Abstract
CONTEXT
The only treatment proven to be efficacious for long-term sustained weight loss for morbid obesity is bariatric surgery.
OBJECTIVE
To create a decision analytic model to estimate the balance between treatment risks and benefits for patients with morbid obesity.
DESIGN
Decision analytic Markov state transition model with multiple logistic regression models as inputs.
PATIENTS or OTHER PARTIPANTS
Data from the 2005 National Inpatient Survey were used to calculate in-hospital mortality risk associated with bariatric surgery and then adjusted for 30-day mortality. To calculate excess mortality associated with obesity, we used the 1991–1996 National Health Interview Survey linked to the National Death Index. Bariatric surgery was assumed to influence mortality only through its impact on the excess mortality associated with obesity and the efficacy of surgery was estimated from a recent large observational trial.
INTERVENTION
Gastric bypass surgery versus no surgical treatment.
MAIN OUTCOME MEASURE
Life expectancy.
RESULTS
Our base case, a 42 year-old female with a BMI of 45 kg/m2, gained an additional 2.95 years of life expectancy with bariatric surgery. No surgical treatment was favored in our base case when the 30-day surgical mortality exceeded 9.5% (baseline 30-day mortality=0.2%) or when the efficacy of bariatric surgery for reducing mortality decreased to 2% or less (baseline efficacy=53%).
CONCLUSIONS
The optimal decision for individual patients varies based on the balance of risk between perioperative mortality, excess annual mortality risk associated with increasing BMI, and the efficacy of surgery; however, for the average morbidly obese patient, gastric bypass improves life expectancy.
Introduction
Morbid obesity continues to be a growing problem in the United States affecting 5.1% of the population1 with an associated increase in direct health care costs of over 11 billion dollars2. Available evidence indicates that dietary, behavioral, and drug treatment options frequently fail to result in sustained, clinically meaningful weight loss in patients with morbid obesity3–5. Given the increasing prevalence of morbid obesity and the lack of viable alternative treatments, the annual demand for bariatric surgery has increased dramatically over the past 12 years from 16,800 cases in 19926 to an estimated 205,000 cases in 20077.
While no large-scale RCTs have compared bariatric surgery with intensive medical management for the morbidly obese, there is evidence from large controlled clinical trials and numerous case series that bariatric surgery is currently the only effective therapy for promoting clinically significant weight loss and improving obesity-associated conditions among adults with a BMI ≥ 40 kg/m28–12. Bariatric surgery has also been shown to be cost-effective when compared to non-operative weight loss interventions13.
Several retrospective cohort studies and one prospective study suggest that bariatric surgery also improves survival12, 14–16. In the largest study to date, Adams and colleagues matched 7925 subjects who had gastric bypass with 7925 controls identified through the Bureau of Motor Vehicles in Utah14. After a mean follow-up of 7.1 years, adjusted mortality was decreased by 40% in the group having surgery.
However, bariatric surgery is not without risk. The 30-day mortality rate following bariatric surgery has been reported to range from 0 to 2%8, 17, but the risk for select subgroups of patients may be much higher18–20. One retrospective cohort study in 16,155 people who underwent bariatric surgery found that the mortality rate at 1 year was 4.6%, and that mortality rates were greater for those aged 65 years or older compared with younger people at 1 year18. In a case series of 1,067 patients, patients older than 55 years of age had a 3-fold increase in mortality20.
While bariatric surgery has been proven to be efficacious and cost-effective in reducing obesity and obesity-associated conditions, clinical trials have not clearly identified characteristics of the ideal surgical candidate. One must consider tradeoffs between early surgical risk and long term efficacy. Clearly, when surgical risk is low and life expectancy following surgery is long, the decision to have bariatric surgery may be straightforward. However, when the surgical risk is higher and the patient has less time to realize the benefits of weight loss, the decision to have bariatric surgery is more difficult. Morbidly obese patients considering bariatric surgery would benefit from a better understanding of the uncertainties associated with the procedure and its impact on weight loss. Therefore, our goal was to better characterize obesity-related mortality and the risks of bariatric surgery, and then create a decision analytic model to estimate the balance between treatment risks and benefits for patients with morbid obesity across a variety of clinical scenarios.
Methods
We developed a decision-analytic Markov state transition model to evaluate two clinical strategies in morbidly obese patients: bariatric surgery versus no surgical treatment. We chose to model only gastric bypass since that is the most common procedure in the U.S, accounting for over 65% of cases19. The decision model was constructed using Decision Maker®22 and all other analyses were conducted using SAS version 9.1 (Cary, NC).
For our base case analyses, we chose a 42 year-old female with a BMI of 45 kg/m2 and a 44 year-old male with a BMI of 45 kg/m2, since these could be considered “average” surgical patients. We also performed analyses for a series of BMI, age and gender categories.
