Abstract
Previous studies have established a link between adverse early life events and subsequent disease vulnerability. The present study assessed the long-term effects of neonatal maternal separation on the response to Theiler's murine encephalomyelitis virus infection, a model of multiple sclerosis. Balb/cJ mouse pups were separated from their dam for 180-min/day (180-min MS), 15-min/day (15-min MS), or left undisturbed from postnatal days 2–14. During adolescence, mice were infected with Theiler's virus and sacrificed at days 14, 21, or 35 post-infection. Prolonged 180-min MS increased viral load and delayed viral clearance in the spinal cords of males and females, whereas brief 15-min MS increased the rate of viral clearance in females. The 15-min and 180-min MS mice exhibited blunted corticosterone responses during infection, suggesting that reduced HPA sensitivity may have altered the immune response to infection. These findings demonstrate that early life events alter vulnerability to CNS infection later in life. Therefore, this model could be used to study gene-environment interactions that contribute to individual differences in susceptibility to infectious and autoimmune diseases of the CNS.
Keywords: Theiler's virus, Maternal separation, Handling, HPA axis, Multiple sclerosis
Introduction
Human epidemiological studies suggest that adverse early environments predispose individuals to a variety of chronic diseases later in life (Anda et al. 2008; Barker 1997, 1998; Boynton-Jarrett et al. 2008; Danese et al. 2007; DeBellis et al. 1999; Dong et al. 2003, 2004; Felitti et al. 1998; Melchior et al. 2007; Seckl 1997; Walker et al. 1999). Animal studies also indicate that exposure to stressors or glucocorticoids (GC) during early life alters the programming of neuroendocrine and neuroimmune systems (Avitsur et al. 2006; Bakker et al. 1997, 2000, 2001; Brunson et al. 2001; Catalani et al. 2000; Coe and Lubach 2003; Hodgson and Knott 2002; Liu et al. 1997, 2000; Marchetti et al. 2001; Sanchez et al. 2001). Moreover, it is well established that the neuroendocrine and neuroimmune systems are mutually regulatory and that the bi-directional communication between these systems may determine the impact of stressors on the onset and progression of infectious and autoimmune diseases (Biondi and Zannino 1997; Maier and Watkins 1998). However, the effects of early life stress on predisposition to viral infection and autoimmune disease have not been intensively studied.
Multiple sclerosis is an inflammatory-autoimmune disease that is modulated by stress. Clinical studies indicate that stress precipitates disease onset, clinical relapses, and lesion development (Ackerman et al. 2002; Grant et al. 1989; Mohr et al. 2000; Sibley 1997; Warren et al. 1982). Although the etiology of multiple sclerosis remains uncertain, epidemiological studies suggest that viral infection during adolescence may be an initiating factor in genetically susceptible individuals (Acheson 1977; Alter et al. 1986; Hernan et al. 2001; Kurtzke et al. 1995; Sospedra and Martin 2005). Psychological stress has also been shown to have a profound impact on animal models of multiple sclerosis, such as Theiler's virus infection (Campbell et al. 2001; Johnson et al. 2004, 2006; Meagher et al. 2007a, b; Mi et al. 2004, 2006a, b; Sieve et al. 2004, 2006; Steelman et al. 2009; Welsh et al. 2004; Young et al., in press) and Experimental Autoimmune Encephalomyelitis (EAE; Bukilica et al. 1991; Dowdell et al. 1999; Griffin and Whitacre 1990; Griffin et al. 1993; Levine and Saltzman 1987; Levine et al. 1962; Stephan et al. 2002; Teunis et al. 2002; Whitacre et al. 1998). Although several studies have shown that early life stress can alter the course of EAE (Laban et al. 1995a, b; Stephan et al. 2002; Teunis et al. 2002), the impact on Theiler's virus infection remains unexplored.
Theiler's murine encephalomyelitis virus (TMEV) is a Picornavirus that causes a biphasic disease of the CNS in susceptible strains of mice. During the acute phase, the virus infects neurons and glia cells in the brain and spinal cord, causing encephalitis and polio-like symptoms (Campbell et al. 2001; Johnson et al. 2004, 2006; Lipton 1975; Meagher et al. 2007a, b; Njenga et al. 1997; Sieve et al. 2004). During the chronic phase, an inflammatory demyelinating disease is observed with lesions and behavioral impairments that are remarkably similar to multiple sclerosis (Aubert et al. 1987; Lipton 1975; McGavern et al. 1999; Sieve et al. 2004; Young et al., in press). Importantly, the virus must persist in the CNS beyond the resolution of the acute phase in order to cause the later demyelinating phase of disease.
The long-term effects of early life stress on vulnerability to infectious and autoimmune diseases are well documented (Avitsur et al. 2006; Coe and Lubach 2003; Dube et al. 2009; Lewis et al. 2000; Teunis et al. 2002). Previous studies indicate that exposure to maternal separation permanently alters the “programming” of many regulatory systems, including the CNS, endocrine, and immune systems (Ackerman et al. 1988; Avitsur et al. 2006; Caldji et al. 2000a, b; Coe and Lubach 2003; Francis et al. 1999a, b; Kalinichev et al. 2002; Laudenslager et al. 1985; Liu et al. 1997; Stephan et al. 2002; Teunis et al. 2002). For example, maternal separation of rodent pups during the first 2 weeks of life induces alterations in behavioral and limbic-hypothalamic–pituitary–adrenal (LHPA) axis reactivity to stress that persist throughout life (Bhansali et al. 2007; Ladd et al. 2000; Parfitt et al. 2004; Plotsky and Meaney 1993; Romeo et al. 2003, 2004; Schmidt et al. 2002, 2003; Veenema et al. 2007). Recent studies have also shown that maternal separation increases vulnerability to EAE (Stephan et al. 2002; Teunis et al. 2002), influenza virus infection in lung (Avitsur et al. 2006), and stroke (Craft et al. 2006). These findings are consistent with recent epidemiological studies on the impact of early life stress on subsequent autoimmune disease vulnerability (Dube et al. 2009). While the phenomenon of early environmental programming is now well established, it is unclear whether early life stress alters vulnerability to CNS infection and multiple sclerosis.
The present study investigated the effects of neonatal maternal separation stress on neuroimmune, endocrine, and behavioral responses to Theiler's virus infection in adolescent male and female mice. Previous research has shown that prolonged maternal separation enhances HPA and fear responses to stressors (Plotsky and Meaney 1993; Ladd et al. 2000; Liu et al. 2000), whereas brief maternal separation has a blunting effect (Levine 1957; Meaney et al. 2000; Meaney 2001). To examine the impact on Theiler's virus infection, Balb/cJ male and female pups were exposed to prolonged maternal separation (180-min MS), brief maternal separation (15-min MS), or an undisturbed control condition for the first 2 weeks of life. Mice were intracranially infected with Theiler's virus on postnatal day 28, which corresponds to the onset of puberty in mice. Following infection with Theiler's virus during adolescence, mice were sacrificed at days 14, 21, or 35 post-infection (pi) for determination of CNS viral titers and tissue weights (adrenals, lymph nodes, thymus, spleens). In addition, the effects of maternal separation on corticosterone (CORT) and behavioral responses to acute TMEV infection were assessed.
Methods
Subjects
Balb/cJ male and female pups were bred in our animal colony (breeders acquired from Jackson Labs; Bar Harbor, ME). Balb/cJ mice were used because prior studies indicated that genetically similar Balb/cByJ and Balb/c mice exhibit a pattern of behavioral and HPA axis changes that resemble the pattern observed in handled and maternally separated rats (Anisman et al. 1998; Bhansali et al. 2007; Hawks et al. 1999). Although Balb/cByJ mice are resistant to TMEV, Balb/cJ's are susceptible (Nicholson et al. 1994). Pregnant dams were individually housed and checked twice daily for the presence of litters. The birth date was designated pnd 0. Within 24 h of birth, litters were sexed and culled to 8 pups. Litters were housed with dams in standard plastic cages with sawdust shavings for bedding and nestlets. Mice were maintained on ad lib food and water, receiving a breeders diet (9% fat) prior to weaning and normal lab chow thereafter. Mice were maintained on a 12:12 h light: dark cycle (lights off at 18:00 h) in a colony room with an ambient room temperature maintained at 25°C. All animal care protocols were in accordance with NIH Guidelines for Care and Use of Laboratory Animals and were approved by the Texas A&M University Laboratory Animal Care and Use Committee.
Neonatal procedure
On pnd 1, litters were randomly assigned to 1 of 3 neonatal treatments, 180-min MS, 15-min MS, and control (Meaney and Aitken 1985; Plotsky and Meaney 1993), which occurred daily (0800–1100) between pnd 2 and 14. (1) Prolonged maternal separation involved removing the mother and then the pups from their home cage for 180 min (180-min MS). The mother was placed in a holding cage during the separation, while the litter was placed into a translucent container with clean bedding housed within a larger isolation chamber maintained at 31°C and at 60–80% humidity. Following separation, the pups were returned to their home cage followed by their mothers. (2) Brief maternal separation involved the same procedure, however, the duration of separation was only 15 min (15-min MS). (3) Control non-handled pups were left undisturbed until pnd 10, when all litters had their first cage cleaning. To minimize disruption of infant-maternal interactions, mice were not handled except during neonatal treatments and cage changing. During cage changing, some old bedding and the nest were transferred to the new cage to minimize novelty stress. All mice were weaned on pnd 21 and housed with same-sex litter mates (2–4 mice/cage).
Experimental designs
Figure 1 provides a schematic of the experimental procedures used in two experiments conducted on separate groups of mice. Experiment 1 used a 3 (neonatal condition) × 2 (sex) × 3 (sacrifice time) between groups factorial design (N = 201) to evaluate the impact on clinical signs of infection, body weight, tissue weight (adrenal glands, lymph nodes, thymus, spleens) and CNS viral titers. Pups were subjected daily to either 180-min maternal separation (MS), 15-min MS, or control conditions between pnd 2–14. During adolescence they were infected with TMEV on pnd 28 and then sacrificed at days 14, 21, and 35 pi. Experiment 2 used a 3 (neonatal condition) × 2 (sex) repeated measures design (N = 76) to evaluate the impact on CORT, exploratory behavior, signs of illness and hind limb motor impairment over days pi.
Fig. 1.
