Abstract
Whereas the ability of oestradiol and insulin-like growth factor-1 (IGF1) to afford neuroprotection against ischemia-induced neuronal death in young female and male rodents is well established, the impact of IGF1 in middle-aged animals is largely unknown. This study assessed the efficacy of oestradiol and IGF1 in reducing neuronal death after transient global ischemia in middle-aged female rats following an 8-week hormone withdrawal. Rats were ovariohysterectomized (OVX) and implanted 8 weeks later with an osmotic mini-pump delivering IGF1 or saline into the lateral ventricle. Some rats also received physiological levels of oestradiol by subcutaneous pellet. Two weeks later, rats were subjected to global ischemia or sham operation. Surviving hippocampal CA1 neurones were quantified. Ischemia produced massive CA1 cell death compared to sham-operated animals evident at 14 days. Significantly more neurones survived in animals treated with either oestradiol or IGF1, but simultaneous treatment produced no additive effect. IGF1, an endogenous growth factor, may be a clinically useful therapy in preventing human brain injury, with neuroprotective equivalence to oestradiol but without the harmful side effects.
Keywords: global ischemia, oestrogens, IGF1, neuroprotection
Introduction
Post-menopausal women are particularly susceptible to cardiovascular and neurovascular disease, especially stroke (1). Quantification of stroke injury and outcomes can be very difficult; approximately 70% of patients have motor deficits, 40% have sensory deficits, and upwards of 40% may have speech, swallowing, and/or visual impairments (2). While some studies show sex differences in the severity of stroke damage, with women suffering more severe strokes (3) and higher rates of handicap and disability at 6 months (4), other data refute this (5). Longitudinal and prospective studies reveal conflicting results concerning the relationship of current and/or past use of estrogens and combined hormone therapy to the incidence of stroke (6, 7). More importantly, little is known about the modulation of ischemic brain damage in women taking estrogens or combined hormone therapy at the time of stroke.
In humans and animals, transient global ischemia induces selective, delayed neuronal cell death of pyramidal neurones in the CA1 hippocampus (8). The exact mechanisms underlying cell death are still not completely understood (9, 10).In peripubertal and young adult rodents (male and female), estrogens afford neuroprotection in experimentally induced models of focal and global ischemia (11, 12). Few studies have assessed these effects in older rodents. Oestradiol significantly reduces brain injury following middle cerebral artery occlusion (MCAO, a stroke model) in middle-aged (9–11 months) (13) and reproductively senescent (16 months) (14, 15) female rats. Recent experiments in our laboratory extended these findings in older rodents to a global ischemia model. Oestradiol retains its neuroprotective actions after global ischemia in middle-aged female rats even if they have been deprived of hormones for a prolonged period (8 weeks) prior to insult (16).
Insulin-like growth factor-1 (IGF1) plays critical roles in somatic and vascular growth, glucose homeostasis, and brain development (17, 18). The IGF1 system is altered after brain injury. Endogenous IGF1 mRNA is locally upregulated in the damaged area of the brain after inhalational ischemia injury (19) with altered expression of IGF1 binding proteins (20). Exogenous IGF1 is neuroprotective in animal models of global ischemia including 4-vessel occlusion (21) 2-vessel occlusion with hypotensive ischemia (22), and focal ischemia (23). However, none of these ischemic models tested older animals. Chronic IGF1 treatment in senescent male rats increases neurogenesis in the dentate gyrus, providing some evidence that the aged brain retains responsiveness to the actions of IGF1 (24). Functionally, IGF1 decreases age-related deficits in working and reference memory tasks in rats (25). The molecular mechanisms underlying IGF1 neuroprotection are incompletely understood. IGF1 may work in concert with oestrogens to reduce hilar neuronal death induced in vivo by systemic kainic acid (26) and by ischemic (27) insults. Reflecting robust communication between the two systems, oestradiol promotes expression of IGF1, its receptor, and binding proteins (28, 29). Serum levels of IGF1 and oestradiol covary, and decline with age in both rodents (30) and humans (31).
Whereas IGF1 has been suggested to be neuroprotective in younger animals after ischemia, to date, no study has assessed whether IGF1 confers protection against transient global ischemia in middle-aged animals. In this study, we hypothesized that ovarian hormone-deprived, middle-aged female rats would retain their ability to respond to IGF1 as well as oestradiol when administered for 2 weeks prior to induction of global ischemia. We also assessed the possibility that chronic administration of IGF1 might potentiate the neuroprotection achieved by administering physiological levels of oestradiol alone.
Materials and methods
Animals
Animals were treated in accordance with the principles and procedures of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Animals were maintained in a temperature and light-controlled environment with a 12 hr light/dark cycle and housed two-three per cage with continuous access to food and water. Animals were weighed at the beginning of all surgical procedures and again at time of perfusion.