Decision Model Structure
The model incorporates a 30-day cycle length and a lifelong time horizon. Prior to entering the Markov simulation, patients in the bariatric surgery branch face the 30-day risk of surgery-related mortality. During the first monthly cycle of the simulation, all patients enter either a post-operative state or a non-surgical morbidly obese state. During each monthly cycle patients face a mortality risk that is based upon their BMI, surgical status, age and gender. Outcomes are evaluated using non-quality adjusted life years because long-term data on changes in quality of life are not currently available for these populations.
Assumptions
Major simplifying assumptions included: bariatric surgery influences mortality only through its impact on the excess mortality associated with obesity, and for the subjects not receiving surgery, BMI category did not change over time. We explored these assumptions through sensitivity analyses.
Model Inputs
Mortality due to Obesity
Age-, gender- and BMI- specific risk of death was calculated using the publically available National Health Interview Survey (NHIS) linked to the National Death Index. The NHIS is a nationally representative yearly survey conducted by the National Center for Health Statistics to gauge the health of the civilian non-institutionalized U.S. population. Mortality follow-up was through December 31, 2002 and is based on a probabilistic match with each NHIS participant.
We combined the datasets from 1991 through 1996 and the resulting dataset was then weighted to be a nationally representative sample. We used this dataset to develop a multivariable logistic regression model that predicts 5-year mortality based upon age, gender and BMI. Since we were most interested in fitting the model to the subset of the population that was overweight or obese, we limited the analysis to subjects with a BMI greater than 25.
The logistic regression model was used to estimate the excess mortality associated with obesity. We calculated the annual death rate for specific examples and compared them to the average weight, age, and gender matched death rate to derive the excess annual mortality associated with BMI. We used a BMI of 26 as the referent as this was the average BMI in the NHIS dataset between 1991 and 1996. In the decision model we added the excess mortality rate associated with obesity to the calculated mortality rates for an average weight population based upon age and gender.
Bariatric Surgery Risk
A second logistic regression model was developed to calculate the in-hospital mortality risk associated with bariatric surgery using data from the 2005 National Inpatient Survey. The National Inpatient Survey (NIS) is an administrative dataset and does not contain height or weight information so we included only age and gender as variables in the final model. Since in-hospital mortality has been shown to underestimate 30-day mortality by a factor of 2 to 315, we adjusted upwards the probability of death calculated using the logistic regression model by a factor of 3.0. This is a conservative estimate that biases the model against gastric bypass surgery. We explored this factor in sensitivity analyses.
Efficacy of Bariatric Surgery
To determine the impact of surgery on survival, we used a retrospective cohort study of gastric bypass with matched controls published by Adams, et al.14 We calculated the efficacy of surgery from this seven year study correcting for age and gender by calculating the average survival rate for the surgical and nonsurgical arms of the study. We then subtracted the expected survival for an age and gender matched cohort using life tables. Comparing the resultant differences between the surgical group and nonsurgical group yields the effect of surgery on the mortality associated with obesity.
In the model, the mortality risk reduction resulting from surgery was multiplied by the excess mortality rate associated with obesity. In this way, surgery could only impact mortality associated with obesity and not improve survival beyond that of the normal weight population.
Results
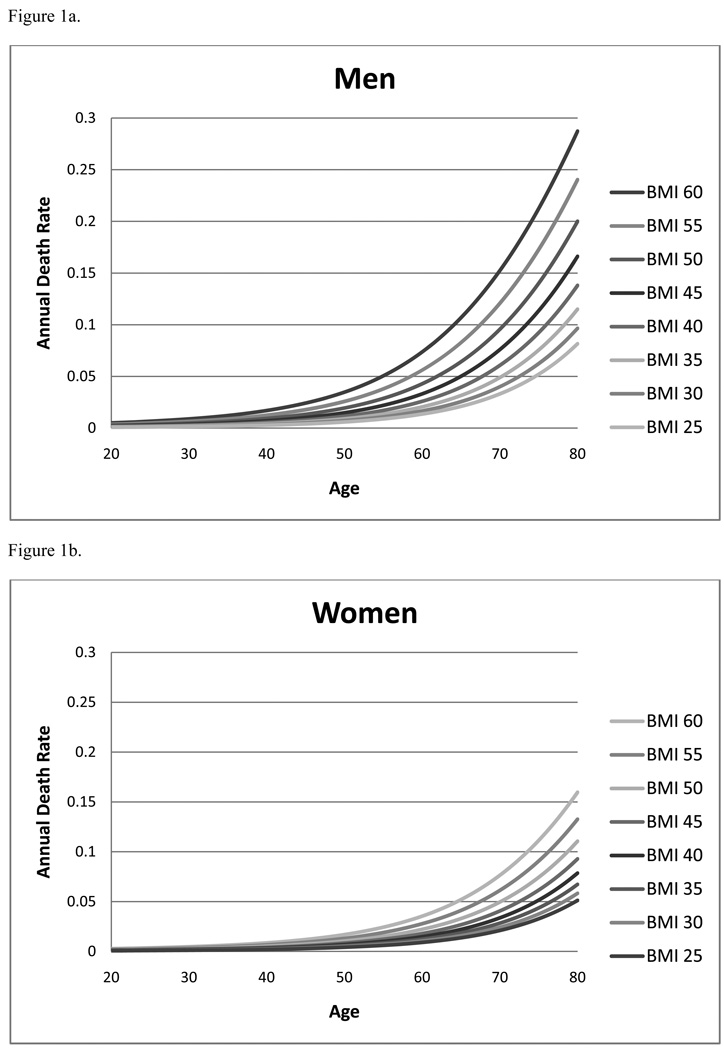
Over 399,000 subjects from the NHIS dataset were included in the final calculation of excess mortality from obesity. The average age was 44.6 years and 46% of the sample was male. The median BMI was 26 kg/m2. The final multivariable logistic regression model predicting mortality based upon age, gender and BMI incorporated seven terms: BMI, BMI2, age, age2, gender, gender*BMI, age*gender. The fit to the data was good, (Hosmer-Lemeshow goodness-of-fit p-value greater than 0.05, c statistic 0.83). Model estimates of annual death rates, stratified by gender, are presented in Figure 1.
