Abstract
Purpose
To compare the HDR point B to pelvic lymph node dose using 3D-planned brachytherapy for cervical cancer.
Materials and Methods
Patients with FIGO Stage IB-IIIB cervical cancer received 70 tandem HDR applications using CT-based treatment planning. The obturator, external and internal iliac lymph nodes (LN) were contoured. Per fraction (PF) and combined fraction (CF) right (R), left (L), and bilateral (Bil) nodal doses were analyzed. Point B dose was compared with LN dose-volume histogram (DVH) parameters by a paired t-test and Pearson correlation coefficients.
Results
The mean PF and CF doses to point B were R 1.40 Gy ±0.14 (CF: 7 Gy), L 1.43 ±0.15 (CF: 7.15 Gy), and Bil 1.41 ±0.15 (CF: 7.05 Gy). The correlation coefficients between point B and the D100, D90, D50, D2cc, D1cc, and D0.1cc LN were all less than 0.7. Only the D2cc to the obturator and the D0.1cc to the external iliac nodes were not significantly different from the point B dose. Significant differences between R and L nodal DVHs were seen, likely related to tandem deviation from irregular tumor anatomy.
Conclusions
With HDR brachytherapy for cervical cancer, the per fraction nodal dose approximates a dose equivalent to teletherapy. Point B is a poor surrogate for dose to specific nodal groups. 3D-defined nodal contours during brachytherapy provide a more accurate reflection of delivered dose, and should be part of comprehensive planning of the total dose to the pelvic nodes, particularly when there is evidence of pathologic involvement of nodes.
Keywords: HDR brachytherapy, cervical cancer, pelvic lymph nodes, point B
Introduction
Brachytherapy has been an integral component of cervical-cancer treatment for almost 100 years. Numerous studies have established the importance of brachytherapy in pelvic control and disease-specific survival. Inferior survival rates and more complications were observed in patients who received higher doses of external-beam radiation and concomitantly lower intracavitary doses.1,2
The standard dosimetric systems, including the Manchester, Paris, and Stockholm techniques, have a long and impressive record in the treatment and cure of cervical cancer. As originally described in 1938, the Manchester technique introduced a radium loading system designed to deliver a homogeneous dose distribution to a defined zone of tissue, known as the paracervical triangle.3 The point of limiting dose tolerance within the paracervical triangle was designated as point A, defined as 2 cm lateral to the central uterine canal and 2 cm from the mucous membrane of the lateral fornix in the axis of the uterus. Point B was designated as 5 cm from midline at the level of point A, and represented the dose delivered to the obturator lymph node, often the first echelon of metastatic spread.4 Point B was routinely recorded to calculate the cumulative dose to the pelvic sidewall delivered by brachytherapy and external-beam radiotherapy.
Subsequently, Gilbert Fletcher developed an afterloading system using mg-hr of radium. The Fletcher technique also defined a lymphatic trapezoid based upon bony landmarks on orthogonal films. The reference points of the lymphatic trapezoid represented the major lymph node chains, including the mid-external iliac, low common iliac, and para-aortic lymph nodes. These nodal groups were reported in the Fletcher system.5 The International Commission on Radiation Units and Measurements (ICRU) 38 recommends standard reporting of the lymphatic trapezoid and pelvic reference points.6 The ABS guidelines for high-dose-rate brachytherapy for cervical cancer recommend recording the brachytherapy component of the dose to the pelvic lymph nodes based on the ICRU 38 pelvic points, but not using point B for reporting or estimating nodal dose.7
With the advent of 3D imaging and treatment planning, evaluation of dose distributions to assess target coverage and spare normal tissue structures is feasible.8,9,10 Furthermore, CT imaging is feasible and reflective of OAR dose,11 and may also permit a more accurate description of dose delivered to the pelvic lymph nodes compared to point dosimetry. To examine the relationship between point B dose and the dose delivered to the pelvic nodal chains, we analyzed a series of HDR brachytherapy applications for cervical-cancer patients treated using CT-based treatment planning.
Materials and Methods
Seventy tandem-based HDR applications of 5–5.5 Gy for 5 fractions were performed on 14 patients with FIGO Stage IB-IIIB cervical cancer at Brigham and Women’s Hospital/Dana-Farber Cancer Institute from July 2005 to May 2007. The applicator type was determined by the initial extent of disease and the clinical and/or radiographic response to external-beam radiotherapy. All applicators were CT-compatible (Nucletron, Veenendaal, The Netherlands), and included tandem and ovoid applicator for 33 cases, tandem and ring applicator for 30 fractions, and tandem and cylinder applicator for 7 HDR treatments. Prior to imaging, 40 cc of barium was placed into the rectum and 60 cc of 1:10 hypaque contrast was instilled into the bladder.