Hormone deprivation
Adult female Sprague-Dawley rats (retired breeders, 9–11 months; Charles River Laboratories, Inc., Wilmington, MA) weighing between 300–500 g were bilaterally ovariohysterectomized (OVX) under isoflurane anesthesia (5% for induction, 2% for maintenance in 70% N2O:30% O2; Baxter Healthcare Corporation, Deerfield, IL). As in our previous work (16), we chose retired breeders instead of virgin females to more accurately represent the typical middle-aged woman with at least one previous pregnancy.
Intracerebroventricular (icv) cannula and oestradiol pellet placement
Eight weeks after OVX, under isoflurane anesthesia, animals were secured in a stereotaxic apparatus. After drilling a burr hole over the right lateral ventricle (1.5 mm lateral, 0.8 mm posterior to bregma), a 0.31 mm diameter cannula (Alzet Brain Infusion Kit, Durect Corporation, Cupertino, CA) connected by polyethylene tubing to a mini-osmotic pump (Alzet Model 2004; Durect Corporation) was inserted to a depth of 3.5 mm. The cannula was secured with dental cement and fixed to anchoring screws. The cannulae and pumps were filled 40 hr prior to surgical placement with either IGF1 (100 µg/ml in sterile saline; GroPep, Thebarton, Australia) or sterile saline (vehicle). Using a pump infusion rate of 0.25 µl/hr, IGF1 was released at a dose of 600 ng/day, totaling 16.8 µg over the 28-day study period. During the same surgical session, some animals received 0.1 mg oestradiol pellets (60-day sustained release; Innovative Research of America, Sarasota, FL) inserted subcutaneously beneath the dorsal surface of the neck. Because these pellets have previously been shown by our laboratory to be neuroprotective in global ischemia (16, 27, 32), they were specifically used for purpose of comparison.
Global ischemia
Two weeks after cannula and pellet placement, animals underwent four vessel global ischemia or sham ischemia surgery as previously described (33). Briefly, under isoflurane anesthesia, the vertebral arteries were coagulated bilaterally through a midline occipital-suboccipital incision between the first and second cervical vertebral bodies using bipolar electrocautery (Stage 1). Twenty-four hr later following an overnight fast, transient global ischemia was induced by bilateral occlusion of the carotid arteries with aneurysm clamps for 10 min followed by reperfusion (Stage 2). Sham-operated rats underwent all procedures except carotid artery occlusion. Rectal temperature was maintained at 37±0.5°C during ischemia by a heating lamp. Pupil dilation was monitored throughout the 10-min clamping period. A total of 33 animals died during induction of global ischemia. Additionally, 1 animal was excluded from analysis because she woke up during the carotid artery clamp.
Histological analysis
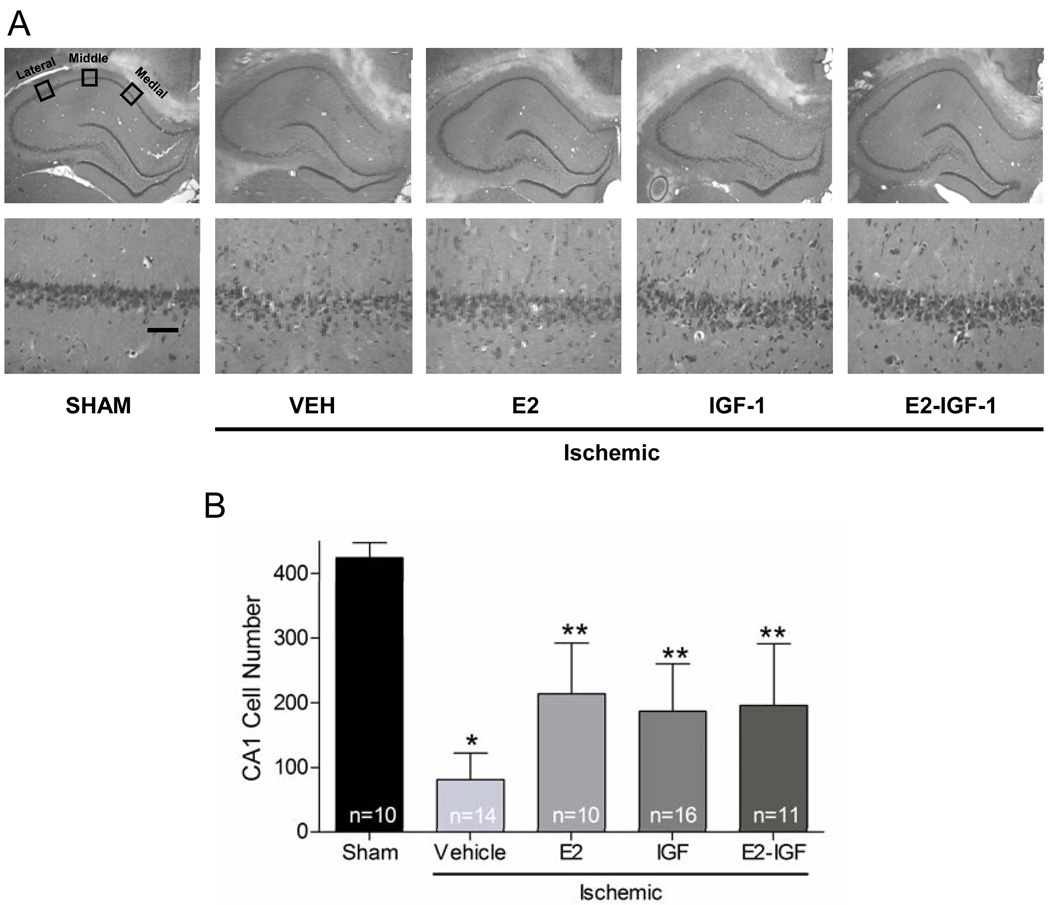
