Abstract
Viral gene delivery for spinal cord injury (SCI) is a promising approach for enhancing axonal regeneration and neuroprotection. An understanding of spatio-temporal transgene expression in the spinal cord is essential for future studies of SCI therapies. Commonly, intracellular marker proteins (e.g., EGFP) were used as indicators of transgene levels after viral delivery, which may not accurately reflect levels of secreted transgene. This study examined transgene expression using ELISA after viral delivery of D15A, a neurotrophin with BDNF and NT-3 activities, at 1, 2, and 4 weeks after in vivo and ex vivo delivery using lentiviral, adenoviral, and retroviral vectors. Further, the inflammatory responses and viral infection patterns after in vivo delivery were examined. Lentiviral vectors had the most stable pattern of gene expression, with D15A levels of 536 ± 38 and 363 ± 47 pg/mg protein seen at 4 weeks after the in vivo and ex vivo delivery, respectively. Our results show that protein levels downregulate disproportionately to levels of EGFP after adenoviral vectors both in vivo and ex vivo. D15A dropped from initial levels of 422 ± 87 to 153 ± 18 pg/mg protein at 4 weeks after in vivo administration. Similarly, ex vivo retrovirus-mediated transgene expression exhibited rapid downregulation by 2 weeks post-grafting. Compared to adenoviral infection, macrophage activation was attenuated after lentiviral infection. These results suggest that lentiviral vectors are most suitable in situations where stable long-term transgene expression is needed. Retroviral ex vivo delivery is optional when transient expression within targeted spinal tissue is desired, with adenoviral vectors in between.
Keywords: spinal cord injury, gene therapy, lentivirus, adenovirus, retrovirus
Axonal regeneration in the adult central nervous system (CNS) depends on a critical balance between growth-promoting and -inhibiting cues (Chen et al., 2000). Neurotrophic factors have a potent survival, neuroprotective and growth-promoting activity on neurons both in vitro and in vivo (Davies, 2000; Tobias et al., 2003), making them an obvious therapeutic candidate for neurodegenerative and traumatic disorders (Nagatsu, 2002; Racine et al., 2002). The major obstacle for the development of such therapies is the exclusion of the therapeutic molecule from the CNS due to the selectivity of the blood–brain barrier (Pardridge, 2002), as well as fast plasma clearance (Dittrich et al., 1994). Further, undesirable side effects of systemic delivery of neurotrophins have been shown (Tuszynski and Blesch, 2004). To avoid these side effects, direct delivery of neurotrophic factors into the CNS using osmotic-pumps was employed (Olson et al., 1992). However, pump failure, variable stability, limited diffusion, and the risk of infection are possible complications limiting the application of this approach (Oudega and Hagg, 1996; Tan and Aebischer, 1996).
Recent advances in molecular biology offer several possibilities for therapeutic gene delivery including purified plasmids (Piccirillo and Prud’homme, 2003), cationic lipid-mediated delivery (Felgner et al., 1995), particle bombardment-mediated gene transfer (gene gun) (Jiao et al., 1993), receptor-mediated endocytosis (Chen et al., 1994), and antisense oligonucleotides (Tomita and Morishita, 2004). These non-viral methods have several advantages including safety, ease of synthesis/modification, and unlimited transgene size. They are complicated, however, by cytotoxicity, low efficiency, and limited target cell population (Tomita and Morishita, 2004).
The use of viral vectors as an approach for gene therapy has been studied in many CNS disorders including Parkinson’s disease (Lundberg et al., 1996), Huntington’s disease (Emerich et al., 1996), Alzheimer’s disease (Rosenberg et al., 1988), and Tay-Sachs disease (Lacorazza et al., 1996). In spinal cord injury (SCI), both ex vivo and in vivo approaches employing genetically modified fibroblasts (Nakahara et al., 1996; Tuszynski et al., 2003), astrocytes (Smith et al., 1996), and olfactory ensheathing cells (Ruitenberg et al., 2003) have been used to deliver neurotrophic factors to enhance neuronal survival, axonal growth, and remyelination (McTigue et al., 1998; Tuszynski et al., 1998). Nonetheless, significant therapeutic efficacy is negated due to downregulation of transgene activity soon after delivery (Andersen et al., 1992), vector cytotoxicity (Hermens and Verhaagen, 1998), or host immune response directed against the transgene and viral proteins (Blomer et al., 1996). Further, widespread acceptance of viral vector-mediated gene therapy as a therapeutic tool in the treatment of CNS disease is complicated by the unknown long-term safety of their use.
In this study, we examined the temporal expression pattern of D15A, a mutant human NT-3 that binds trkB and trkC and has both BDNF and NT3 activities, after its viral-mediated delivery into the spinal cord (Urfer et al., 1994; Strohmaier et al., 1996; Cao et al., 2005), as well as the viral induced inflammatory responses after lentiviral, adenoviral, and retroviral gene delivery in the spinal cord.
MATERIALS AND METHODS
Lentiviral Vector Production
The preparation and use of all viral vectors was carried out in bio-safety Level 2 laboratories with the approval of the Institutional Biosafety Committee of the University of Louisville. We used a third generation lentiviral vector (Invitrogen, Carlsbad, CA; for vector maps see www.invitrogen.com/content/sfs/vectors/plenti6v5dtopo_map.pdf), that was produced according to the manufacturer’s instructions. Briefly, we utilized the cDNA coding for D15A, a human NT-3 mutant that binds and activates both trkB and trkC (Urfer et al., 1994). A 780-bp fragment, containing a Kozak consensus sequence was modified using PCR to include the sequence CACC at the 5′ end. These four nucleotides are complimentary to the overhang sequence GTGG in the pLenti6/V5-D-Topo vector, a requirement for the Topo cloning reaction, the cloning site is located downstream of a CMV promoter sequence. Viral particles were produced by transient transfection of 293FT cells (Naldini et al., 1996) with the Virapower packaging mix (Invitrogen) containing the following plasmids: pLP1 (gag/pol), pLP2 (Rev), pLP/VSVG, and pLenti6/V5-D-Topo/D15A, or pLenti6/V5-GW/LacZ. Forty-eight hours after transfection, supernatants were collected and filtered. High-titer viral stocks were obtained by ultracentrifugation at 16,000 rpm for 90 min and the pellet was re-suspended in phosphate buffered saline (PBS) and virus stored at −80°C until use.
Adenoviral Vector Production
The cDNA encoding D15A was cloned into the E1-deleted Ad5C1-I-GFP (D15A expression is under CMV promoter control; vector maps are available at www.stratagene.com/vectors/maps/pdf/pshuttle-IRES-hrGFP-1.pdf) and replication-deficient Ad5-D15A adenovirus was isolated as described previously (Gao et al., 1998). The titer obtained was 1.1 × 1011 transforming units (TU)/ml. Before surgery, virus was diluted using PBS to either 1 × 105, 5 × 105, or 1 × 106 TU/μl.
Retroviral Vector Production
The D15A cDNA was cloned into MSCV-I-GFP (provided by M.F. Roussel, Dept. of Genetics and Tumor Cell Biology, St. Jude Children’s Research Hospital, Memphis, TN) and LZRSpBMN-I-GFP (kindly provided by G. Nolan, Stanford University; for a map of the LZRSpBMN-I-GFP vector see http://www.stanford.edu/group/nolan/) shuttle vectors. D15A expression in both MSCV-I-GFP and LZRSpBMN-I-GFP retroviruses is under the control of the 5′ LTR. Viral particles were produced by transient transfection of MSCV-I-GFP and VSV-G plasmids into HEK/293 cells. Stable LZRSpBMN-I-GFP producer lines were obtained by transfection into Φnx cells (Pear, 1997; Pear et al., 1993) and also provided by Dr. Nolan. Media were collected, filtered, and viral titer quantified. Virus was concentrated by ultracentrifugation as described previously (Burns et al., 1993).
Tetracycline-Inducible Retroviral System
Tet-inducible retrovirus was produced according to the manufacturer’s instructions (Clontech, Palo Alto, CA; for vector maps see http://orders.clontech.com/clontech/techinfo/vectors/vectorsR-S/prevTRE.shtml). Briefly, after cloning of the D15A cDNA into pRev-TRE vector in which D15A expression is under the control of a minimal CMV promoter, the two-virus system was produced by stably transfecting PT67 cells (NIH/3T3-based cell line expressing 10A1 viral envelope) with pRev-TRE-D15A to produce the first virus. PT67 cells were selected with 0.2 mg/ml hygromycin for 7–10 days. The regulator virus was produced by stably transfecting PT67 cells with the pRevTet-On plasmid followed by G418 selection (0.4 mg/ml) for 5–7 days. Target cells used in the ex vivo experiment were infected with the pRevTet-On virus and subjected to G418 selection (0.4 mg/ml) for 5–7 days, then infected with pRev-TRE-D15A virus and further subjected to hygromycin selection (0.2 mg/ml) for another 7–10 days in vitro. Before doxycycline induction in vitro, the media was changed to DMEM supplemented with tetracycline-free fetal bovine serum (FBS, Clontech).