Figure 1.
Figure 1a. Annual predicted death rate for men by age and BMI category
Figure 1b. Annual predicted death rate for women by age and BMI category
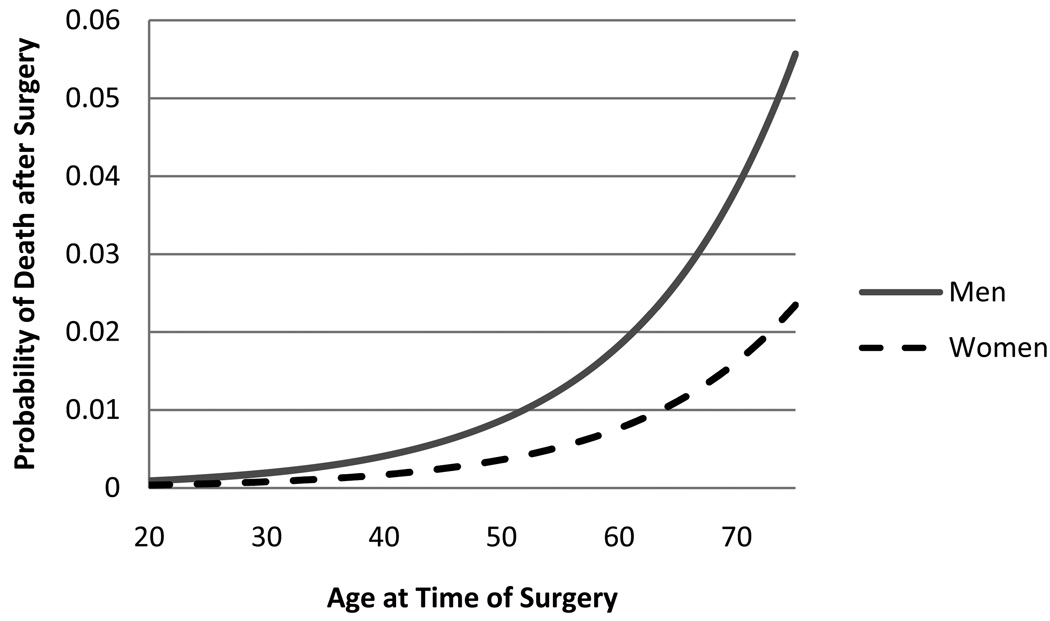
We included 23,281 subjects from the NIS dataset to calculate in-hospital mortality of patients having bariatric surgery. Overall 0.13% of patients died during their hospitalization in 2005. The results of the logistic regression model predicting in-hospital mortality are presented in Figure 2, adjusted for 30-day mortality. Male patients and older patients had higher 30-day mortality from bariatric surgery.
Figure 2.
30-day predicted probability of death after bariatric surgery for men and women by age.
Model Calibration and Validation
To evaluate the model calibration, we compared predicted outcomes from our model with the control group described in the study by Adam et al14. Since the control arm of the Adams study is only used in the calculation of the efficacy term for the decision model and is not used in the non-surgical arm of the decision model, using the results of the decision model in the non-surgical arm provide an unbiased comparison. The control group in this study consisted of 7,925 subjects with an average age of 39 years, 84% of whom were female. 4.1% of the control group died during an average follow-up of 7.1 years. Using these input parameters, our model predicted that 4.16% would die at 7.1 years without gastric bypass surgery.
Markov Model Results
In our base case analysis for an average 42 year-old female with a BMI of 45, bariatric surgery resulted in a gain of 2.95 years of life expectancy (35.03 years versus 32.08 years). No surgical treatment was the preferred strategy in our base case when 30-day surgical mortality exceeded 9.5% (baseline 30-day mortality =0.2%) or when the efficacy of bariatric surgery decreased to 2% or less (baseline efficacy=53%).