Two weeks after ischemia, animals were euthanized by deep isoflurane anesthesia. Blood was collected by cardiac puncture for measurement of oestradiol levels. Each rat was perfused transcardially with 0.9% heparinized saline solution followed by 10% buffered formalin phosphate (Fisher Scientific, Pittsburgh, PA). Brains were subsequently removed, placed in formalin at 4°C, washed, fixed in 30% sucrose in PBS at 4°C for 48–72 hr, and then frozen at -80°C. Coronal sections (20 µm) were cut at the level of the dorsal hippocampus (3.3–4.0 mm posterior from bregma) and 4 sections (at 140 µm intervals) per animal were mounted and stained with haematoxylin and eosin. Medial, middle, and lateral sectors from the CA1 region of the left and right hippocampus were photographed at 40× magnification using a Nikon microscope and digital camera. As previously described (also see Figure 1), these 3 sectors correspond to the area medial from CA2 neurones (lateral sector), at the apex of the CA1 (middle sector) and on the upswing of CA1 in an area clearly distinct from the subiculum (medial sector) (34). Digital images were opened in Adobe Photoshop, and the number of viable pyramidal neurones in each of these three 250 µm × 250 µm regions of interest was counted in both the left and right hippocampus. Viable neurones had rounded cell bodies and clearly visible nucleoli. Pyknotic and shrunken neurones were not counted. Counts were summated over right and left hemispheres and represent the total cell count of all 4 sections for each of the 3 sectors. Experimenters were blinded to treatment conditions at the time of neurone counting.
Figure 1.
Oestradiol and IGF1 provided significant protection against CA1 cell loss. (A) Representative photomicrographs from animals receiving sham surgery or ischemia treated with vehicle, oestradiol, IGF1, or oestradiol + IGF1 are shown at low (top, 4×) and high (bottom, 40×) magnification. Viable neurones were counted in 3 sectors (lateral, middle, and medial) in 4 haematoxylin and eosin stained sections of the dorsal hippocampus. Scale bar, higher magnification (40×) is 50 µm. (B) Data represent the grand sum (mean ± S.D.) of 3 counting sectors over both the right and left hemispheres. * Global ischemia induced significant CA1 cell loss in vehicle-treated compared to sham-operated animals (p<0.001). ** Treatment of ischemic animals with oestradiol, IGF1, or oestradiol + IGF1 provided significant neuroprotection compared to vehicle-treated ischemic animals (p<0.001).
Confirmation of drug delivery and cannula placement
At perfusion, each pump was examined to ensure that no saline or IGF1 remained in the pump reservoir. This was verified in all animals. Additionally, all brains were inspected during sectioning on the cryostat to ensure appropriate location of the cannula track in the right lateral ventricle.
Serum oestradiol assay
Tubes containing whole blood were placed on ice (10 min) and then stored at 4°C for 24 hr. Tubes were centrifuged at 300 × g for 5 min. Serum was collected and stored (−80°C) until analyzed. Serum hormone levels were measured by radioimmunoassay using the Coat-A-Count oestradiol assay (Siemans; Los Angeles, CA). All assays were performed in duplicate, and the mean value reported. The sensitivity of detection is 8 pg/ml. The inter- and intra-assay coefficients of variance are 8.1% and 7.0%, respectively, at the average oestradiol levels in our study.