Determination of Viral Titer and Vector Bioactivity
To determine the viral titer, a serial dilution of the viral stock was applied to 2 × 105 293T and NIH3T3 cells. The dilution that resulted in 15% or less GFP+/LacZ+ cells after 48 hr was used for calculations. Lentivirus-D15A titer was measured according to the manufacturer’s instructions; briefly, serial dilutions (10−2 to 10−8) of viral stocks were applied to 2 × 105 NIH3T3 cells, 4 days later blasticidin 3 μg/ml was added, and cells were maintained for 10 days. Cells were washed in PBS and stained with crystal violet. Blue-stained colonies were counted to determine the titer. Viral titers were routinely 1–3 × 108 TU/ml. Before surgery, virus stock was diluted with sterile saline to 1 × 105 TU/μl, 5 × 105 TU/μl, or 1 × 106 TU/μl for injection into the spinal cord. All viral vectors used for in vivo delivery were matched for volume and titer before delivery. All viral vectors used in the ex vivo and in vitro experiments were matched for titer before use. Bioactivity in conditioned media of infected NIH3T3 cells was tested by examining neurite outgrowth (Fig. 1D–F) from trkC-expressing PC12 cells (Tsoulfas et al., 1993; Cao et al., 2005), recombinant human (rh) NT-3 was used to generate the standard curve. Bioassay data were consistent with D15A levels determined by ELISA (R&D Systems, Minneapolis, MN).
Fig. 1.
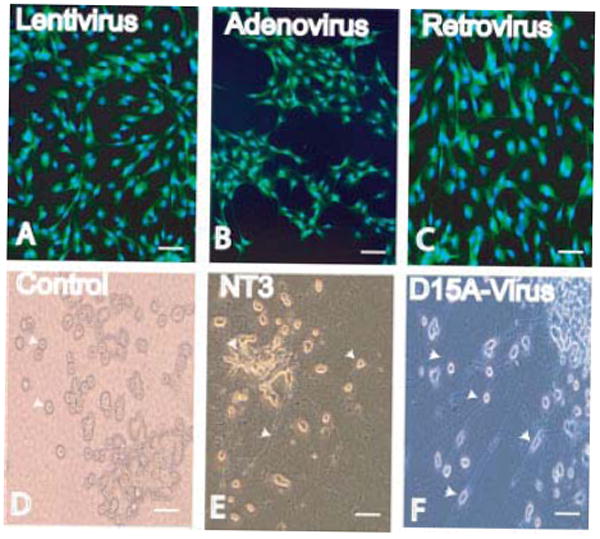
Schwann cells were infected in vitro with either lentivirus (A), adenovirus (B), or retrovirus (C) (MOI = 20). Cells were stained for EGFP and counterstained with Hoechst 33342. TrkC-expressing PC12 cells (arrow heads in D) were used to determine the bioactivity of D15A. Recombinant human (rh) NT-3 was added (100 pg/ml–1 ng/ml) to the media of PC12 cells for 3–4 days, neurite outgrowth from PC12 cells (arrow heads in E) was compared to that of conditioned media of infected NIH3T3 cells added at a concentration range of 10–100%, bioactive D15A in media was identified by examining neurite outgrowth (arrowheads in F) from PC12 cells. Conditioned media at a concentration of 75–100% were similar to an NT-3 concentration of 500 pg/ml–1 ng/ml. Control PC12 cells (D) maintained on DMEM did not show any neurite outgrowth. There was no difference between conditioned media collected from NIH3T3 cells infected with lentivirus, adenovirus, or retrovirus. Scale bar = 40 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
NT-3 ELISA was carried out according to manufacturer’s instructions. Briefly, 96-well microplates (Costar, Cam-bridge, MA) were coated with 100 μl/well of monoclonal mouse anti-NT-3 antibody (0.5 μg/ml; R&D Systems) diluted in PBS buffer overnight at room temperature. The plates were then incubated for 1 hr at room temperature with blocking solution (1% BSA in PBS). With interceding washes (0.05% Tween 20 in PBS, pH 7.4), the plates were subject to sequential 2 hr incubations at room temperature with double aliquots of conditioned media, cell, and protein extracts from spinal cords, or recombinant human NT-3 (rhNT-3; 0–2,000 pg/ml; R&D Systems), biotinylated polyclonal goat anti-NT-3 antibody (200 ng/ml; R&D Systems). The plates were then incubated sequentially for 20 min in Streptavidin-HRP (R&D Systems), and 1:1 mixture of H2O2 and tetramethylbenzidine (R&D Systems). The color reaction was stopped (50 μl/well of 2N H2SO4; R&D Systems) and absorbance at 450 nm was measured using a Molecular Devices Spectramax 384+ (Sunnyvale, CA). Using serial dilutions of known amounts of rhNT-3, this color reaction yielded a linear standard curve from 62.5–2,000 pg.
Preparation of Purified Populations of Schwann Cells
Schwann cells (SC) cultures were isolated as described (Morrissey et al., 1991; Xu et al., 1999). Briefly, sciatic nerves were dissected from adult rats anaesthetized with pentobarbital sodium 40 mg/kg, I.P. After the epineurium and connective tissue were removed, nerves were cut into 1 mm2 explants and placed in 35-mm Corning tissue culture dishes (Baxter, Stone Mountain, GA) containing Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, NY) supplemented with 10% FBS. The explants were transferred to new culture dishes every 7 days with fresh medium. After 3–5 weeks, explants were incubated overnight in dispase 1.25 U/ml (Boehringer Mannheim Biochemicals, Indianapolis, IN), 0.05% collagenase (Worthington Biochemicals Corp., Freehold, NJ) and 15% FBS in DMEM at 37°C in 5% CO2. The following day, explants were dissociated and cells were plated onto poly-l-lysine-coated 100 mm dishes in DMEM/10% FBS, containing 20 mg/ml pituitary extract (BTI, Stoughton, MA) and 2 mM forskolin (Sigma, St. Louis, MO).
Preparation and Transduction of Cells In Vitro
Other cell types used in the study (NIH/3T3, HEK 293FT, PT67, and Φnx) were maintained in DMEM supplemented with 10% FBS. RN33B cells (Whittemore and White, 1993) were grown at 33°C in DMEM/F12 supplemented with 5% FBS, and 250 μg/ml G418.
For transduction of cells for in vitro viral experiments, cells were seeded into 6-well plates at a density of 105 cells/well. Cells were pre-treated with 4–6 μg/ml polybrene (Sigma) for 30–60 min (Manning et al., 1971), then infected for 6–8 hr at a multiplicity of infection (MOI) of 20, resulting in 80–90% infection of the cells. Infection media were then replaced with fresh media. Forty-eight hours later, conditioned media was collected for ELISA.
Surgical Procedures
A total of 150 adult, female, Sprague-Dawley rats (150–175 g) were housed 1 per cage with free access to food and water under 12-hr light:dark cycle. All surgical procedures were approved by and carried out according to the guidelines of the Institutional Animal Care and Use Committee and the Institutional Biosafety Committee at the University of Louisville. Animals were anesthetized with pentobarbital (40 mg/kg IP) and placed into a stereotaxic frame (Kopf Instruments, Tujunga, CA). The spinal cord was exposed with a laminectomy at the level of T8, the dura incised to expose the cord, and a total volume of 1 μl of the viral stocks (1 × 105 or 5 × 105 TU), PBS, or cell suspension (1 × 105 cell/μl) was injected into the cord using a glass micro-pipette (outer diameter 10–20 μm for viral injection and 25–40 μm for the cell suspension) attached to a pico spritzer (Parker Instrumentation, Fairfield, NJ). Injections were made in the ventrolateral white matter of the cord (1 μl/injection) and the glass pipette remained in place for 2–3 min after injection. The incision was closed in layers. For host immune modulation, we used transient immuno-blockade of lymphocytic cells by a combination treatment of purified monoclonal antibodies against the rat CD4 and CD45 (OX-38 and OX-22; Pharmingen, San Diego, CA) lymphocytic receptors. Animals in the adenovirus + mAb group received 50 μg (IP) of the combined anti-sera 24 hr before and 24 hr after the adenovirus administration (Romero and Smith, 1998).
Tissue Processing
At 1, 2, and 4 weeks after viral injection, the rats were anesthetized deeply with pentobarbital and transcardially perfused with 200 ml 0.1 M phosphate buffer (PB) (pH = 7.4). For ELISA (n = 5/group), a 1-cm segment of the spinal cord spanning the injection site was removed, homogenized in lysis buffer (20 mM Tris-HCl pH 8.0, 137 mM NaCl, 1% NP40), and centrifuged at 10,000 rpm for 5–7 min at 4°C to remove tissue debris. Protein concentration in each sample was estimated using the Pierce BCA Protein Assay (Pierce Chemical Co., Rockford, IL). Because D15A is a modified NT-3 with antibody binding epitopes preserved, a two-site NT-3 ELISA (R&D Systems) was used to determine D15A levels in the samples.
For tissue prepared for immunohistochemistry (n = 5/group), PB perfusion was followed by 250–300 ml of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). A 1-cm segment of the spinal cord spanning the injection site was removed and post-fixed for 2 hr at 4°C, then transferred to 30% sucrose in 0.1 M PB for cryoprotection until sectioned on a cryostat (Leica CM3050, Nussloch, Germany) at 20–25 μm and mounted on microscope slides. The sections were rinsed in phosphate buffered saline (PBS), blocked, and permeabilized in PBS containing 10% normal donkey serum (NDS) and 0.3% Triton X-100 for 1 hr at room temperature, and incubated in primary antibody overnight at 4°C, with 5% NDS, and 0.25% Triton X-100 in PBS. Polyclonal rabbit anti-glial fibrillary acidic protein antibody (anti-GFAP; 1:100, Chemicon, Temecula, CA), was used to identify astrocytes and monoclonal antibody adenomatous polyposis coli (anti-APC) (1:100; Oncogene, San Diego, CA) to identify oligodendrocytes. Monoclonal antibody anti-NeuN (1:100; Chemicon), and polyclonal antibody anti-βIII tubulin (1:100; Promega, Madison, WI) were used to identify neurons. Monoclonal antibodies anti-CD4 and anti-CD8 (1:100; Pharmingen, San Diego, CA) were used to identify T lymphocytes. Microglia (both ramified and reactive) and macrophages were stained using monoclonal antibodies anti-OX-42 and anti-ED1 (1:500; Chemicon), respectively, as previously described (Graeber et al., 1988, 1989; Koshinaga and Whittemore, 1995). The next day, sections were rinsed in PBS and incubated in either fluorescein-conjugated or rhodamine-conjugated Fab′ fragment secondary antibodies from donkey (1:100; Jackson Laboratories, Baltimore, MD). Slides were washed, mounted, and examined using a Nikon TE300 fluorescent microscope and photographed with an Olympus Fluview confocal microscope. Figures were assembled using Adobe Photoshop and Illustrator software (San Jose, CA).
