Abstract
Bone morphogenetic proteins (BMPs) play diverse roles in many aspects of skeletal development and bone homeostasis. During endochondral ossification, tight regulation of BMP activity is required to assure proper survival, proliferation and differentiation of skeletal progenitor cells into chondrocytes and osteoblasts. BMP3, a structurally divergent member of the BMP family, acts as a negative regulator of bone formation by limiting BMP signal transduction. In this study, we focus on the chick limb where we find BMP3 has a unique localization pattern with strong expression in the developing perichondrium. Overexpression of BMP3 in chick wing bud at the onset of chondrogenesis, using replication competent retrovirus, reduces BMP signaling leading to increased cell proliferation and delayed cell differentiation, resulting in expanded skeletal elements and joint fusions. Our results suggest that BMP3 expression in the perichondrium may serve to regulate cartilage cell proliferation by modulating the levels of BMP signaling, thus ensuring proper endochondral ossification.
Keywords: BMP, BMP3, ActRIIB, perichondrium, chick limb
INTRODUCTION
Bone morphogenetic proteins (BMPs), members of the transforming growth factor-β superfamily of structurally related secreted growth factors, are expressed throughout the developing skeleton where they influence cell type specification, cell differentiation, and apoptosis during endochondral ossification. In the skeleton, the primary way BMPs affect their target cells is by binding to receptor complexes consisting of combinations of type I (Alk2, Alk3, or Alk6) and type II (BMPR-II or ActRIIA, ActRIIB) serine-threonine kinases that then activate the canonical BMP-specific Smad signaling pathway (R-Smads 1,5,8), or the MAPK pathway (Derynck and Zhang, 2003). As BMP receptors are present in various combinations on virtually all skeletal cell types, BMP activity must be tightly regulated both spatially and temporally to ensure proper bone development (Canalis et al., 2003). One way in which precise control of BMP signals occurs is through the formation of BMP ligand and antagonist complexes that prevent BMPs from engaging their receptors. Antagonists such as noggin, chordin, gremlin, and follistatin are secreted into the extracellular space where they bind with high affinity to BMPs, and dampen BMP signaling (Balemans and Van Hul, 2002). Mice lacking noggin exhibit massive cartilage overgrowth and failure to form joints identifying BMPs as regulators of chondrocyte proliferation and highlighting the importance of controlling BMP signaling during skeletal development (Brunet et al., 1998).
Another level of control of BMP activity in the skeleton appears to come from BMP3, a molecule structurally related to BMPs but without osteoinductive potential (Bahamonde and Lyons, 2001; Daluiski et al., 2001; Hino et al., 2004). BMP3 is not a classic TGF-β–like signaling molecule as it primarily binds ActRIIB (Allen-dorph et al., 2007), a receptor common to both the BMP and activin signaling pathways, but is unable to activate receptor regulated Smads (Gamer et al., 2005). The net result of the BMP3-ActRIIB interaction is a decrease in BMP and/or activin signaling depending on the cell context (Gamer et al., 2005). In the mammalian skeleton, BMP3 serves to reduce BMP activity as mice lacking BMP3 exhibit increased bone mass while those overexpressing BMP3 in bone show delayed endochondral ossification with spontaneous rib fractures (Daluiski et al., 2001; Gamer et al., 2006). Both of these phenotypes appear to be due to changes in the differentiation of osteo-progenitor cells into secretory osteoblasts, with loss of BMP3 increasing the formation of mature osteoblasts and gain of BMP3 delaying osteoblast maturation (Tsuji et al., 2006; Gamer et al., 2006).
Here, we report the expression pattern of BMP3 in the chick limb and show that BMP3 localizes to the developing perichondrium, outlining the future cartilage elements, but is not expressed by condensing mesenchymal cells. We find that misexpression of BMP3 in the chick limb reduces BMP signaling, enhancing chondrocyte proliferation before hypertrophic chondrocyte differentiation, thus widening skeletal elements and producing joint fusions. Our results suggest that perichondrial BMP3 may regulate chondrocyte proliferation by modulating the level and timing of BMP signaling through ActRIIB, therefore ensuring proper formation of individual skeletal elements during bone formation.