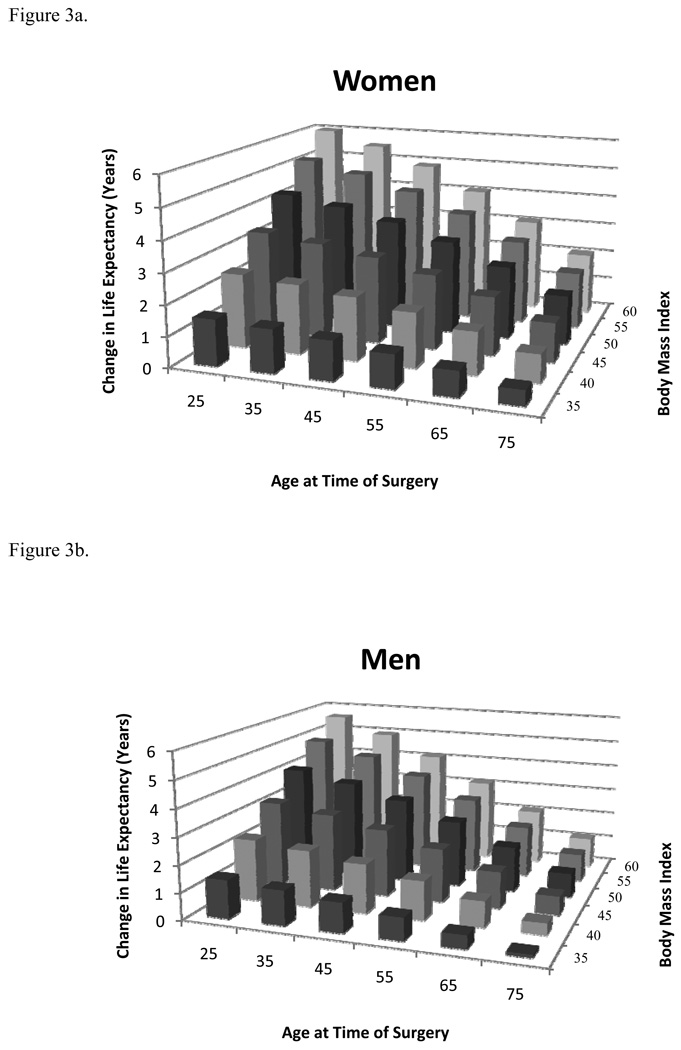
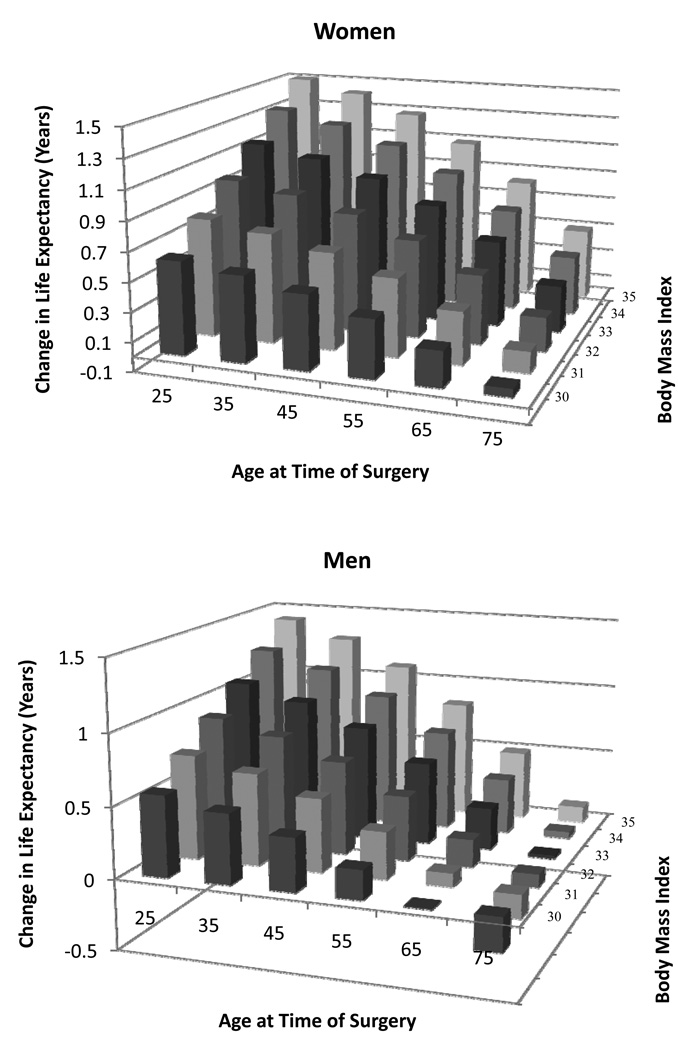
Additional sensitivity analyses revealed that younger women with higher BMI’s gained the most life expectancy from bariatric surgery (Figure 3a). For women with a BMI of 45 kg/m2, surgery results in a gain of at least 1 year of life expectancy until age 80. Women with a BMI of 40 kg/m2 gain at least 1 year of life expectancy until age 74, while women with a BMI of 35 kg/m2 the gain less than one life-year after age 60.