Data analysis
In total, 96 animals were used in the experiment; 33 animals died as a result of ischemia and 1 animal woke up. Animals that died were distributed across treatment groups (7 vehicle, 7 oestradiol, 6 IGF1, 12 oestradiol +IGF1, 1 sham). In addition, histological quantification of cells could not be performed in 1 animal due to poor freezing quality in that brain. Oestradiol levels were evaluated by Kruskall Wallis and post-hoc with Dunn’s Multiple Comparison tests as they were not normally distributed. Differences in cell counts among treatment groups were evaluated by ANOVA followed by Tukey’s HSD post-hoc testing as appropriate. Cell counts for individual sham groups (vehicle n=5 and IGF1 n=5) were first evaluated by Mann Whitney U-test; because they did not differ, they were combined into a single sham group. Sham cell counts were then compared to cell counts in ischemic animals by ANOVA as described above. We did not include sham-operated females treated with oestradiol alone, because our previous work in middle-aged females treated in an identical fashion showed that oestradiol does not alter cell counts in sham animals (cite DeButte-Smith et al., 2009). Similarly, because neither oestradiol nor IGF1 alone altered cell counts in sham-operated animals, we did not do sham operations on animals with the combined treatment. Body weights and weight changes were analyzed by 2-way, mixed repeated-measures ANOVA with subsequent post-hoc evaluation by Bonferroni's multiple comparison tests. A two tailed p <0.05 was considered statistically significant. All analyses were performed used STATA SE v 8 (StataCorp LP, College Station, TX).
Results
Oestradiol levels
Plasma oestradiol levels confirmed the efficacy of OVX as well as delivery of physiological levels of oestradiol. All animals had serum collected for hormone assay. However, some samples did not have enough serum for the assays to be preformed. Animals that received oestradiol pellets (with or without IGF1 infusion) had significantly higher oestradiol levels at perfusion (z=4.338, p<0.001). There were no differences in oestradiol levels between the oestradiol and the oestradiol +IGF1 groups (Table 1). Oestradiol levels obtained in animals with and without pellets were consistent with levels observed in middle-aged female rats that underwent OVX with subsequent oestradiol replacement in our previous experiments (16).
Table 1.
Serum oestradiol values as a function of treatment group: Animals receiving oestradiol pellets had significantly higher serum oestradiol levels than animals who did not receive pellets. Both oestradiol and oestradiol +IGF1 treated animals were also statistically different from the IGF1 alone (p<0.01). Oestradiol values in the oestradiol and oestradiol -IGF1 groups also did not differ (p>0.05) from each other.
| Treatment | Oestradiol pg/ml) | P value |
|---|---|---|
| Vehicle (n=18) | 16.5 ± 12.5 | N/A |
| Oestradiol (n=5) | 38.5 ± 8.6 | < 0.01 |
| IGF1 (n=19) | 14.0 ± 8.9 | > 0.05 |
| Oestradiol + IGF1 (n=9) | 28.8 ± 7.4 | < 0.05 |
Values are mean serum oestradiol levels ± SD. P values are relative to vehicle controls. Serum oestradiol values were not available for every rat as a result of inadequate serum volume for analysis. Values reported in the table for each treatment group include data from both ischemic and sham-operated animals.
Weights
Body weight differed over time (F(4,48)= 99.3, p<0.001). Animals experienced a significant increase in weight in the 8 weeks between OVX and cannula insertion (p<0.001), consistent with the loss of endogenous oestradiol after OVX. Animals lost weight after cannula insertion and after Stage 1 of ischemia (p<0.001), likely due to surgical stress; this weight loss stabilized and did not change between Stage 2 of ischemia and perfusion (p>0.05) (Table 2). Weight changes (%) over time did not differ as a function of IGF1 or hormone treatment (F(12,48)=1.732, p>0.05).
Table 2.
Impact of OVX and surgical treatment on animal body weight: Animals gained significant body weight between OVX and cannula insertion. Although animals lost weight after ischemia, this weight stabilized by the time of perfusion.
| Treatment Group | Weight 1 (g) | Weight 2 (g) | Weight 3 (g) | Weight 4 (g) | Weight 5 (g) |
|---|---|---|---|---|---|
| Vehicle (n=13) | 374.1 ± 69.2 | 469.8 ± 83.4* | 435.1 ± 80.7** | 413.8 ± 84.4*** | 420.0 ± 67.4 |
| Oestradiol (n=10) | 396.7 ± 56.5 | 477.6 ± 69.6* | 431.1 ± 67.8** | 412.1 ± 69.1*** | 399.8 ± 66.0 |
| IGF1 (n=12) | 388.1 ± 45.5 | 477.3 ± 48.2* | 439.7 ± 46.5** | 416.1 ± 43.7*** | 417.1 ± 42.7 |
| Oestradiol + IGF1 (n=8) | 381.8 ± 38.7 | 463.6 ± 41.0* | 431.9 ± 38.4** | 401.5 ± 35.1*** | 394.1 ± 15.3 |
| Sham (n=10) | 381.8 ± 67.8 | 447.9 ± 70.6* | 428.2 ± 62.7** | 400.6 ± 64.7*** | 403.5 ± 59.5 |
Values represent mean body weight (g) ± S.D at the time of OVX (Weight 1), cannula/pellet insertion (Weight 2), Stage 1 (Weight 3) and Stage 2 (Weight 4) of ischemia, and at the time of perfusion (Weight 5).
p<0.001 vs Weight 1;
p<0.001 vs Weight 2;
p<0.001 vs Weight 3.