Following insertion of the HDR applicator, the applicator was fixed to a brachytherapy board and a CT was obtained. Transverse images of the pelvis were acquired in 1.25-mm increments on a dedicated CT simulator (High Speed CT/I, GE Medical Systems, Milwaukee, WI) for CT-based treatment planning (PLATO brachytherapy planning system, Nucletron). A radiation oncologist contoured an estimate of the high-risk clinical target volume (HR-CTV) and of the OAR on each axial CT image. The HR-CTV was defined by clinical exam findings and by a pelvic MRI obtained prior to the first brachytherapy fraction. Previously published guidelines describe the contouring technique for CT-based treatment planning, which included the entire cervix, parametria, or extent of disease at the time of brachytherapy; the superior border of the tumor or cervix was determined by the pre-implantation MRI.11 The OAR, including the bladder, rectum, and sigmoid, were visualized based on the presence of contrast.
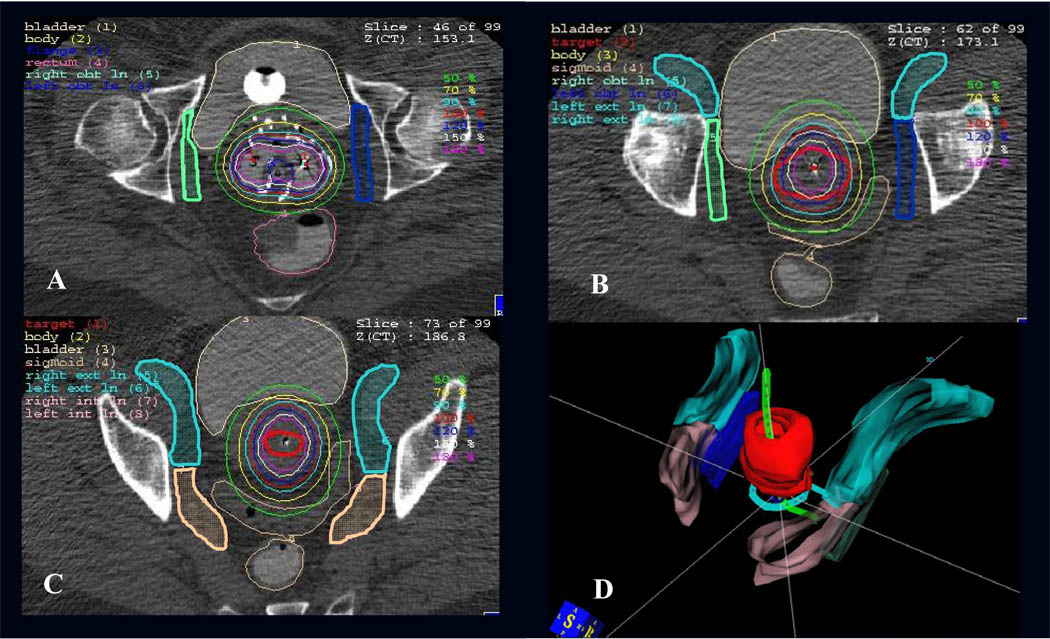
The pelvic lymph node contours were developed in conjunction with a genitourinary radiologist, based on modified Radiation Therapy Oncology Group (RTOG) definitions of pelvic nodal groups for Intensity Modulated Radiation Therapy (IMRT)12 and the published radiographic literature.13,14 Based on CT anatomy, the obturator nodal contour encompassed the obturator space, which lies within a triangle between the external and internal iliac vessels. As shown in Figure 1a–b, the obturator contour encompassed a region 2–2.5 centimeters in length that began inferiorly at the level of the fovea of the femoral head, and extended superiorly to the roof of the acetabulum. The lateral margin was the internal obturator muscle along the pelvic sidewall. The contour did not extend into adjacent muscle, bone, or OAR. The external iliac nodal contour included the external iliac artery and vein, with a 7–10-mm vessel margin to encompass the medial, anterior, and lateral subgroups of the external iliac chain, as shown in Figure 1b–c. The contour began inferiorly at the roof of the acetabulum before the external iliac vessels pass through the femoral canal. Superiorly, the external iliac contour extended to the bottom of the sacroiliac joint, as limited by the number of acquired images. The anterior border extended anterolaterally along the iliopsoas muscle to include the lateral external iliac nodes. The internal iliac nodal group encompassed the internal iliac artery and vein and their subsequent branches, as shown in Figure 1c. The superior extent of the internal iliac nodal chain was the bottom of the sacroiliac joint, and the lateral border extended along the pelvic sidewall.
Figure 1.
Axial CT images with sample contours of the obturator nodal group extending from the fovea of the femoral head inferiorly (A) to the acetabular roof superiorly (B). The external iliac chain encompassed the vessels before entry into the femoral canal (B). The external and internal iliac contours extended superiorly to the bottom of the SI joint (C). Panel D shows a 3D reconstruction of the HR-CTV (red) and pelvic nodal contours with a tandem and ring applicator.
