Abstract
Rationale
Atherosclerosis is initiated by blood flow patterns that activate inflammatory pathways in endothelial cells. Activation of inflammatory signaling by fluid shear stress is highly dependent on the composition of the subendothelial extracellular matrix. The basement membrane proteins laminin and collagen found in normal vessels suppress flow-induced p21 activated kinase (PAK) and NF-κB activation. By contrast, the provisional matrix proteins fibronectin and fibrinogen found in wounded or inflamed vessels support flow-induced PAK and NF-κB activation. PAK mediates both flow-induced permeability and matrix-specific activation of NF-κB.
Objective
To elucidate the mechanisms regulating matrix-specific PAK activation.
Methods and Results
We now show that matrix composition does not affect the upstream pathway by which flow activates PAK (integrin activation, Rac). Instead basement membrane proteins enhance flow-induced protein kinase A (PKA) activation, which suppresses PAK. Inhibiting PKA restored flow-induced PAK and NF-κB activation in cells on basement membrane proteins, whereas stimulating PKA inhibited flow-induced activation of inflammatory signaling in cells on fibronectin. PKA suppressed inflammatory signaling through PAK inhibition. Activating PKA by injection of the PGI2 analog iloprost reduced PAK activation and inflammatory gene expression at sites of disturbed flow in vivo, whereas inhibiting PKA by PKI injection enhanced PAK activation and inflammatory gene expression. Inhibiting PAK prevented the enhancement of inflammatory gene expression by PKI.
Conclusions
Basement membrane proteins inhibit inflammatory signaling in endothelial cells via PKA-dependent inhibition of PAK.
Keywords: Shear stress, extracellular matrix, protein kinase A, p21 activated kinase, NF-κB
Introduction
Atherosclerosis, a chronic inflammatory disease of artery walls, originates as regions of local endothelial cell dysfunction characterized by enhanced permeability, inflammatory gene expression, and turnover1. While classic risk factors for atherosclerosis, including hypercholesterolemia, hyperglycemia, and smoking, occur throughout the vasculature, atherosclerotic plaques preferentially form at vessel curvatures, branch points, and bifurcations where blood flow is of lower magnitude and exhibits complex features including turbulence, oscillations, separation and reattachment, which we term disturbed flow2. Thus, flow patterns critically regulate the local susceptibility to atherosclerosis.
In vitro, laminar flow inhibits endothelial activation and turnover, whereas disturbed flow induces inflammatory signaling, enhanced turnover and other features of atherosclerosis-susceptible regions of arteries3. Interestingly, laminar and disturbed flow both initially activate NF-κB4 and JNK5, intercellular adhesion molecule-1 (ICAM-1) expression6, and enhanced permeability7. However, in laminar flow, these events decrease at later times as the cells align in the direction of flow, whereas in disturbed flow, these events remain elevated3. Thus, the inability of cells to adapt to disturbed flow may mediate the differential cellular responses to these two flow patterns.
Integrins mediate an important subset of the endothelial response to flow. Shear stress stimulates integrin activation8, 9, conversion from a low affinity to a high affinity state, which triggers new integrin-matrix binding; integrins thereby regulate both flow-induced endothelial cell alignment and proinflammatory gene expression10. Individual integrin heterodimers differ in both their ligand preferences and signaling responses, allowing cells to differentiate between matrix substrates. Endothelial cells are normally on a basement membrane comprised mainly of laminins, collagen and other glycoproteins. In contrast, during inflammation, angiogenesis or wound healing, provisional matrix proteins such as fibronectin and fibrinogen are deposited beneath the endothelium11. In in vitro flow models, flow-induced proinflammatory signaling (NF-κB)11, proinflammatory gene expression11, and endothelial permeability7 are highly activated in endothelial cells on fibronectin or fibrinogen but not in cells on basement membrane proteins. Indeed, these pathways are actively suppressed in cells on collagen. In vivo, fibronectin is deposited beneath the endothelium at atherosclerosis-prone regions of arteries. Thus, matrix remodeling may play a critical role in atherogenesis.
The p21-activated kinase (PAK) family of Ser/Thr kinases regulate cell growth, migration, cytoskeletal organization and gene expression12. Shear stress stimulates PAK activation in cells on fibronectin but not in cells on basement membrane proteins or collagen7, and PAK mediates matrix-specific activation of the proinflammatory transcription factor NF-κB13, 14, inflammatory gene expression13, and increased endothelial permeability7 by flow. PAK is activated in athero-prone regions in vivo, which correlates with areas of fibronectin deposition and inflammatory gene expression7. However, the mechanisms mediating matrix-specific PAK activation remain unknown.
PAK is activated by the small GTPases Rac and Cdc42, 12 and suppressed by PAK inhibitory proteins (ex. nischarin, hPIP1)15, 16, by dephosphorylation by phosphatases (PP2A, POPX1/2)17,18, and by phosphorylation by protein kinase A (PKA)19. We therefore set out to elucidate the mechanism of differential PAK activation on different matrix proteins. Our results identify PKA as the critical mediator of matrix-specific PAK activation and hence proinflammatory signaling through NF-κB.
Materials and Methods
Briefly, bovine aortic endothelial cells (BAECs) and human aortic endothelial cells (HAECs) on glass slides coated with specific matrix proteins were exposed to laminar or oscillatory flow using parallel plate flow chambers. Rac activation was assayed by affinity pulldown using GST-p21 binding domain (GST-PBD). Rac and Cdc42 were inhibited with dominant negative N17-Rac and N17-Cdc42 transfected using Lipofectamine 2000. PKA activation was determined by cAMP quantification, an in vitro kinase assay, and an affinity pulldown approach utilizing GST-PKI20. PAK activation was measured by Western blotting using an antibody to phospho-Ser141. NF-κB activation was measured by phosphorylation of the p65 subunit and by p65 nuclear translocation. ICAM-1 expression was measured by Western blotting and quantitative real time PCR. In vivo studies were carried out using either 10 week old or 36 to 38 week old male C57Bl/6J mice in which the effects of PKA activation or inhibition on PAK phosphorylation and inflammatory gene expression was determined. Detailed materials and methods are provided in the online supplement.
