Abstract
A flurry of recent studies has suggested the importance of the actin regulator coronin-1A in lymphocyte development. Now, mutations in this regulator are shown to cause immunodeficiency in both mice and humans.
The Cataract Shionogi (CTS) strain of mouse was initially described in 1968 as having cataracts and micropthalmia, and later reported to have profound peripheral T cell deficiency (Ptcd)1. The nature of this defect has, however, remained unclear. In this issue of Nature Immunology, Cyster and colleagues map the genetic basis of the T cell deficiency in CTS mice and show it is caused by a point mutation in the gene for an actin-regulating protein, Coronin-1A, (Coro1a)2. They performed a mapping cross between CTS and C57BL/6 mice and found that cataracts and microphthalmia failed to segregate with Ptcd. They further mapped the Ptcd locus to a 950 kb DNA interval on chromosome 7, which contained 37 open reading frames, including Coro1a. Sequencing revealed a G→A mutation in exon 2 of Coro1a that resulted in a glutamic acid substitution for a highly conserved lysine at position 26. They confirmed that this E26K mutation causes Ptcd by complementation with Coro1a+/− mice, where the wild-type allele could complement the phenotype but the null allele could not.
In collaboration with Chris Goodnow’s group, they also report a novel mutant mouse strain Koyaanisqatsi (Koy). This mouse, discovered in an ethylnitrosourea (ENU)-mutagenesis screen, was found to lack peripheral T cells; and sequencing of Coro1a in the Koy strain revealed a G→A substitution in exon 7, leading to an Asp→Ala mutation at residue 278. Thymocytes from Koy mice have greatly reduced amounts of Coronin-1A protein, suggesting that D278A is a Coronin-1A hypomorph.
Turning their attention to human severe combined immunodeficiency (SCID) patients, they screened 16 people with primary T cell immunodeficiency for mutations in CORO1A. They found a single patient with a two base pair deletion in exon 3, which resulted in a frameshift mutation and undetectable Coronin-1A expression, suggesting that this particular T−B+NK+ (T cell-deficient, B cell- and NK cell sufficient) SCID was caused by loss of CORONIN-1A.
Interestingly, another mutation in Coro1a was recently identified as a disease-suppressing allele in the MRL mouse model of lupus autoimmune disease3. In this case, suppression of disease by the ‘LMB3’ allele was associated with peripheral T cell deficiency, and impaired T-dependent B cell responses. The LMB3 allele was found to encode a C→T transition at residue 784, converting a glutamine to a stop codon (Q262X), again leading to undetectable Coronin-1A protein. All of these studies reveal that mutations in Coronin-1A cause profound peripheral T cell lymphopenia.
Two groups had recently created deficient mice by gene targeting4,5. The major defect of Coro1a null T cells is impaired survival at the final stage of thymic maturation (Fig. 1) leading to peripheral T cell deficiency. Coro1a−/− mice have normal numbers of ‘immature SP’, suggesting that positive selection is generally intact, but reduced numbers of ‘mature SP’. Like Coro1a−/− mice, mice with the LMB3 null allele or the Koy hypomorph mutation have a similar problem with reduced mature SP thymocytes2,3. Thymic progenitors from these mice die at the mature SP stage, as judged by upregulation of Annexin V2–4.However, whether thisis due to F-actin accumulation4 or an impaired Ca++ response5 is controversial.
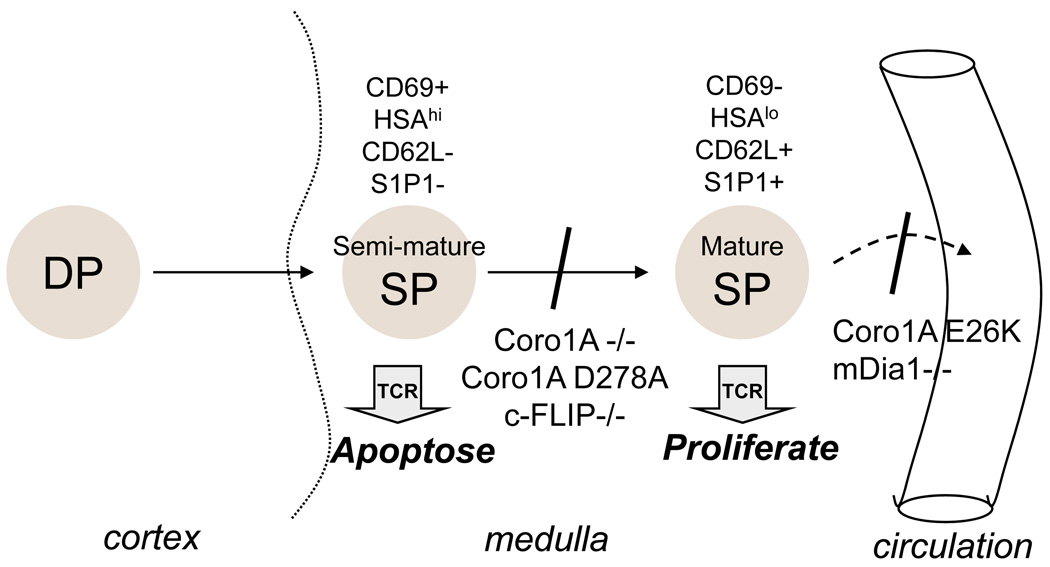
Figure 1. Effect of Coronin-1A mutants on development and emigration from the thymus.