Morphologic Analysis
To quantify the numbers of ED1+ macrophages, sections were analyzed using a 20× objective and the appropriate UV-filter on a Nikon E600 microscope. Starting from the center of the injection, five sections from each animal over a distance of 2 mm were examined and ED1+ cells were counted using Imagepro Plus software (Media Cybernetics, San Diego, CA) in an entire 20× field.
Statistical Analysis
One-way ANOVA was used to compare control values with experimental groups followed by Tukey’s post-hoc comparison test. A P-value of <0.05 is considered statistically significant.
RESULTS
In Vitro Infection Using Lentivirus, Adenovirus, and Retrovirus
Multiple cell lines were infected in vitro with various D15A viral vectors to detect the differences in gene expression levels between different viral delivery systems. Infection efficiencies were 80–90% for all viruses as confirmed by immunohistochemistry (Fig. 1). Table I shows that although similar MOI were used, some differences in protein expression levels were noted. D15A protein levels were relatively higher after infection with both lentivirus (62–103-fold) and adenovirus (41–175-fold) as compared to the two retroviruses LZRS (35–93-fold) and MSCV (35–53-fold). This suggests that differences exist either in the cellular response to viral infection or in internal regulation of gene expression between cells. Consistent with that suggestion, retroviral expression was driven off the 5′ LTR and the lentivirus and adenovirus from the CMV promoter. D15A levels were significantly higher (P < 0.001) in the RN33B group than other viral groups tested or uninfected cells. There was no statistically significant difference between the NIH3T3 cells and Schwann cells.
TABLE I.
D15A Expression in Conditioned Media of Schwann Cells, NIH3T3, and RN33B Cells After Viral Infection In Vitro
| No virus | Lentivirus | Adenovirus | LZRS retrovirus | MSCV retrovirus | |
|---|---|---|---|---|---|
| Schwann cells | 41.95 ± 10.73 | 3108.20 ± 641.51* | 2742.91 ± 568.76* | 1578.69 ± 288.08 | 2278.12 ± 541.61 |
| Fold induction | 75 | 66 | 38 | 55 | |
| NIH 3T3 | 49.36 ± 12.67 | 5061.83 ± 704.09 | 8587.98 ± 630.80* | 4570.17 ± 784.54 | 2612.86 ± 484.58 |
| Fold induction | 103 | 175 | 93 | 53 | |
| RN33B | 244.72 ± 110.23* | 15273.42 ± 1529.2* | 10040.42 ± 2378.69* | 7346.98 ± 852.78* | 8590.45 ± 731.39* |
| Fold induction | 62 | 41 | 30 | 35 |
In Schwann cells, D15A levels in the lentiviral and adenoviral-infected cells were not different from those in the MSCV retroviral-infected group but were significantly higher than no virus and LZRS retrovirus. In NIH 3T3 cells, D15A levels in adenoviral-infected cells was significantly higher than all other groups. MSCV infected cells, D15A levels were significantly lower than all other viral infected groups. In RN33B cells, D15A levels were significantly higher than other cell types in all groups tested. D15A levels in the Lentiviral infected RN33B cells were significantly higher than all other viruses. All cells were seeded at 1 × 105 cell/well. Data are expressed as mean pg D15A/24 hr/ml ± SD (n = 6).
P < 0.001.
Retrovirus Shows Rapid Downregulation of Transgene Expression After Ex Vivo Delivery
Schwann cells were infected in vitro with matched MOI (20) using lentivirus-D15A, adenovirus-D15A, retrovirus LZRS-D15A, and retrovirus-EGFP. After resuspension in culture media, 1 μl containing 1 × 105 cells, with pre-injection viability of 90–95%, was injected into the thoracic spinal cord. Transgene expression was evaluated at 1, 2, and 4 weeks (Fig. 2). There was no statistically significant difference between the normal cord and EGFP-infected Schwann cell grafted groups at all three time points. The group that received lentiviraly-infected Schwann cell grafts showed significantly higher (P < 0.001) expression levels than normal cord, EGFP-, and LZRS-infected cells at 1, 2, and 4 weeks, as well as adenovirally-infected cells at 4 weeks. There was no significant difference between groups that received lentivirally-infected and adenovirally-infected Schwann cells at either 1 or 2 weeks post-grafting. In contrast, grafts of adenovirally-infected Schwann cells showed some down-regulation over time, although D15A levels at 1 week were not significantly different from the 2-week level in groups that received both adenovirally-infected and lentivirally-infected Schwann cells. At 4 weeks, D15A levels were significantly (P < 0.001) lower in the animals that received adenovirally-infected cell grafts than at 1 and 2 weeks, whereas in those that received lentivirally infected cell grafts D15A levels were not significantly different at 2 and 4 weeks. Downregulation in the animals that received retrovirally-infected cell grafts was rapid, with significantly (P < 0.001) lower D15A levels (20-fold) at 2 and 4 weeks post-grafting.
Fig. 2.
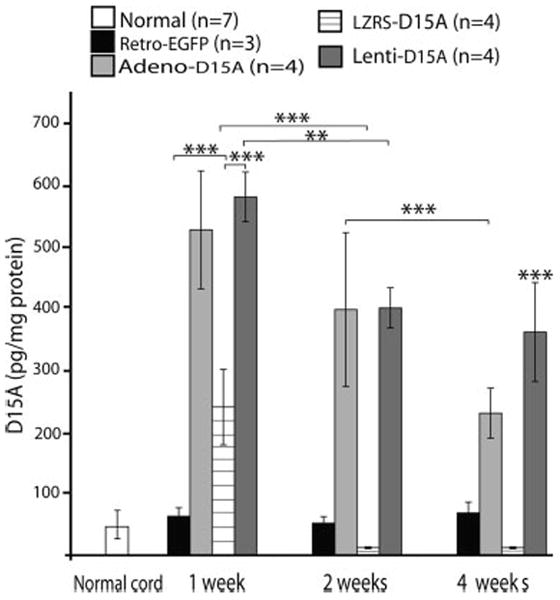
Temporal expression of D15A in the spinal cord after ex vivo gene delivery using lentivirus, adenovirus, EGFP, and retrovirus. Schwann cells were infected with similar MOI (20) and 48 hr later 1 × 105 cells were transplanted into the spinal cord. There was no significant difference between normal spinal cord and EGFP-infected groups at any of the three time points. D15A expression was significantly higher (P < 0.001) in the lentiviral-infected group than normal, EGFP, and retrovirus infected groups at 1 and 2 weeks. No significant difference was found between lentiviral and adenoviral infected groups at 1- and 2-weeks post-transplantation. At 4 weeks, D15A expression was significantly higher (P < 0.001) in the lentiviral-infected group than all other groups. In the adenoviral-infected group, D15A levels were significantly lower (P < 0.001) at 4 weeks. Downregulation of D15A in the retroviral-infected group was rapid with significantly lower (P < 0.001) D15A levels at 2 and 4 weeks compared to that of 1 week. Although the lentiviral-infected group showed some downregulation with D15A levels significantly lower (P < 0.01) at 2 weeks, D15A levels plateaued and were not significantly different at 2 and 4 weeks. Data shown are the mean ± SD (n = 4).
Dose Dependency of Transgene Expression In Vivo
To compare levels of protein expression in response to different concentrations of adenovirus and lentivirus, we tested a low 1 × 105 TU/μl (Huber et al., 2000; Jakobsson et al., 2003) and a moderately high 5 × 105 TU/μl (Byrnes et al., 1995) dose. Expression of D15A with both constructs was 3-fold higher in the 5 × 105 TU/μl group at 1 week. Although not strictly 1:1, gene expression was significantly (P < 0.001) dependent on the viral titer. There was no statistically significant difference in D15A levels between the adenovirus and lentivirus infected groups when the same viral titers were used (Fig. 3).
Fig. 3.
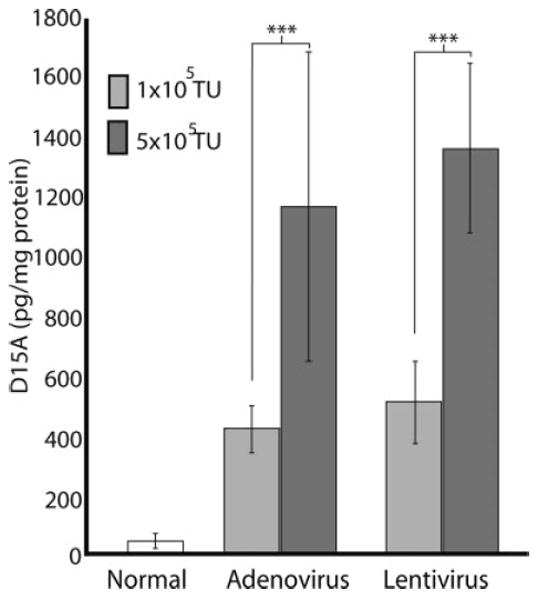
D15A protein expression is dependent on viral titer. One microliter of 1 × 105 TU/μl or 5 × 105 TU/μl of virus was injected into the spinal cord. One week after injection, expression of D15A was significantly (P < 0.001) higher than normal in all groups tested. D15A levels were significantly higher after high titer adenoviral (5 × 105 TU/μl, n = 3; P = 0.001) and lentiviral injections (5 × 105 TU/μl, n = 3/group; P < 0.001) than after the low titer injections (1 × 105 TU/μl, n = 6/group). D15A expression was not significantly different between titer matched adenoviral and lentiviral injected groups. Data shown are mean ± SD.