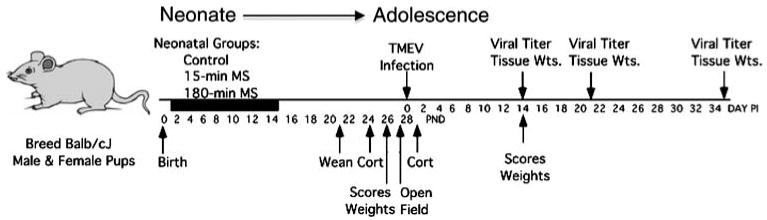
Experimental procedures. Balb/cJ litters were assigned to 1 of 3 neonatal conditions, which occurred daily (8–11:00 am) between pnd 1 and 14: (1) 180-min of maternal separation (180-min MS), (2) 15 min of maternal separation (15-min MS), or (3) undisturbed (Control). Pups were weaned on pnd 21 and housed with same-sex litter mates. To evaluate the long-term effects of maternal separation on reactivity to mild stress, plasma CORT responses to mild handling stress were assessed before infection on pnd 24. Prior to infection, behavioral scores and body weights were assessed on pnd 26, while open field exploratory behavior was assessed on pnd 27. On pnd 28, all mice were intracranially inoculated with 5 × 104 pfu of the BeAn strain of Theiler's virus (TMEV) (day 0 pi). The impact of maternal separation on the CORT response to TMEV infection was assessed on day 1 pi. In Experiment 1, behavioral scores and body weights were assessed every 3–6 days until day 14 pi. These mice were sacrificed at days 14, 21, and 35 pi for CNS viral titer plaque assays and tissue weights. The number of mice in each group is as follows: Control (day 14 n = 24, day 21 n = 23, day 35 n = 18), 15 min MS (day 14 n = 19, day 21 n = 19, day 35 n = 21) and 180 min MS (day 14 n = 26, day 21 n = 20, day 35 n = 18). Experiment 2, behavioral scores and body weights were collected every 2–5 days pi until day 24 pi. The number of mice in each group is as follows: Control n = 38, 15 min MS n = 60, and 180 min MS n = 38
Open field exploratory behavior
To determine whether the neonatal conditions induced long-lasting changes in anxiety-like and motor behavior, exploratory behavior was tested in a novel open-field 1 day prior to infection. Four Accuscan open-field chambers (Omnitech), equipped with two banks of eight photocells on each wall were used to measure vertical activity. Prior research suggests that vertical activity is sensitive to anxiety, because it is reduced by administration of anti-anxiety drugs, such as chlordiazepoxide (Choleris et al. 2001). These chambers are interfaced with a digital multiplexor (Coulbourn E61-58). Testing was conducted in the dark between 1,500 and 1,900. White noise (64 dB) was continually present to mask extraneous disturbances.
Corticosterone determination
The long-term effects of maternal separation on CORT responses to mild handling stress were assessed 4 days prior to infection and on day 1 pi. After the thigh was shaved and anesthetic cream (EMLA, St. Joseph, MO) applied, blood was collected from the saphaneous vein. Mice were individually transported to a nearby room and hand restrained for approximately 3–4 min while blood was collected. Plasma was stored at −80°C until analyzed using a CORT RIA (ICN Biomedicals, Costa, Mesa, CA).
Theiler's virus
The BeAn strain of Theiler's virus (obtained from Dr. H.L. Lipton, Department of Neurology, Northwestern University, Chicago, IL.) was propagated and amplified in BHK-21 cells. The culture supernatant containing infectious virus was aliquoted, titrated and stored at −80°C before use (Welsh et al. 1987). Mice were anesthetized on day 0 pi/pnd 28 with isofluorane anesthesia (IsoSol from Vedco, #58-1825-2/R2, St. Joseph, MO) and injected intracranially (i.c.) into the right posterior cerebral cortex with 5 × 104 pfu of the BeAn strain of Theiler's virus in a 20 μl volume as previously described (Welsh et al. 2004).
Behavioral signs of TMEV infection
The effects of maternal separation on clinical signs of encephalitis (Campbell et al. 2001; Johnson et al. 2004; Sieve et al. 2004) and hind limb impairment (Johnson et al. 2004, 2006; Meagher et al. 2007a, b) were assessed in Experiments 1 and 2.
Clinical scale
In Experiment 1, a subset of mice in each condition (control n = 62, 15-min MS n = 52, 180-min MS n = 66) were scored every 3–6 days pi on a scale from 0 to 5, where 0 indicates a healthy animal and 1–5 represent gradually increasing signs of hind limb weakness, motor impairment, and hunching as follows: 1—one leg weak; 2—one leg some hind limb impairment; 3—both legs some hind limb impairment, one leg some hind limb impairment and one leg weak, or one leg moderate hind limb impairment and hunching; 4—both legs moderate hind limb impairment; 5—both legs prominent hind limb impairment and hunching. This scale was derived by observing the natural progression of hind limb motor impairment and encephalitis-like hunching during the first few weeks of acute TMEV infection in Balb/cJ mice. In Experiment 2, two scales were employed to separately measure signs of hind limb impairment and encephalitis using more precise operational definitions.
Hind limb impairment scale
In Experiment 2, hind limb impairment was scored at baseline and at dpi 1, 3, 5, 7, 10, 14, 17, 21, and 24. The hind limb impairment score is a composite of weakness (ability to cling) and coordination subscales. Weakness was scored by examining the ability of mice to cling to an inverted metal cage grid as follows: 0 = no weakness; 1 = over or under grasping 10–50% of time; 2 = the same as 1, but more than 50% of time; 3 = began dangling off the grid, but upper thigh muscles appeared flexed, more than 10% of time; 4 = same as 3, except dangled lower and thigh muscles appeared extended; 5 = the entire hind end of animal slipped off grid at least two times and animal had difficulty in reattaching; 6 = no constructive use of limb. Coordination was scored as follows: 0 = no coordination problems; 1 = repeated, but successful attempts to place foot (10–20% of time), middle toes may have bent against grid; 2 = repeated, but successful attempts to place foot with toes closed (20–100% of time), sliding instead of stepping for distances greater than 1 cm with toes closed; 3 = similar to 2, but with an open rather than closed toes; 4 = repeated, directed but unsuccessful attempts to place foot with toes open (20–100% of time), may have seen undirected opening and closing of grip, but not on grid; 5 = no directed movements, but thigh muscles had tone when tested by lightly pulling away from body when not on grid, dragged limb to side or behind when ambulating in home cage; 6 = as in 5, but with no muscle tone. The overall score was based on the progression through these two subscales. Lower overall scores were associated with only weakness subscale observations, while at an overall score of 3, the coordination subscale dominated observations. An overall score of 6 was complete paralysis with no muscle tone. Each hind limb was assessed independently and reported as a combined score. This scale has inter-rater reliability (r = 0.9) between trained raters blind to condition (Johnson et al. 2004).
Encephalitis scale
To make comparisons with our previous restraint stress studies (Campbell et al. 2001), mice in Experiment 2 were scored on a 0–4 scale that assessed the degree of encephalitis-like symptoms on several dimensions including hunched posture, piloerection, grooming, lethargy and sunken eyes. For each dimension, the animal received a rating from 0 to 6. This scale was derived by observing the natural progression of behavioral signs of encephalitis in Theiler's virus infected mice subjected to restraint stress (Campbell et al. 2001; Welsh et al. 2004; Sieve et al. 2004). Similar to Johnson et al. (2004), Balb/cJ mice did not develop the entire range of symptoms previously observed in stressed CBA and SJL mice, but they did exhibit hunching. Therefore, the hunching subscale was analyzed separately.
Tissue collection
Brains, spinal cords, thymus, spleens, and adrenals were dissected and weighed prior to being stored at −80°C. Adrenal, thymus, cervical and inguinal lymph nodes, and spleen weights were assessed to determine the long-term physiological impact of the neonatal stress conditions during TMEV infection (Selye 1936).
Viral titer assay
Titers of Theiler's virus in the CNS are maximal at 2 weeks pi and by 4 weeks pi the virus is cleared to non-detectable levels in resistant strains of mice and low levels in susceptible strains of mice (Welsh et al. 1987, 1989). To evaluate the impact of maternal separation on CNS viral clearance during adolescence, subsets of male and female mice were sacrificed by an ip overdose of pentobarbital at days 14, 21 and 35 pi. Brains and spinal cords were removed, homogenized with a glass homogenizer, sonicated to release the virus from the cells, and stored at −80°C. The viral content of the supernatant from the homogenized brains and spinal cords were determined by plaque assay on L2 cells (Welsh et al. 1987). The plates were incubated at 37°C in a 5% CO2 incubator for 72 h. Then, plaque forming units (pfu) were visualized using crystal violet and calculated per gram of harvested tissue (Welsh et al. 1987).
Statistical analyses
Data are presented as means ± SEM. Data were analyzed using analysis of variance (ANOVA). Duncan's New Multiple Range Test and mean comparisons were used for post hoc analyses. A p-value of 0.05 or less was considered significant in all cases.
Results
Open field activity
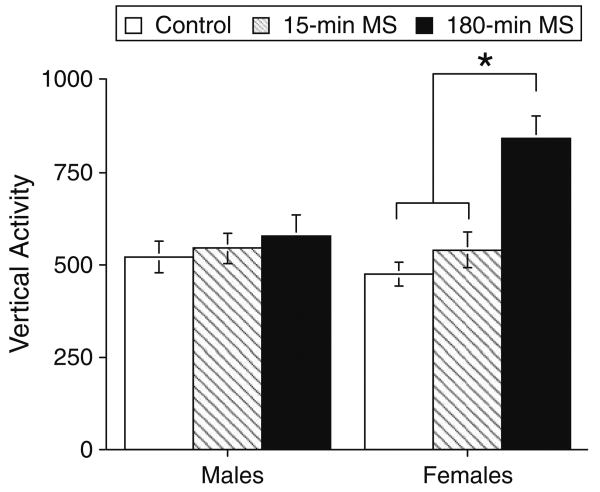
Female mice from the 180-min MS condition exhibited increased vertical exploratory behavior when compared to the neonatal control and 15-min MS conditions when tested 1 day prior to infection (Fig. 2). An ANOVA entering neonatal condition and sex as between group variables confirmed a significant effect of neonatal condition, F(2, 94) = 10.81, p < .0001. Although the effect of sex failed to reach significance, F(1,94) = 3.45, p = .07, there was a significant interaction between neonatal condition and sex, F(2, 94) = 6.03, p < .003. Post hoc mean comparisons indicated that this effect was attributable to increased vertical activity in the 180-min MS females compared to the other groups, p < .001.
Fig. 2.