RESULTS AND DISCUSSION
Expression of BMP3 During Chick Limb Development
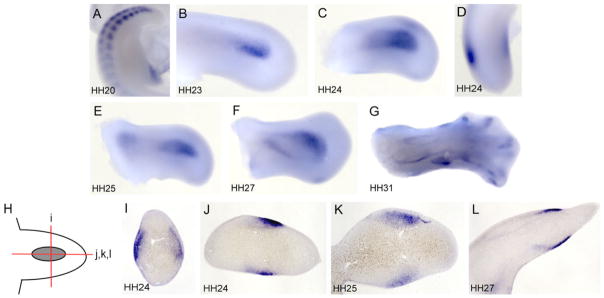
We examined the temporal and spatial expression pattern of BMP3 by whole-mount in situ hybridization. At stage 19–20 when distinct limb buds can be seen, BMP3 was strongly expressed in the developing somites in the posterior of the embryo but was not yet expressed in the limb region (Fig. 1A). Transcripts for BMP3 were first detected in the limb bud at stage 22–23 in the proximal dorsal and ventral mesenchyme (Fig. 1B). At stage 24, the BMP3 expression domain widens and expands distally in the limb mesenchyme and appears stronger on the dorsal versus the ventral side (Fig. 1C,D,I). Histological analysis of the whole-mount in situs revealed that BMP3 transcripts were found in subepidermal mesenchymal cell layers in the dorsal and ventral margins of the limb bud where they start to prefigure the future cartilage elements (Fig. 1I,J). BMP3 transcripts were not detected in the central core mesenchyme that will condense to form the leg bones (Fig. 1I,J,). At stage 25–27, BMP3 expression is seen in both the proximal and distal mesenchyme where it appears to outline where the stylopod (femur) and zuegopod (tibia, fibula) are forming (Fig. 1E,F,K,L). Later in skeletal development at stage 31, BMP3 expression can be distinctly seen in the perichondrium and forming bone collar of the cartilage rudiments of the leg and foot as well as in the tips of the digits (Fig. 1G).
Fig. 1.
Expression of BMP3 during normal chick limb development. A–L: Whole-mount in situ hybridization of BMP3 expression in developing chick legs (A–G) and histological sections of the whole-mount limbs (H–L). In cross-sections, dorsal is to the left and in transverse sections it is at the top. Hamburger and Hamilton (HH) stages are indicated. A: At stage 20, BMP3 is expressed in the developing somites but not in the hindlimb bud. B: At stage 23, BMP3 is expressed in the dorsal and ventral mesenchyme. C: At stage 24, BMP3 expression broadens and expands distally. D: Anterior view of stage 24 leg bud. BMP3 transcripts are excluded from the central core mesenchyme. E: At stage 25, BMP3 is expressed in both the proximal and distal mesenchyme. F: At stage 27, BMP3 expression outlines where the future zuegopod will form. G: At stage 31, BMP3 is expressed in the perichondrium and forming bone collar of the developing bones in the leg and foot and in the digit tips. H: Schematic diagram of a BMP3 hybridized limb bud showing the approximate levels of the cross- and transverse sections shown in I–L. I: Cross-section through stage 24 leg bud showing subepidermal expression of BMP3 in the dorsal and ventral limb mesenchyme and not in the central core mesenchyme. J: Transverse section of stage 24 leg bud. K: Transverse section of stage 25 leg bud. BMP3 continues to be expressed in the subepidermal mesenchyme and begins to prefigure the cartilage elements. L: Transverse section of stage 27 leg bud. BMP3 is expressed in the developing perichondrium that outlines the forming leg bones. BMP, bone morphogenetic protein.
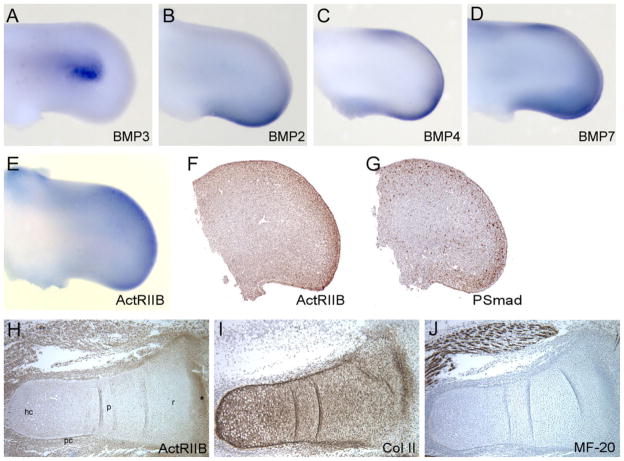
During early limb development, there is very little overlap in the expression of BMP3 and other BMPs thought to be important for skeletal development. For example, transcripts for BMP2, 4, and 7 are first detected in the limb bud mesoderm at stage 17 (Geetha-Loganathan et al., 2006), which is almost a day earlier than when we first see BMP3 expression (Fig. 1B). In the stage 23–24 limb, BMPs 2, 4, and 7 are strongly expressed in the anterior and posterior distal mesenchyme as well as the apical ectodermal ridge (AER) while BMP3 expression is confined to the dorsal and ventral mesenchyme of the central region (Fig. 2). Because BMP3 is thought to antagonize BMP signaling, it may be necessary for these factors to have mutually exclusive expression patterns and timing for proper skeletal formation to occur. Later in skeletal development, however, the expression pattern of BMP3 overlaps with BMPs 2, 4, 5, and 7, particularly in the perichondrium flanking the cartilage elements (Pathi et al., 1999; Geetha-Loganathan et al., 2006).
