Abstract
Epithelial-mesenchymal transformation (EMT) is the primary mechanism for the disappearance of medial edge epithelia (MEE) during palate fusion. This phenotype transition is highly regulated by growth factors, extracellular matrix, cell surface receptors, and a variety of intracellular signaling. Phosphatidylinositol-3 (PI-3) kinase regulates cytoskeleton reorganization, cell migration, and transforming growth factor (TGF) β–regulated EMT. Therefore, we investigated the role of PI-3 kinase in EMT during palatal fusion in vitro. Palatal shelves from embryonic (E) 13.5 day mouse embryos were collected and cultured for up to 72 hr. A specific PI-3 kinase inhibitor, LY294002, was added to the medium at concentrations of 100 ηM, 1 µM, and 10 µM. The fate of midline epithelia was traced by carboxyfluorescence labeling and analyzed by confocal microscopy. Harvested tissues were also processed for immunohistochemical analysis of a specific marker for basal lamina (laminin). Palatal fusion stages were scored on a scale of 1 to 5, with 1 equal to complete nonfusion and 5 equal to complete fusion. The mean fusion score (MFS) was calculated for each treatment group. Palatal shelves fused after 72 hr of culture in control and 100 ηM LY294002 inhibitor-treated groups, with MFS of 4.67 and 4.5, respectively. Laminin was absent in the midline and epithelia transformed into mesenchyme. However, when cultured palates were treated with 1 and 10 µM LY294002, MEE persisted in the midline and the basal lamina remained intact after 72 hr. The MFS was significantly less in the 1 and 10 µM LY294002-treated tissues at 2.08 and 1.33, respectively. Our results demonstrate that EMT during palatal fusion in vitro is dependent on PI-3 kinase activity.
Keywords: palate, epithelial-mesenchymal transformation (EMT), TGF-β3, palatal fusion score, PI-3 kinase, confocal microscopy
INTRODUCTION
Cleft palate is one of the most common craniofacial birth defects. It results from the failure of fusion between the two secondary palatal shelves. Defects in palatal growth, shelf elevation, and medial edge epithelia (MEE) disappearance can all result in cleft palate. In the investigation of MEE disappearance and mesenchymal confluence, several studies suggested that most of the MEE, if not all, go through epithelial-mesenchymal transformation (EMT; Fitchett and Hay, 1989; Shuler et al., 1992; Martinez-Alvarez et al., 2000). During this phenotype change, MEE lose cell-cell adhesion, change cell shape, degrade the basement membrane, and migrate into mesenchyme. Many biological agents, including growth factors, extracellular matrix, intracellular signaling molecules, and transcription factors (Hay, 1995), are implicated in this phenotype transition. Specifically, during palate fusion, TGFβ3 has been established as an essential growth factor inducing EMT during palatal fusion.
The binding of TGFβ3 to its cell surface receptor initiates a series of intracellular signaling events. Some of the downstream signaling molecules are in the Smad family (Whitman, 1998; Attisano and Wrana, 2000; Miyazono, 2000). Although Smad expression was detected during palatal fusion (Cui et al., 2000), Smads were not essential factors for EMT. As an alternative downstream effecter to TGFβ signaling, phosphatidylinositol-3 kinase (PI-3 kinase) is involved in actin reorganization, matrix metalloproteinase (MMP) production, and cell mobility (Metzner et al., 1996; Sugiura and Berditchevski, 1999). Recently, it was demonstrated that LY294002, a specific inhibitor of PI-3 kinase, completely blocked TGFβ-mediated C-terminal phosphorylation of Smad2 and cell migration and partially blocked EMT in mammary epithelial cell culture (Bakin et al., 2000). The current study tested whether blocking PI-3 kinase activity by LY294002 would result in the failure of EMT and palate fusion in vitro.