Some animals were not weighed at the time of perfusion and therefore could not be included in the repeated measures analysis.
Cell survival
As expected, the number of surviving CA1 neurones differed among treatment groups (F(4,60)=38.53, p<0.001). Global ischemia produced massive cell death of CA1 pyramidal neurones in vehicle-infused animals (80% cell death) compared to sham-operated animals (Tukey’s, p<0.001) (Figure 1A). There were no differences in cell counts in sham groups receiving vehicle or IGF1 (n=5 per group; z=0.419, p=0.675); therefore, these groups were combined for further analyses into one “Sham” group. In ischemic animals treated chronically with either oestradiol or IGF1, the number of surviving CA1 pyramidal neurones was higher than in ischemic control animals, but lower than in sham-operated animals (Tukey’s, p<0.001 for all comparisons) (Figure 1B). Oestradiol or IGF1 administered alone provided comparable protection (50% and 56% cell death compared to shams, respectively). Although the combination of oestradiol and IGF1 provided neuroprotection (54% cell death compared to sham animals), the effects were not additive relative to treatment with either agent alone (Tukey’s, p>0.05).
Discussion
The present study demonstrates the novel finding that chronic infusion of IGF1 into the brain is neuroprotective against global ischemia in middle-aged female rats subjected to long-term ovarian hormone deprivation. In fact, intracranial IGF1 application was equivalent to systemically administered oestradiol in its protective efficacy. Although we did not measure circulating levels of IGF1, plasma IGF1 levels are lower in older rats than in young rats, a phenomenon known to accompany chronologic and reproductive aging in rodents and humans (35). We have observed that oestradiol modestly but significantly reduces serum IGF1 levels in middle-aged females (unpublished observations). Moreover, hypothalamic IGF1 levels decrease in middle-aged female rats, demonstrating local alterations in the brain IGF1 system (36). Therefore, our study indicates that middle-aged female rats are able to respond to chronically administered IGF1 after what we assume to be age-related reductions in tissue and serum levels of IGF1.
Our findings that oestradiol is neuroprotective in middle-aged female rats subjected to global ischemia, even after an 8-week OVX interval, confirms our previous experiments (16). These findings help to answer lingering clinical dilemmas related to estrogen replacement in peri- and post-menopausal women. Discrepancies in human data regarding the cardiovascular and neurovascular consequences of estrogen therapy may have their roots in the timing of therapy (6, 7). The presence of a hormone-free interval, the absolute age of patients, and the influence of other metabolic risk factors are all potential confounders in interpreting the results of clinical trials including, most recently the Women’s Health Initiative (7). Our findings clearly show that a long hormone-free interval does not reduce the ability of either oestradiol or IGF1 to provide histological neuroprotection, that is, a reduction in ischemia-induced neuronal death. The data therefore suggest that in appropriately chosen postmenopausal women, such as those with no medical contraindications for the use of oestradiol, hormone treatment may still provide substantial neurological benefit after global ischemia (e.g., due to cardiac arrest).
As noted above, we consistently find that oestradiol is neuroprotective in middle-aged female rats, even when hormone administration is delayed for 8 weeks after OVX (present study and (16)). In contrast, oestradiol does not appear to afford protection in middle-aged female gerbils (37). Species differences between gerbils and rats in overall and brain region-specific sensitivity to oestradiol may contribute to this discrepancy. Additionally, the middle-aged gerbils used in our previous study were nulliparous, and we have no data on the endocrine history of those animals. Thus, differences in parity, the nature of prior estrogen exposure and/or duration of estrogen deprivation may be important factors. Our results also differ from those in young mice treated for 1 week with oestradiol and then subjected to focal ischemia (MCAO). Oestradiol was neuroprotective in this model if administered immediately after OVX but not when delayed by 10 weeks after OVX (38). Additionally, in 12-month old female rats subjected only to OVX, oestradiol initiated immediately or 3 months after but not 10 months after a hormone free interval prevented spatial memory task deficits associated with aging (39). Differences in the mechanisms underlying neuronal death after focal and global ischemia and with aging as well as differences in hormone action in the cortex (major site of cell loss after MCAO) and hippocampus (major site of cell loss after transient global ischemia) may explain why oestradiol continues to be protective after long-term OVX in animals subjected to global ischemia. For example, acute inflammation contributes significantly to the extent of brain injury in focal ischemia. Although oestradiol administered 1 week after OVX reduces expression of local and peripheral inflammatory cytokines, oestradiol administered 10 weeks after OVX has little or no effect on these markers (38). This opens the possibility that inflammation plays a significant role in focal ischemia.