For initial treatment planning, a standard loading pattern was used to create a pear-shaped isodose distribution that delivered the prescription dose to point A. For 51 brachytherapy applications, point A was defined as 2 cm superior to the external os or flange and 2 cm lateral to the intrauterine tandem. In 4 patients in whom 3D imaging and clinical examination showed no residual disease but significant shrinkage of the uterus and cervix after external-beam radiotherapy, point A was designated specifically as 2 cm superior to the os or flange, and 1.5 cm lateral from the tandem in 14 applications. The one remaining patient had 5 applications with point A at a variable width of approximately 2.5 cm in order to cover the tumor volume adequately. Point B was defined as 2 cm above the os or flange and 5 cm lateral from midline for all cases. Source dwell times were initially weighted to minimize dose to the OAR, and optimized for coverage of the HR-CTV.
Dose-volume histograms (DVHs) were generated for the HR-CTV, rectum, bladder, sigmoid, and for the six pelvic nodal groups, including the right and left obturator group, and the external and internal iliac chains. The dose constraint for the OAR was based on the D2cc dose to the bladder (90 Gy), rectum, and sigmoid (70–75 Gy). DVH parameters represented the dose received by 100%, 90%, or 50% of the volume (D100, D90, D50), and the nodal dose received by 0.1 (D0.1cc) or 2 cc (D2cc) of tissue. The DVH parameters were selected to represent the minimum, maximum and median doses to the nodal volumes, as well as standard reporting parameters for normal tissues (D1cc, D2cc) and target volumes (D90). The fractional brachytherapy dose was reported separately and also as an average dose from right and left sides for point A, point B, the obturator group, the external iliac chain, and the internal iliac chain. Treatment was delivered using an iridium-192 afterloading system (Nucletron).
Using a 2-sided paired t-test, point B dose was compared with the DVH parameters for each nodal group. To examine the relationship of point B dose with the DVH parameters, Pearson correlation coefficients were determined. Of the 70 HDR fractions, one data set was corrupted; therefore, 69 applications form the final data set for analysis.
Results
The median patient age was 54 years (range, 29–84 years). The FIGO clinical stage was 1B1 in 1 patient, IIA in 6 patients, IIB in 4 patients, and IIIB in 3 patients. Tumor histology was squamous in 8 patients, adenosquamous in 2 patients, adenocarcioma in 2 patients, and poorly differentiated carcinoma in 2 patients. The median pretreatment tumor diameter was 4.4 cm (range, 3–6.2 cm) measured by MRI in 12 patients, CT in 1 patient, and clinical exam in 1 patient. The median tumor size prior to intracavitary brachytherapy was 1.8 cm (range, 1.3–2.2 cm) measured by MRI. Twelve patients had a PET scan as part of their staging evaluation, of which 8 patients had PET-positive nodal disease. The median dose to the pelvis was 4500 cGy (range, 4320–5940 cGy). Thirteen patients received concurrent platinum-based chemotherapy. All patients received a total of 5 HDR brachytherapy applications. The median tandem length for all cases was 6.0 cm, and the median ovoid, ring, and cylinder size was 3.0 cm. The median prescribed dose was 5.5 Gy (range, 5.0–5.5 Gy), and the median dose to point A was 5.5 Gy (range, 3.49–6.26 Gy). The bilateral (Bil) median dose to point B for all 69 analyzed cases was 1.37 Gy (range 0.90–1.75 Gy), or 26% of the prescription dose.
The mean volume of the HR-CTV was 23.3 ± 8.6 cm3. For HDR applications with point A at 2 cm, the mean volume of the HR-CTV was significantly greater than for applications with point A at 1.5 cm, 26.1 cm3 versus 16.1 cm3, respectively (p<0.0001). As shown in Table 1, the mean D90 was greater than the median prescription dose for all groups, and the mean volume receiving the prescription dose (V100) was greater than 90%.
Table 1.