Results
Regulation of PAK activators
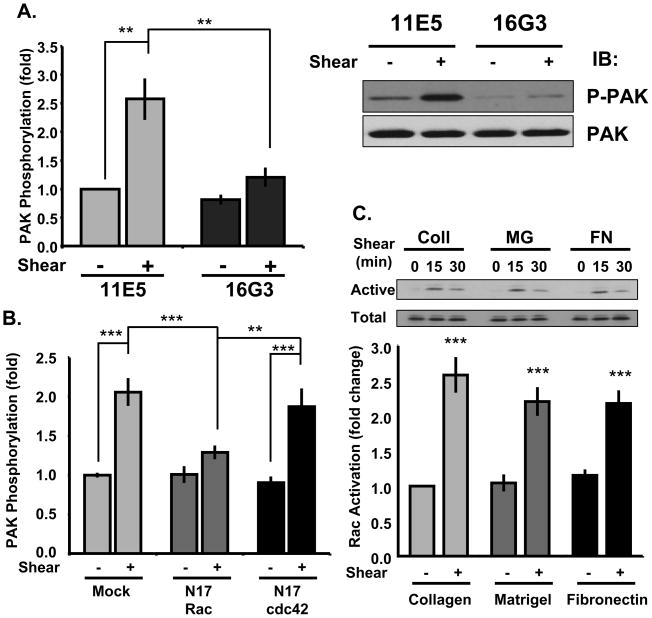
To determine the mechanism of matrix-specific PAK activation, we first investigated the upstream pathway by which shear stress activates PAK in endothelial cells (ECs) on fibronectin. Shear stress stimulates integrins, leading to activation of Rac and Cdc42, both of which can activate PAK10, 21. To determine if flow-induced activation of PAK requires new integrin ligation, we pretreated ECs on fibronectin with either the fibronectin blocking (16G3) or a control nonblocking anti-fibronectin antibody (11E5) for 30 minutes. This short term treatment does not significantly disrupt preexisting adhesions or induce cell rounding10, 21. Pretreatment with 16G3 but not 11E5 significantly inhibited flow-induced PAK activation (Figure 1A), indicating that shear stress stimulates PAK through integrin ligation.
Figure 1. Analysis of the PAK upstream pathway.
(A) BAECs on fibronectin were treated with fibronectin-blocking (16G3, 50 μg/ml) or nonblocking (11E5, 50 μg/ml) antibodies for 30 minutes. Cells were sheared for 15 minutes and PAK phosphorylation was determined by Western blotting for phospho-Ser141. Results are normalized to total protein. n = 3. (B) ECs expressing N17-Rac or N17-Cdc42 plated on a fibronectin matrix were sheared for 15 minutes. PAK activation was determined as described. n = 3–5. (C) ECs plated on collagen (Coll), matrigel (MG), or fibronectin (FN) were sheared for the indicated times and Rac activity was determined by affinity pulldown assays. Rac levels in the pulldown at the 0 and 15 minute time points were normalized to total Rac levels in whole cell lysates. n = 4–6. ** p < 0.01; *** p < 0.001 by multiple comparisons ANOVA.
To address the requirement for Rac and Cdc42,. ECs on fibronectin were transfected with dominant negative N17-Rac or N17-Cdc42. Transfection efficiencies were~70 to 80% (Online Figure I), which allowed biochemical analysis of the entire cell population. Only N17-Rac prevented flow-induced PAK activation (Figure 1B), indicating that Rac is the critical small GTPase in this pathway. To determine whether shear stress-induced Rac activation is matrix-specific, we plated ECs on collagen I, fibronectin, or diluted basement membrane protein (matrigel), which, under these conditions, coats the slides similarly to fibronectin without forming a gel5, 7. After 4h, cells formed a confluent monolayer, at which time they were sheared for 15 or 30 minutes. Surprisingly, flow activated Rac equally well on basement membrane protein and fibronectin (Figure 1C). Thus, the pathway upstream of PAK is matrix-independent, consistent with previous data that flow-induced reactive oxygen species production13, which is thought to be regulated by Rac22, also occurs independently of matrix composition.
Flow-induced PKA activation is matrix dependent
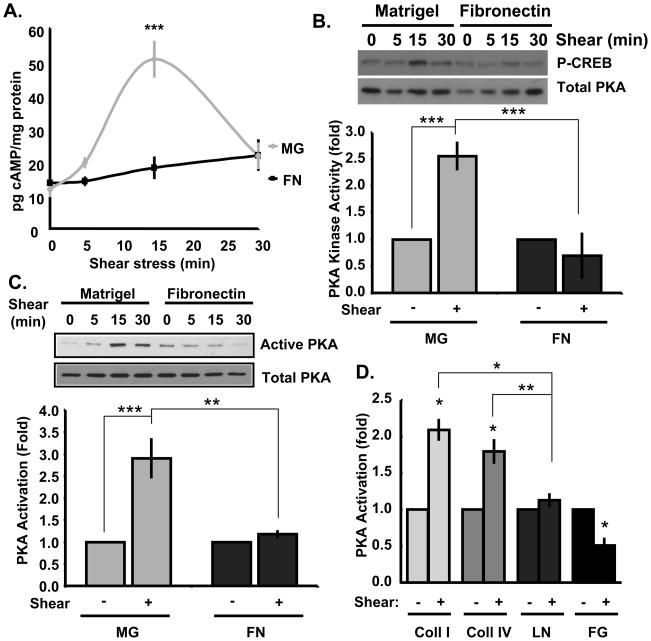
We next investigated possible PAK inhibitory pathways. PKA can directly phosphorylate and suppress PAK19 and has known anti-inflammatory effects23, 24. To investigate this pathway, cells on matrigel or fibronectin were sheared and cAMP was assessed. Flow stimulated a transient increase in cAMP in cells on matrigel but not on fibronectin (Figure 2A). We next assayed PKA using an in vitro kinase assay (verified using the PKA activator Br-cAMP and the PKA inhibitory peptide PKI; Online Figure II). PKA activity increased >2.5-fold in cells on matrigel but showed no change on fibronectin (Figure 2B). An affinity pull-down assay with the PKA pseudosubstrate PKI also showed an increase in ECs on matrigel but not on fibronectin (Figure 2C). Thus, flow stimulates cAMP and PKA on basement membrane protein but not on fibronectin.
Figure 2. Matrix-specific PKA activation.