Most T cells arise from CD4+CD8+ double positive (DP) progenitors that reside in the cortex of the thymus. After positive selection, cells migrate to the medulla and differentiate into CD4+ or CD8+ single positive (SP) cells. There are two general stages of SP differentiation. The semi-mature SP is susceptible to apoptosis when stimulated via the TCR, but this is lost as cells become mature SP, which undergo proliferation when stimulated. Coronin-1A-deficient (Coro1a-KO) cells undergo apoptosis at the mature SP stage, as do cells with a D278A Coronin-1A mutation, deficiency in c-FLIP (c-FLIP-KO), or LMB3 mice (Coronin-1A Q262X). In contrast cells with an E26K mutation of Coronin-1A survive normally as SP thymocytes, but fail to emigrate from the thymus into the circulation. This is similar to cells lacking mDia1 (Diablo) expression. Exactly where the defect is in cells of the T−B+NK+ SCID patient (Coronin-1A P83X) is not clear. However, all of these mutations cause peripheral T cell deficiency.
One of the interesting things about the studies of Cyster and colleagues is that the Coronin-1A E26K point mutation does not cause apoptosis at this stage, rather mature SP are viable and accumulate in the thymus (Fig. 1)2. The fact that the E26K mutant did not precisely phenocopy the null mutants allowed the authors to make important conclusions about the role of Coronin-1A in T cell survival and signaling.
First, the E26K mutant shows a modestly impaired TCR stimulated Ca2+ flux similar to null cells, suggesting that a defective Ca2+ flux does not cause the survival defect. The Ca2+ flux observed in mutant thymocytes2 5, was not as impaired as that in peripheral T cells3 5. This may be a biologically interesting observation, or it may have to do with the compromised viability and function of the minor population of cells that made it to the periphery in these mice. As with many mutants that affect lymphocyte development, a good way to move forward is probably with inducible deficiency, where lymphocytes are allowed to develop and emigrate normally, then signaling, survival, and apoptosis can be studied after induced deletion of the gene.
Second, Cyster and colleagues also report that thymocytes heterozygous for the E26K mutation and the null allele survive, but still have F-actin accumulation. This is surprising because it suggests that F-actin accumulation alone does not cause cell death either. So the precise cause of the survival defect in Coro1a−/− mice remains incompletely understood. This will be an important issue to resolve because the immature to mature SP transition is critical during T cell development. This is the stage where progenitors go from apoptosis susceptibility to proliferation competence. Given this, it is surprising that there is little known about the molecular factors involved in survival and differentiation at this final step. Mice lacking the apoptosis inhibitory molecule, c-FLIP, have a defect at this stage6, but it is unclear what the apoptosis initiating signals are.
What is clear, however, is that all Coronin-1A mutants have motility defects2–4. The Coronin-1A E26K mutant cells are lymphopenic not because mature T cells fail to survive, but because they fail to egress from the thymus. Mutant cells have good viability and normal expression of S1P1 and chemokine receptors that are known to be involved in thymic emigration; but they do not migrate in response to S1P or chemokines. Furthermore they have impaired entry and exit from lymph nodes in adoptive transfers, and showed reduced general motility by two photon microscopy. Biochemically, Coronin-1A E26K was more efficient than WT Coronin-1A at inhibiting the actin-nucleation activity of the actin nucleator protein Arp2/3, and it failed to localize to the leading edge of migrating cells2. Thus the emigration defect seems to arise from an actin-remodeling defect downstream of chemokine-lipid receptor signaling. This is consistent with the emigration defect observed in mice lacking another actin-nucleating protein called mDia1 (DIABLO) 7.
Interestingly, both mDIA1 and Coronin-1A-deficient mutants seem to develop relatively normally up to the semi-mature SP stage (Fig. 1), despite the fact that motility and chemokine responsiveness is impaired earlier, such as in DP thymocytes. This is curious, because T cell development requires progenitor entry from the blood into the thymus, and involves several chemokine dependent migratory events within the thymus8. Whether these earlier processes are impaired in subtle ways, or are truly less dependent on actin remodeling activity remains to be determined.
References
- 1.Yagi H, et al. Immune deficiency of cataract Shionogi (CTS) mouse. II. Impaired in vivo T cell-mediated immune response. Immunol Invest. 1990;19:493–505. doi: 10.3109/08820139009052975. [DOI] [PubMed] [Google Scholar]
- 2.Shiow LR, et al. Coronin-1A is mutated in a thymic egress defective mouse strain and a T-B+NK+ SCID patient. Nat Immunol. 2008 doi: 10.1038/ni.1662. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haraldsson MK, et al. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28:40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foger N, Rangell L, Danilenko DM, Chan AC. Requirement for coronin 1 in T lymphocyte trafficking and cellular homeostasis. Science. 2006;313:839–842. doi: 10.1126/science.1130563. [DOI] [PubMed] [Google Scholar]
- 5.Mueller P, et al. Regulation of T cell survival through coronin-1-mediated generation of inositol-1,4,5-trisphosphate and calcium mobilization after T cell receptor triggering. Nat Immunol. 2008;9:424–431. doi: 10.1038/ni1570. [DOI] [PubMed] [Google Scholar]
- 6.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakata D, et al. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahama Y. Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol. 2006;6:127–135. doi: 10.1038/nri1781. [DOI] [PubMed] [Google Scholar]