and coffee in healthy community living men and women. Health Psychology. 1985;4:323–335. doi: 10.1037//0278-6133.4.4.323. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: The effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, Leischow S. Experimental model of smoking re-exposure: Effects on relapse. Psychopharmacology. 1992;108:495–500. doi: 10.1007/BF02247427. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine and Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Daeppen J, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Ducholz KK, Raimo E, Schuckit MA The Collaborative Study Group on the Genetics of Alcoholism. Clinical correlates of cigarette smoking and nicotine dependence in alcohol dependent men and women. Alcohol and Alcoholism. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. Journal of Studies on Alcohol. 1990;51:130–135. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Beylotte F, Scott M, Saladin ME, Randell CL, Anton RF. Cross-reactivity to alcohol and smoking cues. Alcoholism: Clinical and Experimental Research. 2000;24:147a. [Google Scholar]
- Ellingstad TP, Sobell LC, Sobell MB, Cleland PA, Agrawal S. Alcohol abusers who want to quit smoking: Implications for clinical treatment. Drug and Alcohol Dependence. 1999;54:259–264. doi: 10.1016/s0376-8716(98)00180-x. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington D.C: American Psychiatric Press; 1995. [Google Scholar]
- Garvey A, Bliss R, Hitchcock J, Heinold J, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Gilbert DF, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Exp Clin Psychopharmacol. 1999;7:174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Glautier S, Clements K, White JA, Taylor C, Stolerman IP. Alcohol and the reward value of cigarette smoking. Behavioral Pharmacology. 1996;7:144–154. [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou PS, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Bigelow GR, Liebson I. Facilitation of human tobacco self-administration by ethanol: A behavioral analysis. Journal of Experimental and Analytical Behavior. 1976;25:279–292. doi: 10.1901/jeab.1976.25-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver SB, Rohsenow DJ, Colby SM, Dey AN, Abrams DB, et al. Interrelationship of smoking and alcohol dependence. Journal of Studies on Alcohol. 1995;56:202–206. doi: 10.15288/jsa.1995.56.202. [DOI] [PubMed] [Google Scholar]
- Hariharan M, Van Noord T, Greden JF. A high performance liquid chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clinical Chemistry. 1988;34:724–729. [PubMed] [Google Scholar]
- Hatsukami DK, Hurghes JF, Pickens RW, Svikis D. Tobacco withdrawal symptoms: An experimental analysis. Psychopharmacology. 1984;84:231–236. doi: 10.1007/BF00427451. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Lexau G, Nelson D, Pentel PR, Sofuoglu M, Goldman A. Effects of cotinine on cigarette self-administration. Psychopharmacology. 1998;138:184–189. doi: 10.1007/s002130050661. [DOI] [PubMed] [Google Scholar]
- Hayford KE, Patten CA, Rummans TA, Schroeder DR, Offord KP, et al. Efficacy of bupropion for smoking cessation in smokers with a former history of major depression or alcoholism. British Journal of Psychiatry. 1999;174:173–178. doi: 10.1192/bjp.174.2.173. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking volunteers without histories of alcoholism. Psychopharmacology. 1984;82:1–5. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacology Biochemistry and Behavior. 1997;57(3):419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Treatment of smoking cessation in smokers with past alcohol/drug problems. Journal of Substance Abuse Treatment. 1993;10:181–187. doi: 10.1016/0740-5472(93)90043-2. [DOI] [PubMed] [Google Scholar]
- Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Eberman KM, Croghan IT, Offord KP, Davis LJ, et al. Nicotine dependence treatment during inpatient treatment for other addictions: a prospective intervention trial. Alcoholism: Clinical & Experimental Research. 1994;(4):867–872. doi: 10.1111/j.1530-0277.1994.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Istvan J, Matarazza JD. Tobacco, alcohol, and caffeine use: A review of their interrelationships. Psychological Bulletin. 1984;95:301–326. [PubMed] [Google Scholar]
- Juliano LM, Stitzer ML. Subjective and physiological reactions to smoking a nicotine or de-nicotinized cigarette after a brief period of abstinence. SYM 2B Society for Research on Nicotine and Tobacco. 2003 [Google Scholar]
- Kalman D, Morissette SB, George TP. Co-morbidity of smoking in patients with psychiatric and substance use disorders. American Journal on Addictions. 2005;14:106–123. doi: 10.1080/10550490590924728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DW, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;27:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcoholism: Clinical & Experimental Research. 2002;26:827–835. [PubMed] [Google Scholar]
- King AC, Meyer PJ. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacology Biochemistry and Behavior. 2000;66:563–572. doi: 10.1016/s0091-3057(00)00258-6. [DOI] [PubMed] [Google Scholar]
- King AC, Epstein AM. Alcohol dose-dependent increases in smoking urge in light smokers. Alcoholism: Clinical and Experimental Research. 2005;29:547–552. doi: 10.1097/01.alc.0000158839.65251.fe. [DOI] [PubMed] [Google Scholar]
- Kouri EM, McCarthy EM, Faust AH, Lukas SE. Pretreatment with transdermal nicotine enhances some of ethanol’s acute effects in men. Drug and Alcohol Dependence. 2004;75:55–65. doi: 10.1016/j.drugalcdep.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Curry S, Gordon JR. A longitudinal analysis of unaided smoking cessation. Journal of Consulting & Clinical Psychology. 1988;56:715–720. doi: 10.1037//0022-006x.56.5.715. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale Alcoholism. Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Palmieri SL. Cigarette smoking by women: Interactions with alcohol use. Psychopharmacology. 1987;93:8–15. doi: 10.1007/BF02439579. [DOI] [PubMed] [Google Scholar]
