Abstract
Cigarette smoking has been suggested as a risk factor for several periodontal diseases. It has also been found that smokers respond less favorably than non-smokers to periodontal therapy. Previous work in our lab has shown that nicotine inhibits human gingival cell migration. Since myofibroblasts play an important role in wound closure, we asked if nicotine affects gingival wound healing process by regulating myofibroblast differentiation. Human gingival fibroblasts (HGFs) from two patients were cultured in 10% fetal bovine serum cell culture medium. Cells were pretreated with different doses of nicotine (0, 0.01, 0.1, and 1 mM) for 2 h, and then incubated with transforming growth factor beta (TGF-β1) (0, 0.25, 0.5, and 1 ng/ml) with or without nicotine for 30 h. The expression level of α-smooth muscle actin (α-SMA), a specific marker for myofibroblasts, was analyzed by Western blots, immunocytochemistry, and real-time polymerase chain reaction (real-time PCR). Phosphorylated p38 mitogen-activated protein kinase (Phospho-p38 MAPK) activity was analyzed by Western blots. TGF-β1 induced an increase of α-SMA protein and mRNA expression, while nicotine (1 mM) inhibited the TGF-β1-induced expression of α-SMA but not β-actin. Nicotine treatment down-regulated TGF-β1-induced p38 MAPK phosphorylation. Our results demonstrated for the first time that nicotine inhibits myofibroblast differentiation in human gingival fibroblasts in vitro; supporting the hypothesis that delayed wound healing in smokers may be due to decreased wound contraction by myofibroblasts.
Keywords: human gingival fibroblasts, myofibroblasts, α smooth muscle actin, TGFβ, p38 MAP kinase
Myofibroblasts are contractile cells that share characteristics of fibroblasts and smooth muscle cells [Tomasek et al., 2002]. One of the major features of myofibroblast differentiation is the expression of smooth muscle cell markers. These markers include α-SMA, γ-SMA, smooth muscle 22 (SM22), calponin, smoothelin, meta-vinculin, h-caldesmon [Halayko and Solway, 2001]. Among these, α-SMA has been suggested as the most reliable marker of myofibroblast differentiation [Gabbiani, 2003]. Also, Dr. Masur's lab demonstrated that myofibroblast differentiation was cell density dependent in corneal fibroblasts. They found that the lower the cell density when cells were seeded in culture, the more cells differentiated into myofibroblasts. However, the myofibroblast phenotype was not terminal. The cells lost the myofibroblast phenotype when cultured in high density [Masur et al., 1996], or when stimulated with fibroblast growth factor (FGF) [Maltseva et al., 2001].
Myofibroblasts are different from fibroblasts in two aspects. One is that myofibroblasts have contractile stress fibers that terminate at special adhesion complexes called fibronexi. Another characteristic is that myofibroblasts connect to each other through gap junctions and form multicellular contractile units during contraction. In contrast, fibroblasts do not have these characteristics [Tomasek et al., 2002]. Compared to dermal fibroblasts, human myofibroblasts from implants inserted subcutaneously also responded differently to cytokines. The growth rate and cell surface area of myofibroblasts did not increase in response to TGF-β1 and interferon gamma (IFN-γ), as significantly as fibroblasts, but myofibroblast cells increased collagen production and contractile capacity [Moulin et al., 1998]. However, other studies found that TGF-β1 promoted myofibroblast morphological and functional differentiation. It has been shown that TGF-β1 enhanced the formation of structural elements and expression of α-SMA in myofibroblasts, thereby increasing the contractile force [Vaughan et al., 2000].
Granulation tissue contraction is necessary for efficient and rapid wound closure. The contractile force generated by myofibroblasts is attributed to contractile stress fibers. In addition to being a marker for myofibroblast differentiation, α-SMA is proposed to play an important role in generating myofibroblastic force [Serini and Gabbiani, 1999; Tomasek et al., 2002]. Using deformable silicone substrates and a three dimensional collagen lattice, a correlation between α-SMA expression level and fibroblast contraction force was found. In addition, fibroblasts transfected with α-SMA cDNA had increased contractile ability [Hinz et al., 2001].
Several factors, including cytokines and extracellular matrix modulate myofibroblast differentiation. TGF-βs has been suggested as important inducers of myofibroblast differentiation. All three isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3) are able to stimulate myofibroblast formation in vitro, whereas TGF-β3 acts as a negative regulator of the myofibroblastic phenotype in vivo. Different extracellular matrix components may cause these results [Serini and Gabbiani, 1999]. In addition, myofibroblast differentiation could also be generated independent of TGF-βs. For example, the plasmid containing the cDNA of a 8 kDa protein, P311, induced myofibroblast differentiation when transfected into fibroblasts [Pan et al., 2002].
Although Smad-dependent pathways are known to mediate TGF-β signaling, there is evidence that TGF-β may transmit signals through Smad-independent pathways [Akhurst and Derynck, 2001; Yue and Mulder, 2001; Moustakas et al., 2002; Miyazono et al., 2003; Shi and Massague, 2003]. In addition, TGF-β1 signaling pathways had been shown to crosstalk with other signaling pathways such as mitogen-acitvated protein kinase (MAPK) pathways and phosphatidylinositol-3 kinase (PI3-kinase) pathways [Cheng and Grande, 2002; Kim and Letterio, 2003].
It has been found that smokers respond less favorably than non-smokers to periodontal therapy [Ah et al., 1994; Kaldahl et al., 1996]. In addition, nicotine has been implicated in delayed wound healing [Campanile et al., 1998]. However, it is not clear if nicotine inhibits wound healing by regulating wound contraction, which is an important step that brings the wound margins together. In addition, we did not find any research on the effects of nicotine on myofibroblast differentiation in oral tissues. In this project, we hypothesized that nicotine inhibited myofibroblast differentiation. In addition, we hypothesized that nicotine alters differentiation by regulating TGF-β1 signaling pathways.
MATERIALS AND METHODS
Tissue Culture
Human gingival tissues from two healthy nonsmokers were collected with Institutional Review Board approval and tissues were cultured in low glucose Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), and 1% antimycotic/antibiotic (GIBCO™ Invitrogen Corporation, Carlsbad, CA). Tissues were incubated at 37°C in a humidified gas mixture (5% CO2 and 95% air) and medium was changed every 24–48 h. Human gingival fibroblasts (HGFs) grew out of the tissue after 1 week in culture. Cells were passaged using 0.25% trypsin solutions (GIBCO™ Invitrogen Corporation) and plated in new tissue culture flasks (Falcon™, BD Biosciences Discovery Labware, Bedford, MA). Cells were passaged when they were confluent. Passages 3–6 were used in all of the experiments.