BAECs plated on matrigel (MG) or fibronectin (FN)-coated slides were exposed to onset of laminar flow for the indicated times. (A) cAMP concentration was determined by competitive ELISA assay and normalized to total protein. n = 4–6. (B) Kinase activity in PKA immunoprecipitates was determined in a kinase assay using GST-CREB as substrate and antibodies specific for phosphorylated CREB. Results at the 0 and 15 minute time points were normalized to PKA levels in the immunoprecipitates and for total CREB. n = 3. (C) PKA activity was determined by affinity pulldown using GST-PKI. Active PKA in the pulldowns was normalized to total PKA in cell lysates using the 0 and 15 min. time points. n = 3. (D) ECs plated on slides coated with collagen I (Coll I), collagen IV (Coll IV), laminin (LN), or fibrinogen (FG) were sheared for 15 minutes, and PKA activity determined by GST-PKI pulldown. n = 3–5. * p < 0.05, ** p < 0.01, *** p < 0.001 by multiple comparisons ANOVA.
Matrigel contains several matrix proteins as well as growth factors. We therefore assayed PKA activity in ECs on purified basement membrane proteins (collagen I, collagen IV, laminin). We also examined the provisional matrix protein fibrinogen, which is deposited into the subendothelial matrix in wounds and at early stages of atherosclerosis11. In ECs on collagen I or collagen IV, PKA activation increased ~2-fold after flow (Figure 2D), whereas cells on laminin showed no change and in cells on fibrinogen, PKA signaling decreased. These results suggest that collagens are the main component of matrigel that promote PKA activation in response to flow.
PKA regulates PAK activation
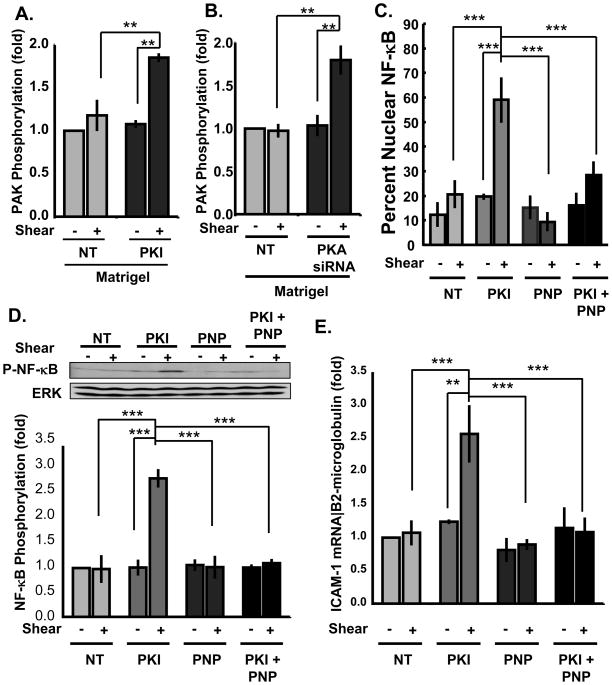
We next tested if basement membrane-associated PKA signaling prevents flow-induced PAK activation. ECs on matrigel were treated with membrane-permeable PKA inhibitory peptide (myristoylated PKI14–22) for 15 minutes, and PAK activation was determined. PKI rescued flow-induced PAK activation on matrigel (Figure 3A) to near levels in cells on fibronectin (Figure 1A). siRNA against the PKA Cα catalytic subunit (~70% knockdown; Online Figure III) similarly restored flow-induced PAK activation in cells on matrigel (Figure 3B). These results show that PKA activation suppresses PAK on basement membranes.
Figure 3. PKA inhibitors rescue inflammatory signaling on MG.
(A) BAECs plated on matrigel were treated with the cell permeant PKA inhibitory peptide PKI (20 μM for 15 min.). Cells were then sheared for 15 minutes and PAK assayed as in Fig 1. n = 3. (B) ECs transfected with siRNA against the PKC Cα catalytic subunit (~70% knockdown) were plated on matrigel and sheared for 15 min. PAK was assayed as above. n = 3. (C,D) ECs plated on matrigel were treated with PKI (20 μM for 15 min.), the PAK inhibitory peptide (PNP; 20 μg/ml for 60 min.) or both. Cells were then sheared for 30 minutes and NF-κB nuclear translocation (C) and phosphorylation on Ser536 (D) were determined. Western blots were normalized to total protein, and representative blots are shown. n = 3. For nuclear translocation, more than 100 cells were counted for each condition and scored as positive or negative for nuclear NF-κB. NF-κB nuclear translocation was averaged from three independent experiments. (E) HAECs were plated on matrigel were treated with PKI (20 μM for 15 min.), the PAK inhibitory peptide (PNP; 20 μg/ml for 60 min.) or both and then sheared for 3 hours. ICAM-1 mRNA expression was determined using quantitative real time PCR and normalized toβ2-microglobulin mRNA. ** p < 0.01, *** p < 0.001 by multiple comparisons ANOVA.
Effects on inflammatory signaling
Low PAK activity mediates the inhibition of flow-induced NF-κB activation and inflammatory gene expression in ECs on basement membrane proteins11, 13. We therefore investigated whether PKA suppresses flow-induced NF-κB activation through PAK inhibition. When ECs on matrigel were treated with PKI flow-induced NF-κB activation was restored as indicated by nuclear translocation (Figure 3C; Online Figure IV) and phosphorylation of p65 on Ser536 (Figure 3D). Rescue of NF-κB in PKI-treated cells was blocked by a cell-permeant PAK inhibitory peptide (PNP)13, 25 (Figure 3C/D),indicating PAK was involved. Additionally, expression of the NF-kB-dependent gene ICAM-1 in HAECs on matrigel showed similar dependence on PAK and PKA (Figure 3E). Taken together, these results indicate that PKA activation in cells on matrigel suppresses NF-κB by inhibiting PAK.