Our initial observations with lentiviral-LacZ injections showed an area of necrosis and tissue destruction (data not shown). We were, however, able to eliminate much of that by removing serum from the media during viral preparation in later experiments. These findings are in agreement with previous reports showing a focal necrosis associated with the point of lentiviral injection that was always greater than PBS injection (Zhao et al., 2003). We also saw a decrease in NeuN immunoreactivity in the rat spinal cord after lentiviral injection. Baekelandt et al. (2003) have observed a similar decrease in the rat brain and confirmed that this decrease was not associated with neuronal death or apoptosis. These adverse effects are not due to the vector transduction per se but seem to be a result of components of the culture system used in viral preparation, which has been similarly observed in clinical trials for SCID and HIV patients (Selvaggi et al., 1997; Tuschong et al., 2002). Several purification methods were suggested to eliminate this immune response including sucrose gradient ultracentrifugation, although this is associated with a decrease in viral titers, and the elimination of serum from culture system (Baekelandt et al., 2003), which in our hands successfully reduced the tissue damage initially observed. Virus prepared under serum-free conditions was used for all data presented in this study.
Lentivirus produces a mild inflammatory response after in vivo delivery. We evaluated the inflammatory response after the in vivo viral delivery for both lentivirus and adenovirus, as well as after injection of PBS. Lentivirus produced very mild ED1+ cellular infiltration that was confined to the injection site (Fig. 4A). Adenoviral injections induced massive ED1+ cellular infiltration that was distributed over multiple spinal segments (Fig. 4C) both rostral and caudal to the injection site, at 1-week post-injection. Intraperitoneal injection of purified monoclonal antibodies against the rat CD4 and CD45 (OX-38 and OX-22) lymphocytic receptors reduced the inflammatory response (Fig. 4B). The number of ED1+ cells at 1 week after lentivirus injection was not significantly different from animals that received PBS or adenovirus injection combined with monoclonal antibodies OX22/OX38 (Fig. 4F). When higher dose virus (1 × 106 TU/μl) was used, ED-1+/OX42+ cellular infiltration was also similar in the lentivirus and adenovirus combined with mAb treated groups (Fig. 5K,L).
Fig. 4.
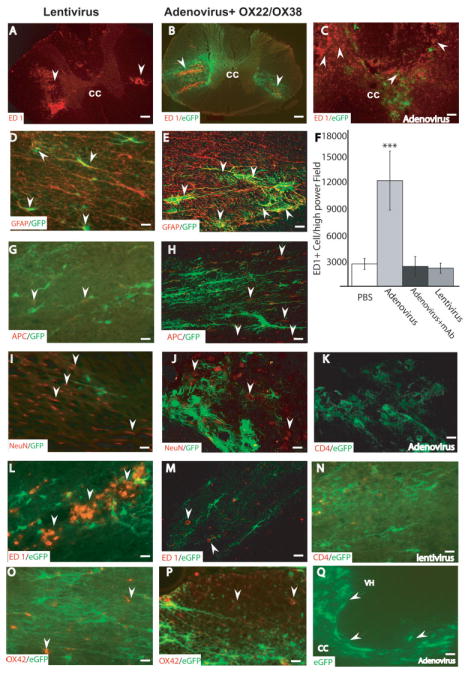
Inflammatory response and cell-specific infection by low titer lentiviral and adenoviral vectors in the spinal cord. Photomicrographs taken within 2 mm from the injection site show representative sections after; lentivirus (A,D,G,I,L,N,O), adenovirus + mAb OX22/ OX38 (B,E,H,J,K,M,P,Q) and adenovirus (C) injections. In both lentivirus (A) and antibody-treated adenovirus groups (B) ED1+ cells were limited to the area surrounding the injection site (arrows), whereas in the non-antibody treated adenovirus group (C) ED1+ cells were widespread over the entire gray and white matter of the spinal cord. ED1+ cells were counted (F) in five sections 2 mm from the injection site from each animal. Cells were counted using Image-pro Plus software in an entire 20× field. The number of ED1+ cells in the adenovirus group were significantly higher (P < 0.001) than all other groups. There was no statistically significant difference among PBS, lentivirus, and adenovirus + mAb groups. Data shown are the mean ± SD, (n = 7/group). To identify cellular phenotypes infected with low titer (1 × 105 TU/μl) lentivirus and adenovirus, sections were double-labeled for EGFP (green) and the cell-specific markers; neurons (NeuN), oligodendrocytes (APC), astrocytes (GFAP), microglia/macrophages ED-1/OX42, and T lymphocytes (CD4). Examination using confocal microscopy showed that astrocytes (arrows) were infected with both viruses (D,E). In contrast, APC+ oligodendrocytes (G,H) and NeuN+ neurons (I,J) (arrows) were not infected after both viral injections. A decrease in NeuN immunoreactivity was also seen after lentiviral injection but not after combined adenoviral injection and immune suppression. CD4+ T-cells were not seen 1 week after low titer adenoviral injection with the OX22/OX38 immunosuppression (K) or lentiviral injection (N). ED1+ macrophages (L,M) and OX42+ reactive microglia (O,P; arrows) were not infected after either viral injection. EGFP labeling was detected at 4 weeks after adenoviral injection with OX22/OX38 immunosuppression (arrows in Q). Data are representative of five independent animals for each experimental group. Scale bar = 200 μm (in A,B); 20 μm (in D–P), and 80 μm (C,Q).
Fig. 5.
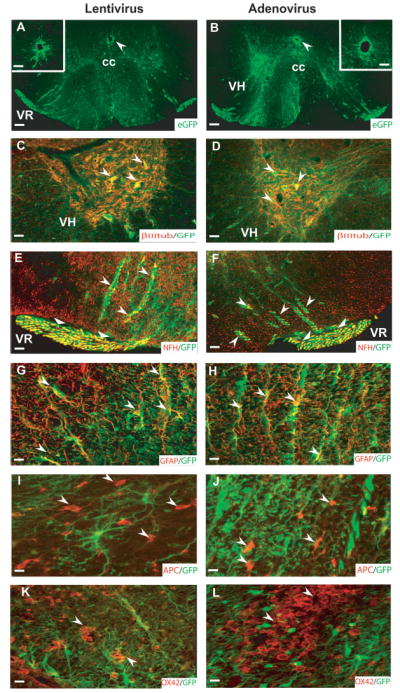
Identification of cell types infected with high titer lentivirus and adenovirus. EGFP labeling was found in both the gray and white matter of the spinal cord after lentiviral (A,C,E,G,I,K) and adenoviral (B,D,F,H,J,L) injections. Ependymal cells surrounding the central canal were infected by both types of viruses that could be clearly seen at high magnifications (arrows in A,B; insets in A and B are from adjacent sections). EGFP labeling was localized in βIII tubulin+ neurons (C,D; arrows), NFH+ axons in the ventral white matter (E,F; arrows) and the ventral rootlets (E,F; triangular arrow heads) associated with motor neurons as well as GFAP+ astrocytes (G,H; arrows). In contrast, neither oligodendrocytes (I, J; arrows) nor reactive microglia/macrophages (K,L; arrows) were labeled with EGFP. Scale bar = 200 μm (A,B); 40 μm (C,F and insets in A,B); 20 μm (in G,H,K,L); 13 μm (I,J). cc, central canal; VH, ventral horn; VR, ventral root. Data are representative of n = 5 animals/group.
Lentivirus and Adenovirus Infect Mainly Astrocytes After Low Titer Injection
Histologic evaluation of the viral tropism in the spinal cord after in vivo delivery of low titer (1 × 105 TU/μl) lentiviral and adenoviral + mAb OX22/OX38 injections showed double-labeled cells that were mainly GFAP+ astrocytes (Fig. 4D,E). No APC+ oligodendrocytes (Fig. 4G,H) or NeuN+ neurons (Fig. 4I,J) were infected. Few ED1+ and OX42+ microglia were seen in the area of injection (Fig. 4L,M,O,P). No CD4+ T-cell lymphocytes (Fig. 4K,N) were seen at 1 week after lentiviral injection. The rostrocaudal extension of EGFP labeling was 3.0 ± 0.5 mm, indicating limited diffusion of virus after low titer injection. Four weeks after low titer adenoviral + mAb OX22/OX38 injection EGFP+ cells were still seen in the spinal cord (Fig. 4Q).
High Titer Viral Injections
To evaluate viral tropism after high dose viral administration, we injected 1μl containing 1 × 106 TU (Blomer et al., 1997; Hermens and Verhaagen 1997) of either lentivirus or adenovirus + mAb OX22/OX38 into the thoracic spinal cord. One week after viral injection, EGFP labeling was spread over the entire ventral segment of the spinal cord (Fig. 5A,B). Cells resembling ependymal cells surrounding the central canal were also infected (inset in Fig. 4A,B). βIII tubulin+ neurons (Fig. 5C,D) in the ventral horn of the gray matter of the thoracic spinal cord were infected with both viruses. In addition, EGFP+/NFH+ axons were observed both in the ventral white matter and in the ventral roots (Fig. 5E,F). The majority of infected cells in both the white and gray matter of the spinal cord were GFAP+ astrocytes (Fig. 5G,H). No APC+ oligodendrocytes (Fig. 5I,J) were seen infected after lentiviral or adenoviral injection. ED1+ cells infiltrating the injection site were seen (data not shown) and were similar to OX-42+ (Fig. 5K,L) cellular infiltrates. None of the ED1+/OX-42+ infiltrating macrophages/microglia were infected with either virus. The extent of EGFP labeling was 8.0 ± 3.0 mm after high titer injection indicating substantial diffusion of viral particles.