TGF-β1 and Nicotine Treatment
To analyze the effect of different concentrations of TGF-β1 on myofibroblast differentiation, subconfluent HGFs cultured in 60 × 15 mm Petri dishes (Falcon™, BD Biosciences Discovery Labware) were washed and cultured in cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) for 2 h, and then changed to cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) containing 0, 0.25, 0.5, or 1 ng/ml TGF-β1 (Sigma, St. Louis, MO). HGFs were cultured for 30 h and cell lysates were collected for Western blot analysis.
To analyze the effect of different concentrations of nicotine on TGF-β1 induced myofibroblast differentiation, subconfluent HGFs cultured in 60 × 15 mm Petri dishes were washed and pretreated with or without 0.01, 0.1, or 1 mM nicotine hemisulfate salt (Sigma) in cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) for 2 h, and then changed to cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) containing 0.5 ng/ml TGF-β1, and 0.01, 0.1, or 1 mM nicotine. HGFs were cultured for 30 h and cell lysates were collected for Western blot analysis and real-time PCR.
To analyze the effect of 1 mM nicotine on different concentrations of TGF-β1 induced myofibroblast differentiation, subconfluent HGFs cultured in 60 × 15 mm Petri dishes were washed and pretreated with or without 1 mM nicotine hemisulfate salt in cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) for 2 h, and then changed to cell culture medium (low glucose DMEM, 0.1% FBS, 1% antimycotic/antibiotic) containing 0, 0.25, 0.5, or 1 ng/ml TGF-β1 and 1 mM nicotine. HGFs were cultured for 30 h and cell lysates were collected for Western blot analysis.
Western Blot
HGFs were homogenized in lysis buffer (50 mM Tris pH 7.5, 10 mM MgCl2, 0.3M NaCl, 2% octylphenyl-polyethylene glycol) with 1× protease inhibitor cocktail, 1× phosphatase inhibitor cocktail I and II (Sigma). The proteins were quantified using Folin Lowry assays and stored at –20°C prior to Western blot analysis. 10–20 μg protein lysates were separated on precast 7.5%–15% acrylamide gels (Bio-Rad, Hercules, CA) under denaturing conditions and transferred to Immobilon-P membranes (Millipore Corporation, Billerica, MA). Blots were rehydrated and blocked with 5% milk (Bio-Rad) or 5% bovine serum albumin (BSA) (Sigma) in Tris-buffered saline/0.1% Tween-20 (TBST) for 1 h at room temperature, then incubated with primary antibodies in 5% milk or 5% BSA in TBST overnight at 4°C. Antibodies and dilutions were: anti-α-SMA mouse monoclonal antibody (1: 1,000) (Sigma); anti-p38 (pTpY180/182) MAPK polyclonal antibody (1: 1,000) (BioSource International, Inc., Camarillo, CA). Membranes were washed with TBST several times and incubated with horse radish peroxidase (HRP)-conjugated goat-anti-mouse or goat-anti-rabbit secondary antibody (1: 2,500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 1 h at room temperature, then detected using the femtoLUCENT® (Geno Technology, Inc., St. Louis, MO) procedure. A Kodak Image Station (Kodak Digital Science™) was used to detect chemiluminescence.
RNA Extraction
ULTRASPEC™ RNA Isolation System (Biotecx Laboratories, Inc., Houston, TX) was used for RNA extraction. Briefly, cell culture medium was removed and 1 ml Ultraspec™ RNA solution (Biotecx Laboratories, Inc.) was added to lyse the cells in the culture dishes. Cell lysates were passed several times through a pipette and were transferred immediately into a microfuge tube. The homogenate was stored at 48C for 5 min to permit the complete dissociation of nucleoprotein complex. Then chloroform (0.2 ml) (Shelton Scientific, Inc., Shelton, CT) was added into each microfuge tube. The homogenate was centrifuged at 12,000g (4°C) for 15 min. The aqueous phase was transferred to a fresh tube. An equal volume of chloroform was added to the aqueous phase and the centrifuge spin was repeated. The aqueous phase was transferred to a fresh tube with isopropanol (450 μl) (Sigma). The samples were stored at –20°C overnight. The samples were centrifuged at 12,000g (4°C) for 10 min. The supernatant was removed and the RNA pellet was washed twice with 75% ethanol (1 ml) by vortexing and subsequent centrifugation for 5 min at 7,500g (4°C). The pellet was briefly dried under a vacuum. The RNA pellet was dissolved in 50 μl RNase free water (Sigma-Aldrich) by vortexing and incubation at 65°C for 5 min. RNA quantitation and integrity were assessed by Agilent Technologies 2100 Bioanalyzer and RNA 6000 Pico Assay (Agilent Technologies, Palo Alto, CA).
cDNA Synthesis
Reverse transcription was performed using the First-Strand synthesis kit for reverse transcription-polymerase chain reaction (RT-PCR) (Ambion, Inc., Austin, TX). In all reactions, 0.37 μg of total RNA was used as a template. dNTP mix (4 μl) (2.5 mM each dNTP) and random decamer (2 μl) were added and mixed. The mixture was heated 3 min at 85°C, then tubes were removed to ice. The following components were added: 2 μl of 10× RT-PCR buffer (100 mM Tris-HCl, pH 8.3/500 mM KCl/15 mM MgCl2), 1 μl of placental RNase inhibitor, and 1 μl of Moloney–Murine Leukemia Virus (M-MLV) Reverse Transcriptase. All components were mixed and incubated at 42°C for 1h, and then incubated at 92°C for 10 min to inactivate the reverse transcriptase. In subsequent real-time PCR reactions, 1 μl of this volume was used for PCR amplification of α-SMA cDNA.
Real-Time PCR
cDNA preparations were obtained by reverse transcription of HGF mRNAs. α-SMA cDNA (accession number: NM_001613) was amplified using the forward primer (5′-CCAAGCACTGTCAGGAAT-3′) and the reverse primer (5′-AGGCAGTGCTGTCCTCTT-3′), obtaining an amplified fragment of 66 bp. β-actin cDNA (accession number: BC013380) was amplified using the forward primer (5′-CGAGGACTTTGATTGCAC-3′) and reverse primer (5′-TATCACCTCCCCTGTGTG-3′), obtaining an amplified fragment of 155 bp. Various dilutions of cDNA template (100–10–4) were used to obtain a standard curve. A 1:10 dilution of cDNA was chosen as the best template concentration. Various concentrations of the 18S internal standard primer (Ambion, Inc.) and α-SMA primers (Invitrogen™ Life technologies, Carlsbad, CA) were analyzed. The optimum 18S primer concentration was found to be 200 nM and the optimum α-SMA forward and reverse primer concentration was found to be 150 nM each. For real-time PCR reaction, total volume for each reaction was 20 μl, 1 μl of template, and 10 μl of SYBR Green Master Mix (Stratagene, La Jolla, CA) were used in each reaction. 18S internal standard was used as a normalizer. PCR cycling programs were set as follows: after an initial denaturation step of 10 min at 95°C, the PCR procedure consisted of 40 cycles of 15 s at 95°C, 1 min at 55°C, and 1 min at 72°C. The amplified product was incubated for dissociation curve, which was 1 min at 95°C, ramping down to 55°C at a rate of 0.2°C/s. The temperature was increased by 0.5°C/cycle for 81 cycles, beginning at 55°C and ending at 95°C. The duration of each cycle was 30 s.