The impact of maternal separation and sex on vertical exploratory behavior in a novel open field before infection on pnd 27. Control Males n = 20, 15 min MS Males n = 21, 180 min MS Males n = 16, Control Females n = 15, 15 min MS Females n = 15, 180 min MS Females n = 13. Statistically significant differences are denoted by asterisks (p < .05)
Corticosterone
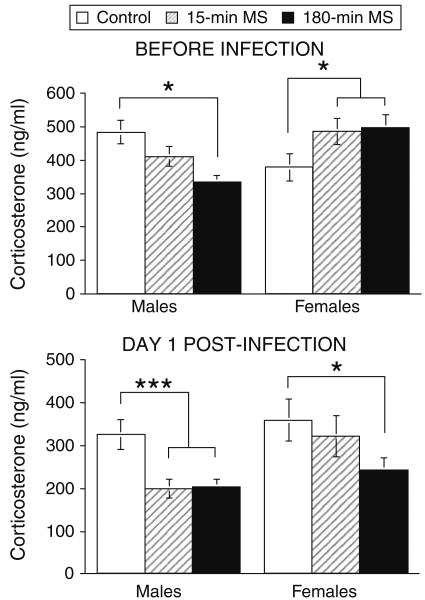
Figure 3 depicts the effects of maternal separation on plasma concentrations of CORT before infection on pnd 24 (top panel) and on day 1 pi (bottom panel) following mild handling stress in Experiment 2. Although a no-stress control group was not included in the present study, CORT levels are elevated relative to a no-stress control group (mean = 210 ng/m) from a previous study that used mice of the same age and strain tested at the same time (Johnson et al. 2004). An ANOVA was conducted on pre-infection CORT levels entering neonatal condition and sex as between group variables. The main effects of neonatal condition, F(1,68) = .991, p > .05, and sex, F(1,68) = .584, p > .05, were not significant. However, there was a significant neonatal condition by sex interaction, F(2,68) = 5.06, p < .009, suggesting that the effect of maternal separation on CORT levels prior to infection depended on sex. Post hoc mean comparisons indicated that the 180-min MS females showed higher CORT levels in response to mild handling stress when compared to the neonatal control females, p < .05, whereas the 15-min MS females did not differ from controls, p < .05. In contrast, the 180-min MS males showed a blunted CORT response to handling stress relative to control males. The reduction observed in the 15-min MS males failed to reach significance, p = .06. No other differences were significant prior to infection.
Fig. 3.
Effect of neonatal maternal separation and sex on plasma CORT levels following a mild handling stress in male and female mice before infection on pnd 24 (top panel) and after infection on day 1 pi (bottom panel). Control Males n = 15, 15 min MS Males n = 19, 180 min MS Males n = 11, Control Females n = 8, 15 min MS Females n = 8, 180 min MS Females n = 11. Statistically significant differences are denoted by asterisks (p < .05)
An ANOVA conducted on day 1 pi CORT levels revealed a significant main effect of neonatal condition, F(2, 67) = 5.6, p < .006. Mean comparisons revealed that both the 15-min and 180-min MS mice exhibited lower pi CORT levels compared to controls, p < .05. A significant effect of sex, F(1,67) = 5.075, p < .03, indicated that females tended to have higher CORT levels than males. No other differences were significant. To examine the effect of infection and time, an additional ANOVA was conducted on CORT levels entering pre-infection and post-infection CORT levels as a repeated measure variable, and neonatal condition and sex as between group variables. The interaction between neonatal condition and sex failed to reach significance, F(2, 66) = 2.896, p = .06. However, the effect of time/infection was significant, F(1, 66) = 51.80, p < .0001, indicating that CORT levels were reduced on day 1 pi compared to pre-infection CORT levels. This effect was qualified by significant interactions between neonatal condition and time/infection, F(2, 66) = 3.38, p < .04, and between neonatal condition, sex, and time/infection, F(2, 66) = 3.70, p < .03. Mean comparisons revealed that males in the 15-min and 180-min MS conditions exhibited decreases in CORT on day 1 pi compared to controls, p < .05. In contrast, females in the 15-min MS exhibited did not show decreases in CORT, but females in the 180-min MS condition exhibited blunted CORT levels compared to controls, p < .05. These results suggest that both brief and prolonged maternal separation blunted the CORT response to mild stress following TMEV infection in males, whereas only prolonged separation blunts this response in females.
Body weight
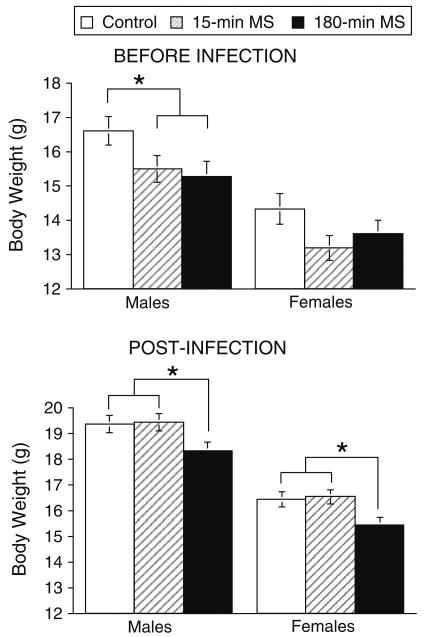
Figure 4 depicts the effects of maternal separation on mean body weights before and after infection in Experiment 1. Prior to infection, it appears that both the 15-min and 180-min MS groups weighed slightly less than the neonatal control group. Post-infection only the 180-min MS mice exhibited reduced body weight gain compared to the 15-min MS and control mice. ANOVA conducted on pre-infection baseline weights found a significant effect of neonatal condition, F(2, 105) = 4.339, p > .02. Post hoc mean comparisons verified that both the 15-min and 180-min MS mice weighed less at baseline compared to control mice. Although there was a significant effect of sex, F(1,105) = 29.97, p < .0001, indicating that females weighed less than males, the interaction between neonatal condition and sex was not significant, F(2,105) = .33, p > .05. Analyses of post-infection mean body weights collected between days 1 and 14 pi revealed significant main effects of neonatal condition, F(2,187) = 8.529, p < .0003, and sex, F(1,187) = 142.16, p < .0001. However, the neonatal condition by sex interaction was not significant, F(2,187) = .001, p > 05. Post hoc mean comparisons revealed that the 180-min MS mice weighed less than both the 15-min MS and undisturbed control mice, p < .05.
Fig. 4.
Effect of neonatal maternal separation and sex on body weights in male and female mice before infection on pnd 26 (top panel) and mean body weight post-infection across days 1–14 pi (bottom panel). Control Males n = 33, 15 min MS Males n = 34, 180 min MS Males n = 32, Control Females n = 35, 15 min MS Females n = 26, 180 min MS Females n = 33. The results are expressed as the mean body weight in grams ± SEM. Statistically significant differences are denoted by asterisks (p < .05)
Behavioral signs of infection
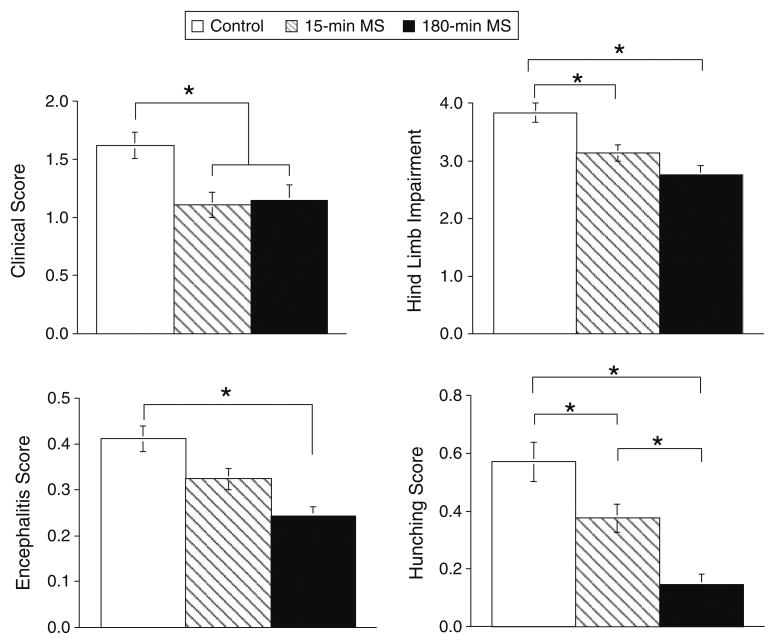
Figure 5 depicts the effects of neonatal condition on post-infection measures of clinical scores from Experiment 1 (top left panel), as well as hind limb impairment (top right panel), encephalitis score (bottom left panel), and hunching scores (bottom right panel) from Experiment 2. Although maternal separation did not induce the full range of clinical signs previously observed following restraint stress (Campbell et al. 2001), significant differences were observed between neonatal conditions on all measures.
Fig. 5.
The impact of maternal separation on post-infection measures of hind limb impairment and illness. Experiment 1 (top left) clinical scores from Control n = 62, 15 min MS n = 52, and 180 min MS n = 66. Experiment 2 (top right, bottom panels) Control n = 26, 15 min MS n = 33, 180 min MS n = 19. Statistically significant differences are denoted by asterisks (p < .05)
Clinical scores
Both the 15-min and 180-min MS conditions tended to reduce clinical scores in males and females in Experiment 1 (Fig. 5, top left panel). An ANOVA conducted on the mean clinical score between day 0 and 14 pi in Experiment 1 confirmed a significant effect of neonatal condition, F(2,174) = 4.46, p < .01. However, the effect of sex, F(1,174) = 0.544, p > .05, and interaction between sex and neonatal condition, F(2,174) = 0.67, p > .05, were not significant. Post hoc mean comparisons confirmed that both the 15-min MS and 180-min MS conditions exhibited less severe signs of illness compared to controls, p < .05.
Hind limb impairment
The effects of neonatal condition and sex on hind limb impairment collapsed over days pi are shown in the top right panel of Fig. 5. Prior to infection, no differences in hind limb impairment were observed across groups, all Fs < 1.32, p > .05. Following infection, there were significant effects of neonatal condition, F(2,70) = 6.814, p < .002, sex, F(1,70) = 7.76, p < .007, and day pi, F(8,560) = 6.75, p < .0001. Mean comparisons indicated that the 180-min MS condition resulted in lower hind limb impairment scores than the undisturbed control condition. In addition, males tended to show greater levels of impairment compared to females, and hind limb impairment gradually increased over days pi. There were significant interactions between day pi and neonatal condition, F(16,560) = 2.59, p < .0006, and day pi and sex, F(8,560) = 2.56, p < .01, which were attributable to the delayed onset and attenuation of hind limb impairment in the 180-min MS condition, an effect which was more pronounced in females than males.