RESULTS AND DISCUSSION
Analysis of Palatal Fusion Stages In Vitro
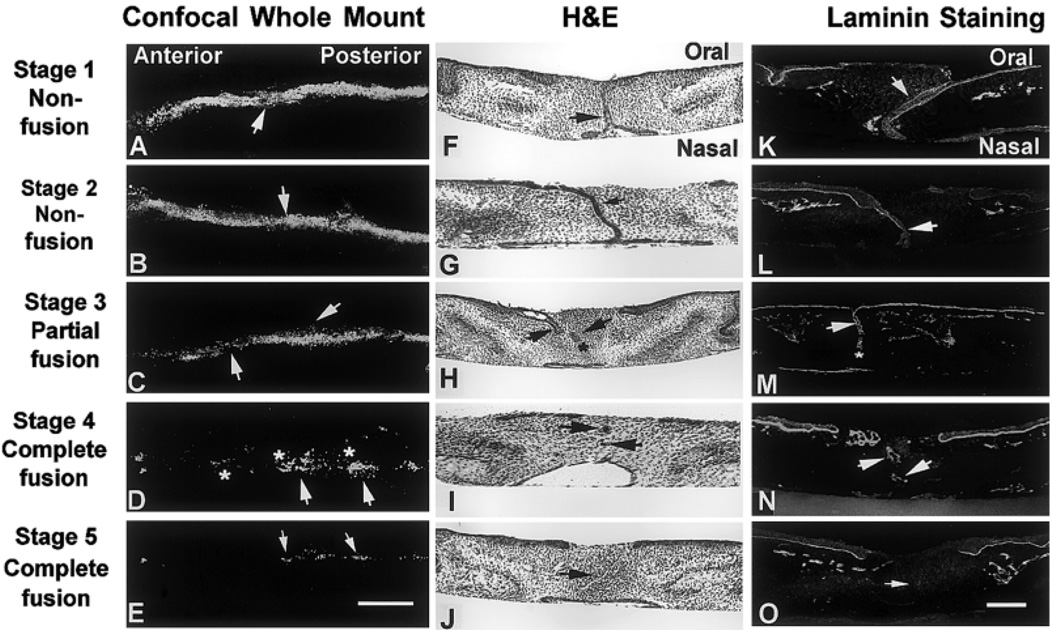
The palates went through different degrees of EMT and fusion after 72 hr in vitro. Five stages of fusion (1–5) were scored according to histomorphologic observations, including confocal analysis of 5 (and 6) carboxy 2,7′-dichlorofluorescein diacetate succinimidyl ester (CCFSE), hematoxylin and eosin (H&E) -stained paraffin sections, and laminin immunohistochemical staining (Fig. 1; Table 1). Single optical confocal images obtained from the middle of the tissues were analyzed for fusion. The location, continuity, and intensity of CCFSE-labeled MEE were compared anterior–posterior in the horizontal plane (Fig. 1A–E). Nonfused or partially fused samples had more intense, continuous, or even two epithelial layers of CCFSE-labeled cells (Fig. 1A–C), whereas the fused palates had very discrete labeling in the fusion zone (Fig. 1D,E), indicating that EMT had occurred in these palates. Palatal samples were also processed as individual cross-sections for H&E staining, and MEE were analyzed in the coronal plane (Fig. 1F–J). The amount of persistent MEE and degree of mesenchymal confluence were compared between treatment groups. To establish that the basement membrane had completed degradation, cross-sections were analyzed by immunohistochemical localization of laminin, a marker for basal lamina (Fig. 1K–O). The amount and continuity of laminin were greater in non- or partially fused samples.
Fig. 1.
Five stages of palate fusion in vitro as observed from confocal analysis of whole mount palates (A–E), hematoxylin and eosin (H&E) staining (F–J), and laminin immunohistochemical studies (K–O). Characteristics of each stage in the three morphologic formats are explained in Table 1. Scale bar = 200 µm in E (applies to A–E), in O (applies to F–O).
TABLE 1.