Indirect Immunocytochemistry and Phalloidin Staining
High density of HGFs were passaged into 8-well Lab-tek chamber slides (Electron Microscopy Sciences, Hatfield, PA) and cultured in cell culture medium (low glucose DMEM, 10% FBS, 1% antimycotic/antibiotic) for 1 day. Cells were serum starved overnight and pre-treated with or without 1 mM nicotine in serum free cell culture medium (low glucose DMEM, with 1% antimycotic/antibiotic) for 2 h. TGF-β1 (0 or 0.5 ng/ml) was added to experimental cell culture medium (+/– nicotine) and cells were cultured for 30 h. The medium was removed; the cells were rinsed and then fixed with 4% paraformaldehyde/phosphate buffered saline (PBS) for 15 min. After washing with PBS, cells were permeabilized with 0.1% Triton X-100 for 2 min. The cells were washed with PBS and blocked with 3% bovine serum albumen (BSA) in PBS for 15 min, washed with PBS, and incubated with α-SMA mouse monoclonal antibody (1:50) (Sigma) for 1 h at 37°C. Negative control groups were incubated with PBS instead of primary antibodies. After rinsing with PBS, the primary antibodies were detected by secondary antibodies conjugated with Alexa Fluor® 488 Goat-anti-Mouse (1:100) (Molecular Probes, Eugene, OR) for 1 h at room temperature. Cells were rinsed with PBS and stained with Alexa Fluor® 546 phalloidin (1:20) (Molecular Probes) for 30 min at room temperature and washed with PBS. The cells were then mounted in Slowfade™ medium, coversliped and viewed using confocal microscopy (Leica TCS-SP2, Germany).
Confocal Laser Scanning Microscopy
Immunocytochemistry experiments were analyzed with the Leica upright TCS-SP2 confocal microscope, equipped with argon and helium-neon lasers with excitation wavelengths of 488, 543, and 633 μm. The cells were analyzed with a 10× air objective lens and a 63× water objective lens. Complete Z-series through each treatment group (n = 3–5 fields) was obtained with a 1 μm step. Maximum projected images were produced from representative fields. Confocal images were arranged with Adobe PageMaker.
RESULTS
TGF-β1 Induced Myofibroblast Differentiation
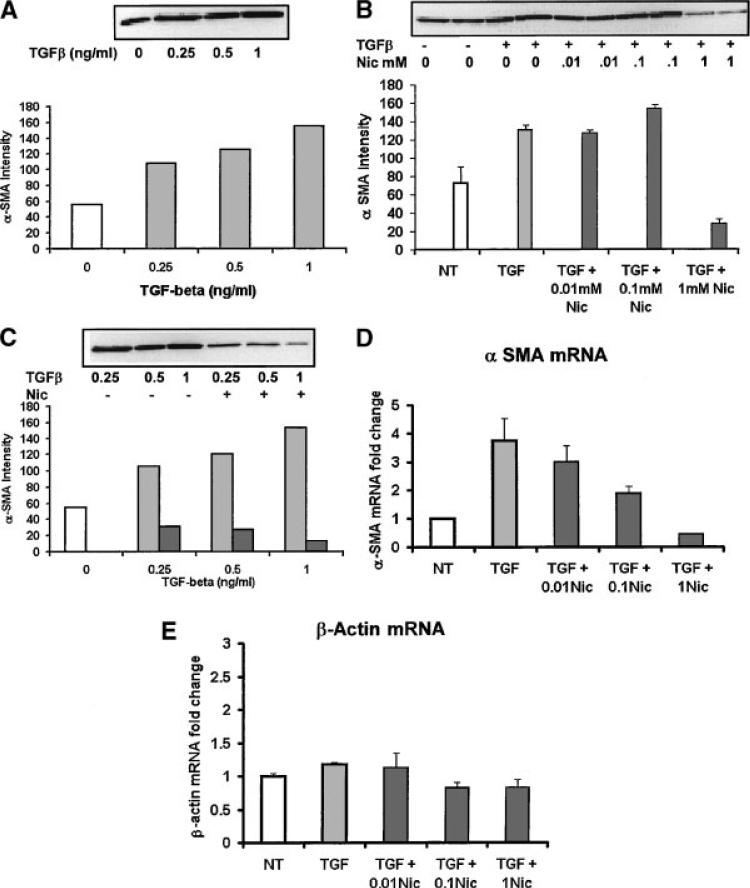
To verify if TGF-β1 induces myofibroblast differentiation in HGFs, subconfluent HGFs were treated with different concentrations (0.25, 0.5, 1 ng/ml) of TGF-β1, and equal amounts of protein lysate were analyzed using Western blots for α-SMA (Fig. 1A). α-SMA was expressed in untreated control HGFs (Fig. 1A, open bar); however, TGF-β1 up-regulated α-SMA protein expression in a dose-dependent manner. Densitometric analysis demonstrated that α-SMA expression was increased approximately twofold at 0.25 ng/ml, 2.2-fold at 0.5 ng/ml, and threefold at 1 ng/ml TGF-β1 (Fig. 1A, graph).
Fig. 1.
A: Western blot analysis of α-SMA expression after different TGF-β1 treatments. Subconfluent human gingival fibroblasts (HGFs) were stimulated with 0 (open bars), 0.25, 0.5, or 1 ng/ml TGF-β1 (gray bars) for 30 h. Whole cell lysates were equally loaded onto a 7.5% SDS–PAGE and α-SMA expression was determined. Densitometric analysis showed that TGF-β1 up-regulated α-SMA protein expression in a dose dependent manner. B: Subconfluent HGFs were pretreated with 0, 0.01, 0.1, or 1 mM nicotine (black bars) for 2 h and then 0.5 ng/ml TGF-β1 for 30 h. Duplicate samples were equally loaded and α-SMA detected on Western blots. TGFβ (TGF) (gray bar) had twofold more α-SMA than control untreated cells (NT open bar) and similar amounts as 0.01 or 0.1 mM nicotine treated cells (black bars). One millimolar of nicotine inhibited TGF-β1 induced α-SMA expression to below NT cell levels. C: HGFs were pre-treated with 1 mM nicotine (black bars) for 2 h before stimulation with 0.25, 0.5, or 1 ng/ml TGF-β1 (gray bars). Equal amounts of cell lysate were used for α-SMA Western blot analysis. Densitometric analysis showed that nicotine down-regulated TGF-β1-induced α-SMA expression at all concentrations of TGFβ. D: HGFs were pre-treated with or without differentiation concentrations of nicotine (black bars) and then treated with 0.5 ng/ml TGF-β1 as described for the Western blot analysis. α-SMA mRNA expression level was determined by using specific primers. The expression level of α-SMA mRNA in control untreated cells (NT open bar) was set at 1. TGF-β1 treatment increased the α-SMA mRNA expression 3.5-fold (gray bar). The cells pre-treated with 0.01 or 0.1 mM nicotine still had an increase of 3.1 or 3.2-fold respectively compared to non-treated controls. However, the cells pre-treated with 1 mM nicotine decreased the α-SMA mRNA expression levels to 0.6 compared to non-treated controls. Error bars equal the standard deviation between three experiments. E: TGFβ had little effect on β-actin mRNA and 0.01 mM nicotine was similar to TGFβ treated cells. Nicotine treatment at 0.1 and 1 mM decreased β-actin mRNA slightly.