Temporal Profile of Lentiviral- and Adenoviral-Mediated Transgene Expression After In Vivo Delivery to the Spinal Cord
We chose the lower viral titer (1 × 105 TU/μl) to study the temporal expression of D15A after viral-mediated delivery in vivo. Titer/volume matched vector stocks were injected into the thoracic spinal cord and gene expression was evaluated 1, 2, and 4 weeks later (Fig. 6). ELISA for D15A showed that although all groups were significantly higher (P < 0.001) than the basal levels of NT-3, there was no significant difference between groups at 1-week post-injection. Lentiviral transgene delivery showed a stable pattern of gene expression. D15A levels in the lentivirus group were not significantly different at 1, 2, and 4 weeks, with D15A levels being significantly higher than both adenovirus groups at 4 weeks. Adenoviral delivery of D15A showed significant downregulation with expression levels gradually decreasing at 2 weeks (P < 0.001) and 4 weeks (P < 0.05). D15A levels in the adenovirus group were significantly (P < 0.001) lower than the adenovirus + OX22/OX38 group at 2 weeks and both lentivirus (P < 0.001) and adenovirus + OX22/OX38 (P < 0.001) at 4 weeks.
Fig. 6.
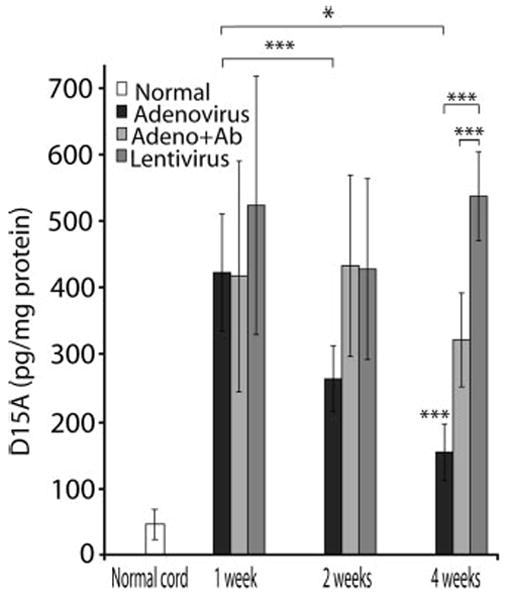
Temporal expression of D15A in the spinal cord after in vivo gene delivery. Lentiviral delivery resulted in robust and stable gene expression for up to 4 weeks post-injection. At 4 weeks post-injection, the D15A levels were significantly higher than both adenoviral infected groups (P < 0.001). In contrast, adenoviral infection showed significant downregulation of D15A at 2 (P < 0.001) and 4 weeks (P < 0.05) post-infection. Treatment of animals with mAb OX-22/OX-38 successfully reduced the downregulation of adenoviral-delivered D15A expression compared to the non-OX22/OX38 treated groups that was significantly lower (P < 0.001) than lentivirus and adenovirus + mAb groups at 4 weeks. Data represent the mean ± SD (n = 5/experimental group).
Immune Suppression Eliminates Downregulation After Adenoviral Transgene Delivery In Vivo
In light of our histologic data indicating that the OX22/OX38 monoclonal antibody treatment reduces the inflammatory response noted after adenoviral transgene delivery, we hypothesized that treating animals in the adenovirus group with OX22/OX38 mAb 24 hr before and after viral injection would result in stabilization of gene expression and minimize the downregulation of D15A expression. Our in vivo results confirmed that the use of immuno-blockade successfully reduces downregulation of D15A after adenovirus injection. At 2 weeks post-injection, D15A levels were significantly higher (P < 0.05) than adenovirus alone, but were not different from lentivirus (Fig. 6). At 4 weeks post-infection, D15A levels were significantly lower (P < 0.001) than the lentivirus group, but they were significantly higher (P < 0.001) than the adenovirus alone group.
Inducible D15A Expression After Ex Vivo Delivery With Tet-on Vectors
Constitutive expression of transgenes may not always be desirable, as the ability to turn a gene on or off at specific times may be therapeutically beneficial. To address the feasibility of this approach, we used the tetracycline-inducible, Tet-on retroviral system. Schwann, RN33B, and NIH3T3 cells were infected in vitro with the two virus Tet-inducible system. After antibiotic selection for 1 week, induction of gene expression with the addition of doxycycline to the culture media showed robust gene expression in vitro with increasing concentrations of doxycycline. Although, D15A levels increased about ~50-fold over basal expression levels with 6 μg/ml of doxycycline and was significantly (P < 0.05) higher than baseline expression, no significant difference was found between 2, 4, and 6 μg/ml doxycycline. Baseline expression was 40 μg/1 × 105 cells/ml/24 hr showing that the system is somewhat leaky with respect to its trans regulation (Fig. 7A). In the initial phase of this experiment, we noted a wide range of variation of gene expression levels in vitro, with between 5–25-fold increase observed after the doxycycline induction (data not shown). However, selecting stable clones using G418 for up to 2 weeks resulted in clones that exhibited stable transgene integration and expression.
Fig. 7.
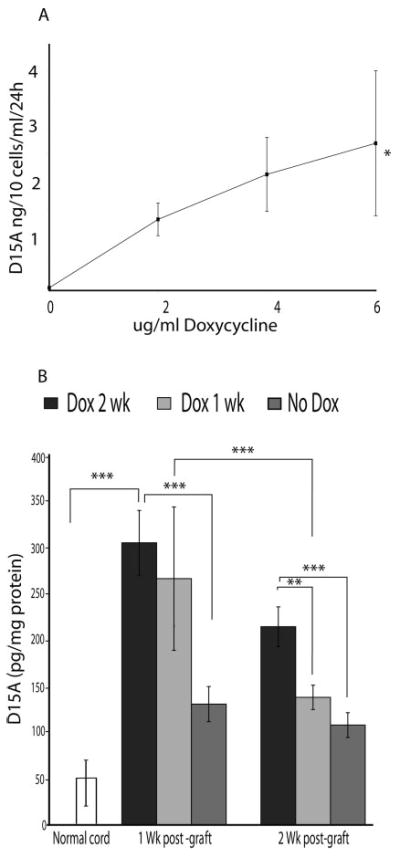
Induction of D15A expression using doxycycline (Dox) in vitro (A) and in vivo (B). A: NIH/3T3 cells were transduced in vitro using the two virus (pRev-Tre D15A and pRev-Tre Tet-on) tetracycline inducible Tet-on system. After antibiotic selection for 1 week in vitro, increasing Dox concentrations were added to the culture media. No significant difference was found between 2, 4, and 6 μg/ml Dox concentrations. D15A levels at 6 μg/ml Dox were significantly higher than baseline expression (P < 0.05). Data shown are the mean ± SD (n = 5/experimental group). B: 1 × 105 D15A-infected RN33B cells were injected into the spinal cord. After the addition of 1 mg/ml Dox to the animal’s drinking water, D15A levels at 1 week were significantly higher than both normal (open bars, P < 0.001) and no-Dox (dark gray bars, P < 0.001) groups. In one group of animals, Dox administration was stopped after 1 week, which resulted in a significant drop in D15A levels at 2 weeks (light gray bars, P < 0.001). At 2 weeks, D15A levels in the group that received Dox for 2 weeks were significantly higher than normal (black bars, P < 0.001), no-Dox (P < 0.001) and animals that received Dox for only 1 week (P < 0.01). Basal expression levels were detectable and were significantly higher (P < 0.05 at 1 week and P < 0.01 at 2 weeks) than in control animals indicating incomplete transcriptional regulation in the absence of Dox. Data shown are the mean ± SD (n = 4).
For ex vivo delivery of D15A, we injected 1 × 105 cell/μl RN33B cells into the spinal cord. Animals were given doxycycline (1 mg/ml) in their drinking water for up to 2 weeks. D15A expression in the spinal cord increased significantly, ~7-fold at 1 week (Fig. 7B). There was no statistically significant difference in D15A expression between 1 and 2 weeks in animals that received doxycycline for 2 weeks. In one group, doxycycline administration was stopped after 1 week (light gray bars), a significant reduction (P < 0.01, 2-fold) in D15A expression at 2 weeks was observed. At 2 weeks post-grafting, D15A levels (black bars) were significantly higher than normal (P < 0.001, 5-fold), no-doxycycline (dark gray bars) (P < 0.001, 2-fold) and animals that received doxycycline for only 1 week (P < 0.01, 1.5-fold). Note that, at 1 and 2 weeks, levels of D15A in the absence of doxycycline are significantly higher at 1 week (P < 0.05, 3-fold) and 2 weeks after the doxycycline induction (P < 0.01, 2.5-fold) than the control values, again reflecting the inherent leakiness of this expression system.
DISCUSSION
Transfer of therapeutically beneficial genes to the CNS is an experimental concept that potentially can be tailored and applied to a variety of CNS disorders. This approach may be designed according to specific therapeutic requirements. In diseases characterized by a chronic and progressive course (e.g., Parkinson’s disease), a sustained administration of the therapeutic molecule (Costantini et al., 2000; Johansen et al., 2002) may be appropriate, whereas for other disorders a transient delivery may be applied. The purpose of the current study was to evaluate various viral delivery techniques, to better understand the temporal pattern of gene expression, as well as the inflammatory response that follows viral injection, with specific attention to the spinal cord as a unique CNS environment.