Encephalitis scores
The lower left panel of Fig. 5 depicts the effects of MS and sex on behavioral signs on TMEV-induced illness and encephalitis collapsed across time pi. No differences in scores were observed prior to infection, all Fs < 1.322, p > .05. Thus, an ANOVA was conducted on post-infection scores entering neonatal condition and sex as between group factors and day pi as a repeated measure. Neonatal condition, F(2,70) = 6.81, p < .002, and sex, F(1,70) = 7.76, p < .007, significantly altered encephalitis scores. Post hoc comparisons indicated that mice in the 180-min MS condition exhibited lower scores compared to control mice. Moreover, males tended to have higher scores compared to females. There was a significant effect of day pi, F(8,560) = 6.75, p < .0001, indicating that scores gradually increased over time. In addition, there were significant interactions between day pi and neonatal condition, F(16,560) = 2.60, p < .0006, and day pi and sex, F(8,560) = 2.56, p < .05. These interactions were largely attributable to the delayed onset of illness behaviors in the 180-min MS condition, which was more pronounced in females than males.
Hunching scores
The lower right panel of Fig. 5 presents the effects of maternal separation and sex on TMEV-induced hunching collapsed across time pi. To facilitate comparisons across Experiments 1 and 2, the data are presented as the mean ± SEM for each neonatal condition collapsed across sex and day pi. Prior to infection, there were no preexisting differences in scores, all Fs < 1.428, p > .05. An ANOVA conducted on post-infection scores entered neonatal condition and sex as between group factors and day pi as a repeated measure. Neonatal condition, F(2,70) = 9.62, p < .0002, significantly altered hunching scores. Post hoc comparisons indicated that mice in the 180-min MS condition exhibited lower scores compared to 15-min MS and control mice, p < .05. In addition, the 15-min MS mice had lower scores compared to controls, p < .05. There was a significant effect of day pi, F(13,910) = 13.18, p < .0001, and significant interactions between day pi and neonatal condition, F(26,910) = 3.94, p < .0001, and day pi, neonatal condition, and sex, F(26,910) = 1.99, p < .002. These differences were attributable to a gradual increase in scores over time, as well as the delayed onset of hunching in the 15-min and 180-min MS conditions, which was more pronounced in females than males.
Impact of maternal separation on adrenal gland, lymph node, and spleen weights
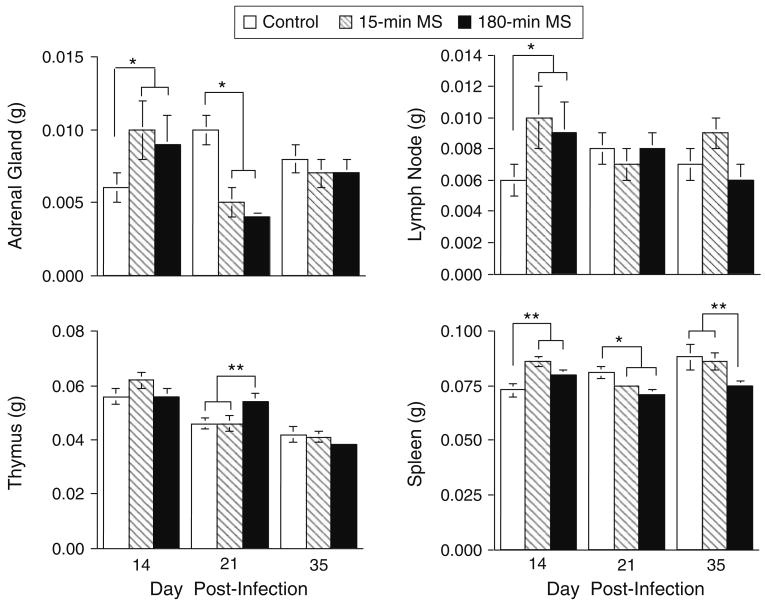
As shown in Fig. 6, both the 15-min and 180-min MS conditions produced an initial increase in adrenal, lymph node, and spleen weights at day 14 pi, suggesting a more intense physiological stress response to infection. A series of ANOVAs was conducted on tissue weights entering neonatal condition, sex, and time of sacrifice (day pi) as between group variables.
Fig. 6.
Effect of neonatal maternal separation on adrenal, lymph node, thymus, and spleen weights in mice sacrificed at days 14, 21, and 35 pi. Data are expressed as mean ± SEM and statistically significant differences are denoted by asterisks (p < .05)
Adrenal glands
Analyses of adrenal weights indicated a significant interaction between neonatal condition and day pi, F(4, 167) = 3.84, p < .005. Mean comparisons clarified that this effect was attributable to weight increases in the 15-min and 180-min MS mice compared to controls at the day 14 pi time point, p < .03. This was followed by the reverse pattern at day 21 pi, when weights increased in the control mice relative to the 15-min and 180-min MS mice, p < .01.
Spleen
Analyses of spleen weights revealed a significant effect of neonatal condition, F(2, 181) = 4.06, p < .02, and day pi, F(1,181) = 3.48, p < .03. Similar to the adrenal weight data, the interaction between neonatal condition and day of sacrifice was significant, F(4, 181) = 4.311, p < .002. Mean comparisons indicated that the spleens of the 15-min and 180-min MS mice weighed more than those of controls at day 14 pi, p < .05. On day 21 pi, control spleens were heavier than the 180-min MS spleens, but not the 15-min MS spleens, p < .05. On day 35 pi, the control and 15-min MS spleen were heavier compared to the 180-min MS spleens, p < .05.
Lymph nodes
Although an ANOVA conducted on lymph node weights failed to find a significant effect of neonatal condition when all sacrifice time points were included, mean comparisons conducted on the dpi 14 sacrifice time point revealed that both the 15-min and 180-min MS groups had significantly enlarged lymph nodes when compared to controls, p < .05.
Thymus
Thymus weight analyses revealed a significant effect of sex, F(1,181) = 77.397, p < .001, reflecting greater thymus weights in females compared to males. A significant effect of day pi, F(2,181) = 35.45, p < .0001, and a day pi by sex interaction, F(2,181) = 4.29, p < .01, was attributable to a decrease in weights over time, an effect that was more pronounced in females than males. Although the interaction between day pi and neonatal condition failed to reach significance, F(4,181) = 2.15, p = .08, mean comparisons revealed that the 180-min MS mice had heavier thymuses compared to control and 15-min MS mice on day 21 pi, p < .05.
No other differences in tissue weights were significant. Analyses of organ weights expressed as a percent of body weight yielded the same significant pattern of results for thymus, adrenal, and spleen, but the effect in lymph node was attenuated. Taken together, these gross changes in tissue weights suggest that maternal separation alters endocrine and immune function during TMEV immune challenge later in life.
CNS viral titers
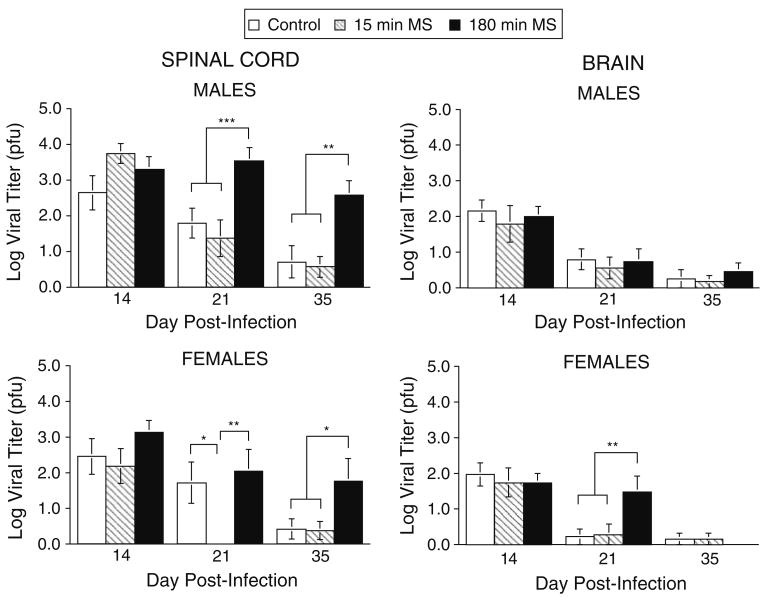
Figure 7 depicts the effect of the neonatal conditions on viral titer in spinal cord (left panel) and in brain (right panel) in male (top panels) and female (bottom panels) mice sacrificed on days 14, 21 and 35 pi. It is evident that exposure to prolonged maternal separation (180-min MS) disrupted viral clearance from the spinal cord mice infected with TMEV during adolescence. Although a similar pattern was observed in brain, the impact of maternal separation appears attenuated and viral titers in brain are generally reduced compared to spinal cord.
Fig. 7.
The impact of maternal separation on viral titer (log10) in the spinal cord (left panels) and brain (right panels) of male (top panels) and female mice (bottom panels) sacrificed at days 14, 21, and 35 pi. Control (day 14 n = 25, day 21 n = 23, day 35 n = 18), 15 min MS (day 14 n = 19, day 21 n = 19, day 35 n = 21) and 180 min MS (day 14 n = 34, day 21 n = 20, day 35 n = 20). Data are expressed as mean ± SEM. Statistically significant differences are denoted by asterisks (p < .05)
An ANOVA performed on spinal cord viral titer entering neonatal condition, sex, and day pi as between group variables verified that neonatal condition significantly altered spinal cord viral titers, F(2,170) = 15.474, p < .0001. In addition, there was an effect of day pi, F(2,170) = 26.57, p < .0001, which reflected the general decline in viral titers over time. Importantly, this effect was qualified by a significant interaction between neonatal condition and day pi. This interaction suggests that neonatal condition determined the rate of viral clearance over time. A significant main effect of sex, F(1, 170) = 10.30, p < .002, revealed that males tended to have higher viral titers (mean viral titer collapsed over day pi = 2.273) compared to females (mean = 1.673). However, sex did not interact with neonatal condition or day pi, all Fs < 1.55, p > .05. Post hoc tests performed on spinal cord viral titers collapsed over day pi and sex showed that titers in the 180-min MS condition were elevated compared to the 15-min MS and neonatal control conditions, all p's < .05. In addition, mean comparisons conducted at each time point indicated that the 180-min MS groups had elevated viral titers compared to the 15-min MS and neonatal control conditions at days 21 and 35 pi, ps < .0001. The 15-min MS mice also showed more rapid viral clearance at day 21 pi when compared to both the 180-min MS and neonatal control conditions, ps < .02. However, this effect was attributable to the 15-min MS female mice on day 21 pi, which showed a marked reduction in viral titers relative to both the 180-min MS and control conditions, p < .005.