Nicotine Inhibited TGF-β1 Induced Myofibroblast Differentiation
Nicotine has been shown to inhibit wound healing processes. In addition, serum nicotine concentrations correlate with the degree of smoking. A correlation has been established between the smoking frequencies, with higher serum nicotine concentrations from patients that are heavy smokers. It is not clear if nicotine inhibits wound healing by regulating myofibroblast differentiation. Therefore, we determined the effect of different concentrations of nicotine on TGF-β1-induced myofibroblast differentiation.
Different concentrations of nicotine (0.01, 0.1, and 1 mM) were used to pre-treat the HGFs for 2 h before TGF-β1 stimulation. Interestingly, HGF cells pre-treated with low nicotine concentrations, 0.01 or 0.1 mM, had TGF-β1 induced α-SMA expression levels similar to non-nicotine groups (Fig. 1B). However, HGF cells treated with 1 mM nicotine had down-regulated α-SMA protein levels (Fig. 1B). Densitometric analysis showed that α-SMA was 4.7-fold less in 1 mM nicotine pre-treated groups (Fig. 1B, graph). In addition, cells pre-treated with 1 mM nicotine had lower α-SMA expression compared to untreated controls. Therefore, we concluded that nicotine (1 mM) inhibited myofibroblast differentiation.
To determine if nicotine had a different effect at different concentrations of TGF-β1, HGFs were pretreated with 1 mM nicotine before TGF-β1 (0.25, 0.5, 1 ng/ml) stimulation (Fig. 1C). Nicotine down-regulated TGF-β1-induced α-SMA protein expression at all tested concentrations (Fig. 1C). Densitometric analysis showed that α-SMA expression in cells pre-treated with 1 mM nicotine was 3.3-, 4.2-, and 11-fold less, respectively, compared to 0.25, 0.5, and 1 ng/ml TGF-β1 treated cells (Fig. 1C, graph). This experiment confirmed that nicotine inhibited α-SMA at different doses of TGF-β1.
We further determined if α-SMA mRNA was increased by TGF-β1 and if nicotine inhibited TGF-β1 induced α-SMA mRNA expression. Total RNA was isolated from HGFs cultured under different experimental treatments. RNA quality and quantity were analyzed. A successful total RNA run was obtained. Using real-time PCR, the expression level of α-SMA mRNA in control groups was set at 1. TGF-β1 treatment increased the α-SMA mRNA expression to 3.7-fold (Fig. 1D). The cells pre-treated with 0.01 or 0.1 mM nicotine increased α-SMA mRNA 3.0- or 1.8-fold, respectively (Fig. 1D). However, the cells pre-treated with 1 mM nicotine decreased the α-SMA mRNA expression levels to 0.4 compared to untreated control cells, and over eightfold less compared to TGF-β1 treated cells (Fig. 1D). Therefore, we concluded nicotine (1 mM) inhibited TGF-β1 induced myofibroblast differentiation by blocking α-SMA transcription. In addition, TGF-β1 treatment increased β actin mRNA expression and higher doses of nicotine (0.1 and 1 mM) slightly decreased β actin mRNA expression levels (Fig. 1E). The α-SMA primers did not cross react with β actin cDNA (data not shown).
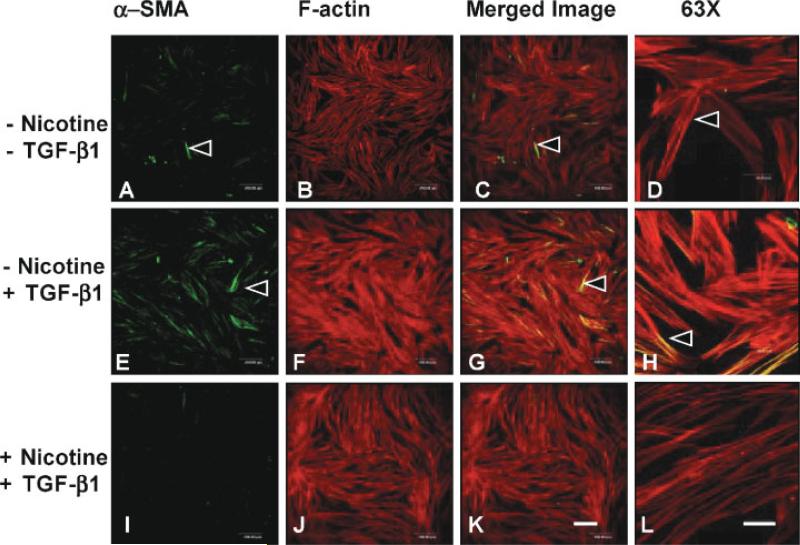
Myofibroblast differentiation is cell density and culture condition dependent [Masur et al., 1996], and more myofibroblasts differentiated in lower density culture. In addition, fetal bovine serum in cell culture medium may also induce myofibroblast differentiation. Our preliminary data using cells cultured from low density HGFs found that even untreated control groups had α-SMA expression. Therefore, in an immunocytochemistry study, HGFs were seeded at high density for 24 h, serum starved, pre-treated with or without 1 mM nicotine, and then treated with or without TGF-β1. The cells were fixed after 30 h. Intracellular localization of F-actin and α-SMA were examined using confocal microscopy. The average cell thickness was 10–15 μm. Complete Z-series through the cells were projected (MAX) into a single image (step = 1 μm). Isolated cells expressing α-SMA (green) were observed among the confluent untreated control cell layer (Fig. 2A–D). While in the TGF-β1 treated group large numbers of cells stained positively for α-SMA (Fig. 2E–H). On the contrary, nicotine (1 mM) pre-treated cells had very few α-SMA positive staining areas (Fig. 2I–L). In addition, compared to untreated controls and nicotine treated cells; the TGF-β1 treated cells had condensed F-actin staining (Fig. 2E–H). The same treatment groups were examined using higher magnification (Fig. 2D, H,L). The cells in TGF-β1 treated groups were larger and more spread than the spindle shaped cells in the untreated control group (Fig. 2D). The TGF-β1 treated cells had more and thicker stress fibers, and α-SMA staining could be seen clearly. In addition, some of the α-SMA overlapped with F-actin (Fig. 2H). While in nicotine pre-treated groups, cells were spindle shaped, F-actin staining was thin, and there was no α-SMA staining (Fig. 2L). These results demonstrated that TGF-β1 induced the morphological differentiation of myofibroblasts and α-SMA expression, whereas 1 mM nicotine inhibited these TGF-β1-induced changes.