Although IGF1 and oestradiol given alone produced comparable levels of histological neuroprotection, their effects were not additive. We hypothesized that dual treatment might synergize, because there is substantial cross-talk between the two systems (28). The pathways by which oestradiol and IGF1 provide neuroprotection may converge. Both activate the MAPK pathway as well as PI3K-dependent phosphorylation of Akt (40). Therefore, if co-administration of oestradiol and IGF activate the same molecular pathways, administration of the two agents together would not be expected to achieve greater protection. Alternatively, there may be a limit to the degree of histological protection that any pretreatment can achieve in middle-aged animals. All three pretreatments produced approximately 50% cell survival, which may represent such a limit. Finally, higher doses of IGF1 may be needed to produce greater protection alone or to synergize with oestradiol.
Natural declines in both endogenous oestradiol (41) and IGF1 (42).predict increased cardiovascular mortality and morbidity. However, the use of estrogen therapy for neuroprotection in older postmenopausal women is controversial because it can precipitate neurovascular and cardiovascular events (6, 7). Thus, IGF1 therapy is a potential alternative. Higher levels of endogenous IGF1 after ischemic stroke in humans are associated with improved functional outcome (43). In fact, among hospitalized patients, IGF1 levels are decreased in stroke victims and inversely correlate with mortality at 3 and 6 months (44). This suggests that exogenous supplementation of IGF1 is a biologically plausible avenue for clinical trials.
Human data so far show IGF1 to be safe and well tolerated. Acute, systemic IGF1 administration has been attempted for treatment of septic ICU patients with no apparent harmful side effects (45). Systemic IGF1 therapy has been proposed for patients with chronic heart failure due to its potential cardiovascular benefits (46). There is some precedent for large scale human trials with IGF1 therapy for cognitive benefit. Although inconsistent results were reported, chronic, systemically delivered IGF1 has been tested in human trials for the treatment of amyotropic lateral sclerosis, with minimal toxicity (47). The reported lack of benefit may be related to the systemic route of administration; intranasal delivery of IGF1 may better deliver active drug to the central nervous system (48). Experimentally, intranasal IGF1 decreased infarct volume in adult male rats after MCAO (49). Recently, intranasal IGF1 decreased infarct volumes and increased open field activity in mice subjected to MCAO (50). In this study, intranasal IGF1 delivery to the brain occurred more quickly, and IGF1 reached higher peak levels than with other delivery routes (i.v, i.p, s.c.). Thus, intranasal IGF1 delivery may be more practical than systemic administration for protection against brain injury in humans. Collectively, these data suggest that chronic, centrally administered IGF1 may be a viable, non-toxic therapy to reduce neuronal damage after global ischemic events in humans.
Acknowledgments
Authors gratefully acknowledge research support by DHHS grant R01 AG027702 awarded to AME and by the Department of Neuroscience at AECOM. The authors would also like to thank Drs. Nanette Santoro and Gohar Zeitlian for assistance with oestradiol assays.
Footnotes
Disclosure Statement. There are no actual or potential conflicts of interest for any author.
Contributor Information
Michael L. Traub, Email: traubml@yahoo.com.
Maxine De Butte-Smith, Email: mdebutte-smith@mail.wtamu.edu.
R. Suzanne Zukin, Email: zukin@aecom.yu.edu.
Anne M. Etgen, Email: etgen@aecom.yu.edu.