Stages of Palatal Fusion In Vitroa
| CCFSE labeling | H&E staining | Laminin analysis | |
|---|---|---|---|
| Stage 1 | |||
| Nonfusion | Intense CCFSE labeling in the midline; MEE did not thin into a single seam (arrows, Fig. 1A) | Two layers of MEE persistent (arrow, Fig. 1F) | Two layers of basal lamina continue with oral epithelia (arrow, Fig. 1K) |
| Stage 2 | |||
| Nonfusion | Similar to Fig. 1A, but two distinctive layers of MEE was NOT observed (arrow, Fig. 1B) | An epithelial seam continuous in the midline (arrow, Fig. 1G) | Laminin reaction beneath the epithelial seam but discontinuous (arrow, Fig.1L) |
| Stage 3 | |||
| Partial Fusion | Intense labeling in midline, but broken areas observed; some MEE cells seemed to migrate into mesenchyme (arrows, Fig.1C) | Mesenchymal confluence was achieved in places (asterisk), but large epithelial islands remained (arrows, Fig. 1H) | Positive laminin was detected next to the remaining MEE (arrow), but not in the confluent area (asterisk, Fig. 1M) |
| Stage 4 | |||
| Complete fusion | Islands of MEE were observed (arrows); discrete CCSFE-stained cells migrated away from the midline (asterisks, Fig. 1D) | Only small epithelial islands remained (arrows, Fig. 1I) | Laminin staining was only observed next to the remaining epithelial islands (arrows, Fig. 1N) |
| Stage 5 | |||
| Complete fusion | Only discontinuous CCFSE-stained cells were detected in the fusion zone (arrows, Fig. 1E) | No epithelia persist in the midline (arrow, Fig. 1J) | Laminin was NOT detectable throughout fusion zone arrow, Fig. 1O) |
CCFSE, 5 (and 6) carboxy 2,7′-dichlorofluorescein diacetate succinimidyl ester; H&E, hematoxylin and eosin; MEE, medial edge epithelia.
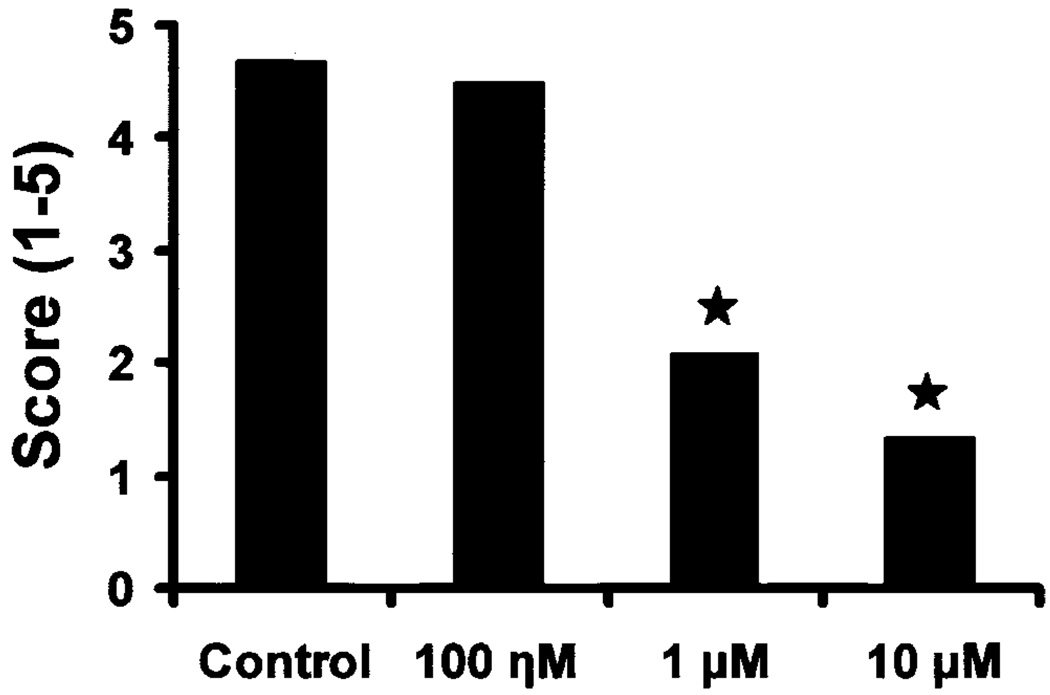
Each cultured palate was scored from 1 to 5 (Table 2), and the mean fusion score (MFS) was determined for the palates cultured for 72 hr. The MFS was calculated by multiplying the palate stage (1–5) times the number of embryos for each stage, and dividing the sum by the total number of embryos in that group (Table 2; Fig. 2). For example, to calculate the MFS for the controls the following calculation was made: (2)(3)+(4)(4)+(18)(5)/24 = 4.67. Groups with higher fusion scores had MFSs closer to 5. MFS was compared between groups by using Kruskal-Wallis test, and a P value of less than 0.01 was considered statistically significant.