Summary of the mean target coverage parameters for the high-risk CTV
| HR-CTV (cm3) | D90 (Gy) | V100 (%) | |
|---|---|---|---|
| All patients | 23.3 ± 8.6 | 5.58 ± 0.64 | 91.3 ± 6.2 |
| Point A at 2 cm | 26.1 ± 8.0 | 5.64 ± 0.65 | 91.0 ± 6.5 |
| Point A at 1.5 cm | 16.1 ± 2.3 | 5.60 ± 0.51 | 92.5 ± 4.5 |
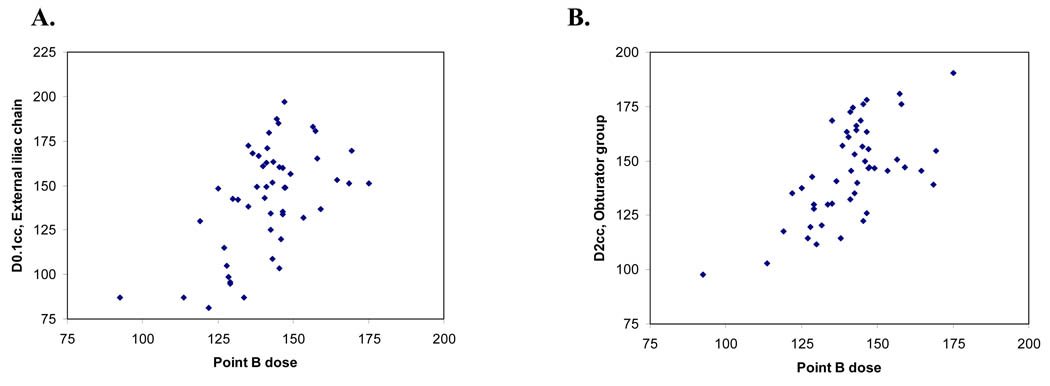
For the 50 applications with point A at 2 cm, the mean bilateral dose to point B was 1.41 ± 0.15 Gy (1.40 Gy R, 1.43 Gy L). The DVH parameters and correlation coefficients for the pelvic nodal groups are summarized in Table 2. Similar results were obtained when all 69 cases were analyzed together (Table 3). The correlation coefficients were all less than 0.7, indicating a relatively low degree of correlation. The majority of values were significantly different from point B on t-test, except for the obturator nodal group and external iliac chain with point A designated at 2 cm. The mean D2cc for the obturator group was 1.45 ± 0.21 Gy, (p=0.19), which was significantly correlated with point B dose [correlation coefficient (cor)= 0.62, p<0.0001], as shown in Table 2 and Figure 2A. The D100, D90, D50, D1cc and D0.1cc for the obturator group were statistically different from point B dose (paired t-test, all p<0.0001). For the external iliac chain, the mean D2cc was 1.10 ± 0.26 Gy, which was not equivalent to point B dose (t-test, p<0.0001), although there was a significant correlation (cor=0.57, p<0.0001). The DVH parameter for the external iliac chain that most closely represented point B dose was the D0.1cc. The mean D0.1cc was 1.42 ± 0.30 Gy (t-test, p=0.79) with a correlation coefficient of 0.56, p<0.0001 (Table 2 and Figure 2B). For the internal iliac nodes, the mean D2cc was 1.52 ± 0.29 Gy (t-test, p=0.007, cor=0.39 (p=0.005) and the mean D0.1cc was 1.92 ± 0.38 Gy (t-test, p<0.0001), cor=0.32 (p=0.025). This study did not identify a DVH parameter for the internal iliac chain that represented point B dose.
Table 2.
DVH parameters and correlation coefficients for the obturator, external iliac and internal iliac nodal groups representing 50 brachytherapy applications with point A at 2 cm.
| Right mean dose (Gy) |
Cor | p value |
t-test | Left mean dose (Gy) |
Cor | p value | t-test | Bilateral Mean dose (Gy)* |
Cor | p value | t-test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obturator nodal group | ||||||||||||
| D100 | 0.59± 0.17 | 0.37 | 0.009 | <0.0001 | 0.75±0.26 | 0.62 | <0.0001 | <0.0001 | 0.67±0.17 | 0.66 | <0.0001 | <0.0001 |
| D90 | 0.85±0.22 | 0.24 | 0.09 | <0.0001 | 1.03±0.29 | 0.58 | <0.0001 | <0.0001 | 0.94±0.18 | 0.60 | <0.0001 | <0.0001 |
| D50 | 1.13±0.30 | 0.09 | 0.51 | <0.0001 | 1.36±0.36 | 0.48 | 0.0005 | 0.18 | 1.25±0.19 | 0.51 | 0.0002 | <0.0001 |
| 2cc | 1.30±0.31 | 0.13 | 0.37 | 0.03 | 1.59±0.45 | 0.49 | 0.0004 | 0.005 | 1.45±0.21 | 0.62 | <0.0001 | 0.19 |
| 1 cc | 1.43±0.36 | 0.09 | 0.53 | 0.62 | 1.73±0.48 | 0.46 | 0.0008 | <0.0001 | 1.58±0.22 | 0.56 | <0.0001 | <0.0001 |