Fig. 2.
Expression of BMPs, ActRIIB, and P-Smads in chick limb bud at stage 23/24 and day 7.5. A–E: Whole-mount in situ hybridization of legs for the genes (A) BMP3, (B) BMP2, (C) BMP4, (D) BMP7, (E) ActRIIB. F: Section immunohistochemistry for ActRIIB in stage 24 leg bud. G: Section immunohistochemistry for P-Smad 1, 5, 8 in stage 23 leg bud. H: Section immunohistochemistry for ActRIIB in day 7.5 distal tibia of hindlimb. I: Section immunohistochemistry for Collagen II to mark cartilage cells and chondrocytes in day 7.5 distal tibia. J: Section immunohistochemistry for MF-20 to mark differentiated muscle in day 7.5 leg. hc, hypertrophic chondrocytes; p, proliferating chondrocytes; pc, perichondrium; r, round, resting chondrocytes; BMP, bone morphogenetic protein. The asterisk marks the developing joint.
We next looked at the localization pattern of ActRIIB, the binding partner for BMP3, and a type II BMP receptor used by BMP2, 4, and 7 for signaling to determine whether these factors were coexpressed in developing limb. At stage 23, ActRIIB transcripts are found throughout the limb mesenchyme surrounding the developing cartilages (Fig. 2E) and appear to be expressed near BMP3 in the dorsal and ventral mesenchyme (Fig. 2A,E). ActRIIB expression is strongest in the distal mesenchyme under the AER where it appears to overlap with BMP2, 4, and 7 transcripts (Fig. 2B–E). We then analyzed the expression pattern of ActRIIB in detail in chick hindlimbs from early condensing cartilage stages (stage 24–25) to differentiated skeletal elements (day 7.5–9) using immunohistochemistry. At stage 24/25, ActRIIB was found throughout the limb mesenchyme with higher levels of the receptor localizing to the mesenchyme around the condensing central cartilage (Fig. 2F). At day 7.5–8, ActRIIB was expressed in the developing cartilage, the surrounding perichondrium and mesenchyme as well as the muscle (which is marked by MF-20 staining; Fig. 2H–J). In the forming skeletal elements, ActRIIB was detected in the differentiating chondrocytic layers (overlapping with Col II staining), including the round cells in the epiphysis, the proliferating, flattened cells in the metaphysis, and the hypertrophic chondrocytes in the diaphysis (Fig. 2H). ActRIIB was also expressed in the joint spaces and the presumptive articular cartilages at the ends of the developing skeletal elements (Fig. 2H). At later stages when bone is forming (day 9–10), ActRIIB continues to be expressed in a similar pattern in the chondrocytes and articular cartilage as well as in the perichondrium and muscle (data not shown). This localization pattern for ActRIIB receptor protein in the mesencyhme, perichondrium and cartilage of the developing chick limb matches ActRIIB expression using in situ hybridization (Nohno et al., 1993; Merino et al., 1999).
Finally, to localize activated BMP signaling in the stage 23/24 limb bud, we analyzed the expression pattern of phospho-Smad 1, 5, 8 using immunohistochemistry. The majority of phospho-Smad 1, 5, 8 positive cells were located in the anterior and posterior distal mesenchyme, the region where transcripts for BMP 2,4,7 and ActRIIB were found, indicating that ActRIIB is available in the limb bud for BMP signaling (Fig. 2G). Interestingly, in the areas of dorsal and ventral mesenchyme where BMP3 and ActRIIB are both expressed, there were fewer phospho-Smad 1, 5, 8 positive cells, in keeping with the idea that BMP3 may negatively regulate BMP signaling.
Skeletal Defects Caused by Overexpression of BMP3
To study the function of BMP3 in the chick, human BMP3 was cloned into the replication competent avian retroviral vector (RCAS), viral particles produced in DF1 cells and concentrated. To verify that the virus produced BMP3 protein, conditioned media from DF1 cells transfected with RCAS-BMP3 or RCAS-alkaline phosphatase (RCAS-AP) as a control were analyzed using Western blots and anti-BMP3 antibody. Figure 3A shows that an approximately 30-kDa band was detected in conditioned media from cells transfected with RCAS-BMP3, whereas no band was seen in conditioned media from RCAS-AP infected cells.
Fig. 3.