TABLE 2.
Number of Fused Palatal Shelves in Different Treatment Groups and Mean Fusion Scoresa
| Number of palates at different fusion stages |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 48 hr |
72 hr |
||||||||||||
| Nonfusion |
Partial fusion |
Complete fusion |
Nonfusion |
Partial fusion |
Complete fusion |
||||||||
| 1 | 2 | 3 | 4 | 5 | Total | 1 | 2 | 3 | 4 | 5 | Total | MFS | |
| Control | 0 | 0 | 3 | 3 | 0 | 6 | 0 | 0 | 2 | 4 | 18 | 24 | 4.67 |
| 100 ηM | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 3 | 5 | 14 | 22 | 4.50 |
| 1 µM | 2 | 3 | 1 | 0 | 0 | 6 | 3 | 6 | 4 | 0 | 0 | 13 | 2.08 |
| 10 µM | 4 | 1 | 1 | 0 | 0 | 6 | 9 | 2 | 1 | 0 | 0 | 12 | 1.33 |
MFS, mean fusion score.
Fig. 2.
Mean fusion scores of palates after 72 hr of culture in different treatment groups (Table 2). There was no significant difference between controls and 100 ηM LY294002-treated palates. However, the 1 and 10 µM LY294002-treated tissues were significantly different than controls (*P ≤ 0.01).
Palatal Shelves Cultured in Control Conditions Completely Fused in 72 Hr
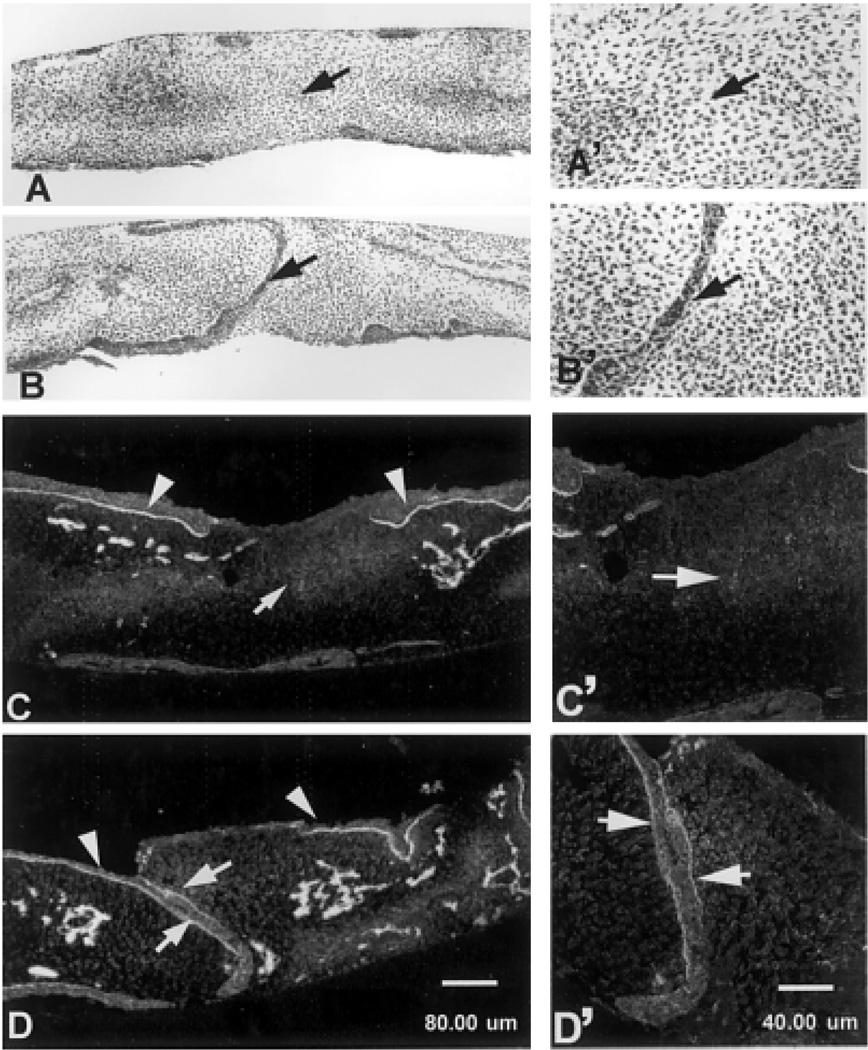
Eighteen embryonic palatal (Table 2) samples were completely fused with no epithelium remaining (stage 5), as observed by confocal microscopy. An additional four palates achieved stage 4 fusion, with small epithelial islands in the midline (Fig. 1D). Disappearance of midline epithelia in palates cultured for 72 hr was confirmed in H&E-stained paraffin sections (Fig. 3A). No continuous epithelia remained in the midline region (arrow, Fig. 3A). We also observed epithelial loss in the medial region of the upper surface. These epithelia may have had MEE origin and “moved” to the oral surface due to tissue manipulation during culture. The “moved” MEE went through EMT without the contact with opposing epithelia as previously reported by other groups (Smiley and Mato, 1975; Tyler, 1975). Laminin expression was