Fig. 2.
Nicotine inhibited TGF-β1 increased α-SMA and actin stress fibers in densely cultured HGFs. α-SMA expression was located by a specific antibody and detected by Alexa Fluor® 488 secondary antibody (green). A confocal z series was projected into a single image to demonstrate the intracellular distribution of HGFs immunolabeled with α-SMA (green) and F-actin (phalloidin, red). The cells were plated densely in lab-tek slides and either pre-treated with (I–L) or without (A–H) 1 mM nicotine and stimulated with 0.5 ng/ml (E–L) TGF-β1. In untreated control groups (A–D), there were isolated cells stained positive for α-SMA (A–D). In TGF-β1 treated groups, a large number of the cells were α-SMA positive (E–H). However, in nicotine pre-treated cells, very few cells stained positive for α-SMA (I–L). Scale bar = 200 μm (A–C, E–G, I–K). Higher magnification of the projected confocal fields demonstrated that untreated control cells were spindle shaped and were not α-SMA positively stained (D). In TGF-β1 treated groups, cells were larger, actin stress fibers were thicker than in other two groups, and α-SMA staining was clearly demonstrated (H). In contrast, in nicotine pre-treated groups, cells were not stained positive for α-SMA, and actin fibers were thin (L). Scale bar = 40 μm (D, H, L).
Nicotine Inhibited TGF-β1-Induced p38 MAPK Activity
It was shown that either Smad2 or Smad3 could activate the α-SMA enhancer [Cogan et al., 2002]. In addition, fibroblasts transfected with Smad2 had up-regulated α-SMA mRNA expression and altered cell phenotype [Evans et al., 2003]. In our study, active Smad2 could not be detected after 30 h of TGF-β1 treatment in the presence or absence of nicotine. Interestingly, nicotine increased phosphor-Smad2 in response to TGF-β1 in the first hour (data not shown).
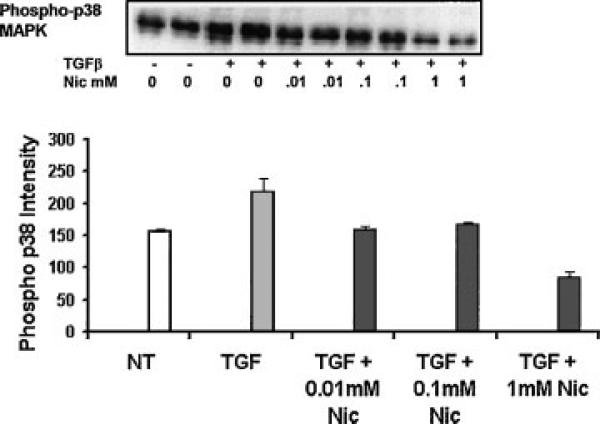
It has been shown that p38 MAPK was activated by TGF-β1 in hepatic stellate cells, and p38 MAPK phosphorylated Smad3 and promoted the nuclear translocation of Smad3/Smad4 [Furukawa et al., 2003]. In addition, inhibition of p38 MAPK by SB203580 and SB 202190 decreased α-SMA expression in pancreatic stellate cells [Masamune et al., 2003]. Therefore, we determined if nicotine inhibited myofibroblast differentiation by down-regulating p38 MAPK activity. HFGs were pre-treated with different concentrations of nicotine and then stimulated with TGF-β1 with or without nicotine for 30 h. Phosphospecific antibodies were used to detect the p38 MAPK activity (Fig. 3). Densitometric analysis showed that compared to untreated control group, TGF-β1 treatment up-regulated p38 MAPK activity 1.4-fold. Both 0.01 and 0.1 mM nicotine inhibited TGF-β1-induced p38 MAPK activity, and phospho-p38 MAPK expression levels were the same as for the untreated control. In 1 mM nicotine treated cells, phosph-p38 MAPK expression levels were reduced to half those of untreated controls (Fig. 3, graph).
Fig. 3.
Nicotine decreased TGF-β1-induced p38-MAPK activity. HGFs were pretreated with different concentrations of nicotine (0.01, 0.1, or 1 mM) for 2 h (black bars) and then treated with TGF-β1 (0.5 ng/ml) with or without nicotine (gray bar). Phosphor-p38 MAPK was detected by Western blots as previously described. Densitometric analysis showed that TGF-β1 treatment up-regulated p38-MAPK activity compared to untreated cells (NT open bar), while nicotine inhibited TGF-β1-induced p38-MAPK activity (black bars). Error bars equal the standard deviation between three experiments.
DISCUSSION
Cigarette smoking, tobacco chewing, and snuff using are prevalent forms of nicotine addiction in the United States. Smoking has been shown as a major risk factor for the onset and development of periodontal diseases [Rivera-Hidalgo, 2003], and a delayed healing response subsequent to periodontal therapies has been demonstrated in smokers [Grossi et al., 1996]. However, it is not clear if nicotine inhibits the wound healing process by delaying wound contraction. Myofibroblasts are the major cells that lead to wound contraction to bring wound margins together. Therefore, in the present study, we investigated the effects of nicotine on myofibroblast differentiation in HGFs.
Myofibroblasts can be occasionally found in normal tissues. However, dramatically increased numbers of myofibroblasts appear during the wound healing processes [Hakkinen et al., 2000]. It has been found that myofibroblast differentiation occurs in HGFs in vitro [Arora and McCulloch, 1999], and our results confirmed that upon TGF-β1 stimulation HGFs could differentiate into myofibroblasts. We showed that the TGF-β1 concentration correlated with myofibroblast differentiation, the higher the concentration of TGF-β1, the more myofibroblast differentiation as measured by α-SMA expression. Our results also demonstrated that there was α-SMA expression even in non-treated HGF control groups. This was not surprising since myofibroblast differentiation was induced by culture conditions. Primary cultured rat lung fibroblasts had increased α-SMA expression in culture with serum in a time-dependent manner [Roy et al., 2001]. In addition, myofibroblast differentiation was cell density dependent. The lower the cell density when seeded in culture, the more myofibroblasts differentiated [Masur et al., 1996]. The HGFs we used for the experiments were cultured in 10% FBS cell culture medium for 3–5 days; growth factors in FBS might cause the myofibroblast differentiation before TGF-β1 treatment.