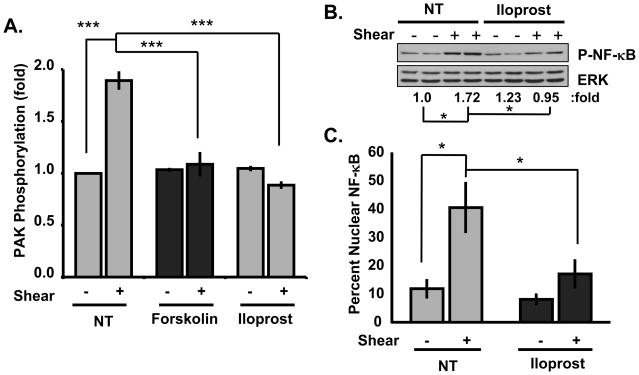
To further test this idea, ECs on fibronectin were treated with forskolin, a direct activator of adenylate cyclase26, or iloprost, a prostacyclin analog that stimulates the Gαs-dependent activation of adenylate cyclase27. Both agents blocked flow-induced PAK activation on fibronectin (Figure 4A). These agents may also stimulate signaling through the Epac/Rap1 pathway, however, the Epac activating compound 8-pCPT-2′-O-Me-cAMP did not inhibit PAK activation on fibronectin (Online Figure V), implicating PKA as the primary regulator. Furthermore, pre-treatment with iloprost also inhibited flow-induced NF-κB phosphorylation (Figure 4B) and nuclear translocation (Figure 4C; Online Figure VI).
Figure 4. PKA activators inhibit inflammatory signaling on fibronectin.
(A) BAECs plated on fibronectin were treated with forskolin (10 μM) or iloprost (1 μM) for 30 minutes. Cells were sheared for 15 minutes and PAK was assayed as in Figure 1. n = 3–6. (B,C) ECs plated on fibronectin were treated with iloprost (1 μM for 30 min.), sheared for 30 min., and (B) NF-κB phosphorylation on Ser536 and (C) nuclear translocation were determined as in Fig 3. n = 3. * p < 0.05, *** p < 0.001 by multiple comparisons ANOVA.
PKAin oscillatory flow
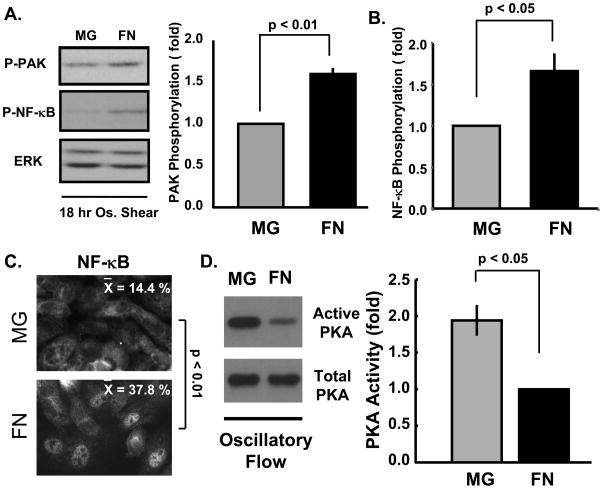
To investigate whether the matrix-specific events observed with onset of laminar shear apply to disturbed flow, ECs were exposed to oscillatory flow (±5 dynes/cm2) for 18h. Assays for PAK, NF-κB, and PKA activation showed enhanced PAK and NF-κB activity on fibronectin compared to matrigel (Figure 5A/B/C). In contrast, oscillatory flow increased PKA activity ~2-fold in cells on matrigel compared to fibronectin (Figure 5D).
Figure 5. Matrix-specific signaling in oscillatory flow.
BAECs plated on matrigel or fibronectin were exposed to oscillatory flow for 18 hours. Activation of (A) PAK and (A,B) NF-κB were determined by Western blotting with phospho-specific antibodies as previously described. n = 3–4. (C) Cells were fixed and NF-κB nuclear translocation was determined as in previous figures. Representative images are shown. n = 3 (D) PKA activity was determined using the GST-PKI affinity beads. Active PKA in the pulldown was normalized to total PKA in the lysate. n = 3. Statistical method was student’s T-test.
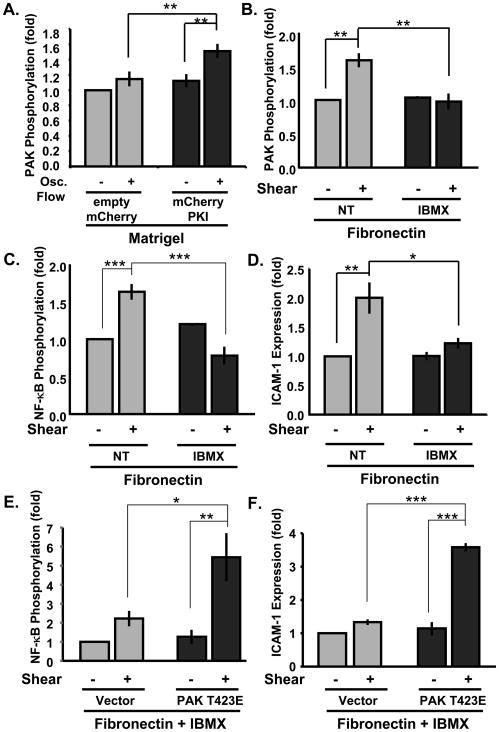
We next asked whether matrigel-associated PKA activation also mediates suppression of PAK activation and NF-κB-dependent gene expression. ECs were transfected with mCherry-tagged PKI, then plated on matrigel and exposed to oscillatory flow for 18 hours. Despite 40–50% transfection efficiencies, PKI significantly increased PAK activation in oscillatory flow compared to control vector (Figure 6A). Additionally, HAECs on fibronectin were sheared in the presence of the phosphodiesterase (PDE) inhibitor IBMX to stimulate cAMP accumulation and PKA activation. Forskolin and iloprost were not used in these experiments due to the amounts required to reach the target concentration in the large volume of flow medium. IMBX (100 μM) completely inhibited PAK activation (Figure 6B), NF-κB phosphorylation (Figure 6C) and ICAM-1 expression (Figure 6D) by oscillatory flow. Constitutively active PAK (T423E) restored NF-κB activation (Figure 6E) and ICAM-1 expression (Figure 6F) by oscillatory flow in the presence of IBMX. Taken together, these data show that, similar to onset of laminar shear, basement membrane protein suppresses inflammatory activation of ECs by oscillatory flow via PKA activation and PKA-dependent PAK inhibition.
Figure 6. PAK mediates anti-inflammatory PKA signaling in oscillatory flow.