| 0.1 cc | 1.70±0.44 | 0.06 | 0.68 | <0.0001 | 2.02±0.54 | 0.43 | 0.002 | <0.0001 | 1.86±0.26 | 0.49 | <0.0001 | <0.0001 |
| External iliac nodes | ||||||||||||
| D100 | 0.20±0.09 | 0.26 | 0.07 | <0.0001 | 0.26±0.10 | 0.57 | <0.0001 | <0.0001 | 0.23±0.07 | 0.53 | <0.0001 | <0.0001 |
| D90 | 0.44±0.13 | 0.29 | 0.04 | <0.0001 | 0.49±0.13 | 0.56 | <0.0001 | <0.0001 | 0.46±0.11 | 0.49 | 0.0003 | <0.0001 |
| D50 | 0.74±0.22 | 0.31 | 0.03 | <0.0001 | 0.83±0.23 | 0.59 | <0.0001 | <0.0001 | 0.79±0.18 | 0.56 | <0.0001 | <0.0001 |
| 2 cc | 1.04±0.33 | 0.31 | 0.03 | <0.0001 | 1.16±0.35 | 0.56 | <0.0001 | <0.0001 | 1.10±0.26 | 0.57 | <0.0001 | <0.0001 |
| 1 cc | 1.12±0.35 | 0.29 | 0.04 | <0.0001 | 1.27±0.38 | 0.55 | <0.0001 | 0.001 | 1.20±0.28 | 0.56 | <0.0001 | <0.0001 |
| 0.1 cc | 1.35±0.40 | 0.27 | 0.06 | 0.32 | 1.50±0.42 | 0.53 | <0.0001 | 0.15 | 1.42±0.30 | 0.56 | <0.0001 | 0.79 |
| Internal iliac nodes | ||||||||||||
| D100 | 0.66±0.27 | 0.15 | 0.30 | <0.0001 | 0.75±0.24 | 0.54 | <0.0001 | <0.0001 | 0.70±0.17 | 0.50 | 0.0002 | <0.0001 |
| D90 | 0.87±0.29 | 0.16 | 0.27 | <0.0001 | 1.00±0.28 | 0.50 | 0.0002 | <0.0001 | 0.94±0.18 | 0.51 | 0.0001 | <0.0001 |
| D50 | 1.14±0.38 | 0.13 | 0.38 | <0.0001 | 1.29±0.35 | 0.44 | 0.0014 | 0.003 | 1.21±0.22 | 0.46 | 0.0008 | <0.0001 |
| 2 cc | 1.45±0.45 | 0.13 | 0.36 | 0.48 | 1.60±0.46 | 0.36 | 0.01 | 0.007 | 1.52±0.29 | 0.39 | 0.005 | 0.007 |
| 1 cc | 1.56±0.50 | 0.12 | 0.42 | 0.03 | 1.72±0.50 | 0.34 | 0.02 | 0.0001 | 1.64±0.32 | 0.37 | 0.009 | <0.0001 |
| 0.1 cc | 1.83±0.60 | 0.10 | 0.51 | <0.0001 | 2.00±0.59 | 0.30 | 0.03 | <0.0001 | 1.92±0.38 | 0.32 | 0.025 | <0.0001 |
| Point B dose | 1.40±0.14 | 1.43±0.15 | 1.41±0.15 | |||||||||
Key: Cor=correlation coefficient
Table 3.
DVH parameters and correlation coefficients for the obturator, external iliac and internal iliac nodal groups for all 69 brachytherapy applications.
| Right mean dose (Gy) |
Cor | p value |
t-test | Left mean dose (Gy) |
Cor | p value | t-test | Bilateral Mean dose (Gy)* |
Cor | p value | t-test | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Obturator nodal group | ||||||||||||
| D100 | 0.60±0.18 | 0.27 | 0.02 | <0.0001 | 0.70±0.25 | 0.61 | <0.0001 | <0.0001 | 0.65±0.17 | 0.59 | <0.0001 | <0.0001 |
| D90 | 0.85±0.23 | 0.23 | 0.06 | <0.0001 | 0.97±0.29 | 0.61 | <0.0001 | <0.0001 | 0.91±0.19 | 0.58 | <0.0001 | <0.0001 |
| D50 | 1.16±0.32 | 0.12 | 0.32 | 0.0006 | 1.29±0.37 | 0.53 | <0.0001 | 0.32 | 1.23±0.22 | 0.51 | <0.0001 | 0.0003 |
| 2cc | 1.36±0.38 | 0.08 | 0.50 | 0.35 | 1.53±0.44 | 0.49 | <0.0001 | 0.0001 | 1.44±0.25 | 0.47 | <0.0001 | 0.0001 |
| 1 cc | 1.50±0.44 | 0.07 | 0.59 | 0.002 | 1.66±0.48 | 0.48 | <0.0001 | <0.0001 | 1.58±0.28 | 0.44 | 0.0002 | <0.0001 |
| 0.1 cc | 1.80±0.57 | 0.05 | 0.72 | <0.0001 | 1.96±0.54 | 0.45 | 0.0001 | <0.0001 | 1.88±0.35 | 0.36 | 0.002 | <0.0001 |
| External iliac nodes | ||||||||||||
| D100 | 0.21±0.09 | 0.15 | 0.22 | <0.0001 | 0.24±0.10 | 0.59 | <0.0001 | <0.0001 | 0.22±0.08 | 0.46 | <0.0001 | <0.0001 |