We have chosen to compare lentiviral and adenoviral vectors because of their pantropic nature in the nervous system after in vivo delivery. Other viral vectors used currently for gene therapy, HSV and AAV, are mainly neurotropic (Geller et al., 1997; Buning et al., 2003) in vivo.
We show that high titer injections of adenovirus and lentivirus into the CNS are both pantropic transducing mostly neurons and astrocytes after parenchymal injection, consistent with previous reports (Le Gal La Salle et al., 1993; Huber et al., 2000). Ependymal cells are also infected in our study after parenchymal injections (Fig. 4A,B) and after intra-ventricular injection by others (Bajocchi et al., 1993). Oligodendrocytes have been only transduced in vitro (Ohashi et al., 1995). In our hands, no oligodendrocytes were infected in vivo after viral administration. After lentiviral injection in the mouse substantia nigra, 45% of cells were neurons and 17% were astrocytes (Bensadoun et al., 2000), whereas in the rat brain the majority of transduced cells were neurons (Naldini et al., 1996).
The use of a lentiviral vector pseudotype with VSV-G (Burns et al., 1993) gives this system an extremely broad host range in the CNS (Blomer et al., 1997; Kordower et al., 1999) making it most suitable for delivery of therapeutic molecules for different parts of the CNS where different populations of CNS neurons and glia exist. Watson et al. (2002) used pseudotype lentivirus with Mokola, lymphocytic choriomeningitis virus (LCMV), and VSV-G and showed that similar populations were infected by all three types both in vitro and in vivo, with Mokola pseudotype-virus being most efficient when injected in mouse striatum and hippocampus. In contrast, no transduction resulted from injection of Ebola-pseudotype virus. Englund (2000) and Falk (2002) have also shown limited efficiency of lentiviral vectors in mice. Although many studies support the notion that VSV-G-coated lentivirus is neurotropic in the brain (Blomer et al., 1997; Kordower et al., 1999), Jakobsson et al. (2003) provided evidence that VSV-G allows transduction of both glial and neuronal cells and the promoter dictates in what cell type the transgene will be expressed. The present study detected no difference in the ability of lentivirus and adenovirus to infect Schwann cells and other cell lines in vitro. This contrasts with others who have shown adenovirus to be superior for infection of murine neural stem cells (Falk et al., 2002). These discrepancies are likely due to murine-specific differences that limit lentivirus nuclear entry and integration leading to decreased transgene production (Johansson et al., 1999; Englund et al., 2000; Falk et al., 2002).
Lentiviral injection into the spinal cord resulted in stable transgene expression with minimal inflammatory response and tissue damage making this system ideal for situations that require long term delivery of therapeutic molecules to the CNS. βgal+ (data not shown) and EGFP+ cells were detected as far as 3–4 mm from the injection site for up to 4 weeks post-injection (the longest time point evaluated). In previous studies, the extent of βgal staining ranged from 1 mm from the injection site after a 1 μl injection of 3 × 108 TU/ml (Bensadoun et al., 2000) to several millimeters away from the injection site. In all cases, long-term expression of the transgene was seen for periods ranging from 60 days (Zhao et al., 2003) to 6 months post-injection (Blomer et al., 1997), with retrograde transport of the virus after injection in the spinal cord, to the substantia nigra and the nigrostriatal (Blomer et al., 1997) .
Not unexpectedly, protein levels after adenovirus-mediated transgene delivery were dose-dependent, although not strictly 1:1. Transgene expression also downregulated after the administration of low titers of adenovirus (1 × 105 TU/μl). Others have used moderate (5 × 106 TU/μl) (Romero and Smith, 1998) to high titers (1 × 109 TU/μl) (Zhou et al., 2003) in an effort to achieve higher expression of transgene. Although we have only studied the temporal expression after low dose virus delivery, we would anticipate the downregulation after higher doses to be comparable to that observed here, albeit with a more intense inflammatory response. Previous studies have shown that at higher concentrations, the majority of CNS infected cells cease transgene expression within 2 weeks after administration or degenerate due to an immune inflammatory response (Hermens and Verhaagen, 1997; Peltekian et al., 1997). This downregulation of adenovirus driven expression has also been observed by others employing it in both ex vivo (Blits et al., 1999) and in vivo (Zhou et al., 2003) approaches. Non-integrating adenoviral vectors are almost certainly lost during cell replication, but adenovirus expression can be persistent in grafted post-mitotic cells (Boer et al., 2001).
Monoclonal antibodies against CD4 and CD45 T lymphocyte receptors successfully reduced transgene downregulation after adenoviral administration, contributing to significantly higher D15A expression for up to 4 weeks post-infection. This is in agreement with previous work by Romero and Smith (1998). Several other strategies have been developed to avoid this downregulation and inflammation, including the use of immune suppressors (cyclosporine and cyclophosphamide) (Dai et al., 1995), or monoclonal antibodies to CD4, CD40, and TCR (Guerette et al., 1996; Scaria et al., 1997; Zsengeller et al., 1997). This strategy likely eliminates only one cause for the downregulation after adenoviral gene delivery as later downregulation may still occur due to a delayed immune response, or cell death.
Although promoter silencing can be a cause for downregulation of transgene production after viral gene delivery, it is not in our opinion the reason for the noted differences in transgene expression patterns between lentivirus and adenovirus noted in the present study, as both lentiviral and adenoviral vectors used a CMV promoter to drive D15A expression. Also down-regulation after in vivo adenoviral delivery has been reversed, at least in part, by modifying the animal’s immune response using monoclonal antibodies against CD4 and CD45 T lymphocyte receptors. These data are consistent with an immune-mediated removal of infected cells in the presence of activated T cells.
One significant finding of this study shows that although EGFP levels were histologically detectable 4 weeks after adenoviral injection, the downregulation of D15A assessed by ELISA was greater than what would have been predicted by the qualitative levels of EGFP. These data indicate that using marker proteins as the sole measure to assess the temporal expression after viral injection is problematic. Most studies have relied on his-tochemical or immunohistologic methods to analyze the duration of transgene expression in neural cells (Bennett et al., 1994; Akli et al., 1996) leading to difficulties of interpretations and variable conclusions. For example, although βgal has been seen up to 2 months after adenoviral injection (Byrnes et al., 1995), others showed rapid downregulation after in vivo and ex vivo studies using both βgal histochemistry (Blits et al., 1999) and NT-3 ELISA (Zhou et al., 2003). Using RT-PCR to detect GDNF mRNA, Baumgartner and Shine (1998) showed adenoviral-driven gene expression in the spinal cord at 3 weeks, but no detectable levels at 6 weeks after adenoviral injection. Collectively, these results indicate that direct assessment of the relevant protein should be employed to measure transgene expression.
The ex vivo gene transfer results using the LZRS retrovirus with a constitutive 5′ LTR promoter in Schwann cells showed rapid downregulation of gene expression by 2 weeks post-grafting. These findings are in agreement with previous reports, which have shown variable transgene downregulation independent of either the promoter or cell type used (Liu et al., 1999; Johansen et al., 2002). This downregulation and variability is believed to be due to the random integration into the host genome, and the issue has been raised about possible complications related to permanently modifying the host genotype, triggering insertional mutagenesis and the activation of protooncogenes in host cells (Kohn et al., 2003; Noguchi, 2003). This would likely be a very rare event, however, and the rapid downregulation of transgene expression could prove useful when only transient spinal cord expression is desired.
We show robust, doxycycline-dependent induction of gene expression ex vivo with the tetracycline-regulated promoter. Basal expression levels were detectable and were significantly higher than control animals, however, indicating incomplete transcriptional regulation in the absence of doxycycline, highlighting one problem related to the use of this system. Blesch et al. (2001) have shown efficient gene expression for a period of 3 months in vivo using a tet-off system, whereas others reported varying basal expression using tet-on system in vitro (Paulus et al., 2000) and marked downregulation immediately after transplantation into the rat striatum (Johansen et al., 2002). Our findings are in line with the latter study, in that we report significant basal expression both in vitro and ex vivo. The basal expression level observed in vitro as well as in vivo in the present study may be explained by multiple factors including, but not limited to, enhancer elements in the promoter, low affinity binding of the activator to the tetracycline responsive element (TRE) in the absence of doxycycline, or basal activity of the promoter (Johansen et al., 2002).
In summary, the results of the present study indicate that: 1) lentiviral-mediated gene delivery is best suited for situations when long-term delivery of a transgene is needed, whereas ex vivo retroviral delivery system is more appropriate for transient delivery of therapeutic molecules, with adenoviral-driven expression intermediate; 2) the use of transgene-specific assays to determine the protein level after delivery are more reliable and biologically relevant than the use of marker proteins as indicators of transgene levels; and 3) protein levels are dependent on viral titer, as greater concentrations of the therapeutic molecules can be obtained by increasing viral dose. The caveat to this later point is that as viral loads increase, so does the extent of the inflammatory response. Taken together, these results provide further support for the use of viral vectors for gene transfer of therapeutic molecules aimed at enhancing regeneration after SCI. Moreover, results indicate that multiple viral delivery approaches may be used singly or in combination to achieve a targeted temporal, as well as spatial, delivery of transgene to the injured CNS.
Acknowledgments
We would like to thank D.A. Burke for her assistance with statistical analyses, R.M. Howard for his assistance in virus preparation, L. Zhang for his assistance in cell culture, and A. Puckett for his assistance with animal care. Supported by National Institutes of Health grants NS38665 (S.R.W., P.T.), RR15576 (S.R.W.), NS36350 (X.M.X.), Norton Healthcare, the Kentucky Spinal Cord and Head Injury Research Trust, the Commonwealth of Kentucky Research Challenge for Excellence (S.R.W., X.M.X.), and The Daniel Heumann Fund for Spinal Cord Research (X.M.X.).