References
- 1.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6(12):1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 2.Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- 3.Olsen TS, Dehlendorff C, Andersen KK. Sex-related time-dependent variations in post-stroke survival--evidence of a female stroke survival advantage. Neuroepidemiology. 2007;29(3–4):218–225. doi: 10.1159/000112464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Carlo A, Lamassa M, Baldereschi M, Pracucci G, Basile AM, Wolfe CD, Giroud M, Rudd A, Ghetti A, Inzitari D. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke. 2003;34(5):1114–1119. doi: 10.1161/01.STR.0000068410.07397.D7. [DOI] [PubMed] [Google Scholar]
- 5.Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, Cheung AM. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. 2005;36(4):809–814. doi: 10.1161/01.STR.0000157662.09551.e5. [DOI] [PubMed] [Google Scholar]
- 6.Grodstein F, Stampfer MJ, Manson JE, Colditz GA, Willett WC, Rosner B, Speizer FE, Hennekens CH. Postmenopausal estrogen and progestin use and the risk of cardiovascular disease. N Engl J Med. 1996;335(7):453–461. doi: 10.1056/NEJM199608153350701. [DOI] [PubMed] [Google Scholar]
- 7.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Yamashima T, Tonchev AB, Borlongan CV. Differential response to ischemia in adjacent hippocampalsectors: neuronal death in CA1 versus neurogenesis in dentate gyrus. Biotechnol J. 2007;2(5):596–607. doi: 10.1002/biot.200600219. [DOI] [PubMed] [Google Scholar]
- 9.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4(5):399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 10.Zukin R, Jover T, Yokota H, Calderone A, Simionescu M, Lau C-Y. Molecular and Cellular Mechanisms of Ischemia-Induced Neuronal Death. In: Mohr J, Choi D, Grotta J, Weir B, Wolf P, editors. Stroke: Pathophysiology, Diagnosis, and Management. Philadelphia: Churchill Livingstone; 2004. pp. 829–854. [Google Scholar]
- 11.Gibson CL, Gray LJ, Murphy SP, Bath PM. Estrogens and experimental ischemic stroke: a systematic review. J Cereb Blood Flow Metab. 2006;26(9):1103–1113. doi: 10.1038/sj.jcbfm.9600270. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GE, Merchenthaler I, Zup SL. Neuroprotection by ovarian hormones in animal models of neurological disease. Endocrine. 2006;29(2):217–231. doi: 10.1385/ENDO:29:2:217. [DOI] [PubMed] [Google Scholar]
- 13.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142(1):43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 14.Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- 15.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24(10):1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 16.De Butte-Smith M, Gulinello M, Zukin RS, Etgen AM. Chronic estradiol treatment increases CA1 cell survival but does not improve visual or spatial recognition memory after global ischemia in middle-aged female rats. Horm Behav. 2009;55(3):442–453. doi: 10.1016/j.yhbeh.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson MF, Aberg MA, Nilsson M, Eriksson PS. Insulin-like growth factor-I and neurogenesis in the adult mammalian brain. Brain Res Dev Brain Res. 2002;134(1–2):115–122. doi: 10.1016/s0165-3806(02)00277-8. [DOI] [PubMed] [Google Scholar]
- 18.Yakar S, Wu Y, Setser J, Rosen CJ. The role of circulating IGF-I: lessons from human and animal models. Endocrine. 2002;19(3):239–248. doi: 10.1385/ENDO:19:3:239. [DOI] [PubMed] [Google Scholar]
- 19.Gluckman P, Klempt N, Guan J, Mallard C, Sirimanne E, Dragunow M, Klempt M, Singh K, Williams C, Nikolics K. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182(2):593–599. doi: 10.1016/0006-291x(92)91774-k. [DOI] [PubMed] [Google Scholar]
- 20.Guan J, Williams C, Gunning M, Mallard C, Gluckman P. The effects of IGF-1 treatment after hypoxic-ischemic brain injury in adult rats. J Cereb Blood Flow Metab. 1993;13(4):609–616. doi: 10.1038/jcbfm.1993.79. [DOI] [PubMed] [Google Scholar]
- 21.Guan J, Bennet L, George S, Wu D, Waldvogel HJ, Gluckman PD, Faull RL, Crosier PS, Gunn AJ. Insulin-like growth factor-1 reduces postischemic white matter injury in fetal sheep. J Cereb Blood Flow Metab. 2001;21(5):493–502. doi: 10.1097/00004647-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Zhu CZ, Auer RN. Intraventricular administration of insulin and IGF-1 in transient forebrain ischemia. J Cereb Blood Flow Metab. 1994;14(2):237–242. doi: 10.1038/jcbfm.1994.30. [DOI] [PubMed] [Google Scholar]
- 23.Kooijman R, Sarre S, Michotte Y, De Keyser J. Insulin-Like Growth Factor I. A Potential Neuroprotective Compound for the Treatment of Acute Ischemic Stroke? Stroke. 2009 doi: 10.1161/STROKEAHA.108.528356. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenwalner RJ, Forbes ME, Bennett SA, Lynch CD, Sonntag WE, Riddle DR. Intracerebroventricular infusion of insulin-like growth factor-I ameliorates the age-related decline in hippocampal neurogenesis. Neuroscience. 2001;107(4):603–613. doi: 10.1016/s0306-4522(01)00378-5. [DOI] [PubMed] [Google Scholar]