(A) BAECs transfected (50% efficiency) with control, empty mCherry vector or mCherry-PKI were plated on matrigel and exposed to oscillatory flow for 18 hours. PAK activation was determined as in Fig 1. n = 4. (B) HAECs plated on fibronectin were exposed to oscillatory flow for 18 hours in the presence of the PDE inhibitor IBMX (100 μM) or vehicle control. (B) PAK phosphorylation and (C) NF-κB phosphorylation were determined. (D) ICAM-1 expression was determined by Western blotting and normalized to total protein. n = 3. (E,F) HAECs transfected with Myc-tagged T423E-PAK or control vector were plated on fibronectin and exposed to oscillatory flow in the presence of IBMX (100 μM) for 18 hours. (E) NF-κB phosphorylation and (F) ICAM-1 expression were determined by Western blotting as previously described. n = 3. * p < 0.05, ** p < 0.01 by multiple comparisons ANOVA.
Activating PKA limits PAK activation in vivo
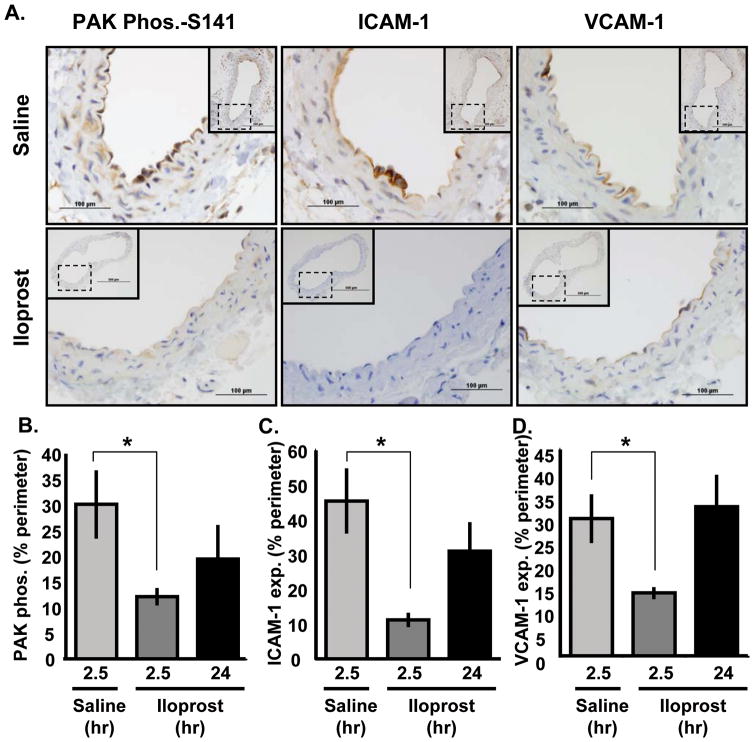
PGI2 analogs, such as iloprost, reduce inflammatory gene expression and vascular permeability both in vitro and in vivo28, 29. We therefore assessed whether iloprost mediates some of its protective effects through PAK inhibition using aged C57Bl/6J mice that show a modest level of chronic inflammation in regions of disturbed flow (e.g., the carotid sinus). Male C57Bl/6J mice (chow diet; 36–38 weeks old) received intraperitoneal injections of iloprost (20 μg; ~1 mg/kg). At 2.5 or 24 hrs post-injection, control mice showed PAK phosphorylation in the EC layer at the atheroprone carotid sinus, concomitant with ICAM-1 and VCAM-1 expression (Figure 7A). In contrast, mice treated with iloprost for 2.5 hrs show a significant reduction in PAK activation (Figure 7B) as well as ICAM-1 (Figure 7C) and VCAM-1 (Figure 7D) expression. PAK activity and ICAM-1 expression remained lower at 24 hours post-treatment; however this effect did not reach statistical significance, mostly likely because iloprost has a short half life in vivo30. Thus, activation of PKA can suppress PAK activation in atheroprone regions of arteries in vivo.
Figure 7. Iloprost reduces PAK activation in areas of disturbed flow in vivo.
(A) C57Bl/6J mice at 36–38 weeks received intraperitoneal injection of iloprost (20 μg; ~1 mg/kg) or saline control. After 2.5 or 24 hours, carotid arteries were collected and analyzed for PAK phosphorylation (Ser141), ICAM-1 expression, and VCAM-1 expression by immunohistochemistry. 40X images show the atheroprone region at the outer wall of the carotid sinus. 10X images show the entire artery (insets). (B,C,D) The percent of the vessel lumen staining positive for (B) phosphorylated PAK, (C) ICAM-1, and (D) VCAM-1 was determined. n = 3. * p < 0.05 by one-way ANOVA.
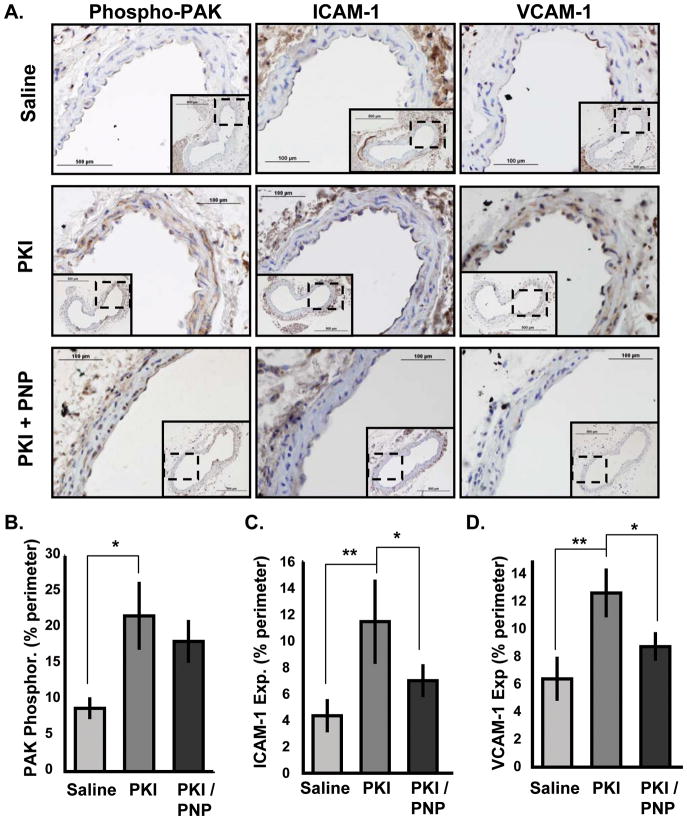
Since the effects of iloprost may result from secondary hemodynamic alterations, we also tested whether PKA inhibition could stimulate PAK activation and inflammation in otherwise healthy mice. Male C57Bl/6J mice (chow diet; 10 weeks old) were given retroorbital injections of PKI (24 μg, ~1.2 mg/kg) with or without the PAK inhibitory peptide (PNP; 50 μg, ~2.5 mg/kg). After 2.5 hrs, PKI treatment significantly increased PAK activation within the carotid sinus compared to control mice along with ICAM-1 and VCAM-1 staining (Figure 8). PNP did not significantly affect PAK activation, consistent with previous reports that it inhibits downstream PAK signaling but not PAK activation directly, but it significantly reversed the effects of PKI on ICAM-1 (Figure 8C) and VCAM-1 (Figure 8D). Taken together, these results suggest that PKA signaling limits inflammation at least in part through PAK inhibition.