An ANOVA conducted on brain viral titers entering neonatal condition, sex, and day pi as between group variables revealed a significant main effect of day pi, F(2,183) = 50.93, p < .0001, but no other main effects or interaction terms were significant, all Fs < 1.236, p > .05. However, mean comparisons conducted on day 21 pi viral titers alone indicated that the 180-min MS condition had higher viral titers than the 15-min MS and control conditions, p < .02. This effect was attributable to the female mice that showed a pronounced effect when analyzed separately, p < .005, whereas males did not, p > .05.
Discussion
The present study examined the effects of neonatal maternal separation on Theiler's virus infection. Mouse pups were separated from their mothers for either 15 or 180 min each day or left undisturbed during the first 2 weeks of life. During adolescence, mice were infected with Theiler's virus and the response to infection was evaluated. Consistent with prior studies the undisturbed control mice cleared the virus from CNS to undetectable levels during the first month of infection (Meagher et al. 2007a, b; Welsh et al. 1987, 1989). In contrast, exposure to the 180-min MS condition resulted in persistent elevations in spinal cord viral titers in both male and female mice on days 21 and 35 pi. While viral titers were lower in the brain than the spinal cord, the 180-min MS condition increased viral titers in the brains of female mice on day 21 pi. These findings suggest that the 180-min MS condition impaired host resistance during infection and delayed the kinetics of viral clearance. Interestingly, the 15-min MS condition reduced viral titers in the spinal cord of female mice on day 21 pi, which is consistent with the long-term protective effects of “handling” observed in previous studies (Anisman et al. 1998; Meaney 2001). Moreover, exposure to the 15-min and 180-min MS conditions produced an increase in adrenal and spleen weights on day 14 pi. Taken together, these findings suggest that the 180-min MS condition induced persistent changes in neuroimmune function that disrupted CNS anti-viral mechanisms later in life resulting in high viral titers, whereas the 15-min MS condition facilitated viral clearance in female mice.
Maternal separation was also found to alter CORT and behavioral responses to handling stress before and after infection. Prior to infection, female mice exposed to 180-min MS condition showed an increase in their CORT response to handling stress compared to 15-min MS and control mice. In contrast, male mice exposed to either 15-min or 180-min MS showed blunted CORT responses to handling stress. After infection, both the 15-min and 180-min MS mice exhibited blunted CORT responses to handling stress on day 1 pi. Altered behavioral signs of illness, including reductions in hind limb impairment, encephalitis-like symptoms, and hunching in both the 15-min and 180-min MS conditions, accompanied these changes in immune and endocrine function. However, only the 180-min MS mice exhibited reduced weight gain post-infection, which may reflect a suppression in food intake or an increase in metabolic rate associated with their more severe infection. In contrast, the 15-min MS mice compensated for their low body weights during infection, which may reflect an increase in food intake associated with their less severe infection. Collectively, these findings suggest that early life stress has long-lasting effects on the immune, endocrine, and behavioral responses to acute TMEV infection.
Relation to prior studies examining the impact of maternal separation on viral infection
Our results suggest that prolonged maternal separation influences vulnerability to Theiler's virus infection later in life. This finding is consistent with a previous report that daily 6-h maternal separation increases viral replication in lung following influenza virus infection in adult C57BL/6 mice (Avitsur et al. 2006). Although Avitsur did not observe a delay in the kinetics of influenza viral clearance, maternal separation was found to increase levels of several pro-inflammatory cytokines and alter the kinetics of the cytokine response to infection in lung. These findings suggest that maternal separation may impair host resistance by disrupting the regulation of the innate immune response to viral infection. Similar to the present study, Avitsur found that maternal separation blunted the CORT response during infection, suggesting that the increase in proinflammatory cytokines in lungs may be related to a disruption of HPA axis feedback regulation. Normally, the HPA axis plays an important role in regulating the immune response to infection, providing negative feedback regulation to suppress the release of proinflammatory cytokines. Following infection, activated immune cells release proinflammatory cytokines and other substances designed to decrease viral replication and promote viral clearance. However, the immune response to infection must be precisely timed and tightly regulated to contain infection while also preventing tissue damage. Under normal conditions the secretion of proinflammatory cytokines by immune cells triggers the HPA axis to release GCs. GCs, such as CORT, function to provide negative feedback regulation to suppress inflammation and optimize the immune response. Thus, the effectiveness of the host response to viral infection depends on the coordination of immune and endocrine responses. This suggests that alterations in HPA axis feedback regulation may have profound effects on the process viral replication, viral clearance, and disease course. The results from the present study, coupled with those of Avitsur et al. (2006), suggest that maternal separation may decrease the sensitivity of the HPA axis to cytokines leading to blunted CORT response. Disruption of negative feedback regulation may result in a shift away from optimal levels of immune activation, thereby reducing the ability of the immune response to fight infection.
Relation to prior studies examining the effect of stress on Theiler's virus infection
The current study is one in a series examining the effects of stress on the neuropathogenesis of Theiler's virus infection. We have previously shown that restraint stress administered during the first 4 weeks of Theiler's virus infection exacerbated the acute CNS viral infection and the chronic demyelinating phase of disease (Campbell et al. 2001; Welsh et al. 2004; Mi et al. 2004, 2006a, b; Sieve et al. 2004, 2006). During the acute phase, restraint increased behavioral signs of infection, weight loss, mortality rates, and viral load in the CNS, while decreasing NK cell lysis, chemokine and cytokine expression, virus specific CD4+ and CD8+ T cell responses, and histological signs of inflammation in the brain (Campbell et al. 2001; Johnson et al. 2004; Lipton 1975; Mi et al. 2004, 2006a, b; Sieve et al. 2004, 2006; Steelman et al. 2009; Welsh et al. 2004). During the chronic phase, previously stressed mice exhibited greater behavioral impairments and increased inflammatory demyelinating lesions in the spinal cord (Sieve et al. 2004; Young et al., in press).
We have also examined the effects of social stress on TMEV infection. Similar to the effects of restraint stress, exposure to repeated social stress immediately prior to infection disrupted viral clearance resulting in a more severe acute (Johnson et al. 2004; Meagher et al. 2007a, b) and chronic phase of the disease (Johnson et al. 2006). However, in contrast to the effects of restraint stress, social stress exacerbated disease by inducing GC resistance and IL-6 proinflammatory cytokine expression, resulting in increased CNS inflammation, behavioral impairment, and autoimmune responses to myelin and virus (Johnson et al. 2006; Meagher et al. 2007a, b). Importantly, we have recently shown that the adverse effects of social stress on TMEV infection can be prevented by intracranial infusions of a neutralizing antibody to IL-6 during the stress exposure period (Meagher et al. 2007a, b). This suggests that the adverse effects of social stress on infection are partially mediated by stress-induced increases in proinflammatory cytokine levels that impair the host response to infection. Therefore, social stress appears to exacerbate disease by inducing an increase in proinflammatory cytokine activity beyond the optimal levels needed to mount an effective immune response to combat CNS infection.
Similar to the effects of restraint and social stress, maternal separation disrupted CNS viral clearance during acute TMEV infection. However, additional research will be needed to elucidate the role of HPA axis and cytokine responses in mediating the relationship between 180-min MS and the disruption of viral clearance. One hypothesis is that maternal separation induces persistent decreases in inflammatory cytokine activity, which dysregulates the immune response to infection. A decrease in CNS inflammatory cell infiltration may account for both the disruption of viral clearance and as well as the decrease in behavioral signs of illness and motor impairment that result from inflammation. Recent histological evidence provides preliminary support for this hypothesis, demonstrating that both the 15-min and 180-min MS conditions reduced signs of meningitis and microgliosis in spinal cord (Meagher et al., in revision). Similar to the effects of restraint stress (Mi et al. 2004, 2006a, b), the decrease in CNS inflammation observed in maternally separated mice may be due to the suppression of chemokine and cytokine expression during early infection. An alternative hypothesis is that maternal separation induces persistent increases in inflammatory cytokine activity, which dysregulates the immune response to infection. This would be consistent with the effects of social stress on TMEV infection (Johnson et al. 2004; Meagher et al. 2007a, b) and the effects of maternal separation on influenza virus infection (Avitsur et al. 2006) and mucosal immunity (Barreau et al. 2004; O'Mahony et al. 2009).
Stressor specificity in CORT and behavioral responses to stress
Although maternal separation blunted the CORT response to the handling stress after infection in both male and female mice, prior to infection there appears to be a sex difference. Specifically, females in the 180-min MS condition exhibited elevated CORT levels compared to the control females, whereas males in the 180-min MS condition exhibited reduced CORT levels compared to control males. If persistent alterations in HPA reactivity to stress play a major role in regulating the immune response to infection during adolescence, then one would expect to see a mapping between the CORT response and viral clearance. However, the sex-dependent nature of these preexisting differences in CORT reactivity to mild stress does not account for the uniform pattern of viral titer results observed in both males and females following infection. Moreover, the blunted CORT response to stress during infection in the 15-min and 180-min MS mice does not account for the selective elevation of spinal cord viral titers observed in the 180-min MS mice. Although additional work is needed to investigate the effects of maternal separation on the HPA axis response in this model, the present findings suggest that the effects of maternal separation on viral titers are not mediated by persistent alterations in HPA reactivity alone. Rather, the development of the neonate's immune system may be permanently altered by exposure to stress during this critical period (Coe and Lubach 2003). While early life events may alter the development of the immune system, this does not mean that HPA axis mediators are not involved. Indeed, they may play an important role during the induction phase.