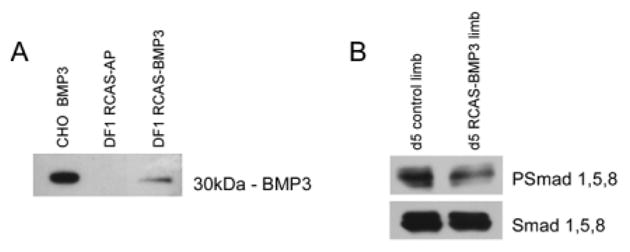
Western blot analysis of bone morphogenetic protein-3 (BMP3) and PSmad protein expression. A: Western blot of conditioned media from DF1 cells infected with replication competent avian retroviral vector (RCAS) -BMP3 or RCAS-AP. A 30-kDa BMP3 band is only detected in the RCAS-BMP3–infected cells. As a positive control, conditioned media from CHO cells transfected with human BMP3 was used (left lane). B: Western blot of extracts from RCAS-BMP3–infected and uninfected limb buds collected on day 5 using anti-PSmad 1, 5, 8 antibody to evaluate BMP signaling. The blot was stripped and anti-Smad 1, 5, 8 antibody was used to detect total Smad 1, 5, 8 protein as a loading control. Limbs infected with BMP3 had decreased levels of PSmad 1, 5, 8 when compared with controls.
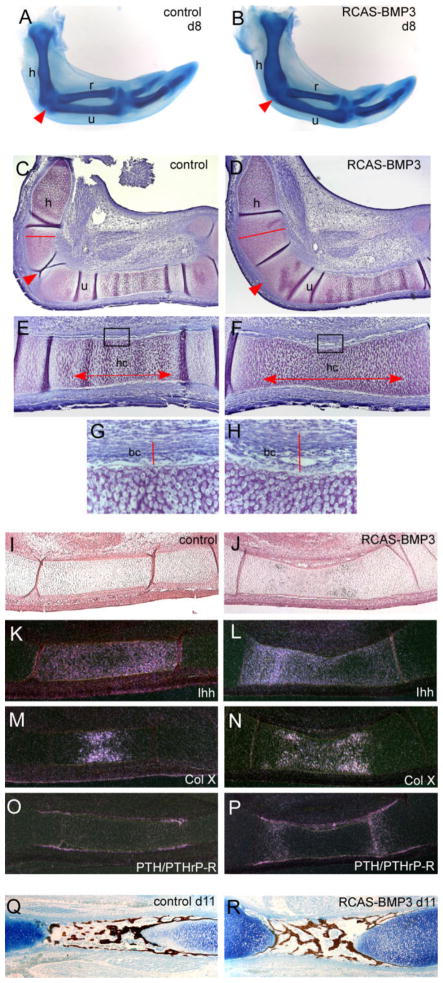
We then injected BMP3 virus into the posterior half of limb buds at stage 22, a time when the condensations of humerus, radius, and ulna are starting to form and are influenced by BMP activity. The contralateral limb served as a control and was injected with either RCAS-AP (which had no effect on the limb) or left uninfected. To determine whether misexpression of BMP3 would alter BMP signaling, we analyzed phospho-Smad 1,5,8 levels in control and RCAS-BMP3 infected limb buds 48 hr after injection (day 5). Limbs infected with RCAS-BMP3 showed reduced levels of phospho-Smad 1,5,8 when compared with controls (Fig. 3B). When we analyzed the formation of the skeletal elements in these wings at later stages by Alcian blue staining, we found that overexpression of BMP3 caused an obvious expansion of the cartilages of the humerus and ulna when compared with the contralateral control (n = 28/30 with widened skeletal elements; Fig. 4A,B). In many cases, the BMP3-infected humeri were almost twice as wide as controls, and the adjacent joint region was not clearly visible and appeared fused (n = 14/30 with altered joint formation).
Fig. 4.
Effects of overexpressing replication competent avian retroviral vector–bone morphogenetic protein (RCAS-BMP3) in chick wings. A,B: Embryos were harvested after 8 days of development and the limb skeleton stained with Alcian blue. BMP3-infected wings have expanded cartilage elements and joint fusions at the junction of the humerus (h), radius (r), and ulna (u) (arrowhead). C,D: Toluidine blue stained sections through day 8 RCAS-BMP3–infected and contralateral control wings. Note the widening of the humerus (compare red line in D with C) and the lack of a joint (arrowhead) in the BMP3 injected cartilages. E,F: Higher-power view of BMP3-infected and contralateral control ulnas. The zone of hypertrophic chondrocytes is notable longer in BMP3 ulnas (compare arrows in F with E). The developing bone collar (black box) also appears wider. G,H: Higher power view of the boxed areas shown in E and F. The developing bone collar of the BMP3-infected ulna has an increased width when compared with the control (compare red line in H with G). I–P: Analysis of molecular markers in BMP3-infected ulnae and their corresponding contralateral controls on day 8 (HH 32) by radioactive section in situ hybridization. I,J: Bright field views of control and BMP3-infected ulna. K,L: Shows the expanded expression domain of Ihh in BMP3 ulna. M,N: Shows the expanded expression domain of collagen X in BMP3 ulna. O,P: Shows the increased expression of PTH/PTHrP receptor in chondrocytes and perichondrium/periosteum of BMP3 ulna. Q,R: von Kossa staining for mineral deposition in control and BMP3-infected ulna on day 11. Sections were counterstained with Alcian blue.