In order to detect if nicotine inhibits myofibroblast differentiation, we used three concentrations of nicotine: 0.01, 0.1, and 1 mM, which equal to 2,240, 22,400, 224,000 ng/ml. These concentrations are much higher than those reported in the blood of smokers. However, it has been found that smokers had a much higher nicotine concentration in saliva than that in blood [Teneggi et al., 2002]. Studies detecting salivary nicotine concentration within half an hour of the last cigarette smoked found that nicotine concentrations in saliva could range from 50 to 4,000 ng/ml [Teneggi et al., 2002]. In addition, nicotine yields correlated with the cigarette brand. Mean nicotine yield could reach as high as 0.91 mg per cigarette [Jarvis et al., 2001]. As one of the first tissues to contact and absorb nicotine, it is possible that oral mucosa and gingiva have much higher nicotine concentrations than in saliva. Indeed, it has been found that cotinine, a metabolite of nicotine, had an approximately six times higher concentration in gingival crevicular fluid compared to saliva. The mean crevicular fluid cotinine concentration was 2,457 ng/ml, while it was 424 ng/ml in saliva [McGuire et al., 1989]. In addition, saliva nicotine concentration in snuff dippers could reach as high as 9.6 mM [Hoffmann and Adams, 1981]. Moreover, nicotine has been found on roots of extracted teeth from smokers [Cuff et al., 1989]. Therefore, we used very high biological nicotine concentrations (2,240 – 224,000 ng/ml) in our study. Interestingly, both Western blot analysis and real-time PCR results demonstrated that only at the highest concentration, 1 mM, did nicotine block TGF-β1-induced myofibroblast differentiation. Therefore, our result demonstrated for the first time that nicotine inhibited myofibroblast differentiation.
Many periodontal research experiments have supported the theory that nicotine inhibits cell proliferation. However, it was controversial which nicotine dosage affected cell growth and actin organization. In in vitro experiments using human gingival fibroblasts (HGFs), it was found that nicotine treatment inhibited HGF proliferation in doses higher than 7.8 mM, and disrupted the microtubule and vimentin cytoskeleton in doses higher than 3.9 mM [Alpar et al., 1998]. Whereas in studies using human periodontal ligament fibroblasts, it was demonstrated that nicotine (≥ 25 μM) inhibited cell proliferation and protein synthesis (≥ 5 mM) in a dose-dependent manner [Chang et al., 2002]. In our study, HGFs were subconfluent or confluent before exposure to nicotine; therefore, the effect of nicotine on cell proliferation was not evaluated. It was observed that F-actin staining was less and stress fibers were thin in nicotine pre-treated cells. Further studies need to be completed to find out if nicotine altered actin organization of serum cultured HGFs; however the mRNA levels for β-actin were slightly decreased. Our data showed that the protein expression level of α-SMA remained the same as non-treatment controls after 2 h of nicotine (1 mM) pre-treatment. However, longer treatment times need to be evaluated to demonstrate the effect of nicotine on the basal level of α-SMA expression. It is well known that the TGF-β1 signaling pathway is important in myofibroblast differentiation, whereas the roles of Smads in this pathway are contradictory. In cutaneous wounding, the number of myofibroblasts was less in Smad3 knockout mice compared to wild-type mice. However, treatment of primary neonatal dermal fibroblasts from Smad3 knockout mice with 5 ng/ml TGF-β1 for 4 days induced α-SMA protein at the same level as fibroblasts from wild-type mice [Flanders et al., 2003]. Furthermore, lung fibroblasts transfected with Smad2, but not Smad3 had up-regulated α-SMA mRNA expression and altered cell phenotypes [Evans et al., 2003]. These data suggested that Smad3 was not required for the differentiation of fibroblasts to myofibroblasts. Interestingly, other studies using fibroblasts found that both Smad2 and Smad3 were translocated to the nuclear compartment 30 min after TGF-β1 treatment. Either Smad2 or Smad3 was able to activate the α-SMA enhancer when an expression plasmid encoding the collaborating Smad4 protein was included [Cogan et al., 2002]. Moreover, transcription factors such as Sp1/3 proteins were able to bind and activate the enhancer of α-SMA. However, the promoter activation by Sp1/3 and Smad proteins was not synergic [Cogan et al., 2002]. Interestingly, in rat kidney interstitial fibroblasts, hepatocyte growth factor blocked TGF-β1-induced α-SMA expression even though Smad 2/3 phosphorylation and their association with Smad4 were not affected. However, activated nuclear translocation was blocked [Yang et al., 2003].
Studies on another marker of myofibroblast differentiation, SM22, have found that Smad3, not Smad2, was required in TGF-β1 signaling to stimulate SM22 transcription. Smad3 bound directly to Smad-binding elements in the SM22 promoter and interacted with a protein complex that is important in smooth muscle cell promoters [Qiu et al., 2003]. Interestingly, SM22 mRNA was up-regulated 2 h after TGF-β1 treatment in Smad3-null embryonic fibroblasts. This result suggested that a Smad3-independent pathway was involved in TGF-β1-induced SM22 transcription [Qiu et al., 2003].
TGF-β signaling pathways interact with other signaling pathways to regulate myofibroblast differentiation. A study using lung fibroblasts found that TGF-β1 up-regulated c-Jun NH2-terminal kinase (JNK), p38 MAPK, and extracellular regulated kinase (ERK) phosphorylation and activity. However, only CEP-1347 (a specific inhibitor of the JNK-mediated signaling pathway), but not SB 203580 or PD 98059 (inhibitors of p38 MAPK and ERK kinase respectively) decreased the TGF-β1-induced myofibroblast differentiation in a dose dependent manner [Hashimoto et al., 2001]. These data suggested that TGF-β1—activated JNK pathway regulated myofibroblast differentiation. However, studies using pancreatic stellate cells showed that inhibition of p38 MAPK by SB 203580 and SB 202190 decreased α-SMA expression [Masamune et al., 2003]. In addition, other molecules correlated with TGF-β signaling in inducing myofibroblast differentiation. ED-A fibronectin, which is an isoform of fibronectin, has been found to be required for TGF-β1 induced α-SMA expression. A blocking antibody (IST-9) to the ED-A domain of cellular fibronectin, inhibited TGF-β1-induced α-SMA expression [Serini et al., 1998]. It was suggested that TGF-β1-induced α-SMA expression resulted from the cooperation of two signaling pathways mediated through TGF-β1 and ED-A fibronectin. However, it is not quite clear how these pathways interacted with each other [Serini et al., 1998].
Using phosphospecific antibody to p38 MAPK, we found nicotine inhibited TGF-β1-induced activation of this signaling molecule even at the lowest dose (0.01 mM). Low concentrations of nicotine decreased the release of TGF-β1 in bovine endothelial cells [Cucina et al., 1999]. This may also explain the down-regulation of TGF-β1-induced p38 MAPK phosphorylation. Further study is needed to analyze the correlation between TGF-β1 and p38 MAPK pathways.