Figure 8. PKA inhibition stimulates PAK activation and inflammation in vivo.
(A) C57Bl/6J mice at 10 weeks old received retroorbital injection of saline, PKI (24 μg, ~1.2 mg/kg) or PKI and the PAK inhibitory peptide (PNP, 50 μg, ~2.5 mg/kg). After 2.5 hours, carotid arteries were collected and analyzed for PAK phosphorylation (Ser141), ICAM-1 expression, and VCAM-1 expression by immunohistochemistry. 40X images show the atheroprone region at the outer wall of the carotid sinus. 10X images show the entire artery (insets). (B,C,D) The percent of the vessel lumen staining positive for (B) phosphorylated PAK, (C) ICAM-1, and (D) VCAM-1 was determined. n = 3. * p < 0.05 by one-way ANOVA.
Discussion
Previous work showed that PAK is activated by flow in cells on fibronectin but not collagen or basement membrane protein, and that it subsequently regulates EC inflammatory gene expression and endothelial monolayer permeability5, 7, 13, 31, 32. We now show, first, that PAK activation by flow is matrix-specific despite equivalent activation of its direct upstream element Rac, suggesting that basement membranes actively suppress PAK. PKA was identified as the critical suppressor of PAK in this system, based on the matrix-specific cAMP production and PKA activation, the restoration of PAK activation in cells on basement membranes by inhibitors of PKA, and the suppression of PAK in cells on fibronectin by activators of PKA. Collagen appears to be the active component of the basement membrane. Furthermore, we show that PKA controls matrix-specific activation of NF-κB and downstream genes through PAK. Lastly, we show that pharmacological activation of PKA inhibits PAK activation at sites of disturbed flow in vivo. Taken together, these data demonstrate that basement membrane proteins inhibit PAK and downstream inflammatory signaling through PKA. These results further suggest that PAK inhibition may mediate a subset of the anti-inflammatory and barrier protective properties ascribed to PKA activators24, 28, 29.
Although PKA has long been associated with the EC responses to flow33, the molecular mechanism of activation is unknown. PKA is classically activated through Gαs-dependent activation of adenylate cyclase. While flow was reported to activate EC G protein-coupled receptors34 and G proteins35, this work implicated Gαi and Gαq subclasses, not Gαs. Furthermore, these G protein-dependent signals affected the early calcium-dependent nitric oxide (NO) production36, but not the late calcium-independent NO production mediated by PKA. Ligation of the collagen-binding integrin α2β1 by the perlecan fragment endorepellin stimulates PKA37, and direct application of mechanical force to β1 integrins can stimulate Gαs-dependent cAMP production and PKA activation38. However, there is currently no information about how integrins affect Gαs signaling.
Recently, PKA signaling was implicated in flow-induced EC alignment in cells plated on the connecting segment-1 (CS-1) fragment of fibronectin, a ligand for integrin α4β139. Flow-inducedα4β1 ligation induced polarized PKA activation, which was required for flow-induced EC alignment 39. However, ECs express high levels of the integrin α5β1, which is the endothelial fibronectin receptor in cells from multiple vascular beds40, 41, whereas expression of α4β1 is less abundant and restricted to a small subset of ECs in vivo42. One potential implication of α4β1-dependent PKA activation is that α4β1 may serve as a protective signaling pathway limiting EC dysfunction induced by the α5β1-fibronectin interaction. In support of this idea, fibronectin stimulated the α5β1-dependent expression of NF-κB target gene MMP-9 in fibroblasts, which was diminished by α4β1 expression43.
The molecular mechanism by which PKA inhibits PAK activation is currently unknown. Previous work (Howe and Juliano, 2000) suggested that PKA phosphorylates PAK directly19 though at an unknown site. Alternatively, PKA could mediate PAK suppression indirectly through activation of eNOS and protein kinase G, which similarly phosphorylates PAK directly44. PKA-dependent PAK suppression could also result from regulation of protein phosphatases. PP2A dephosphorylates PAK limiting its activation18, and PKA can directly stimulate PP2A phosphatase activity45. Future work will be required to determine the mechanism by which PKA inhibits PAK.
Multiple stimuli, including PGI2 and adiponectin, reduce endothelial permeability through PKA signaling24, 28, 46. Proposed mechanisms for PKA-dependent barrier protection include inhibition of Rho47 and activation of Rac28. Active PAK destabilizes EC cell-cell junctions by enhancing cortical actin contractility32 and stimulating VE-cadherin internalization 31, and PAK inhibitors reduce endothelial permeability in vitro 7, 32 and in vivo7 48. Thus, PKA-dependent PAK inhibition may mediate, at least in part, PKA’s barrier protective effects.