- Mintz J, Boyd G, Rose JE, Charuvastra VC, Jarvik MC. Alcohol increases cigarette smoking: a laboratory demonstration. Addictive Behaviors. 1985;10:203–207. doi: 10.1016/0306-4603(85)90001-2. [DOI] [PubMed] [Google Scholar]
- Mitchell SH, deWit H, Zacny JP. Effects of varying ethanol dose of cigarette consumption in healthy normal volunteers. Behavioral Pharmacology. 1995;6:359–365. [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Nides M, Rakos R, Gonzales D, Murray R, Tashkin D, Bjornson-Benson W, Lindgren P, Connett J. Predictors of initial smoking cessaition and relapse through the first two years of the Lung Health Study. Journal of Consulting and Clincial Psychology. 1995;63:60–69. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- Norregaard J, Tonnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine. 1993;22:261–271. doi: 10.1006/pmed.1993.1021. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Patten CA, Martin JE, Owen N. Can psychiatric and chemical dependency treatment units be smoke free? Journal of Substance Abuse Treatment. 1996;13:107–118. doi: 10.1016/0740-5472(96)00040-2. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Meeker J, White W, Wilson W. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behavioral Pharmacology. 2001;12:35–44. doi: 10.1097/00008877-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Fonte C, Sanders M, White W, Wilson A. Effects of training dose and two- versus three-choice testing procedure on nicotine discrimination in humans. Psychopharmacology. 1999;145:418–425. doi: 10.1007/s002130051076. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Levine M, Marcus M, Shiffman S, D'Amico D, Miller A, Keins A, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. Journal of Consulting & Clinical Psychology. 2000;68:176–180. doi: 10.1037/0022-006X.68.1.176. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Saners M, D’Amico D, Wilson A. Nicotine discrimination and self-adminstration in humans as a function of smoking status. Psychopharmacology. 1997;131:361–370. doi: 10.1007/s002130050304. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacology. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Cole PA, Lumley MA, Marks JL, et al. Effects of menstrual phase on nicotine, alcohol, and caffeine intake in smokers. Journal of Substance Abuse. 1994;6:227–234. doi: 10.1016/s0899-3289(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Drug and Alcohol Dependence. 1997;45:21–29. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brauer LH, Behm FM, Cramblett M, Calkins K, Lawhon D. Psychopharmacological interactions between nicotine and ethanol. Nicotine & Tobacco Research. 2004;6:133–144. doi: 10.1080/14622200310001656957. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Wilhelmsen L, Wedel H. Separate and combined effects of smoking and alcohol abuse in middle-aged men. ACTA Medica Scandinavia. 1993;223:111–118. doi: 10.1111/j.0954-6820.1988.tb15774.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Perrott MA, Peters AR. The effects of alcohol on cigarette craving in heavy smokers and tobacco chippers. Psychology of Addictive Behaviors. 2005;19:263–270. doi: 10.1037/0893-164X.19.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Wertz JM, Shiffman SS, Perrott MA. A multi-dimensional analysis of cue-elicited craving in heavy smokers and tobacco chippers. Addiction. 2005;96:1419–1432. doi: 10.1080/09652140120075152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch Gen Psych. 1984;41:879–885. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Shiffman S. A cluster-analysis classification of smoking relapse episodes. Addictive Behaviors. 1986;11:292–307. doi: 10.1016/0306-4603(86)90057-2. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Balabanis M. Associations between alcohol and tobacco. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: From Basic Science to Clinical Practice. Vol. 30. Washington DC: National Institutes of Health; 1995. pp. 17–39. Research Monograph Series. [Google Scholar]
- Shiffman S, Engberg JB, Paty JA, Perz WG, Gnys M, Kassel JD, Hickcox M. A day at a time: Predicting smoking lapse from daily urge. Journal of Abnormal Psychology. 1997;106:104–116. doi: 10.1037//0021-843x.106.1.104. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Fischer LA, Paty J, Gnys M, Hickcox M, Kassel JD. Drinking and smoking: A field study of their association. Annals of Behavioral Medicine. 1994;16:203–209. [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting & Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Followback: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Techniques to assess alcohol consumption. New Jersey: Humana Press; 1993. [Google Scholar]
- Sobell LC, Sobell MB, Kozlowski LT, Toneatto T. Alcohol or tobacco research versus alcohol and tobacco research. British Journal of Addiction. 1990;85:263–269. doi: 10.1111/j.1360-0443.1990.tb03082.x. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Kozlowski LT. Dual recoveries from alcohol and smoking problems. In: Fertig JB, Allen JP, editors. Alcohol and Tobacco: from Basic Science to Clinical Practice. Bethesda, MD: National Institutes of Health; 1995. pp. 207–224. [Google Scholar]
- Stitzer ML, Rand CS, Below GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. J Appl Behav Anal. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST. Potential functions of classical conditioning in drug addiction. In: Drumond DC, Tiffany ST, Glautier SP, Remington B, editors. Addictive Behavior: Cue Exposure, Theory, and Practice. London: Wiley; 1995. pp. 47–71. [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow KE, Batt Rd, editors. Human Metabolism of Alcohol. Vol I. Boca Raton, FL: CRC Press; 1989. pp. 41–56. [Google Scholar]
- Westman EC, Behm FM, Simel DL, Rose JE. Smoking behavior on the first day of a quit attempt predicts long-term abstinence. Archives of Internal Medicine. 1997;157:335–340. [PubMed] [Google Scholar]
- Zimmerman RS, Warheit GJ, Ulbrich PM, Auth JB. The relationship between alcohol use and attempts and success at smoking cessation. Addictive Behaviors. 1990;15:197–207. doi: 10.1016/0306-4603(90)90063-4. [DOI] [PubMed] [Google Scholar]