Our study demonstrated that nicotine (1 mM) inhibited myofibroblast differentiation in a long term incubation (30 h). However, we do not know if nicotine inhibited myofibroblast differentiation by regulating Smad activity. Future studies need to determine if nicotine inhibits Smad activity and if the nicotinic receptor plays a role in this effect. Since nicotine has been found to simulate FGF2 expression and inhibit TGF-β1 expression [Cucina et al., 1999, 2000], and FGF2 plus heparin could reverse TGF-β1 induced corneal myofibroblasts to corneal fibroblasts [Maltseva et al., 2001], future studies need to determine if nicotine inhibits myofibroblast differentiation through FGF2 signaling, and if blocking FGF2 signaling can eliminate nicotine effects.
In addition to altering TGF-β1 signaling pathways, nicotine affects cell attachment, which may in turn have an effect on myofibroblast differentiation. However, the effects of nicotine on cell attachment have been controversial. Studies using HGFs demonstrated that nicotine enhanced the cell attachment to the tissue culture surface [Peacock et al., 1993]. A recent investigation using dermal fibroblasts and cigarette smoke had increased focal adhesions and increased vinculin with decreased migration [Wong and Martins-Green, 2004]. Whereas, using human periodontal ligament fibroblasts, it was found that nicotine inhibited cell attachment in a dose-dependent manner [Giannopoulou et al., 1999]. Similar results were obtained from another study that demonstrated nicotine inhibited periodontal ligament fibroblast proliferation and attachment [James et al., 1999]. While using human dermal fibroblasts, it has been found that even though nicotine treatment did not inhibit the cell attachment to the tooth root surface, the nicotine treated cells had different morphology under scanning electron microscopy. The nicotine treated cells had filopodia projected away from their attachment surface compared to the smooth surfaces in non treatment control cells [Raulin et al., 1988]. The different results may be due to different concentrations of nicotine, different cell types, and different nicotine exposure times.
Myofibroblast-like cells could be found in gingival fibromatoses and oral smooth muscle tumors [Kawakami et al., 1987; Takagi et al., 1991]; however, it has not been determined if myofibroblasts appear during periodontal wound healing process in these cases. Therefore, for future clinical studies, subjects receiving periodontal surgeries including gingival flap surgery and crown lengthening can be assigned into non-smoking, light smoking, and heavy smoking groups depending on the patients’ smoking status, saliva, and gingival crevicular fluid nicotine concentrations. Gingival tissue biopsies could be obtained periodically from wound beds after surgeries or evaluated in a non evasive procedure. Granulation tissue formation and myofibroblast differentiation could be analyzed by histological evaluation and immunohistochemistry studies. Our hypothesis is that nicotine inhibits granulation tissue formation and myofibroblast differentiation in vivo.
In conclusion, our results demonstrated that TGF-β1 induced myofibroblast differentiation in HGFs and nicotine (1 mM) inhibited this myofibroblast differentiation. This is the first study to explore the role of nicotine in myofibroblast differentiation and supports the theory that functioning myofibroblasts contribute to the speed of gingival wound healing.
ACKNOWLEDGMENTS
We thank Sala Senkayi, Jesus Acevedo, Petra Moessner, and Steve Lin for technical assistance. We thank Drs. David Coon and Yi-Shing (Lisa) Cheng and the Periodontics department for assistance obtaining patient tissues.
Grant sponsor: Baylor Oral Health Foundation; Grant sponsor: Tobacco Endowment Fund, Texas A&M University System; Grant number: 304-202850-4013.
REFERENCES
- Ah MK, Johnson GK, Kaldahl WB, Patil KD, Kalkwarf KL. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91–97. doi: 10.1111/j.1600-051x.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Akhurst RJ, Derynck R. TGF-beta signaling in cancer—A double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- Alpar B, Leyhausen G, Sapotnick A, Gunay H, Geurtsen W. Nicotine-induced alterations in human primary periodontal ligament and gingiva fibroblast cultures. Clin Oral Investig. 1998;2:40–46. doi: 10.1007/s007840050042. [DOI] [PubMed] [Google Scholar]
- Arora PD, McCulloch CA. The deletion of transforming growth factor-beta-induced myofibroblasts depends on growth conditions and actin organization. Am J Pathol. 1999;155:2087–2099. doi: 10.1016/s0002-9440(10)65527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanile G, Hautmann G, Lotti T. Cigarette smoking, wound healing, and face-lift. Clin Dermatol. 1998;16:575–578. doi: 10.1016/s0738-081x(98)00041-8. [DOI] [PubMed] [Google Scholar]
- Chang YC, Huang FM, Tai KW, Yang LC, Chou MY. Mechanisms of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodontal Res. 2002;37:279–285. doi: 10.1034/j.1600-0765.2002.01612.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Grande JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood) 2002;227:943–956. doi: 10.1177/153537020222701102. [DOI] [PubMed] [Google Scholar]
- Cogan JG, Subramanian SV, Polikandriotis JA, Kelm RJ, Jr., Strauch AR. Vascular smooth muscle alpha-actin gene transcription during myofibroblast differentiation requires Sp1/3 protein binding proximal to the MCAT enhancer. J Biol Chem. 2002;277:36433–36442. doi: 10.1074/jbc.M203232200. [DOI] [PubMed] [Google Scholar]
- Cucina A, Corvino V, Sapienza P, Borrelli V, Lucarelli M, Scarpa S, Strom R, Santoro-D'Angelo L, Cavallaro A. Nicotine regulates basic fibroblastic growth factor and transforming growth factor beta1 production in endothelial cells. Biochem Biophys Res Commun. 1999;257:306–312. doi: 10.1006/bbrc.1999.0478. [DOI] [PubMed] [Google Scholar]
- Cucina A, Sapienza P, Borrelli V, Corvino V, Foresi G, Randone B, Cavallaro A, Santoro-D'Angelo L. Nicotine reorganizes cytoskeleton of vascular endothelial cell through platelet-derived growth factor BB. J Surg Res. 2000;92:233–238. doi: 10.1006/jsre.2000.5894. [DOI] [PubMed] [Google Scholar]
- Cuff MJ, McQuade MJ, Scheidt MJ, Sutherland DE, Van Dyke TE. The presence of nicotine on root surfaces of periodontally diseased teeth in smokers. J Periodontol. 1989;60:564–569. doi: 10.1902/jop.1989.60.10.564. [DOI] [PubMed] [Google Scholar]