The expansion of the cartilage elements in BMP3-infected limbs could be a result of increased cell proliferation, decreased apoptosis, increased recruitment of chondrocytes or altered chondrocyte differentiation. When we performed histological analysis of day 8 wings, we found that the cartilages of the proximal ulna and humerus were clearly wider in the BMP3-infected limb, and no joint space formed between these skeletal elements (Fig. 4D). In addition, the BMP3-infected cartilages had significantly expanded zones of hypertrophic chondrocytes (Fig. 4C–F). The BMP3-infected ulnas were 37% longer (control ulna = 0.50 ± 0.02 mm; RCAS-BMP3 ulna = 0.79 ± 0.03 mm; n = 4 each; P < 0.0001) and the BMP3-infected humeri were 45% longer (control humerus length = 0.48 ± 0.03 mm; RCAS-BMP3 = 0.87 ± 0.04; n = 4 each; P < 0.0001) when compared with controls. We also noticed that the developing bone collar of BMP3-infected ulna had an increased diameter when compared with the contralateral control (n = 4; Fig. 4G,H). Next, we performed in situ hybridization to monitor chondrocyte differentiation and maturation, using Ihh as a marker for prehypertrophic chondrocytes, collagen X for hypertrophic chondrocytes and PTH/PTHrP receptor for the perichondrium (Fig. 4I–P) We found that BMP3-infected ulnas expressed these markers at the proper time and place but had expanded expression domains when compared with contralateral controls. In BMP3-infected ulnas, more chondrocytes appeared to undergo hypertrophy as indicated by the increased size of the collagen X domain (n = 3; Fig. 4M,N) and there also appeared to be an expansion in the population of prehypertrophic chondrocytes as shown by the extended Ihh (n = 3; Fig. 4K,L) and PTH/PTHrP receptor domains (n = 3; Fig. 4O,P). In addition, BMP3-infected ulnas had expanded expression of PTH/PTHrP receptor in the perichondrium and developing bone collar (n = 3; Fig. 4P). These data confirm our histological observations and suggest that BMP3 causes a broadening of the skeletal elements by expanding the zones of prehypertrophic and hypertrophic chondrocytes and the diameter of the perichondrium. The alterations in the expression domains of these chondrocyte and perichondrial markers did not seem to have negative consequences on subsequent bone formation. von Kossa staining of day 11 wings showed that, although the BMP3-infected ulna was still noticeably wider than the contralateral control, mineralized bone appeared to develop normally (n = 3; Fig. 4Q,R).
Alterations in BMP signaling in the limb have been shown to effect cell proliferation and apoptosis (Pogue and Lyons, 2006). To determine whether overexpression of BMP3 had an effect on cell proliferation, we performed bromodeoxyuridine (BrdU) labeling experiments on RCAS-BMP3–injected wings on day 6. We noted a generalized increase in cell proliferation throughout the limb tissue infected with the BMP3 virus. This effect may reflect the widespread expression of ActRIIB throughout the limb mesenchyme at this stage (Fig. 2E,F,H and Nohno et al., 1993). When we looked specifically at the cartilage elements, we found that more proliferating cells were present within the developing ulna and perichondrium of RCAS-BMP3–injected wings when compared with their contralateral controls (n = 3; Fig. 5). Cell counts of the distal ulna region revealed that there were almost twice as many proliferating cells per cartilage area in the day 6 BMP3-infected limbs (control = 80.7 ± 5.3 vs. RCAS-BMP3 = 157 ± 5.3, n = 3 each; P < 0.001). We also performed terminal deoxynucleotidyl transferase–mediated deoxyuridinetriphosphate nick end-labeling (TUNEL) staining to examine if BMP3-infected cartilages had alterations in the rate of cell death and found no overall changes in the level of apoptosis when compared with controls (n = 3; data not shown).
Fig. 5.