To determine whether the neonatal conditions induced long-lasting changes in anxiety-like behavior, exploratory behavior was tested the day prior to infection. The 180-min MS female mice showed increased vertical activity compared to the other groups. This finding is consistent with prior reports that exposure to 180-min MS increased vertical activity in FVB/JN female mice (Millstein and Holmes 2007) and open field exploratory behavior in C57BL/6 female mice (Romeo et al. 2003). In contrast, the 180-min MS male mice did not exhibit a change exploratory behavior, which is consistent with two recent studies (Millstein and Holmes 2007; Parfitt et al. 2007). Furthermore, the pattern of results for exploratory behavior does not map onto our CORT results. For example, the 180-min MS females exhibit increased CORT reactivity to handling stress on pnd 24 but show decreased exploratory behavior, whereas 180-min MS males exhibit decreased CORT levels but show no change in exploratory behavior. Although previous studies of maternal separation in male rats report corresponding increases anxiety-like behavior and HPA responses to stress (Ladd et al. 2000; Liu et al. 2000; Plotsky and Meaney 1993; Plotsky et al. 2005), studies in mice have yielded mixed results. Several studies in male mice report that 180-min MS increased anxious and depressive behavior (Bhansali et al. 2007; Romeo et al. 2003; Veenema et al. 2007) and CORT responses to mild stress (Parfitt et al. 2004). However, other studies have reported inconsistent effects of 180-min MS on anxietylike behavior using several inbred strains of mice (Millstein et al. 2006; Millstein and Holmes 2007; Parfitt et al. 2007) and blunted CORT responses to mild stress and infection in C57BL/6 mice (Avitsur et al. 2006; Parfitt et al. 2007). Thus, our results are consistent with the latter studies indicating that the 180-min MS condition induces a blunted HPA axis during infection and inconsistent effects on anxiety-like behavior in male mice.
Inconsistencies across rat and mouse studies may be attributed to species differences in neural development and maternal care behavior (Denenberg et al. 1962; Denenberg 1999; Parfitt et al. 2007). The mouse brain develops more rapidly than the rat brain (Whishaw et al. 2001). Thus, the relatively more mature HPA axis of the mouse may not be as sensitive to the effects of maternal separation as is the HPA axis of the rat. Other work suggests that the effects of handling and maternal separation on reactivity to stress may be mediated by alterations in maternal care behaviors following reunion (Anisman et al. 1998; Caldji et al. 1998; Huot et al. 2004; Liu et al. 1997; Pryce et al. 2001). Support for this hypothesis comes from rat studies showing that brief maternal separation (handling) increases maternal care behaviors and blunts HPA axis function (Liu et al. 1997). Moreover, studies of individual differences in maternal care behavior have observed blunted HPA reactivity to stress in the offspring of mothers with high rates of licking, grooming, and arched back nursing of their pups (Caldji et al. 1998; Liu et al. 1997). While the effects of prolonged maternal separation on maternal behaviors have not been systematically investigated, some evidence suggests disruption of maternal care behavior mediates the increase in HPA reactivity observed in rats (Huot et al. 2004; Pryce et al. 2001). In contrast, prolonged maternal separation appears to increase maternal care behaviors in several strains of mice (Millstein and Holmes 2007). Increased maternal care may have a protective effect on reactivity to stress and account for the divergent effects of 180-min MS on behavioral and HPA reactivity in male mice and rats.
Prior theories suggest that stressors may be categorized as primarily cognitive-emotional or “processive” or as primarily physical or “systemic” (Meaney et al. 1996; Plotsky et al. 1993; Herman and Cullinan 1997). The divergent effects of maternal separation in females on CORT responses to handling stress prior to infection compared to CORT responses after infection may be related to this distinction. Exposure to the handling stressor prior to infection represents a processive stressor, but there is reason to believe that TMEV infection contributes to the CORT response after infection. We have previously found that TMEV infection amplifies the CORT response elicited by restraint stress (Young et al. 2008). Specifically, TMEV infection increased CORT levels in restraint stressed mice on days 1 and 2 pi compared to non-infected restraint stressed mice. This suggests that stress interacts with TMEV infection to increase CORT during early infection. Thus, the CORT response to stress after infection has both processive and systemic components. The present findings suggest that maternal separation may have differential effects on the CORT response to processive and systemic stressors, which may reflect differential changes in HPA axis regulation at the pituitary and supra-pituitary level, respectively. Support for this hypothesis is provided by Wigger and Neumann (1999) who observed effects of maternal separation after exposure to a mild emotional stress (plus maze exposure), but not after exposure to a complex physical stressor (forced swimming in cold water). At a neural level, Ladd et al. (1996) has also observed effects of maternal deprivation on the central corticotrophin-releasing hormone (CRH) system, producing an increase in median eminence CRH content, but decreases in CRH binding in the anterior pituitary. In light of these findings, we propose that exposure to maternal separation during the stress hyporesponsive period may differentially alter the programming of processive and systemic neural systems.
Impact of early life stress on the development of the immune system and disease vulnerability
Prior research indicates that early life stress can permanently alter the programming of the nervous system, affecting the HPA axis, autonomic nervous system, neurotransmitters, and the emotional reactivity of offspring, which in turn can alter the neuroregulation of immune function. Moreover, increased susceptibility to infectious and autoimmune diseases has also been linked to persistent HPA axis hypoactivity (e.g., Avitsur et al. 2006; Sternberg et al. 1989); however, the present findings argue against an exclusive role of the HPA axis in determining susceptibility. Rather, we propose that stress during early development may alter disease vulnerability by altering the programming of immune regulatory mechanisms. Support for this view comes from studies in monkeys examining the long-term effects of the early rearing environment on a range of immune responses. Disruption of the early rearing environment resulted in suppressed NK cell activity and decreased antibody responses to vaccines, while at the same time increasing lymphocyte proliferation following mitogen stimulation in juvenile rhesus monkeys (Coe et al. 1992). A later study also observed elevated proliferative activity in monkeys reared by humans in a nursery compared to mother-reared monkeys, whereas abnormally low CD8+ cells were observed in nursery-reared monkeys, resulting in a skewed CD4/CD8 ratio (Lubach et al. 1995). Moreover, alteration in T cell numbers persisted during this 2-year study. These findings suggest that disruption of the early rearing environment altered the set point for T cell numbers, thereby influencing proliferative responses. Although these studies did not examine whether this altered immune profile increased vulnerability to immune-related diseases later in life, other evidence suggests that neonatal maternal separation is related to early onset of symptoms in simian immunodeficiency virus-infected in adult monkeys (SIV; Capitanio and Lerche 1991) and on the course of experimental allergic encephalomyelitis in rats (Laban et al. 1995a, b; Stephan et al. 2002; Teunis et al. 2002). Thus, early rearing environments play an important role in shaping the offspring's immune response and altering vulnerability to infectious and autoimmune diseases later in life.
It is well established that genetic factors contribute to the pathogenesis of multiple sclerosis (Fazekas et al. 1988; FRGMS 1992; Sadovnick et al. 1996; Willer et al. 2003), but the low rate of concordance in monozygotic twins (20–25%) suggests that environmental influences play a critical role. The present study involved environmental challenges during two critical periods in development: stress during the neonatal period and infection during the peri-pubertal period. Recent animal research suggests that the peri-pubertal period is a unique vulnerability period for immune challenges (Laroche et al. 2009). In addition, human epidemiological evidence indicates that multiple sclerosis is associated with viral infection during adolescence (Kurtzke et al. 1995). For example, increasing evidence suggests that exposure to Epstein-Barr virus (EBV) during adolescence plays a key role in determining MS risk (Ascherio and Munger 2007; Hernan et al. 2001; Jilek et al. 2008). Moreover, high levels of EBV-specific CD8+ T-cell responses have been observed early in the course of MS, which supports the hypothesis that EBV infection is linked to disease onset (Jilek et al. 2008). Our findings suggest that early life stress may contribute to individual vulnerability to infection during adolescence by disrupting antiviral mechanisms. Disruption of viral clearance may have important implications for the later demyelinating phase of disease because higher viral load during acute Theiler's virus infection is associated with more severe demyelination (Borrow et al. 1992).
In summary, maternal separation disrupted TMEV clearance from the CNS during adolescence. These effects do not appear to be explained by persistent alterations in CORT responsiveness to stress observed before or after infection. Rather, we propose that early life stress may alter the developmental programming of neuroimmune regulatory mechanisms. The long-term goal of this research program is to elucidate mechanisms by which disruption of early rearing environments affect life-long vulnerability to infectious, inflammatory, and autoimmune diseases. Understanding these mechanisms could lead to interventions that prevent or reverse the deleterious effects of early life stress on disease predisposition.
Acknowledgments
This research was funded by grants from the National Multiple Sclerosis Society RG-3128 and NIH/NINDS R01-NS39569 to C.J.W. and M.W.M and by NIH/NINDS R01-NS060822 awarded to M.W.M. and C.J. W. We would also like to thank Heath McCullough and Patrick Bridegam for their help in conducting the maternal separation procedures and data management.
Contributor Information
M. W. Meagher, Email: M-Meagher@tamu.edu, Department of Psychology, College of Liberal Arts, Texas A&M University, College Station, TX 77843-4235, USA.