| D90 | 0.44±0.12 | 0.23 | 0.06 | <0.0001 | 0.47±0.13 | 0.49 | <0.0001 | <0.0001 | 0.46±0.11 | 0.41 | 0.0005 | <0.0001 |
| D50 | 0.75±0.20 | 0.26 | 0.03 | <0.0001 | 0.80±0.22 | 0.53 | <0.0001 | <0.0001 | 0.77±0.17 | 0.50 | <0.0001 | <0.0001 |
| 2 cc | 1.04±0.30 | 0.26 | 0.03 | <0.0001 | 1.13±0.33 | 0.46 | <0.0001 | <0.0001 | 1.08±0.24 | 0.46 | <0.0001 | <0.0001 |
| 1 cc | 1.13±0.32 | 0.24 | 0.047 | <0.0001 | 1.22±0.35 | 0.44 | 0.0001 | 0.008 | 1.18±0.25 | 0.46 | <0.0001 | <0.0001 |
| 0.1 cc | 1.35±0.37 | 0.23 | 0.06 | 0.37 | 1.45±0.40 | 0.43 | 0.0002 | 0.006 | 1.40±0.27 | 0.46 | <0.0001 | 0.0096 |
| Internal iliac nodes | ||||||||||||
| D100 | 0.66±0.25 | 0.18 | 0.14 | <0.0001 | 0.71±0.23 | 0.52 | <0.0001 | <0.0001 | 0.69±0.16 | 0.50 | <0.0001 | <0.0001 |
| D90 | 0.88±0.28 | 0.19 | 0.12 | <0.0001 | 0.95±0.27 | 0.51 | <0.0001 | <0.0001 | 0.92±0.18 | 0.52 | <0.0001 | <0.0001 |
| D50 | 1.15±0.36 | 0.16 | 0.20 | 0.0009 | 1.23±0.34 | 0.46 | <0.0001 | 0.01 | 1.19±0.22 | 0.47 | <0.0001 | <0.0001 |
| 2 cc | 1.45±0.42 | 0.16 | 0.19 | 0.008 | 1.54±0.46 | 0.36 | 0.002 | 0.0001 | 1.50±0.29 | 0.39 | 0.0009 | <0.0001 |
| 1 cc | 1.57±0.46 | 0.15 | 0.22 | <0.0001 | 1.66±0.51 | 0.35 | 0.003 | <0.0001 | 1.62±0.32 | 0.38 | 0.002 | <0.0001 |
| 0.1 cc | 1.84±0.56 | 0.13 | 0.27 | <0.0001 | 1.94±0.63 | 0.30 | 0.011 | <0.0001 | 1.89±0.39 | 0.33 | 0.006 | <0.0001 |
| Point B dose | 1.31±0.20 | 1.33±0.21 | 1.32±0.21 | |||||||||
Key: Cor=correlation coefficient
Figure 2.
Correlation of (A) Point B dose and D2cc of the obturator group and (B) Point B dose and D0.1cc of the external iliac chain.
For the subset of tandem-based applications with point A at 1.5 cm, the mean point B dose was 1.09 ± 0.15 Gy. The DVH parameters that most closely represented point B dose were D50 for the obturator group (1.09±0.28 Gy, t-test p=0.86), D1cc and D2cc for the external iliac group (1.11±0.19 Gy, t-test p=0.69 and 1.02±0.18 Gy, t-test p=0.08), and D50 for the internal iliac group (1.13±0.24 Gy, t-test p=0.32). When the entire cohort of 69 applications was analyzed, there were no representative DVH parameters identified correlated to point B (Table 3).
We also studied the relationship between point B dose and the dose to the pelvic lymph nodes for the 50 cases with point A at 2 cm only, including 23 tandem and ring and 27 tandem and ovoid applications, as summarized in Table 4. Although the sample size is small and somewhat heterogeneous, the dose to the internal iliac lymph nodes was higher by 0.22–0.29 Gy for the ring applicator when compared by a t-test. The mean D2cc for the obturator group and D0.1cc for the external iliac chain remained equivalent to point B dose when the tandem and ring and tandem and ovoid applications were analyzed individually.
Table 4.
Comparison of DVH parameters and point B dose for the obturator, external iliac and internal iliac nodal groups by applicator type. Data includes 27 tandem and ovoids (T&O) and 23 tandem and rings (T&R) for point A at 2 cm.