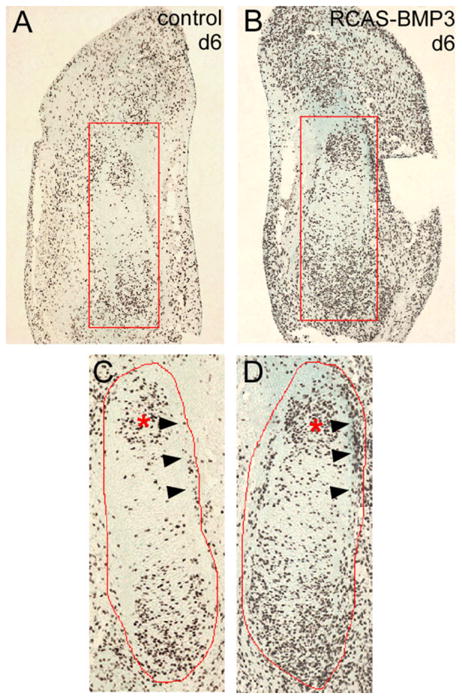
Immunohistochemical analysis of cell proliferation by bromodeoxyuridine (BrdU) labeling. Representative sections from control and replication competent avian retroviral vector–bone morphogenetic protein (RCAS-BMP3) -injected wings collected on day 6. Proliferating cells were labeled with BrdU and detected by antibody staining. The cartilage regions examined are outlined in red. The distal condensing end of the developing ulna is marked by an asterisk, and this region was used for counting the number of proliferating cells in control and BMP3-infected wings. The forming bone collar is marked by arrow heads. A–D: More BrdU-positive cells are present in developing ulna and perichondrium of RCAS-BMP3–infected wings as compared to their contralateral controls.
Misexpression of BMP3 in the developing chick wing at the time cartilage condensations are forming caused an expansion of the resulting skeletal elements. The enlarged cartilages had increased domains of prehypertrophic and hypertrophic chondrocytes, as measured by elongation of the expression domains for collagen X, Ihh, and PTH/PTHrP receptor, as well as a thicker perichondrium near the center of the developing bone. This phenotype appears to be due to an increase in chondrocyte cell proliferation in the RCAS-BMP3–infected skeletal elements. As BMP3 reduces PSmad 1,5,8 levels, we believe BMP signaling in chondroprogenitor cells directly influences their decision to proliferate or differentiate, with decreasing BMP signaling favoring continued proliferation. Continued proliferation, may in turn delay chondrocyte maturation (Pogue and Lyons, 2006).
The phenotype we observe when we modulate BMP3 levels in the chick limb is different from what has been reported for changing the levels of other BMP antagonists at similar stages of limb development and may reflect the unique mode of action of BMP3. Overexpression of noggin in the chick limb caused severe shortening of skeletal elements and inhibited chondrogenesis, leading to a reduced number of hypertrophic chondrocytes (Pathi et al., 1999; Pizette and Niswander, 2000) identifying BMP activity as necessary for the commitment and differentiation of the chondrogenic lineage. The interaction of noggin with BMP ligands prevents them from binding to their receptors effectively blocking signaling by removing the signal. In contrast, BMP3 interacts with the type II BMP receptor, ActRIIB, causing a decrease in BMP signaling through reducing receptors available for signaling (Gamer et al., 2005). We surmise from these differences that ActRIIB is a conduit for BMP signaling involved in chondrocyte proliferation, while the other type II BMP receptors (ActRIIA, BMPR-II) may be more important for other aspects of endochondral ossification. This explanation is in keeping with the observation that overexpression of BMP2 or BMP4 in the chick limb caused a dramatic increase in the volume of cartilage elements, altered their shapes and led to joint fusions through increased recruitment of mesenchymal precursors into the chondrocyte lineage (Duprez et al., 1996) and not to an increase in cartilage cell proliferation as we see for BMP3. In addition, increased expression of BMP2 and BMP4 also caused a delay in chondrocyte hypertrophy and formation of the periosteum (Duprez et al., 1996) phenotypes we did not see in our BMP3 limbs. These differences are probably due to the fact that, while BMP2, BMP3 and BMP4 can all bind to ActRII, only BMPs 2 and 4 can activate signal transduction by means of BMPR-II.
We find the fact that BMP3 localizes to the perichondrium during chondrogenesis intriguing as this structure is thought to play an important role in regulating cartilage growth and differentiation. Removal of the perichondrium/periosteum from chick tibia results in larger bones with increased chondrocyte proliferation and larger zones of chondrocyte hypertrophy (Long and Linsenmayer, 1998; Di Nino et al., 2001). These data suggest that signals from the perichondrium influence the rate of chondrocyte maturation and hypertrophy. Our data suggest that BMP3 expression in the perichondrium could play a role in regulating chondrocyte proliferation and maturation by means of type II BMP receptors (ActRIIB), which are expressed in the chondrocytes of the developing skeletal elements (Fig. 2H; Nohno et al., 1993; Merino et al., 1999). This interplay between the perichondrium/periosteum and the underlying cartilage may be necessary to ensure proper formation of the limb skeleton.