Figure 3.
Figure 3a. Change in life expectancy with bariatric surgery for women at different ages and BMIs.
Figure 3b. Change in life expectancy with bariatric surgery for men at different ages and BMIs.
A 44 year-old man with a BMI of 45 kg/m2 would gain 2.57 additional years of life expectancy (26.82 years versus 24.25 years). No surgical treatment was preferred when the 30-day surgical mortality exceeded 8.6% (baseline 30-day mortality =0.55%) or when the efficacy of bariatric surgery decreased to 3% or less (baseline efficacy=53%).
As with women, younger men with higher BMI’s gained the most life expectancy after bariatric surgery; however, the gain was slightly less for men of all ages and BMI subgroups (Figure 3b). Men with a BMI of 45 kg/m2, gain at least 1 year of life expectancy until age 72. Men with a BMI of 40 kg/m2 gain at least 1 year of life expectancy until age 66, while men with a BMI of 35 kg/m2 gain less than one life-year after age 50.
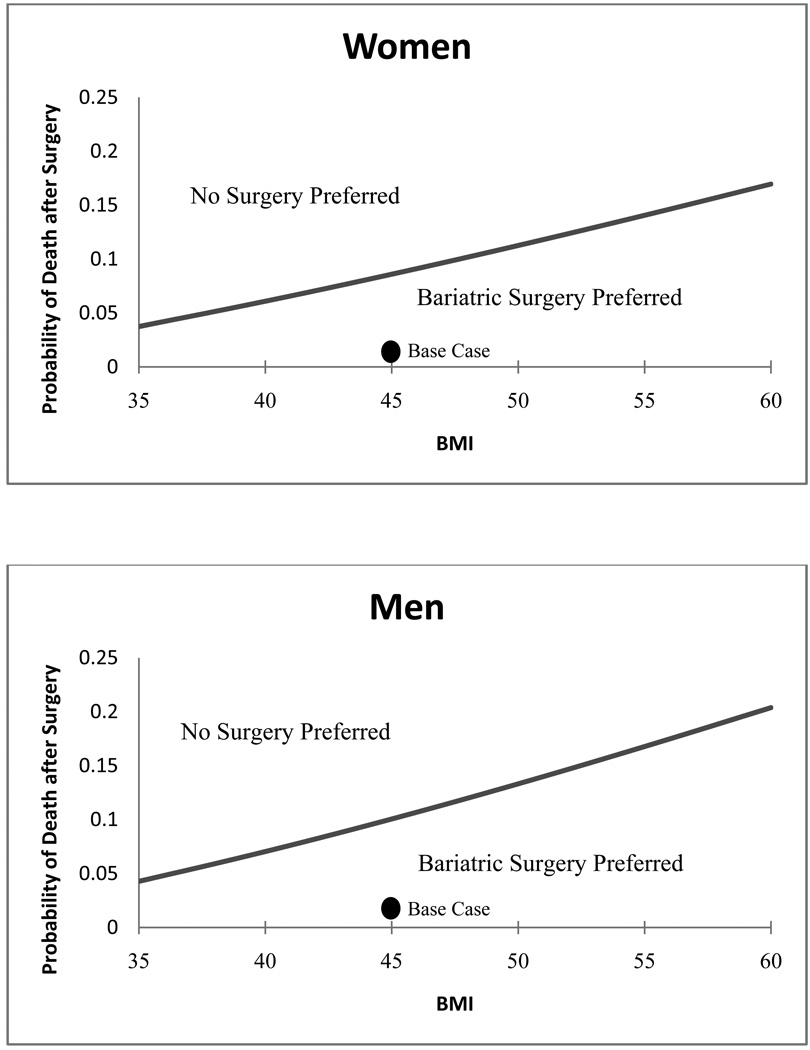
Since variability in surgical mortality may be due to both institutional (e.g. surgical centers) and individual patient characteristics, we examined this parameter closely. As expected, when the 30-day risk of dying from surgery is higher than average, the expected benefit of bariatric surgery is lower. However, the model is only sensitive to this parameter when the treatment decision is a close call (Figure 4).
Figure 4.
2-way sensitivity analysis for 30-day probability of death after surgery for women at age 42 and men at age 44. In the region to the lower right, below the line, where the probability of death is low and body mass index is higher, bariatric surgery is preferred. To the upper left, when the probability of death is higher and the body mass index is lower, no surgery is the preferred strategy. The line indicates the threshold between the two strategies. The dark circles indicate the base case values for each parameter.