observed beneath the surface epithelia (arrowheads, Fig. 3C). However, it was negative in the fusion zone of the control-cultured palates (arrow, Fig. 3C), indicating that the basement membrane was completely degraded. In all sections stained for laminin, additional areas that were not associated with the epithelia also stained (Figs. 1K,L, 3C,D). These areas may be developing blood vessels or other structures. They were consistent in all sections examined regardless of treatment group. In addition, occasionally tooth buds were observed on the oral epithelial surface (Fig. 3D, invagination lateral to the arrowhead).
Fig. 3.
Individual sections of hematoxylin and eosin (H&E; A,A′,B,B′) and laminin stained (C,C′,D,D′) from palatal tissues after 72 hr in control (A,C) or 1 µM LY294002-treated (B,D) medium. The palate completely fused in control medium (A,A′). No epithelia were observed in the midline. In the presence 1 µM LY294002, medial edge epithelia persisted (arrows in B,B′). Laminin was observed beneath the oral surface epithelia in all groups (arrowheads in C,D) and in the mesenchymal areas that may be developing blood vessels. However, laminin was negative in the midline of the control-cultured palate (arrows in C,C′), indicating that the basal lamina had completely degraded in the fused palate. In contrast, laminin was detected in the midline of LY294002-treated groups (arrows in D,D′) lateral to two layers of MEE. Scale bar = 80 µm in D (applies to A–D), 40 µm in D′ (applies to A′–D′).
In the 72-hr control group, the majority of the palates (22 of 24) were either stage 4 or 5 (Table 2). However, there were also two samples only partially fused (stage 3). Large epithelial islands were detected especially in the posterior region of these palates. The MFS of control group after 72 hours of culture was 4.67 (Table 2; Fig. 2). In contrast, no control palates had completely fused after 48 hr culture (Table 2).