EXPERIMENTAL PROCEDURES
Embryos
Embryos were obtained by incubating fertilized White Leghorn chicken eggs (Charles River) at 39°C and staged according to Hamburger and Hamilton (1951).
In Situ Hybridization
Whole-mount in situ hybridization on chick embryos was performed as described in Riddle et al. (1993). Whole-mount embryos were processed, embedded in paraffin, and sectioned as described previously (Nieto et al., 1996). Probes were synthesized using a standard protocol for BMP3 (EST 603535861F1 from ARK-Genomics), BMP2, BMP4, BMP7 (kindly provided by P. Francis-West), and ActRIIB (kindly provided by Tsutomu Nohno). Section in situ hybridization with radiolabeled probes was performed as described (Lanske et al., 1996). Probes for chick Ihh and PTH/PTHrP receptor (Vortkamp et al., 1996) were kindly provided by Cliff Tabin and for chick collagen X (Ninomiya et al., 1986) by Bjorn Olsen.
Immunohistochemistry
For immunostaining, sections of stage 23–35 chick leg buds were microwaved in citrate buffer for antigen unmasking. Sections were blocked in 5% goat serum or 1% horse serum, incubated with primary antibody (P-Smad1,5,8: Cell Signaling Technology; ActRIIB: Santa Cruz Biotechnology; MF-20 [detects sarcomeric myosin] and Collagen II: Developmental Studies Hybridoma Bank) overnight at 4°C and then incubated with the appropriate secondary antibody according to the Vectastain ABC kit (Vector Labs). Color was developed using the diaminobenzidine Chromogen kit (Vector Labs), and sections were counter-stained with hematoxylin.
Retrovirus Construction and Infection
To construct the BMP3 retrovirus, we used the full-length coding sequence from a human BMP3 cDNA that shares 95% amino acid sequence identity with chick BMP3 in the mature domain of the protein. This cDNA was amplified by polymerase chain reaction (PCR) and subcloned into the NcoI and EcoRI sites of the adaptor plasmid pSLAX13 (a gift from Bruce Morgan, MGH). The ClaI insert was subcloned into the chick retroviral vector RCASBP (A) and the correct orientation was confirmed by PCR analysis and sequencing. Concentrated retroviral supernatants were prepared in DF1 cells using the method of Logan and Tabin (1998), and had titers of > 1 × 108 virus particles/ml. Chick limb buds were infected by injecting the concentrated virus in the posterior region of stage 21–22 wing buds.
Western Blot
Conditioned media from DF1 cells transfected with RCAS-alkaline phosphatase or RCAS-BMP3 were collected 72 hr after transfection. Conditioned media from CHO cells transfected with human BMP3 (Gamer et al., 2005) was used as a positive control. Equal amounts of conditioned media were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to nitrocellulose and protein expression was detected using anti-BMP3 antibody (Santa Cruz Biotechnology). For phospho-Smad (PSmad) and Smad protein detection, whole protein lysates from day 5 limb were generated by homogenizing limb buds in Cell Lysis Buffer (Cell Signaling Technology) with 1 mM phenylmethyl sulfonyl fluoride. A total of 50 μg of total protein was separated by SDS-PAGE, transferred to nitrocellulose membranes and blotted with anti-PSmad 1,5,8 (Cell Signaling Technologies) antibody. Blots were then stripped and re-probed with anti-Smad 1, 5, 8 antibody (Santa Cruz Biotechnology). Proteins were visualized by using SuperSignal West Pico Chemiluminescent kit (Pierce).
Morphological and Histological Analysis of Limbs
For whole skeletal preparations, embryos of the appropriate stage were fixed in 4% paraformaldehyde overnight and then stained with Alcian blue as described previously (Gamer et al., 2001). For histology, limbs from embryos of various stages were fixed, embedded in paraffin, sectioned at 8 μm and stained with Toluidine blue. von Kossa staining was performed on sections of day 11 limbs using standard methods.
Cell Proliferation and Apoptosis
Cell proliferation assays of day 6 chick embryos were performed as described previously (Zou et al., 1997) using BrdU-labeling reagent and the Cell Proliferation Kit (Amersham). Cell proliferation was examined in the condensing distal ulna cartilage from multiple adjacent sections (n = 3 control and injected wings) by individually counting the number of BrdU-positive cells present within a comparable cartilage region. Immunohistochemical detection of cells undergoing apoptosis was performed on paraffin embedded control and RCAS-BMP3–injected limb buds on day 6 using the In Situ Cell Death Detection Kit (Roche) according to the manufacturer.
Acknowledgments
We thank John Nove and Lindsey Korepta for technical assistance in making retrovirus.