| T&O Mean dose(Gy) |
T&R Mean dose (Gy) |
Δ (Gy) | t-test | |
|---|---|---|---|---|
| Obturator nodal group | ||||
| D100 | 0.73 ± 0.15 | 0.61 ± 0.17 | −0.12 | 0.010 |
| D90 | 1.00 ± 0.16 | 0.87 ± 0.19 | −0.13 | 0.013 |
| D50 | 1.31 ± 0.17 | 1.19 ± 0.20 | −0.12 | 0.033 |
| 2 cc | 1.50 ± 0.22 | 1.41 ± 0.21 | −0.09 | 0.084 |
| 1 cc | 1.64 ± 0.24 | 1.54 ± 0.23 | −0.10 | 0.068 |
| 0.1 cc | 1.94 ± 0.29 | 1.81 ± 0.25 | −0.13 | 0.066 |
| External iliac lymph nodes | ||||
| D100 | 0.26 ± 0.08 | 0.20 ± 0.05 | −0.06 | 0.004 |
| D90 | 0.49 ± 0.12 | 0.43 ± 0.09 | −0.06 | 0.085 |
| D50 | 0.81 ± 0.20 | 0.76 ± 0.16 | −0.05 | 0.305 |
| 2 cc | 1.12 ± 0.29 | 1.08 ± 0.24 | −0.04 | 0.600 |
| 1 cc | 1.22 ± 0.30 | 1.17 ± 0.25 | −0.04 | 0.526 |
| 0.1 cc | 1.44 ± 0.31 | 1.40 ± 0.28 | −0.04 | 0.602 |
| Internal iliac lymph nodes | ||||
| D100 | 0.68 ± 0.17 | 0.73 ± 0.18 | 0.04 | 0.371 |
| D90 | 0.91 ± 0.18 | 0.96 ± 0.18 | 0.05 | 0.343 |
| D50 | 1.16 ± 0.22 | 1.27 ± 0.21 | 0.11 | 0.064 |
| 2 cc | 1.42 ± 0.28 | 1.64 ± 0.27 | 0.22 | 0.007 |
| 1 cc | 1.53 ± 0.29 | 1.77 ± 0.31 | 0.24 | 0.008 |
| 0.1 cc | 1.79 ± 0.33 | 2.07 ± 0.39 | 0.29 | 0.007 |
| Point B dose | 1.45 ± 0.15 | 1.38 ± 0.14 | −0.07 | 0.088 |
Discussion
This study represents the first dosimetric analysis of HDR fractional dose delivered to the pelvic lymph nodes in 3D-planned brachytherapy for cervical cancer, and demonstrates the importance of lymph node contouring particularly in patients with involved nodes. We found that point B receives a substantial fractional dose by HDR brachytherapy, ranging from 0.9–1.75 Gy. The median point B dose was 1.4 Gy, which represents 25% of the HDR prescription dose and resulted in an average 7 Gy of additional dose to point B from brachytherapy. However, overall, point B was not reflective of the contoured nodal dose. The dose to the contoured lymph nodes ranged from 1.0 to 1.5 Gy per fraction. In patients who have enlarged nodal disease and receive a 3D conformal or IMRT external-beam boost dose of radiation, the contribution of dose to the 3D contoured lymph nodes from brachytherapy should be recorded in order to report the cumulative nodal dose.
Overall, nodal contours more accurately defined nodal dose when compared with point B. Only two DVH parameters correlated with point B, including the D2cc of the obturator group and the D0.1cc of the external iliac chain. For the obturator group, the nodal volume received a minimum dose of 0.67 Gy (mean D100) and a maximum dose of 1.86 Gy (mean D0.1cc). The external iliac chain had a similar dose range, with the minimum dose to the nodal volume as low as 0.23 Gy, and a maximum point dose of 1.42 Gy. Because the superior extent of the iliac chain was designated as the bottom of the SI joint, as mandated by the institutional scanning protocol, the minimum dose may be an overestimate of the dose delivered to the most proximal portion of the external iliac nodal chain. The fractional HDR dose delivered to the internal iliac chain ranged from 0.70–1.92 Gy.
In this cohort of patients, the median dose delivered to point A was equivalent to the prescription dose. As recommended by the Groupe Europeen de Curietherapie-European Society for Therapeutic Radiology and Oncology (GEC ESTRO) working group guidelines published in 2005,8, 9 the treatment planning process began with the standard method of dose prescription to point A, followed by stepwise adjustments to the loading pattern. Coverage of the HR-CTV was assessed, and the isodose distributions were optimized to maximize HR-CTV coverage and spare the OAR. It is important to consider the potential implications of adopting new image-based technology. For example, loading of the full length of the intrauterine tandem in standard loading brachytherapy not only delivers dose to the entire endometrial canal, but may also contribute significantly to delivering dose to the adjacent pelvic lymph nodes. As the coverage of the HR-CTV is optimized, the dose delivered to the pelvic sidewall may change substantially, depending on the size of the treatment volume and its symmetry in relation to the midline. As expected, the median dose to point B with point A at 1.5 cm was significantly less than with point A at 2 cm. Furthermore, the representative DVH parameters for the nodal chains differed when the prescription point was modified. These findings are also subject to change based on applicator type, applicator position, and individual patient anatomy.
The doses to the left and to the right nodal chains were significantly different, reflecting variations in applicator position, primarily resulting from changes secondary to tumor, such as irregular shrinkage during external beam treatment, unilateral parametrial shortening due to tumor invasion, or sidewall fixation. Therefore, for patients with enlarged nodes requiring additional radiation dose to the nodes, contouring the nodes with each fraction of brachytherapy will more accurately assess the dose received, as the location of the applicator may shift between fractions.
When the fractional dose delivered to the nodal chain was analyzed by applicator type, D2cc of the obturator group and D0.1cc of the external iliac chain remained equivalent to point B dose for both the tandem and ring and tandem and ovoid applicators. However, the tandem and ring applicators delivered a higher dose to the internal iliac chain in this subset of patients. This observation may be due to source loading in the more posterior dwell positions of the ring.