Palatal Shelves Failed to Go Through EMT in the Presence of LY294002 In Vitro
The compound LY294002 is a highly selective PI-3 kinase inhibitor that does not affect the activity of other protein kinases such as MAP kinase, PKC, or phosphatidylinositol-4 kinase at 50 µM (Vlahos et al., 1994; Davies et al., 2000), five-fold higher than the concentration used in this study. In the current investigation, palatal culture medium was supplemented with LY294002 at concentrations of 100 ηM, 1 µM, and 10 µM. In an in vitro assay, LY294002 also inhibited casein kinase 2 (CK2) above 6.9 µM (Davies et al., 2000). Therefore, the palates treated with 10 µM LY294002 had both CSK and PI3 kinase inhibited. Interestingly, 1 µM LY294002 also blocked palatal fusion (Table 2).
Palatal shelves cultured with 100 ηM LY294002 progressed through normal EMT (19 of 22 scored 4 or 5), and the palates fused after 72 hr (Table 2). There was no significant difference in MFS (4.5) compared with the control group (Table 2; Fig. 2). However, in the presence of 1 or 10 µM LY294002, no complete fusion was found in the examined tissues (Table 2). Nine nonfusions (3 stage 1 and 6 stage 2) and 4 partial fusions (stage 3) were in the 1 µM inhibitor group; whereas 11 nonfusions (9 stage 1 and 2 stage 2) and 1 partial fusion were observed in the 10 µM LY294002 treatment group (Table 2).
Histochemical observations of LY294002-treated palate cultures indicated that the treatment with PI-3 kinase inhibitor did not interfere with the tissue growth in culture, as the size and thickness of the palatal shelves were similar to the control group (Fig. 3A–D). However, two distinct layers of cuboidal MEE persisted in the fusion zone (arrow, Fig. 3B), and mesenchymal confluence was not achieved. Laminin was detected along the fusion zone beneath two layers of MEE (arrows, Fig. 3D), in continuation with the oral surface epithelia (arrowheads, Fig. 3D). These results further demonstrated that the MEE did not thin into one layer and the intact basal lamina remained in the tissue. Therefore, basement membrane degradation, an essential event during EMT, did not occur in PI-3 kinase–treated palate cultures. In addition, confocal analysis of whole tissues demonstrated that intensely labeled epithelia were almost continuous along the fusion zone in 1 or 10 µM inhibitor-treated tissues (data not shown).
In summary, the MFS for the 1 and 10 µM LY294002 treatment groups were 2.08 and 1.33, respectively (Table 2; Fig. 2). These values were significantly lower than the control and 100 ηM LY294002-treated groups. Therefore, in the presence of LY294002, the palatal fusion was inhibited in a dose-dependent manner. The results suggested PI-3 kinase activity was necessary for inducing and/or maintaining EMT during palatal fusion in vitro.
In mammalian cells, class I PI-3 kinase proteins are composed of a p110 catalytic subunit and a p85 regulatory subunit. This class of PI-3 kinases is the subject of extensive research because of their capability to be activated by a variety of stimuli, such as growth factors, integrins, and the formation of adherens junctions. For example, PI-3 kinase can be activated in response to E-cadherin–mediated cell–cell contact in canine kidney epithelial cells (Pece et al., 1999). Upon activation, PI-3 kinase translocates to the plasma membrane and associates with E-cadherin in the adherens junction complex (Pece et al., 1999). When blocking this outside-in signal transduction with PI-3 kinase inhibitor, LY294002, the adherens junctions were disrupted in mammary epithelial cells (Somasiri et al., 2000). These studies demonstrated that PI-3 kinase was responsible for cellular activity upon cell–cell contact and maintaining cell adhesion. The initiation of palatal fusion involves new desmosome formation to anchor the epithelia from the opposed shelves (Fitchett and Hay, 1989). Therefore, we speculate that the inhibition of PI-3 kinase could result in the failure of forming epithelial adherence and the thinning of the seam into a single layer in our LY294002-treated palate cultures.
As one of the activators of PI-3 kinase, β1 integrin expression significantly increased before and during EMT in lens epithelial cells (Zuk and Hay, 1994). Another study demonstrated that during tumor cell invasion, cytoskeleton rearrangement and MMP2 production upon α3β1 integrin stimulation were dependent on PI-3 kinase activity (Sugiura and Berditchevski, 1999). Because MMPs are necessary for breaking down basement membrane, we speculate that blocking PI-3 kinase activity inhibited cell migration and MMP production downstream of extracellular matrix-integrin signaling.