References
- Akli S, Guidotti JE, Vigne E, Perricaudet M, Sandhoff K, Kahn A, Poenaru L. Restoration of hexosaminidase A activity in human Tay-Sachs fibroblasts via adenoviral vector-mediated gene transfer. Gene Ther. 1996;3:769–774. [PubMed] [Google Scholar]
- Andersen JK, Garber DA, Meaney CA, Breakefield XO. Gene transfer into mammalian central nervous system using herpes virus vectors: extended expression of bacterial lacZ in neurons using the neuron-specific enolase promoter. Hum Gene Ther. 1992;3:487–499. doi: 10.1089/hum.1992.3.5-487. [DOI] [PubMed] [Google Scholar]
- Baekelandt V, Eggermont K, Michiels M, Nuttin B, Debyser Z. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10:1933–1940. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]
- Bajocchi G, Feldman SH, Crystal RG, Mastrangeli A. Direct in vivo gene transfer to ependymal cells in the central nervous system using recombinant adenovirus vectors. Nat Genet. 1993;3:229–234. doi: 10.1038/ng0393-229. [DOI] [PubMed] [Google Scholar]
- Baumgartner BJ, Shine HD. Neuroprotection of spinal motoneurons following targeted transduction with an adenoviral vector carrying the gene for glial cell line-derived neurotrophic factor. Exp Neurol. 1998;153:102–112. doi: 10.1006/exnr.1998.6878. [DOI] [PubMed] [Google Scholar]
- Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- Bensadoun JC, Deglon N, Tseng JL, Ridet JL, Zurn AD, Aebischer P. Lentiviral vectors as a gene delivery system in the mouse midbrain: cellular and behavioral improvements in a 6-OHDA model of Parkinson’s disease using GDNF. Exp Neurol. 2000;164:15–24. doi: 10.1006/exnr.2000.7409. [DOI] [PubMed] [Google Scholar]
- Blesch A, Conner JM, Tuszynski MH. Modulation of neuronal survival and axonal growth in vivo by tetracycline-regulated neurotrophin expression. Gene Ther. 2001;8:954–960. doi: 10.1038/sj.gt.3301480. [DOI] [PubMed] [Google Scholar]
- Blits B, Dijkhuizen PA, Carlstedt TP, Poldervaart H, Schiemanck S, Boer GJ, Verhaagen J. Adenoviral vector-mediated expression of a foreign gene in peripheral nerve tissue bridges implanted in the injured peripheral and central nervous system. Exp Neurol. 1999;160:256–267. doi: 10.1006/exnr.1999.7204. [DOI] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Kafri T, Trono D, Verma IM, Gage FH. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomer U, Naldini L, Verma IM, Trono D, Gage FH. Applications of gene therapy to the CNS. Hum Mol Genet. 1996;5(Spec No):1397–1404. doi: 10.1093/hmg/5.supplement_1.1397. [DOI] [PubMed] [Google Scholar]
- Boer GJ, van Esseveldt KE, Dijkhuizen PA, Hermens WT, te Beek ET, van Heerikhuize JJ, Poldervaart HA, Verhaagen J. Adenoviral vector-mediated expression of neurotrophin-3 increases neuronal survival in suprachiasmatic nucleus grafts. Exp Neurol. 2001;169:364–375. doi: 10.1006/exnr.2001.7683. [DOI] [PubMed] [Google Scholar]
- Buning H, Ried MU, Perabo L, Gerner FM, Huttner NA, Enssle J, Hallek M. Receptor targeting of adeno-associated virus vectors. Gene Ther. 2003;10:1142–1151. doi: 10.1038/sj.gt.3301976. [DOI] [PubMed] [Google Scholar]
- Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes AP, Rusby JE, Wood MJ, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66:1015–1024. doi: 10.1016/0306-4522(95)00068-t. [DOI] [PubMed] [Google Scholar]
- Cao Q, Xu XM, Devries WH, Enzmann GU, Ping P, Tsoulfas P, Wood PM, Bunge MB, Whittemore SR. Functional recovery in traumatic spinal cord injury after transplantation of multi neurotrophin-expressing glial-restricted precursor cells. J Neurosci. 2005;25:6947–6957. doi: 10.1523/JNEUROSCI.1065-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gamou S, Takayanagi A, Shimizu N. A novel gene delivery system using EGF receptor-mediated endocytosis. FEBS Lett. 1994;338:167–169. doi: 10.1016/0014-5793(94)80357-9. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Costantini LC, Bakowska JC, Breakefield XO, Isacson O. Gene therapy in the CNS. Gene Ther. 2000;7:93–109. doi: 10.1038/sj.gt.3301119. [DOI] [PubMed] [Google Scholar]
- Dai Y, Schwarz EM, Gu D, Zhang WW, Sarvetnick N, Verma IM. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci U S A. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AM. Neurotrophins: neurotrophic modulation of neurite growth. Curr Biol. 2000;10:R198–200. doi: 10.1016/s0960-9822(00)00351-1. [DOI] [PubMed] [Google Scholar]
- Dittrich F, Thoenen H, Sendtner M. Ciliary neurotrophic factor: pharmacokinetics and acute-phase response in rat. Ann Neurol. 1994;35:151–163. doi: 10.1002/ana.410350206. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington’s disease. J Neurosci. 1996;16:5168–5181. doi: 10.1523/JNEUROSCI.16-16-05168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund U, Ericson C, Rosenblad C, Mandel RJ, Trono D, Wictorin K, Lundberg C. The use of a recombinant lentiviral vector for ex vivo gene transfer into the rat CNS. Neuroreport. 2000;11:3973–3977. doi: 10.1097/00001756-200012180-00014. [DOI] [PubMed] [Google Scholar]
- Falk A, Holmstrom N, Carlen M, Cassidy R, Lundberg C, Frisen J. Gene delivery to adult neural stem cells. Exp Cell Res. 2002;279:34–39. doi: 10.1006/excr.2002.5569. [DOI] [PubMed] [Google Scholar]
- Felgner PL, Tsai YJ, Sukhu L, Wheeler CJ, Manthorpe M, Marshall J, Cheng SH. Improved cationic lipid formulations for in vivo gene therapy. Ann NY Acad Sci. 1995;772:126–139. doi: 10.1111/j.1749-6632.1995.tb44738.x. [DOI] [PubMed] [Google Scholar]
- Gao GP, Qu G, Faust LZ, Engdahl RK, Xiao W, Hughes JV, Zoltick PW, Wilson JM. High-titer adeno-associated viral vectors from a Rep/Cap cell line and hybrid shuttle virus. Hum Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- Geller AI, Yu L, Wang Y, Fraefel C. Helper virus-free herpes simplex virus-1 plasmid vectors for gene therapy of Parkinson’s disease and other neurological disorders. Exp Neurol. 1997;144:98–102. doi: 10.1006/exnr.1996.6394. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Formation of microglia-derived brain macrophages is blocked by adriamycin. Acta Neuropathol (Berl) 1989;78:348–358. doi: 10.1007/BF00688171. [DOI] [PubMed] [Google Scholar]
- Guerette B, Vilquin JT, Gingras M, Gravel C, Wood KJ, Tremblay JP. Prevention of immune reactions triggered by first-generation adenoviral vectors by monoclonal antibodies and CTLA4Ig. Hum Gene Ther. 1996;7:1455–1463. doi: 10.1089/hum.1996.7.12-1455. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Verhaagen J. Adenoviral vector-mediated gene expression in the nervous system of immunocompetent Wistar and T cell-deficient nude rats: preferential survival of transduced astroglial cells in nude rats. Hum Gene Ther. 1997;8:1049–1063. doi: 10.1089/hum.1997.8.9-1049. [DOI] [PubMed] [Google Scholar]
- Hermens WT, Verhaagen J. Suppression of inflammation by dexamethasone prolongs adenoviral vector-mediated transgene expression in the facial nucleus of the rat. Brain Res Bull. 1998;47:133–140. doi: 10.1016/s0361-9230(98)00042-2. [DOI] [PubMed] [Google Scholar]
- Huber AB, Ehrengruber MU, Schwab ME, Brosamle C. Adenoviral gene transfer to the injured spinal cord of the adult rat. Eur J Neurosci. 2000;12:3437–3442. doi: 10.1046/j.1460-9568.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- Jakobsson J, Ericson C, Jansson M, Bjork E, Lundberg C. Targeted transgene expression in rat brain using lentiviral vectors. J Neurosci Res. 2003;73:876–885. doi: 10.1002/jnr.10719. [DOI] [PubMed] [Google Scholar]
- Jiao S, Cheng L, Wolff JA, Yang NS. Particle bombardment-mediated gene transfer and expression in rat brain tissues. Biotechnology (NY) 1993;11:497–502. doi: 10.1038/nbt0493-497. [DOI] [PubMed] [Google Scholar]
- Johansen J, Rosenblad C, Andsberg K, Moller A, Lundberg C, Bjorlund A, Johansen TE. Evaluation of Tet-on system to avoid transgene downregulation in ex vivo gene transfer to the CNS. Gene Ther. 2002;9:1291–1301. doi: 10.1038/sj.gt.3301778. [DOI] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukemia following gene therapy of X-linked SCID. Nat Rev Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Bloch J, Ma SY, Chu Y, Palfi S, Roitberg BZ, Emborg M, Hantraye P, Deglon N, Aebischer P. Lentiviral gene transfer to the nonhuman primate brain. Exp Neurol. 1999;160:1–16. doi: 10.1006/exnr.1999.7178. [DOI] [PubMed] [Google Scholar]
- Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;12:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- Lacorazza HD, Flax JD, Snyder EY, Jendoubi M. Expression of human beta-hexosaminidase alpha-subunit gene (the gene defect of Tay-Sachs disease) in mouse brains upon engraftment of transduced progenitor cells. Nat Med. 1996;2:424–429. doi: 10.1038/nm0496-424. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G, Robert JJ, Berrard S, Ridoux V, Stratford-Perricaudet LD, Perricaudet M, Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993;259:988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Liu Y, Himes BT, Solowska J, Moul J, Chow SY, Park KI, Tessler A, Murray M, Snyder EY, Fischer I. Intraspinal delivery of neurotrophin-3 using neural stem cells genetically modified by recombinant retrovirus. Exp Neurol. 1999;158:9–26. doi: 10.1006/exnr.1999.7079. [DOI] [PubMed] [Google Scholar]
- Lundberg C, Horellou P, Mallet J, Bjorklund A. Generation of DOPA-producing astrocytes by retroviral transduction of the human tyrosine hydroxylase gene: in vitro characterization and in vivo effects in the rat Parkinson model. Exp Neurol. 1996;139:39–53. doi: 10.1006/exnr.1996.0079. [DOI] [PubMed] [Google Scholar]
- Manning JS, Hackett AJ, Darby NB., Jr Effect of polycations on sensitivity of BALD-3T3 cells to murine leukemia and sarcoma virus infectivity. Appl Microbiol. 1971;22:1162–1163. doi: 10.1128/am.22.6.1162-1163.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11:2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T. Parkinson’s disease: changes in apoptosis-related factors suggesting possible gene therapy. J Neural Transm. 2002;109:731–745. doi: 10.1007/s007020200061. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5:191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi P. Risks and benefits of gene therapy. N Engl J Med. 2003;348:193–194. doi: 10.1056/NEJMp020184. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Watabe K, Sato Y, Saito I, Barranger JA, Eto Y. Successful transduction of oligodendrocytes and restoration of arylsulfatase A deficiency in metachromatic leukodystrophy fibroblasts using an adenovirus vector. Gene Ther. 1995;2:443–449. [PubMed] [Google Scholar]
- Olson L, Nordberg A, von Holst H, Backman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A, et al. Nerve growth factor affects 11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report) J Neural Transm Park Dis Dement Sect. 1992;4:79–95. doi: 10.1007/BF02257624. [DOI] [PubMed] [Google Scholar]
- Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Neurotrophins, neuroprotection and the blood-brain barrier. Curr Opin Investig Drugs. 2002;3:1753–1757. [PubMed] [Google Scholar]
- Paulus W, Baur I, Keyvani K, Senner V. Variability of transcriptional regulation after gene transfer with the retroviral tetracycline system. J Biotechnol. 2000;81:159–165. doi: 10.1016/s0168-1656(00)00291-1. [DOI] [PubMed] [Google Scholar]
- Pear W, Scott M, Nolan GP. Generation of high titre, helper-free retroviruses by transient transfection. In: Robbins P, editor. Methods in molecular medicine: gene therapy protocols. Totowa, NJ: Humana Press; 1997. pp. 41–57. [DOI] [PubMed] [Google Scholar]
- Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltekian E, Parrish E, Bouchard C, Peschanski M, Lisovoski F. Adenovirus-mediated gene transfer to the brain: methodological assessment. J Neurosci Methods. 1997;71:77–84. doi: 10.1016/s0165-0270(96)00128-8. [DOI] [PubMed] [Google Scholar]
- Piccirillo CA, Prud’homme GJ. Gene therapy with plasmids encoding cytokine- or cytokine receptor—IgG chimeric proteins. Methods Mol Biol. 2003;215:153–170. doi: 10.1385/1-59259-345-3:153. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Adams B, Osehobo P, Fahnestock M. Neural growth, neural damage and neurotrophins in the kindling model of epilepsy. Adv Exp Med Biol. 2002;497:149–170. doi: 10.1007/978-1-4615-1335-3_16. [DOI] [PubMed] [Google Scholar]
- Romero MI, Smith GM. Adenoviral gene transfer into the normal and injured spinal cord: enhanced transgene stability by combined administration of temperature-sensitive virus and transient immune blockade. Gene Ther. 1998;5:1612–1621. doi: 10.1038/sj.gt.3300774. [DOI] [PubMed] [Google Scholar]
- Rosenberg MB, Friedmann T, Robertson RC, Tuszynski M, Wolff JA, Breakefield XO, Gage FH. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988;242:1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Plant GW, Hamers FP, Wortel J, Blits B, Dijkhuizen PA, Gispen WH, Boer GJ, Verhaagen J. Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. J Neurosci. 2003;23:7045–7058. doi: 10.1523/JNEUROSCI.23-18-07045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaria A, St. George JA, Gregory RJ, Noelle RJ, Wadsworth SC, Smith AE, Kaplan JM. Antibody to CD40 ligand inhibits both humoral and cellular immune responses to adenoviral vectors and facilitates repeated administration to mouse airway. Gene Ther. 1997;4:611–617. doi: 10.1038/sj.gt.3300431. [DOI] [PubMed] [Google Scholar]
- Selvaggi TA, Walker RE, Fleisher TA. Development of antibodies to fetal calf serum with Arthus-like reactions in human immunodeficiency virus-infected patients given syngeneic lymphocyte infusions. Blood. 1997;89:776–779. [PubMed] [Google Scholar]
- Smith TA, White BD, Gardner JM, Kaleko M, McClelland A. Transient immunosuppression permits successful repetitive intravenous administration of an adenovirus vector. Gene Ther. 1996;3:496–502. [PubMed] [Google Scholar]
- Strohmaier C, Carter BD, Urfer R, Barde YA, Dechant G. A splice variant of the neurotrophin receptor trkB with increased specificity for brain-derived neurotrophic factor. EMBO J. 1996;15:3332–3337. [PMC free article] [PubMed] [Google Scholar]
- Tan SA, Aebischer P. The problems of delivering neuroactive molecules to the CNS. Ciba Found Symp. 1996;196:211–236. doi: 10.1002/9780470514863.ch14. discussion 236–219. [DOI] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- Tomita N, Morishita R. Antisense oligonucleotides as a powerful molecular strategy for gene therapy in cardiovascular diseases. Curr Pharm Des. 2004;10:797–803. doi: 10.2174/1381612043452965. [DOI] [PubMed] [Google Scholar]
- Tsoulfas P, Soppet D, Escandon E, Tessarollo L, Mendoza-Ramirez JL, Rosenthal A, Nikolics K, Parada LF. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993;10:975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- Tuschong L, Soenen SL, Blaese RM, Candotti F, Muul LM. Immune response to fetal calf serum by two adenosine deaminase-deficient patients after T cell gene therapy. Hum Gene Ther. 2002;13:1605–1610. doi: 10.1089/10430340260201699. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Blesch A. Nerve growth factor: from animal models of cholinergic neuronal degeneration to gene therapy in Alzheimer’s disease. Prog Brain Res. 2004;146:441–449. doi: 10.1016/s0079-6123(03)46028-7. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Weidner N, McCormack M, Miller I, Powell H, Conner J. Grafts of genetically modified Schwann cells to the spinal cord: survival, axon growth, and myelination. Cell Transplant. 1998;7:187–196. doi: 10.1177/096368979800700213. [DOI] [PubMed] [Google Scholar]
- Urfer R, Tsoulfas P, Soppet D, Escandon E, Parada LF, Presta LG. The binding epitopes of neurotrophin-3 to its receptors trkC and gp75 and the design of a multifunctional human neurotrophin. EMBO J. 1994;13:5896–5909. doi: 10.1002/j.1460-2075.1994.tb06935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson DJ, Kobinger GP, Passini MA, Wilson JM, Wolfe JH. Targeted transduction patterns in the mouse brain by lentivirus vectors pseudotyped with VSV, Ebola, Mokola, LCMV, or MuLV envelope proteins. Mol Ther. 2002;5:528–537. doi: 10.1006/mthe.2002.0584. [DOI] [PubMed] [Google Scholar]
- Whittemore SR, White LA. Target regulation of neuronal differentiation in a temperature-sensitive cell line derived from medullary raphe. Brain Res. 1993;615:27–40. doi: 10.1016/0006-8993(93)91111-5. [DOI] [PubMed] [Google Scholar]
- Xu XM, Zhang S-X, Li H, Aebischer P, Bunge MB. Regrowth of axons into the distal spinal cord through a Schwann-cell-seeded mini-channel implanted into hemisected adult rat spinal cord. Eur J Neurosci. 1999;11:1723–1740. doi: 10.1046/j.1460-9568.1999.00591.x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Strappe PM, Lever AM, Franklin RJ. Lentiviral vectors for gene delivery to normal and demyelinated white matter. Glia. 2003;42:59–67. doi: 10.1002/glia.10195. [DOI] [PubMed] [Google Scholar]
- Zhou L, Baumgartner BJ, Hill-Felberg SJ, McGowen LR, Shine HD. Neurotrophin-3 expressed in situ induces axonal plasticity in the adult injured spinal cord. J Neurosci. 2003;23:1424–1431. doi: 10.1523/JNEUROSCI.23-04-01424.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsengeller ZK, Boivin GP, Sawchuk SS, Trapnell BC, Whitsett JA, Hirsch R. Anti-T cell receptor antibody prolongs transgene expression and reduces lung inflammation after adenovirus-mediated gene transfer. Hum Gene Ther. 1997;8:935–941. doi: 10.1089/hum.1997.8.8-935. [DOI] [PubMed] [Google Scholar]