One series of low-dose-rate brachytherapy in 30 patients with cervical carcinoma performed CT-imaging and contoured pelvic nodes.15 When the iliac vessels were contoured as surrogates for pelvic nodal groups, the study found that the average dose to point B most closely correlated with the maximum common iliac dose. Point B underestimated the maximum dose rate and overestimated the minimal dose rate to the external and internal nodal chains in most cases, although there was substantial variation among patients in these measures.
In addition to 3D-based brachytherapy, the use of IMRT for dose escalation has been advocated, and in cervical cancer, IMRT as a nodal boost delivers therapeutic doses for unresectable nodal disease.16 Given the high brachytherapy fractional dose to the pelvic lymph nodes, contouring the nodes in patients requiring nodal boosts may be helpful in planning the cumulative nodal dose. Although point B may be a surrogate in some cases, the relatively low correlation coefficient indicates that contouring nodes is a more accurate determinant of nodal dose than the single-point estimate. Future studies are needed to compare clinical outcomes for patients with nodal disease treated with image-guided HDR brachytherapy.
Acknowledgments
Thank you to Cynthia Tanaka for patient data management and handling regulatory issues, Barbara Silver for thoughtful comments and to Desmond O’Farrell and Erlian Lu for treatment planning and assistance with image retrieval.
Footnotes
Presented as an oral presentation at the American Brachytherapy Society-GEC ESTRO World Congress, May 5, 2008
Conflict of Interest Notification: The authors report no potential conflicts of interest.
References
- 1.Coia L, Won M, Lanciano R, Marcial VA, Martz K, Hanks G. The Patterns of Care Outcome Study for cancer of the uterine cervix. Results of the Second National Practice Survey. Cancer. 1990;66(12):2451–2456. doi: 10.1002/1097-0142(19901215)66:12<2451::aid-cncr2820661202>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys. 1999;43(4):763–775. doi: 10.1016/s0360-3016(98)00482-9. [DOI] [PubMed] [Google Scholar]
- 3.Tod MCMW. A dosage system for use in treatment of cancer of the uterine cervix. Br J Radiol. 1938;11:809. [Google Scholar]
- 4.Sakuragi N, Satoh C, Takeda N, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with Stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85(7):1547–1554. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher G. Textbook of Radiotherapy. 3rd ed. Philadelphia: Lea & Febiger; 1980. [Google Scholar]
- 6.Dose and Volume Specification for Reporting Intracavitary Therapy in Gynecology (Report 38): International Commission on Radiation Units and Measurements. 1985
- 7.Nag S, Erickson B, Thomadsen B, Orton C, Demanes JD, Petereit D. The American Brachytherapy Society recommendations for high-dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys. 2000;48(1):201–211. doi: 10.1016/s0360-3016(00)00497-1. [DOI] [PubMed] [Google Scholar]
- 8.Haie-Meder C, Potter R, Van Limbergen E, et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (I): concepts and terms in 3D image based 3D treatment planning in cervix cancer brachytherapy with emphasis on MRI assessment of GTV and CTV. Radiother Oncol. 2005;74(3):235–245. doi: 10.1016/j.radonc.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol. 2006;78(1):67–77. doi: 10.1016/j.radonc.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Potter R, Dimopoulos J, Georg P, et al. Clinical impact of MRI assisted dose volume adaptation and dose escalation in brachytherapy of locally advanced cervix cancer. Radiother Oncol. 2007;83(2):148–155. doi: 10.1016/j.radonc.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Viswanathan AN, Dimopoulos J, Kirisits C, Berger D, Potter R. Computed tomography versus magnetic resonance imaging-based contouring in cervical cancer brachytherapy: results of a prospective trial and preliminary guidelines for standardized contours. Int J Radiat Oncol Biol Phys. 2007;68(2):491–498. doi: 10.1016/j.ijrobp.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Small W, Jr, Mell LK, Anderson P, et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71(2):428–434. doi: 10.1016/j.ijrobp.2007.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Portaluri M, Bambace S, Perez C, Angone G. A three-dimensional definition of nodal spaces on the basis of CT images showing enlarged nodes for pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(4):1101–1107. doi: 10.1016/j.ijrobp.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Taylor A, Rockall AG, Reznek RH, Powell ME. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63(5):1604–1612. doi: 10.1016/j.ijrobp.2005.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Gebara WJ, Weeks KJ, Jones EL, Montana GS, Anscher MS. Carcinoma of the uterine cervix: a 3D - CT analysis of dose to the internal, external and common iliac nodes in tandem and ovoid applications. Radiother Oncol. 2000;56(1):43–48. doi: 10.1016/s0167-8140(00)00176-6. [DOI] [PubMed] [Google Scholar]
- 16.Portelance L, Chao KS, Grigsby PW, Bennet H, Low D. Intensity-modulated radiation therapy (IMRT) reduces small bowel, rectum, and bladder doses in patients with cervical cancer receiving pelvic and para-aortic irradiation. Int J Radiat Oncol Biol Phys. 2001;51(1):261–266. doi: 10.1016/s0360-3016(01)01664-9. [DOI] [PubMed] [Google Scholar]