References
- Allendorph GP, Isaacs MJ, Kawakami Y, Izpisua Belmonte JC, Choe S. BMP-3 and BMP-6 structures illuminate the nature of binding specificity with receptors. Biochem. 2007;46:12238–12247. doi: 10.1021/bi700907k. [DOI] [PubMed] [Google Scholar]
- Bahamonde ME, Lyons KM. BMP3: to be or not to be a BMP. J Bone Joint Surg Am. 2001;83(suppl 1):S56–S62. [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocr Rev. 2003;24:218–235. doi: 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- Daluiski A, Engstrand T, Bahamonde ME, Gamer LW, Agius E, Stevenson SL, Cox K, Rosen V, Lyons KM. Bone morphogenetic protein-3 is a negative regulator of bone density. Nat Genet. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Di Nino DL, Long F, Linsenmayer TF. Regulation of endochondral cartilage growth in the developing avian limb: cooperative involvement of perichondrium and periosteum. Dev Biol. 2001;240:433–442. doi: 10.1006/dbio.2001.0471. [DOI] [PubMed] [Google Scholar]
- Duprez D, Bell EJ, Richardson MK, Archer CW, Wolpert L, Brickell PM, Francis-West PH. Overexpression of BMP-2 and BMP-4 alters the size and shape of developing skeletal elements in the chick limb. Mech Dev. 1996;57:145–157. doi: 10.1016/0925-4773(96)00540-0. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Cox KA, Small C, Rosen V. Gdf11 is a negative regulator of chondrogenesis and myogenesis in the developing chick limb. Dev Biol. 2001;229:407–420. doi: 10.1006/dbio.2000.9981. [DOI] [PubMed] [Google Scholar]
- Gamer LW, Nove J, Levin M, Rosen V. BMP-3 is a novel inhibitor of both activin and BMP-4 signaling in Xenopus embryos. Dev Biol. 2005;285:156–168. doi: 10.1016/j.ydbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Gamer L, Korepta L, Cox K, Rosen V. BMP3 regulates osteoblast differentiation and maturation in the postnatal skeleton. J Bone Miner Res. 2006;211(suppl 1):S33. [Google Scholar]
- Geetha-Loganathan P, Nimmagadda S, Huang R, Scaal M, Christ B. Expression pattern of BMPs during chick limb development. Anat Embryol (Berl) 2006;211(suppl 1):87–93. doi: 10.1007/s00429-006-0129-6. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hino J, Kangawa K, Matsuo H, Nohno T, Nishimatsu S. Bone morphogenetic protein-3 family members and their biological functions. Front Biosci. 2004;9:1520–1529. doi: 10.2741/1355. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LH, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Logan M, Tabin C. Targeted gene misexpression in chick limb buds using replication-competent retroviruses. Methods. 1998;14:407–420. doi: 10.1006/meth.1998.0595. [DOI] [PubMed] [Google Scholar]
- Long F, Linsenmayer TF. Regulation of growth region cartilage proliferation and differentiation by perichondrium. Development. 1998;125:1067–1073. doi: 10.1242/dev.125.6.1067. [DOI] [PubMed] [Google Scholar]
- Merino R, Macias D, Ganan Y, Rodriguez-Leon J, Economides AN, Rodriguez-Esteban C, Izpisua-Belmonte JC, Hurle JM. Control of digit formation by activin signalling. Development. 1999;126:2161–2170. doi: 10.1242/dev.126.10.2161. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Gordon M, van der Rest M, Schmid T, Linsenmayer T, Olsen BR. The developmentally regulated type X collagen gene contains a long open reading frame without introns. J Biol Chem. 1986;261:5041–5050. [PubMed] [Google Scholar]
- Nohno T, Noji S, Koyama E, Myokai F, Ohuchi H, Nishikawa K. Expression patterns of activin receptor IIA and IIB genes during chick limb development. Prog Clin Biol Res. 1993;383:705–714. [PubMed] [Google Scholar]
- Pathi S, Rutenberg JB, Johnson RL, Vortkamp A. Interaction of Ihh and BMP/Noggin signaling during cartilage differentiation. Dev Biol. 1999;209:239–253. doi: 10.1006/dbio.1998.9181. [DOI] [PubMed] [Google Scholar]
- Pizette S, Niswander L. BMPs are required at two steps of limb chondrogenesis: formation of prechondrogenic condensations and their differentiation into chondrocytes. Dev Biol. 2000;219:237–249. doi: 10.1006/dbio.2000.9610. [DOI] [PubMed] [Google Scholar]
- Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Curr Top Dev Biol. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Nove J, Gamer L, Cox K, Rosen V. BMP3 is a mediator of age dependent bone loss in postnatal mice through its action on osteoblast differentiation. J Bone Miner Res. 2006;21:S1–S77. [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin CJ. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]