There may be additional obesity-related comorbid conditions (e.g. diabetes, hypertension) that impact survival; therefore, in any BMI category, some patients may have a higher or lower annual mortality risk. In sensitivity analyses examining this parameter, bariatric surgery becomes more attractive as the mortality of obesity associated conditions increases. This is particularly evident in subgroups where benefit is small (e.g. older age, lower BMI).
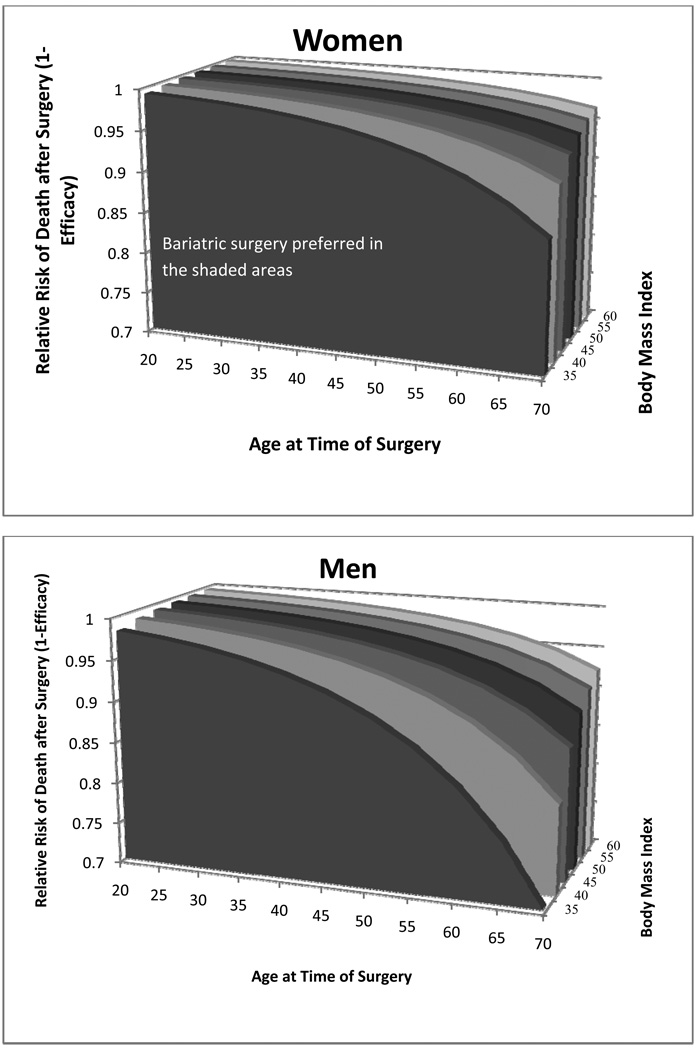
The efficacy of surgery also may vary across patient groups14. Therefore, we examined the impact of changes in surgical efficacy across subgroups, including BMI and gender. Outcomes for men and older patients were more sensitive to this parameter (Figure 5).
Figure 5.
3-way sensitivity analysis for the impact of surgery on the relative risk of death (1-efficacy) at varying ages and BMIs by gender. The shaded areas are where the surgery is the preferred strategy. For higher relative risks of death (or lower efficacies), above the shaded areas, no surgery is preferred. For example, for a 45 year-old woman with a BMI of 40, when the relative risk of death after surgery is greater than 0.98 (efficacy of surgery is less than 0.02), no surgical treatment is the preferred strategy. When the relative risk of death after surgery is less than 0.98 (efficacy of surgery is greater than 0.02), bariatric surgery is the strategy with the greatest gain in life expectancy.
Some studies have suggested that bariatric surgery may be beneficial for patients with a BMI between 30 kg/m2 and 35 kg/m2 21. Therefore, we performed exploratory analyses for patients with a BMI in this range (Figure 6). In these sub-analyses, women benefit from bariatric surgery, however the benefit from surgery is very sensitive to the model parameters. For instance, a 42 year-old women with a BMI of 32 kg/m2 gains 0.8 years of life expectancy, but if the 30-day surgical mortality exceeds 2.4% (baseline 30-day mortality =0.2%) surgery is no longer favored. Recognizing that the efficacy of surgery may be lower in this subgroup, we performed a sensitivity analysis on efficacy within this BMI range. We found that, as efficacy decreases, the benefit of surgery decreases for all ages: although, for a 42 year-old woman with a BMI of 32 kg/m2, surgery remains favored until the efficacy is below 4%. We found similar results for men, but the benefits of bariatric surgery were less.
Figure 6.
Sensitivity analysis exploring effect of bariatric surgery on patients with lower BMIs between 30 and 35.