- 25.Markowska AL, Mooney M, Sonntag WE. Insulin-like growth factor-1 ameliorates age-related behavioral deficits. Neuroscience. 1998;87(3):559–569. doi: 10.1016/s0306-4522(98)00143-2. [DOI] [PubMed] [Google Scholar]
- 26.Azcoitia I, Sierra A, Garcia-Segura LM. Neuroprotective effects of estradiol in the adult rat hippocampus: interaction with insulin-like growth factor-I signalling. J Neurosci Res. 1999;58(6):815–822. doi: 10.1002/(sici)1097-4547(19991215)58:6<815::aid-jnr8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148(3):1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendez P, Wandosell F, Garcia-Segura LM. Cross-talk between estrogen receptors and insulin-like growth factor-I receptor in the brain: cellular and molecular mechanisms. Front Neuroendocrinol. 2006;27(4):391–403. doi: 10.1016/j.yfrne.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Pons S, Torres-Aleman I. Estradiol modulates insulin-like growth factor I receptors and binding proteins in neurons from the hypothalamus. J Neuroendocrinol. 1993;5(3):267–271. doi: 10.1111/j.1365-2826.1993.tb00482.x. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Segura LM, Diz-Chaves Y, Perez-Martin M, Darnaudery M. Estradiol, insulin-like growth factor-I and brain aging. Psychoneuroendocrinology. 2007;32 Suppl 1:S57–S61. doi: 10.1016/j.psyneuen.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Wilshire GB, Loughlin JS, Brown JR, Adel TE, Santoro N. Diminished function of the somatotropic axis in older reproductive-aged women. J Clin Endocrinol Metab. 1995;80(2):608–613. doi: 10.1210/jcem.80.2.7852528. [DOI] [PubMed] [Google Scholar]
- 32.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 33.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke; a journal of cerebral circulation. 1979;10(3):267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 34.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15(11):7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744–752. doi: 10.1210/jcem.78.3.8126152. [DOI] [PubMed] [Google Scholar]
- 36.Miller BH, Gore AC. Alterations in hypothalamic insulin-like growth factor-I and its associations with gonadotropin releasing hormone neurones during reproductive development and ageing. J Neuroendocrinol. 2001;13(8):728–736. doi: 10.1046/j.1365-2826.2001.00686.x. [DOI] [PubMed] [Google Scholar]
- 37.De Butte-Smith M, Nguyen AP, Zukin RS, Etgen AM, Colbourne F. Failure of estradiol to ameliorate global ischemia-induced CA1 sector injury in middle-aged female gerbils. Brain Res. 2007;1153:214–220. doi: 10.1016/j.brainres.2007.03.082. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21(1):107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 40.Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37(1℃3):320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- 41.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- 42.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 43.Bondanelli M, Ambrosio MR, Onofri A, Bergonzoni A, Lavezzi S, Zatelli MC, Valle D, Basaglia N, degli Uberti EC. Predictive value of circulating insulin-like growth factor I levels in ischemic stroke outcome. J Clin Endocrinol Metab. 2006;91(10):3928–3934. doi: 10.1210/jc.2006-1040. [DOI] [PubMed] [Google Scholar]
- 44.Denti L, Annoni V, Cattadori E, Salvagnini MA, Visioli S, Merli MF, Corradi F, Ceresini G, Valenti G, Hoffman AR, Ceda GP. Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am J Med. 2004;117(5):312–317. doi: 10.1016/j.amjmed.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 45.Yarwood GD, Ross RJ, Medbak S, Coakley J, Hinds CJ. Administration of human recombinant insulin-like growth factor-I in critically ill patients. Crit Care Med. 1997;25(8):1352–1361. doi: 10.1097/00003246-199708000-00023. [DOI] [PubMed] [Google Scholar]
- 46.Donath MY, Sutsch G, Yan XW, Piva B, Brunner HP, Glatz Y, Zapf J, Follath F, Froesch ER, Kiowski W. Acute cardiovascular effects of insulin-like growth factor I in patients with chronic heart failure. J Clin Endocrinol Metab. 1998;83(9):3177–3183. doi: 10.1210/jcem.83.9.5122. [DOI] [PubMed] [Google Scholar]
- 47.Sorenson EJ, Windbank AJ, Mandrekar JN, Bamlet WR, Appel SH, Armon C, Barkhaus PE, Bosch P, Boylan K, David WS, Feldman E, Glass J, Gutmann L, Katz J, King W, Luciano CA, McCluskey LF, Nash S, Newman DS, Pascuzzi RM, Pioro E, Sams LJ, Scelsa S, Simpson EP, Subramony SH, Tiryaki E, Thornton CA. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71(22):1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanson LR, Frey WH., 2nd Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008 9 Suppl 3:S5. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood-brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187(1–2):91–97. doi: 10.1016/s0022-510x(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 50.Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. J Neurosurg. 2009 doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]