- Evans RA, Tian YC, Steadman R, Phillips AO. TGF-beta1-mediated fibroblast-myofibroblast terminal differentiation-the role of Smad proteins. Exp Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujii M, Blalock TD, Schultz GS, Sowers A, Anzano MA, Mitchell JB, Russo A, Roberts AB. Interference with transforming growth factor-beta/Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–2257. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology. 2003;38:879–889. doi: 10.1053/jhep.2003.50384. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Giannopoulou C, Geinoz A, Cimasoni G. Effects of nicotine on periodontal ligament fibroblasts in vitro. J Clin Periodontol. 1999;26:49–55. doi: 10.1034/j.1600-051x.1999.260109.x. [DOI] [PubMed] [Google Scholar]
- Grossi SG, Skrepcinski FB, DeCaro T, Zambon JJ, Cummins D, Genco RJ. Response to periodontal therapy in diabetics and smokers. J Periodontol. 1996;67:1094–1102. doi: 10.1902/jop.1996.67.10s.1094. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Uitto VJ, Larjava H. Cell biology of gingival wound healing. Periodontology. 2000;24:127–152. [PubMed] [Google Scholar]
- Halayko AJ, Solway J. Molecular mechanisms of phenotypic plasticity in smooth muscle cells. J Appl Physiol. 2001;90:358–368. doi: 10.1152/jappl.2001.90.1.358. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth Factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med. 2001;163:152–157. doi: 10.1164/ajrccm.163.1.2005069. [DOI] [PubMed] [Google Scholar]
- Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann D, Adams JD. Carcinogenic tobacco-specific N-nitrosamines in snuff and in the saliva of snuff dippers. Cancer Res. 1981;41:4305–4308. [PubMed] [Google Scholar]
- James JA, Sayers NM, Drucker DB, Hull PS. Effects of tobacco products on the attachment and growth of periodontal ligament fibroblasts. J Periodontol. 1999;70:518–525. doi: 10.1902/jop.1999.70.5.518. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Boreham R, Primatesta P, Feyerabend C, Bryant A. Nicotine yield from machine-smoked cigarettes and nicotine intakes in smokers: Evidence from a representative population survey. J Natl Cancer Inst. 2001;93:134–138. doi: 10.1093/jnci/93.2.134. [DOI] [PubMed] [Google Scholar]
- Kaldahl WB, Johnson GK, Patil KD, Kalkwarf KL. Levels of cigarette consumption and response to periodontal therapy. J Periodontol. 1996;67:675–681. doi: 10.1902/jop.1996.67.7.675. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Hasegawa H, Chino T. A transmission electron microscopic study of two cases of oral smooth muscle neoplasm. J Oral Maxillofac Surg. 1987;45:551–555. doi: 10.1016/s0278-2391(87)80022-8. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Letterio J. Transforming growth factor-beta signaling in normal and malignant hematopoiesis. Leukemia. 2003;17:1731–1737. doi: 10.1038/sj.leu.2403069. [DOI] [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Masamune A, Satoh M, Kikuta K, Sakai Y, Satoh A, Shimosegawa T. Inhibition of p38 mitogen-activated protein kinase blocks activation of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2003;304:8–14. doi: 10.1124/jpet.102.040287. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JR, McQuade MJ, Rossmann JA, Garnick JJ, Sutherland DE, Scheidt MJ, Van Dyke TE. Cotinine in saliva and gingival crevicular fluid of smokers with periodontal disease. J Periodontol. 1989;60:176–181. doi: 10.1902/jop.1989.60.4.176. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Suzuki H, Imamura T. Regulation of TGF-beta signaling and its roles in progression of tumors. Cancer Sci. 2003;94:230–234. doi: 10.1111/j.1349-7006.2003.tb01425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin V, Castilloux G, Auger FA, Garrel D, O'Connor-McCourt MD, Germain L. Modulated response to cytokines of human wound healing myofibroblasts compared to dermal fibroblasts. Exp Cell Res. 1998;238:283–293. doi: 10.1006/excr.1997.3827. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Pan D, Zhe X, Jakkaraju S, Taylor GA, Schuger L. P311 induces a TGF-beta1-independent, nonfibrogenic myofibroblast phenotype. J Clin Invest. 2002;110:1349–1358. doi: 10.1172/JCI15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock ME, Sutherland DE, Schuster GS, Brennan WA, O'Neal RB, Strong SL, Van Dyke TE. The effect of nicotine on reproduction and attachment of human gingival fibroblasts in vitro. J Periodontol. 1993;64:658–665. doi: 10.1902/jop.1993.64.7.658. [DOI] [PubMed] [Google Scholar]
- Qiu P, Feng XH, Li L. Interaction of Smad3 and SRF-associated complex mediates TGF-beta1 signals to regulate SM22 transcription during myofibroblast differentiation. J Mol Cell Cardiol. 2003;35:1407–1420. doi: 10.1016/j.yjmcc.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Raulin LA, McPherson JC, 3rd, McQuade MJ, Hanson BS. The effect of nicotine on the attachment of human fibroblasts to glass and human root surfaces in vitro. J Periodontol. 1988;59:318–325. doi: 10.1902/jop.1988.59.5.318. [DOI] [PubMed] [Google Scholar]
- Rivera-Hidalgo F. Smoking and periodontal disease. Periodontol. 2003;32:50–58. doi: 10.1046/j.0906-6713.2003.03205.x. [DOI] [PubMed] [Google Scholar]
- Roy SG, Nozaki Y, Phan SH. Regulation of alpha-smooth muscle actin gene expression in myofibroblast differentiation from rat lung fibroblasts. Int J Biochem Cell Biol. 2001;33:723–734. doi: 10.1016/s1357-2725(01)00041-3. [DOI] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Takagi M, Yamamoto H, Mega H, Hsieh KJ, Shioda S, Enomoto S. Heterogeneity in the gingival fibromatoses. Cancer. 1991;68:2202–2212. doi: 10.1002/1097-0142(19911115)68:10<2202::aid-cncr2820681019>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Teneggi V, Squassante L, Iavarone L, Milleri S, Bye A, Gomeni R. Correlation and predictive performances of saliva and plasma nicotine concentration on tobacco withdrawal-induced craving. Br J Clin Pharmacol. 2002;54:407–414. doi: 10.1046/j.0306-5251.2002.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Vaughan MB, Howard EW, Tomasek JJ. Transforming growth factor-beta1 promotes the morphological and functional differentiation of the myofibroblast. Exp Cell Res. 2000;257:180–189. doi: 10.1006/excr.2000.4869. [DOI] [PubMed] [Google Scholar]
- Wong LS, Martins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: Implications for impaired healing. Wound Repair Regeneration. 2004;12:471–484. doi: 10.1111/j.1067-1927.2004.12403.x. [DOI] [PubMed] [Google Scholar]
- Yang J, Dai C, Liu Y. Hepatocyte growth factor suppresses renal interstitial myofibroblast activation and intercepts Smad signal transduction. Am J Pathol. 2003;163:621–632. doi: 10.1016/S0002-9440(10)63689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]