A. N. Sieve, Department of Psychology, College of Liberal Arts, Texas A&M University, College Station, TX 77843-4235, USA
R. R. Johnson, Department of Psychology, College of Liberal Arts, Texas A&M University, College Station, TX 77843-4235, USA
D. Satterlee, Department of Psychology, College of Liberal Arts, Texas A&M University, College Station, TX 77843-4235, USA
M. Belyavskyi, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA
W. Mi, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA
T. W. Prentice, Department of Psychology, College of Liberal Arts, Texas A&M University, College Station, TX 77843-4235, USA
T. H. Welsh, Jr., Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA; Department of Animal Science, College of Agriculture and Life Sciences, Texas A&M University, College Station, TX, USA
C. J. R. Welsh, Department of Veterinary Integrative Biosciences, College of Veterinary Medicine and Biomedical Sciences, Texas A&M University, College Station, TX, USA
References
- Acheson ED. Epidemiology of multiple sclerosis. Brit Med Bull. 1977;33:9–14. doi: 10.1093/oxfordjournals.bmb.a071407. [DOI] [PubMed] [Google Scholar]
- Ackerman SH, Keller SE, Schleifer SJ, Shindledecker RD, Camerino M, Hofer MA, Weiner H, Stein M. Premature maternal separation and lymphocyte function. Brain Behav Immun. 1988;2:161–165. doi: 10.1016/0889-1591(88)90016-5. [DOI] [PubMed] [Google Scholar]
- Ackerman KD, Heyman R, Rabin BS, Anderson BP, Houck PR, Frank E, Baum A. Stressful life events precede exacerbations of multiple sclerosis. Psychosom Med. 2002;64:916–920. doi: 10.1097/01.psy.0000038941.33335.40. [DOI] [PubMed] [Google Scholar]
- Alter M, Zhang ZX, Davanipour Z, Sobel E, Zibulewski J, Schwartz G, Friday G. Multiple sclerosis and childhood infections. Neurology. 1986;36:1386–1389. doi: 10.1212/wnl.36.10.1386. [DOI] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34:396–403. doi: 10.1016/j.amepre.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Zaharia DM, Meaney MJ, Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Aubert C, Chamorro M, Brahic M. Identification of Theiler's infected cells in the central nervous system of the mouse during demyelinating disease. Microb Pathog. 1987;3:319–326. doi: 10.1016/0882-4010(87)90002-7. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Hunzekerb J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–348. doi: 10.1016/j.bbi.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Bakker JM, Schmidt ED, Kroes H, Kavelaars A, Heijnen CJ, Tilders FJH, Van Rees EP. Effects of neonatal dexamethasone treatment on the hypothalamic-pituitary adrenal axis and immune system of the rat. J Neuroimmunol. 1997;74:69–76. doi: 10.1016/s0165-5728(96)00207-x. [DOI] [PubMed] [Google Scholar]
- Bakker JM, Kavelaars A, Kamphuis PJGH, Cobelens PM, van Vugt HH, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment increases susceptibility to experimental autoimmue disease in adult rats. J Immunol. 2000;165:5932–5937. doi: 10.4049/jimmunol.165.10.5932. [DOI] [PubMed] [Google Scholar]
- Bakker JM, Kavelaars A, Kamphuis PJGH, Zijlstra J, van Bel F, Heijnen CJ. Neonatal dexamethasone treatment induces long-lasting changes in T-cell receptor Vβ repertoire in rats. J Neuroimmunol. 2001;112:47–54. doi: 10.1016/s0165-5728(00)00406-9. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Fetal nutrition and cardiovascular disease in later life. Br Med Bull. 1997;53:96–108. doi: 10.1093/oxfordjournals.bmb.a011609. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, babies, and health in later life. Churchill Livingstone; London: 1998. [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi M, Zannino LG. Psychological stress, neuroimmunomodulation, and susceptibility to infectious disease in animals and man: a review. PNAS. 1997;93:3043–3047. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- Borrow P, Tonks P, Welsh CJR, Nash AA. The role of CD8+ T cells in the acute and chronic phases of Theiler's virus-induced disease in mice. J Gen Virol. 1992;73:1861–1865. doi: 10.1099/0022-1317-73-7-1861. [DOI] [PubMed] [Google Scholar]
- Boynton-Jarrett R, Ryan LM, Berkman LF, Wright RJ. Cumulative violence exposure and self-rated health: longitudinal study of adolescents in the United States. Pediatrics. 2008;122:961–970. doi: 10.1542/peds.2007-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukilica M, Djordjevic S, Maric I, Dimitrijevic M, Markovic BM, Jankovic BD. Stress-induced suppression of experimental allergic encephalomyelitis in the rat. Int J Neurosci. 1991;59:167–175. doi: 10.3109/00207459108985460. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Tannenbaum B, Sharma S, Meaney MJ. Maternal care in infancy influences the development of neural systems mediating fearfulness in the rat. PNAS. 1998;95:5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000a;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000b;22:219–229. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- Campbell T, Meagher MW, Sieve A, Scott B, Storts R, Welsh TH, Welsh CJR. The effects of restraint stress on the neuropathogenesis of Theiler's virus-induced demyelination. I. Acute disease. Brain Behav Immun. 2001;15:235–254. doi: 10.1006/brbi.2000.0598. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Lerche NW. Psychosocial factors and disease progression in simian AIDS: a preliminary report. AIDS. 1991;5:1103–1106. doi: 10.1097/00002030-199109000-00007. [DOI] [PubMed] [Google Scholar]
- Catalani A, Casolini P, Scaccianoce S, Patacchioli FR, Spinozzi P, Angelucci L. Maternal corticosterone during lactation permanently affects brain corticosteroid receptors, stress response and behaviour in rat progeny. Neuroscience. 2000;100:319–325. doi: 10.1016/s0306-4522(00)00277-3. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR. Critical periods of special health relevance for psychoneuroimmunology. Brain Behav Immun. 2003;12:3–12. doi: 10.1016/s0889-1591(02)00099-5. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Schneider ML, Dierschke DJ, Ershler WB. Early rearing conditions alter immune responses in the developing infant primate. Pediatrics. 1992;90:505–509. [PubMed] [Google Scholar]
- Craft T, Zhang N, Glasper E, Hurn P, DeVries A. Neonatal factors influence adult stroke outcome. Psychoneuroendocrinology. 2006;31:601–613. doi: 10.1016/j.psyneuen.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellis MD, Baum AS, Birmaher B, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. Developmental traumatology part I: biological stress systems. Biol Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Commentary: is maternal stimulation the mediator of the handling effect in infancy. Dev Psychobiol. 1999;34:1–3. doi: 10.1002/(sici)1098-2302(199901)34:1<1::aid-dev2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Denenberg VH, Ottinger DR, Stephens MW. Effects of maternal factors upon growth and behavior of the rat. Child Dev. 1962;33:65–71. doi: 10.1111/j.1467-8624.1962.tb05988.x. [DOI] [PubMed] [Google Scholar]
- Dong M, Dube SR, Felitti VJ, Giles WH, Anda RF. Adverse childhood experiences and self-reported liver disease: new insights into the causal pathway. Arch Intern Med. 2003;163:1949–1956. doi: 10.1001/archinte.163.16.1949. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Dowdell KC, Gienapp IE, Stuckman S, Wardrop RM, Whitacre CC. Neuroendocrine modulation of chronic relapsing experimental autoimmune encephalomyelitis: a critical role for the hypothalamic-pituitary-adrenal axis. J Neuroimmunol. 1999;100:243–251. doi: 10.1016/s0165-5728(99)00211-8. [DOI] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. 2009;71:243–250. doi: 10.1097/PSY.0b013e3181907888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazekas F, Offenbacher H, Fuchs S. Criteria for an increased specificity of MRI interpretation in elderly subjects with suspected multiple sclerosis. Neurology. 1988;38:1822–1825. doi: 10.1212/wnl.38.12.1822. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences study. Am J Prev Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor–norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999a;46:1153–1166. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999b;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- French Research Group on Multiple Sclerosis. Multiple sclerosis in 54 twinships: concordance rate is independent of zygosity. Ann Neurol. 1992;32:724–727. doi: 10.1002/ana.410320604. [DOI] [PubMed] [Google Scholar]
- Grant J, Brown GW, Harris T. Severely threatening events and marked life difficulties preceding the onset or exacerbation of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1989;52:8–13. doi: 10.1136/jnnp.52.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin AC, Whitacre CC. Differential effects of stress on disease outcome in experimental allergic encephalomyelitis. FASEB J. 1990;4:2038. [Google Scholar]
- Griffin A, Lo W, Wolny A, Whitacre C. Suppression of experimental autoimmune encephalomyelitis by restraint stress: sex differences. J Neuroimmunol. 1993;44:103–116. doi: 10.1016/0165-5728(93)90273-2. [DOI] [PubMed] [Google Scholar]
- Hawks BW, Plotsky SJ, Garlow SJ. Mouse strain differences in behavior and corticosterone in response to early postnatal separation. Soc Neurosci Abs. 1999;25:616. [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamic-pituitary-adrenal axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Hernan MA, Zhang SM, Lipworth L, Olek MJ, Ascherio A. Multiple sclerosis and age at infection with common viruses. Epidemiology. 2001;12:301–306. doi: 10.1097/00001648-200105000-00009. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Knott B. Potentiation of tumor metastasis in adulthood by neonatal endoxotin exposure: sex differences. Psychoneuroendocrinology. 2002;27:791–798. doi: 10.1016/s0306-4530(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology. 2004;29:279–289. doi: 10.1016/s0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A, Kleeberg J, Goff GL, Pantaleo G, Du Pasquier RA. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–1721. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Storts R, Welsh TH, Jr, Welsh CJ, Meagher MW. Social stress alters the severity of acute Theiler's virus infection. J Neuroimmunol. 2004;148:74–85. doi: 10.1016/j.jneuroim.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Johnson RR, Prentice TW, Bridegam P, Young CR, Steelman AJ, Welsh TH, Welsh CJR, Meagher MW. Social stress alters the severity and onset of the chronic phase of Theiler's virus infection. J Neuroimmunol. 2006;175:39–51. doi: 10.1016/j.jneuroim.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF, Hyllested K, Heltberg A. Multiple sclerosis in the Faroe Islands: transmission across four epidemics. Acta Neurol Scand. 1995;91:321–332. doi: 10.1111/j.1600-0404.1995.tb07015.x. [DOI] [PubMed] [Google Scholar]
- Laban O, Dimitrijevic M, von Hoersten S, Markovic BM, Jankovic BD. Experimental allergic encephalomyelitis in adult DA rats subjected to neonatal handling or gentling. Brain Res. 1995a;676:133–140. doi: 10.1016/0006-8993(95)00106-z. [DOI] [PubMed] [Google Scholar]
- Laban O, Markovic BM, Dimitrijevic M, Jankovic BD. Maternal deprivation and early weaning modulated experimental allergic encephalomyelitis in the rat. Brain Behav Immun. 1995b;9:9–19. doi: 10.1006/brbi.1995.1002. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–1218. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager ML, Capitanio JP, Reite M. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. Am J Psychiatry. 1985;142:862–864. doi: 10.1176/ajp.142.7.862. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience to physiological stress. Science. 1957;126:405–406. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S, Saltzman A. Nonspecific stress prevents relapses of experimental allergic encephalomyelitis in rats. Brain Behav Immun. 1987;1:336–341. doi: 10.1016/0889-1591(87)90036-5. [DOI] [PubMed] [Google Scholar]