PAK signaling is classically associated with cell proliferation and migration12. As such, previous work has established PAK as a key regulator of vascular remodeling during development49, angiogenesis25, and restenosis50. Our work was the first to demonstrate a role for PAK signaling in EC inflammatory activation in atherogenesis. However, the therapeutic potential of long-term PAK inhibition is uncertain. Global PAK inhibitors are likely to induce deleterious side effects; a peptide inhibitor of PAK caused Alzheimer’s-like symptoms in mice presumably due to inhibition of PAK3 in neuronal tissue51. As an alternative approach, identifying endogenous inhibitory pathways, such as PKA, may provide a more selective reduction of PAK activation under defined circumstances. The PDE inhibitor IBMX blocks PAK activation and inflammatory gene expression by oscillatory flow, and PDE4 inhibitors are currently in clinical trials as immunosuppressive agents used to treat asthma and chronic obstructive pulmonary disease52. However, global PKA activation may also produce deleterious side effects, and identifying endothelial cell-specific molecular targets (ex. adenylate cyclase isoforms, PDEs, A kinase anchoring proteins) will likely be required for efficient therapeutic intervention. PKA has been found to inhibit NF-κB in other systems through unknown mechanisms; the current work therefore suggests that inhibition of PAK could play a wider role in this effect.
Novelty and Significance
What Is Known
Vascular regions exposed to disturbed blood flow profiles are more susceptible to atherosclerotic plaque formation.
Basement membrane proteins suppress flow-induced endothelial cell activation while wound-associated matrices such as fibronectin and fibrinogen support flow-induced endothelial cell activation.
Enhanced p21 activated kinase (PAK) signaling in endothelial cells on fibronectin promotes flow-induced endothelial cell activation in both in in vitro and in vivo model systems; however the mechanisms mediating matrix-specific PAK signaling were unknown.
What New Information Does This Article Contribute
Basement membrane proteins enhance flow-induced protein kinase A (PKA) signaling to suppress PAK activation.
Inhibiting PKA reduces the protective effects of the basement membrane on flow-induced PAK activation and inflammation in vitro and at sites of disturbed flow in vivo, whereas activating PKA blocks PAK activation and inflammatory gene expression in both in vitro and in vivo model systems.
Regions of disturbed blood flow do not demonstrate significant endothelial cell activation until fibronectin is deposited into the endothelial cell matrix. In endothelial cells in culture, fibronectin promotes flow-induced endothelial permeability and inflammatory gene expression through a PAK-dependent pathway. PAK activation occurs concomitant with fibronectin deposition in vivo, and PAK inhibitors reduce vascular permeability and inflammation in mouse models of atherosclerosis. However, the mechanisms controlling matrix-specific PAK activation were unknown. By analyzing how flow activates PAK, we now show that matrix composition does not affect the signaling mediators upstream of PAK. Instead, the endothelial cell basement membrane limits PAK activation through enhanced PKA signaling. PKA possesses both barrier protective and anti-inflammatory properties, and PKA can phosphorylate and inhibit PAK. Basement membrane proteins significantly enhance flow-induced PKA signaling. PKA inhibitors promote flow-induced PAK activation and inflammation in cells on basement membrane proteins in vitro and at sites of disturbed flow in young C57Bl/6J mice. Stimulating PKA prevents PAK activation and inflammation in both in vitro flow systems and aged C57Bl/6J mice. These results identify a novel molecular mechanism regulating PAK signaling during endothelial cell activation and provide an alternative mechanism by which PKA exerts its protective effects on vascular physiology.
Supplementary Material
Acknowledgments
The authors thank Alan Howe (University of Vermont) for providing the mCherry-PKI construct and Kathleen O’Connor (University of Texas Medical Branch at Galveston) for the GST-PKI construct and for technical assistance.
Sources of Funding
This work was funded by NIH R01 HL75092 to M.A.S. and by an American Heart Association Scientist Development Grant to A.W.O.
Nonstandard abbreviations and acronyms
- BAEC
Bovine aortic endothelial cell
- CS-1
connecting segment-1
- EC
endothelial cell
- HAEC
human aortic endothelial cell
- ICAM-1
intercellular adhesion molecule-1
- IBMX
isobutylmethylxanthine
- PAK
p21 activated kinase
- PBD
p21 binding domain
- PDE
phosphodiesterase
- PKA
protein kinase A
Footnotes
Disclosures
None
Works Cited
- 1.Ross R. Atherosclerosis--an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [see comment] [DOI] [PubMed] [Google Scholar]
- 2.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals of the New York Academy of Sciences. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 3.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohan S, Mohan N, Sprague EA. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am J Physiol. 1997;273:C572–578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 5.Hahn C, Orr AW, Sanders JM, Jhaveri KA, Schwartz MA. The subendothelial extracellular matrix modulates JNK activation by flow. Circ Res. 2009;104:995–1003. doi: 10.1161/CIRCRESAHA.108.186486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagel T, Resnick N, Atkinson WJ, Dewey CF, Jr, Gimbrone MA., Jr Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J Clin Invest. 1994;94:885–891. doi: 10.1172/JCI117410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr AW, Stockton R, Simmers MB, Sanders JM, Sarembock IJ, Blackman BR, Schwartz MA. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orr AW, Ginsberg MH, Shattil SJ, Deckmyn H, Schwartz MA. Matrix-specific suppression of integrin activation in shear stress signaling. Mol Biol Cell. 2006;17:4686–4697. doi: 10.1091/mbc.E06-04-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO Journal. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzima E, Del Pozo MA, Kiosses WB, Mohamed SA, Li S, Chien S, Schwartz MA. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO Journal. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orr AW, Sanders JM, Bevard M, Coleman E, Sarembock IJ, Schwartz MA. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- 13.Orr AW, Hahn C, Blackman BR, Schwartz MA. p21-activated kinase signaling regulates oxidant-dependent NF-kappa B activation by flow. Circ Res. 2008;103:671–679. doi: 10.1161/CIRCRESAHA.108.182097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins T, Cybulsky MI. NF-kappaB: pivotal mediator or innocent bystander in atherogenesis? Journal of Clinical Investigation. 2001;107:255–264. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alahari SK, Reddig PJ, Juliano RL. The integrin-binding protein Nischarin regulates cell migration by inhibiting PAK. Embo J. 2004;23:2777–2788. doi: 10.1038/sj.emboj.7600291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia C, Ma W, Stafford LJ, Marcus S, Xiong WC, Liu M. Regulation of the p21-activated kinase (PAK) by a human Gbeta -like WD-repeat protein, hPIP1. Proc Natl Acad Sci U S A. 2001;98:6174–6179. doi: 10.1073/pnas.101137298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh CG, Tan EJ, Manser E, Lim L. The p21-activated kinase PAK is negatively regulated by POPX1 and POPX2, a pair of serine/threonine phosphatases of the PP2C family. Curr Biol. 2002;12:317–321. doi: 10.1016/s0960-9822(02)00652-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhan Q, Ge Q, Ohira T, Van Dyke T, Badwey JA. p21-activated kinase 2 in neutrophils can be regulated by phosphorylation at multiple sites and by a variety of protein phosphatases. J Immunol. 2003;171:3785–3793. doi: 10.4049/jimmunol.171.7.3785. [DOI] [PubMed] [Google Scholar]