Discussion
We developed a decision analytic model to evaluate the decision to have gastric bypass surgery for the treatment of morbid obesity. The optimal decision for individual patients varies depending upon the balance of risks between perioperative mortality, excess annual mortality associated with increasing BMI, and the efficacy of surgery; however, for the average morbidly obese patient, gastric bypass surgery increases life expectancy. Younger patients have lower surgical risk and longer time horizon over which to realize the benefits of surgery. For older patients, the gain is smaller, and for some gastric bypass surgery will decrease life expectancy.
The results of our base case are similar to the results of a previously published decision analysis by Pope and colleagues22. Their analysis found that a 40 year-old woman with a BMI of 40 kg/m2 would gain 2.6 years of life expectancy with bariatric surgery and that the absolute life expectancy benefit is similar across age groups. However, we found, the absolute gain in life expectancy is inversely correlated with age.
There are three major differences between the two models which lead to somewhat different conclusions. First, the model by Pope and colleagues assumed that the increase in life expectancy was due to changes in BMI after surgery, placing the patient in a new BMI category with a lower mortality rate; while in our model we made no assumptions about weight loss, rather we used an efficacy term derived from a large prospective cohort study. Secondly, Pope and colleagues estimated the additional mortality associated with obesity by using a large prospective cancer trial and were unable to adjust for the effect of age on mortality. By using the National Health Interview Survey, we were able to use a nationally representative sample fully adjusted for age, gender and BMI across a broader continuum. Finally, the surgical morality risk used by Pope and colleagues was based upon case series and explored in sensitivity analyses. The surgical risk in our model is based upon a logistic regression model derived from the National Inpatient Survey that takes into account patient age and gender. These three factors allow our model to better examine a wide range of patient-specific scenarios and BMI categories.
There are several limitations to our analysis. Since the dataset from NIS is derived from administrative data, it does not include clinical variables, such as BMI, which may be important predictors of surgical mortality. However, the NIS dataset provides the best nationally representative sample of surgical mortality and until more complete datasets become available provides the most generalizable estimates of surgical mortality. A number of obesity-associated conditions may increase operative mortality and conversely may increase the benefits following successful surgery. However, data capturing this level of detail do not currently exist. When such data become available, models like ours will be able to make even more specific recommendations.
The data used to determine the efficacy of surgery, from the study by Adams et al., is from a single state, Utah, and is not from a randomized controlled trial. While this data is not nationally representative and involves selection bias, it is the largest study to date demonstrating the efficacy of gastric bypass surgery.
Another limitation of our analysis is that we did not model long-term complications following surgery, including the need for surgical revision. However, by using efficacy data published by Adams and colleagues, we indirectly accounted for long-term mortality due to surgery. The most common complications, anastomotic stricture, marginal ulcer and internal hernia, are rarely fatal and therefore have limited impact on life expectancy.
Because data describing longitudinal changes in quality of life over time due to changes in body weight are not yet available, we chose to use life expectancy alone as our outcome metric. Gastric bypass surgery has been shown to improve quality of life in the short-term23. Until there are studies demonstrating the durability and stability of the quality of life improvements using preference based utilities suitable for use in a decision analysis, incorporating quality of life adjustments would bias the results of the model towards favoring gastric byapss.
We did not explicitly model deaths due to accident or suicide although it has been reported that patients having bariatric surgery may be at increased risk of these events14. However, we included these deaths in the determination of the efficacy and thus biased the model further against bariatric surgery by systematically underestimating the effect of surgery on obesity related mortality.
It is likely that certain subgroups of patients with high mortality due to obesity associated conditions but with a BMI less than 35 may benefit from gastric bypass. Our sensitivity analyses demonstrated that, for women in particular, there are subgroups of patients with a BMI between 30 and 35 whose survival would improve with surgery. Further research needs to explore the benefit of bariatric surgery in subgroups of patients who may benefit outside of the current guidelines.
The decision analysis presented here is a step forward in understanding optimal patient selection but also highlights some of the areas for which better data are needed. Understanding more about how efficacy of bariatric surgery varies based upon patient characteristics is an important next step because the data necessary to accurately model these outcomes are not currently available. For example, there is currently data on the impact of bariatric surgery on the resolution of diabetes24, but there are no data on the stability of this resolution over time or how this affects long-term mortality. Likewise, it would not be accurate to stratify operative mortality by obesity-associated condition in the decision analysis without including long term mortality projections. Including pre-surgical duration of obesity-associated conditions may also be important, especially in patients with diabetes mellitus: the presence of microvascular or macrovascular complications prior to surgery may impact both surgical risk and the efficacy of surgery.
In conclusion, while not all patients are guaranteed a good outcome, our model indicates that gastric bypass increases life expectancy for most patient subgroups; however, for those at high surgical risk or in whom efficacy of surgery is likely to be low, benefit will be minimal. We believe results of this analysis can be used to better inform both patients’ and physicians’ decisions regarding gastric bypass surgery.