- Levine S, Strebel R, Wenk E, Harman P. Suppression of experimental autoimmune encephalomyelitis by stress. Exp Biol Med. 1962;109:294–298. doi: 10.3181/00379727-109-27183. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Gluck JP, Petitto JM, Hensley LL, Ozer H. Early social deprivation in nonhuman primates: long-term effects on survival and cell-mediated immunity. Biol Psychiatry. 2000;47:119–126. doi: 10.1016/s0006-3223(99)00238-3. [DOI] [PubMed] [Google Scholar]
- Lipton HL. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975;11:1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277(12):1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinephrine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL, Ershler WB. Effects of early rearing environment on immune responses of infant rhesus monkeys. Brain Behav Immun. 1995;9:31–46. doi: 10.1006/brbi.1995.1004. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:85–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marchetti B, Morale MC, Testa N, Tirolo C, Caniglia S, Amor A, Dijkstra CD, Barden N. Stress, the immune system and vulnerability to degenerative disorders of the central nervous system in transgenic mice expressing glucocorticoid receptor antisense RNA. Brain Res Rev. 2001;37:259–272. doi: 10.1016/s0165-0173(01)00130-8. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurological deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Johnson RR, Young EE, Vichaya EG, Harden A, Connor M, Welsh CJR. Interleukin-6 as a mechanism for the adverse effects of SDR on acute TMEV Infection. Brain Behav Immun. 2007a;21:1083–1095. doi: 10.1016/j.bbi.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher MW, Johnson RR, Vichaya EG, Young EE, Lunt S, Welsh CJR. Social conflict exacerbates an animal model of multiple sclerosis. Trauma Violence Abuse. 2007b;8:314–330. doi: 10.1177/1524838007303506. [DOI] [PubMed] [Google Scholar]
- Meagher MW, Sieve AN, Johnson RR, Satterlee D, Johnson RR, Storts R, Welsh TH, Welsh CJR. Early life stress interacts with later restraint stress to alter CNS inflammation and endocrine responses during infection in adult mice in revision. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlanta P, Caldji C, Sharma S, Seckl JS, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neurosci. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Weaver S, Yau J, Chapman K, Seckl JR. Postnatal handling increases the expression of cAMP-inducible transcription factors in the rat hippocampus: the effects of thyroid hormones and serotonin. J Neurosci. 2000;20:3926–3935. doi: 10.1523/JNEUROSCI.20-10-03926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. Am J Epidemiol. 2007;166:966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Belyavskyi M, Johnson RR, Sieve AN, Meagher MW, Welsh CJR. Alterations in chemokine expression following Theiler's virus infection and restraint stress. J Neuroimmunol. 2004;178:59–61. doi: 10.1016/j.jneuroim.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Prentice TW, Young CR, Johnson RR, Sieve AN, Meagher MW, Welsh CJR. Restraint stress decreases virus-induced pro-inflammatory cytokine expression during acute Theiler's virus infection. J Neuroimmunol. 2006a;178:49–61. doi: 10.1016/j.jneuroim.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Mi W, Young CR, Storts R, Steelman A, Meagher MW, Welsh CJR. Stress alters pathogenicity and facilitates systemic dissemination of Theiler's virus. Microb Pathog. 2006b;41:133–143. doi: 10.1016/j.micpath.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Millstein RA, Ralph RJ, Yang RJ, Holmes A. Effects of repeated maternal separation on prepulse inhibition of startle across inbred mouse strains. Genes Brain Behav. 2006;5:346–354. doi: 10.1111/j.1601-183X.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- Mohr DC, Goodkin DE, Bacchetti P, Boudewyn AC, Huang L, Marrietta P, Cheuk W, Dee B. Psychological stress and the subsequent appearance of new brain MRI lesions in MS. Neurology. 2000;55:55–61. doi: 10.1212/wnl.55.1.55. [DOI] [PubMed] [Google Scholar]
- Nicholson SM, Peterson JD, Miller SD, Wang K, Dal Canto MC, Melvold RW. BALB/c substrain differences in susceptibility to Theiler's murine encephalomyelitis virus-induced demyelinating disease. J Neuroimmunol. 1994;52:19–24. doi: 10.1016/0165-5728(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Njenga MK, Asakura K, Hunter SF, Wettstein P, Pease LR, Rodriguez M. The immune system preferentially clears Theiler's virus from the gray matter of the central nervous system. J Virol. 1997;71:8592–8601. doi: 10.1128/jvi.71.11.8592-8601.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho AM, Quigley EMM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–267. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL. Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Res. 2004;1016:111–118. doi: 10.1016/j.brainres.2004.04.077. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Walton JR, Corriveau EA, Helmreich DL. Early life stress effects on adult stress-induced corticosterone secretion and anxiety-like behavior in the C57BL/6 mouse are not as robust as initially thought. Horm Behav. 2007;52:417–426. doi: 10.1016/j.yhbeh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Brain Res Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Meaney MJ. Central and feedback regulation of hypothalamic corticotropin-releasing factor secretion. Ciba Found Symp. 1993;172:59–84. doi: 10.1002/9780470514368.ch4. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Dev Psychobiol. 2001;38:239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Fossella JA, Bateup HS, Sisti HM, Brake WG, McEwen BS. Maternal separation suppresses TGF alpha mRNA expression in the prefrontal cortex of male and female neonatal C57BL/6 mice. Brain Res Dev Brain Res. 2004;152:73–77. doi: 10.1016/j.devbrainres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Sadovnick A, Ebers G, Dyment D, et al. Evidence for the genetic basis of multiple sclerosis. Lancet. 1996;347:1728–1730. doi: 10.1016/s0140-6736(96)90807-7. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Oitzl MS, Levine S, de Kloet R. The HPA system during postnatal development of CD1 mice and the effects of maternal deprivation. Brain Res Dev Brain Res. 2002;139:39–49. doi: 10.1016/s0165-3806(02)00519-9. [DOI] [PubMed] [Google Scholar]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, Oitzl MS. The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci. 2003;21:125–132. doi: 10.1016/s0736-5748(03)00030-3. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Glucocorticoids, fetoplacental 11-hyrdoxysteroid dehydrogenase type 2 and the early life origins of adult disease. Steroids. 1997;62:89–94. doi: 10.1016/s0039-128x(96)00165-1. [DOI] [PubMed] [Google Scholar]
- Selye H. Thymus and adrenals in the responses of organisms to injuries and intoxications. Br J Exp Pathol. 1936;17:234–248. [Google Scholar]
- Sibley WA. Risk factors in multiple sclerosis. In: Raine CS, McFarland HF, Tortellotte WW, editors. Multiple sclerosis: clinical and pathogenetic basis. Chapman & Hall; London: 1997. pp. 141–148. [Google Scholar]
- Sieve AN, Steelman D, Storts R, Young CR, Welsh TH, Welsh CJR, Meagher MW. Chronic restraint stress during early infection exacerbates the subsequent demyelinating phase of Theiler's virus infection. J Neuroimmunol. 2004;155:103–118. doi: 10.1016/j.jneuroim.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Sieve AN, Steelman AJ, Young CR, Storts R, Welsh TH, Welsh CJR, Meagher MW. Sex-dependent effects of chronic restraint stress during early Theiler's virus infection on the subsequent demyelinating disease in CBA mice. J Neuroimmunol. 2006;177:46–62. doi: 10.1016/j.jneuroim.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- Steelman AJ, Dean DD, Young CR, Smith R, Prentice TW, Meagher MW, Welsh CJR. Restraint stress modulates virus specific adaptive immunity during acute Theiler's virus infection. Brain Behav Immun. 2009;23:830–843. doi: 10.1016/j.bbi.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan M, Straub RH, Breivik T, Pabst R, von Hörsten S. Postnatal maternal deprivation aggravates experimental autoimmune encephalomyelitis in adult Lewis rats: reversal by chronic imipramine treatment. Int J Dev Neurosci. 2002;20:125–132. doi: 10.1016/s0736-5748(02)00007-2. [DOI] [PubMed] [Google Scholar]
- Sternberg E, Hill J, Chrousos G, Kamilaris T, Listwak S, Gold P, Wilder R. Inflammatory mediator-induced hypothalamic–pituitary–adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci USA. 1989;86:2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunis MAT, Heijnen CJ, Sluyter F, Bakker JM, Van Dam AMW, Hof M, Cools AR, Kavelaars A. Maternal deprivation of rat pups increases clinical symptoms of experimental autoimmune encephalomyelitis at adult age. J Neuroimmunol. 2002;133:30–38. doi: 10.1016/s0165-5728(02)00351-x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewolda R, Neumanna ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999;107:332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Warren S, Greenhill S, Warren KG. Emotional stress and the development of multiple sclerosis: case-control evidence of a relationship. J Chronic Dis. 1982;35:821–831. doi: 10.1016/0021-9681(82)90047-9. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Tonks P, Nash AA, Blakemore WF. The effect of L3T4 T cell depletion on the pathogenesis of Theiler's murine encephalomyelitis virus infection in CBA mice. J Gen Virol. 1987;68:1659–1667. doi: 10.1099/0022-1317-68-6-1659. [DOI] [PubMed] [Google Scholar]
- Welsh CJR, Blakemore WF, Tonks P, Borrow P, Nash AA. Theiler's murine encephalomyelitis virus infection in mice: a persistent viral infection of the central nervous system which induces demyelination. In: Dimmock N, editor. Immune responses, virus infection and disease. Oxford University Press; Oxford; 1989. pp. 125–147. [Google Scholar]
- Welsh CJ, Bustamante L, Nayak M, Welsh TH, Dean DD, Meagher MW. The effects of restraint stress on the neuropathogenesis of Theiler's virus infection II: NK cell function and cytokine levels in acute disease. Brain Behav Immun. 2004;18:166–174. doi: 10.1016/S0889-1591(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Whishaw I, Metz G, Kolb B, Pellis S. Accelerated nervous system development contributes to behavioral efficiency in the laboratory mouse: a behavioral review and theoretical proposal. Dev Psychobiol. 2001;39:151–170. doi: 10.1002/dev.1041. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Dowdell K, Griffin AC. Neuroendocrine influences on experimental autoimmune encephalomyelitis. Ann N Y Acad Sci. 1998;840:705–716. doi: 10.1111/j.1749-6632.1998.tb09609.x. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiol Behav. 1999;66:293–302. doi: 10.1016/s0031-9384(98)00300-x. [DOI] [PubMed] [Google Scholar]
- Willer C, Dyment D, Risch N, et al. Twin concordance and sibling recurrence rate in multiple sclerosis. Proc Natl Acad Sci. 2003;100:12877–12882. doi: 10.1073/pnas.1932604100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Prentice TW, Satterlee D, McCullough H, Sieve AN, Johnson RR, Welsh TH, Welsh CJR, Meagher MW. Glucocorticoid exposure alters the pathogenesis of Theiler's murine encephalomyelitis virus during acute infection. Physiol Behav. 2008;95:63–71. doi: 10.1016/j.physbeh.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EE, Sieve AN, Vichaya EG, Young CR, Ambrus A, Storts R, Welsh CJR, Meagher MW. Chronic restraint stress during early Theiler's virus infection exacerbates the subsequent demyelinating disease in SJL mice: II. Histological evaluation of CNS disease severity. J Neuroimmunol. doi: 10.1016/j.jneuroim.2010.01.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]