- 19.Howe AK, Juliano RL. Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol. 2000;2:593–600. doi: 10.1038/35023536. [DOI] [PubMed] [Google Scholar]
- 20.Paulucci-Holthauzen AA, O’Connor KL. Use of pseudosubstrate affinity to measure active protein kinase A. Anal Biochem. 2006;355:175–182. doi: 10.1016/j.ab.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Tzima E, Kiosses WB, del Pozo MA, Schwartz MA. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. Journal of Biological Chemistry. 2003;278:31020–31023. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- 22.Yeh LH, Park YJ, Hansalia RJ, Ahmed IS, Deshpande SS, Goldschmidt-Clermont PJ, Irani K, Alevriadou BR. Shear-induced tyrosine phosphorylation in endothelial cells requires Rac1-dependent production of ROS. Am J Physiol. 1999;276:C838–847. doi: 10.1152/ajpcell.1999.276.4.C838. [DOI] [PubMed] [Google Scholar]
- 23.Ollivier V, Parry GC, Cobb RR, de Prost D, Mackman N. Elevated cyclic AMP inhibits NF-kappaB-mediated transcription in human monocytic cells and endothelial cells. J Biol Chem. 1996;271:20828–20835. doi: 10.1074/jbc.271.34.20828. [DOI] [PubMed] [Google Scholar]
- 24.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 25.Kiosses WB, Hood J, Yang S, Gerritsen ME, Cheresh DA, Alderson N, Schwartz MA. A dominant-negative p65 PAK peptide inhibits angiogenesis. Circulation Research. 2002;90:697–702. doi: 10.1161/01.res.0000014227.76102.5d. [DOI] [PubMed] [Google Scholar]
- 26.Seamon KB, Padgett W, Daly JW. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991;260:C1052–1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- 28.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA-and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Bella S, Molteni M, Mocellin C, Fumagalli S, Bonara P, Scorza R. Novel mode of action of iloprost: in vitro down-regulation of endothelial cell adhesion molecules. Prostaglandins. 2001;65:73–83. doi: 10.1016/s0090-6980(01)00131-9. [DOI] [PubMed] [Google Scholar]
- 30.Renaud F, Succo E, Alessi MC, Legre R, Juhan-Vague I. Iloprost and salvage of a free flap. Br J Plast Surg. 1996;49:245–248. doi: 10.1016/s0007-1226(96)90060-0. [DOI] [PubMed] [Google Scholar]
- 31.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 32.Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- 33.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, Jo H. Shear stress stimulates phosphorylation of eNOS at Ser(635) by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–1828. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 34.Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A. 2006;103:15463–15468. doi: 10.1073/pnas.0607224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells. Involvement of G proteins in mechanochemical signal transduction. Circ Res. 1996;79:834–839. doi: 10.1161/01.res.79.4.834. [DOI] [PubMed] [Google Scholar]
- 36.Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol. 1994;267:C753–758. doi: 10.1152/ajpcell.1994.267.3.C753. [DOI] [PubMed] [Google Scholar]
- 37.Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, Zutter MM, Santoro SA, Kim JK, Hook M, Reed CC, Iozzo RV. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through alpha2beta1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alenghat FJ, Tytell JD, Thodeti CK, Derrien A, Ingber DE. Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J Cell Biochem. 2009 doi: 10.1002/jcb.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldfinger LE, Tzima E, Stockton R, Kiosses WB, Kinbara K, Tkachenko E, Gutierrez E, Groisman A, Nguyen P, Chien S, Ginsberg MH. Localized alpha4 integrin phosphorylation directs shear stress-induced endothelial cell alignment. Circ Res. 2008;103:177–185. doi: 10.1161/CIRCRESAHA.108.176354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- 41.Albelda SM, Daise M, Levine EM, Buck CA. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989;83:1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mechtersheimer G, Barth T, Hartschuh W, Lehnert T, Moller P. In situ expression of beta 1, beta 3 and beta 4 integrin subunits in non-neoplastic endothelium and vascular tumours. Virchows Arch. 1994;425:375–384. doi: 10.1007/BF00189575. [DOI] [PubMed] [Google Scholar]
- 43.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fryer BH, Wang C, Vedantam S, Zhou GL, Jin S, Fletcher L, Simon MC, Field J. cGMP-dependent Protein Kinase Phosphorylates p21-activated Kinase (Pak) 1, Inhibiting Pak/Nck Binding and Stimulating Pak/Vasodilator-stimulated Phosphoprotein Association. J Biol Chem. 2006;281:11487–11495. doi: 10.1074/jbc.M600279200. [DOI] [PubMed] [Google Scholar]
- 45.Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc Natl Acad Sci U S A. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lum H, Jaffe HA, Schulz IT, Masood A, RayChaudhury A, Green RD. Expression of PKA inhibitor (PKI) gene abolishes cAMP-mediated protection to endothelial barrier dysfunction. Am J Physiol. 1999;277:C580–588. doi: 10.1152/ajpcell.1999.277.3.C580. [DOI] [PubMed] [Google Scholar]
- 47.Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- 48.Reutershan J, Stockton R, Zarbock A, Sullivan GW, Chang D, Scott D, Schwartz MA, Ley K. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007;175:1027–1035. doi: 10.1164/rccm.200612-1822OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117:4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- 50.Wang D, Paria BC, Zhang Q, Karpurapu M, Li Q, Gerthoffer WT, Nakaoka Y, Rao GN. A role for Gab1/SHP2 in thrombin activation of PAK1: gene transfer of kinase-dead PAK1 inhibits injury-induced restenosis. Circ Res. 2009;104:1066–1075. doi: 10.1161/CIRCRESAHA.109.196691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- 52.Banner KH, Trevethick MA. PDE4 inhibition: a novel approach for the treatment of inflammatory bowel disease. Trends Pharmacol Sci. 2004;25:430–436. doi: 10.1016/j.tips.2004.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.