Acknowledgement Section
Dr. Schauer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schauer, Arterburn, Livingston, Fischer, Eckman
Acquisition of data: Schauer, Livingston
Analysis and interpretation of data: Schauer, Arterburn, Livingston, Fischer, Eckman
Drafting of the manuscript: Schauer
Critical revision of the manuscript for important intellectual content: Arterburn, Livingston, Fischer, Eckman
Statistical analysis: Schauer, Livingston
Administrative, technical or material support: Eckman
Supervision: Schauer, Eckman
Financial Disclosures: Dr. Schauer receives grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (grant 1K23DK075599-01A1).
Funding/Support: This study was funded by the NIH/NIDDK (grant 1K23DK075599-01A1).
Role of the Sponsor: The funding source had no role in the design and conduct of the study; collection, management, analysis and interpretation of data; and preparation, review or approval of the manuscript.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004 Jun 16;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.Arterburn DE, Maciejewski ML, Tsevat J. Impact of morbid obesity on medical expenditures in adults. Int J Obes Relat Metab Disord. 2005 Mar;29(3):334–339. doi: 10.1038/sj.ijo.0802896. [DOI] [PubMed] [Google Scholar]
- 3.Arterburn D. Obesity. Am Fam Physician. 2002 Oct 1;66(7):1279–1280. [PubMed] [Google Scholar]
- 4.Arterburn DE, Crane PK, Veenstra DL. The efficacy and safety of sibutramine for weight loss: a systematic review. Arch Intern Med. 2004 May 10;164(9):994–1003. doi: 10.1001/archinte.164.9.994. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005 Apr 5;142(7):532–546. doi: 10.7326/0003-4819-142-7-200504050-00012. [DOI] [PubMed] [Google Scholar]
- 6.Steinbrook R. Surgery for severe obesity. N Engl J Med. 2004 Mar 11;350(11):1075–1079. doi: 10.1056/NEJMp048029. [DOI] [PubMed] [Google Scholar]
- 7.American Society for Metabolic and Bariatric Surgery. [Accessed September 17, 2008]; http://www.asbs.org/Newsite07/media/fact-sheet1_bariatric-surgery.pdf.
- 8.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005 Apr 5;142(7):547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 9.McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003 Dec 2;139(11):933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- 10.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004 Dec 23;351(26):2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of Weight Status following Laparoscopic Gastric Bypass. Obes Surg. 2006 Sep;16(9):1227–1231. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 12.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007 Aug 23;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 13.Salem L, Devlin A, Sullivan SD, Flum DR. Cost-effectiveness analysis of laparoscopic gastric bypass, adjustable gastric banding, and nonoperative weight loss interventions. Surg Obes Relat Dis. 2008 Jan-Feb;4(1):26–32. doi: 10.1016/j.soard.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007 Aug 23;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 15.Flum DR, Dellinger EP. Impact of gastric bypass operation on survival: a population-based analysis. J Am Coll Surg. 2004 Oct;199(4):543–551. doi: 10.1016/j.jamcollsurg.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 16.Christou NV, Sampalis JS, Liberman M, et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg. 2004 Sep;240(3):416–423. doi: 10.1097/01.sla.0000137343.63376.19. discussion 423–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007 Dec;246(6):1002–1007. doi: 10.1097/SLA.0b013e31815c404e. discussion 1007–1009. [DOI] [PubMed] [Google Scholar]
- 18.Flum DR, Salem L, Elrod JA, Dellinger EP, Cheadle A, Chan L. Early mortality among Medicare beneficiaries undergoing bariatric surgical procedures. Jama. 2005 Oct 19;294(15):1903–1908. doi: 10.1001/jama.294.15.1903. [DOI] [PubMed] [Google Scholar]
- 19.Livingston EH. Procedure incidence and in-hospital complication rates of bariatric surgery in the United States. Am J Surg. 2004 Aug;188(2):105–110. doi: 10.1016/j.amjsurg.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Livingston EH, Huerta S, Arthur D, Lee S, De Shields S, Heber D. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002 Nov;236(5):576–582. doi: 10.1097/00000658-200211000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien PE, Dixon JB, Laurie C, et al. Treatment of mild to moderate obesity with laparoscopic adjustable gastric banding or an intensive medical program: a randomized trial. Ann Intern Med. 2006 May 2;144(9):625–633. doi: 10.7326/0003-4819-144-9-200605020-00005. [DOI] [PubMed] [Google Scholar]
- 22.Pope GD, Finlayson SR, Kemp JA, Birkmeyer JD. Life expectancy benefits of gastric bypass surgery. Surg Innov. 2006 Dec;13(4):265–273. doi: 10.1177/1553350606296324. [DOI] [PubMed] [Google Scholar]
- 23.Puzziferri N, Austrheim-Smith IT, Wolfe BM, Wilson SE, Nguyen NT. Three-year follow-up of a prospective randomized trial comparing laparoscopic versus open gastric bypass. Ann Surg. 2006 Feb;243(2):181–188. doi: 10.1097/01.sla.0000197381.01214.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004 Oct 13;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]