Previous studies have shown that PI-3 kinase activity was necessary for TGFβ-mediated EMT in mammary epithelial cells (Bakin et al., 2000). Because TGFβ3 was essential for the cell-phenotype transition of MEE, it was tempting to speculate that PI-3 kinase was the downstream effecter of TGFβ3 during palate fusion. However, we did not test this direct interaction. A parallel TGFβ-mediated EMT has been shown to include the small GTPase RhoA and its downstream effecter p160ROCK. TGFβ-induced EMT was unaffected by decreased Smad signaling but blocked by inactive RhoA in mammary epithelial cells (Bhowmick et al., 2001). In addition, increased RhoA production upon TGFβ stimulation during EMT was independent of PI-3 kinase activity (Bhowmick et al., 2001).
Future studies in our laboratory will also investigate the downstream signaling pathways of PI-3 kinase that may be directly responsible for EMT. Phosphatidylinositol 3,4,5-trisphosphate (PIP3) is generated upon activation of PI-3 kinase. Along with small GTPases Rac and Rho, PIP3 activates several serine/threonine kinases such as 3-phosphoinostide-dependent protein kinases (PDKs). Integrin linked kinase (ILK) is one of these PDK proteins (Hannigan et al., 1997). Overexpression of ILK in epithelial cells induced anchorage-independent cell growth and loss of E-cadherin expression and EMT (Radeva et al., 1997; Wu et al., 1998). ILK was also implicated in TGFβ-induced fibroblastic conversion of highly metastatic cells (Janji et al., 1999). Therefore, the potential roles of ILK as a signaling molecule downstream of PI-3 kinase need to be evaluated in this EMT model.
EXPERIMENTAL PROCEDURES
Embryos
Timed-pregnant CD1 mice (Harlan Sprague-Dawley, Inc.) were used in these studies. Animals were maintained under standard conditions at a 10hr:14hr light: dark cycle. Female mice were mated overnight, and the day of vaginal plug was timed as day 0. In CD1 mice, the palate shelves elevate above the tongue between embryonic day (E) 13 and E13.5. Therefore, pregnant females were killed by cervical dislocation at day 13.5, and the embryos were dissected out of the amniotic sacs into a dish of Hanks’ balanced salt solution (HBSS; Gibco). The stage of development was determined by using morphologic features.
CCFSE Labeling of Palate Shelves
The 5 (and 6) carboxy 2,7′-dichlorofluorescein diacetate succinimidyl ester (CCFSE; Molecular Probes) was used to label the surface epithelia (Griffith and Hay, 1992). Briefly, 1:500 dilution of 10 mM CCFSE stock solution (in dimethyl sulfoxide, DMSO) was prepared and upper heads of the embryos were exposed to CCFSE for 1 hr at 37°C. For control, tissues were exposed to DMSO under the same conditions.
Organ Culture and Treatment With PI-3 Kinase Inhibitor
Palatal shelves were dissected from E13.5 mouse embryos and placed nasal side down on filters with their medial edges in contact. The tissues were suspended at the air–media (BGJb, Gibco) interface on a triangular-shaped wire grid in an organ culture dish. PI-3 kinase inhibitor-treated groups were supplemented with LY 294002 (Cell Signaling Tech., Beverly, MA) at concentrations of 100 ηM, 1µM, and 10µM. The medium barely covered the apical surface of the tissue. Tissues were incubated at 37°C in a humidified gas mixture (5% CO2 and 95% air), and medium was changed every 24 hr. Experiments were repeated 12 times on a total of 91 embryos (from 14 pregnant mice).
Histology and Immunohistochemistry
Cultured palatal shelves were collected at 72 hr and fixed in freshly prepared 4% paraformaldehyde/phosphate buffered saline (PBS) (pH 7.4) for 30 min. After rinsing in PBS, the tissues were processed for paraffin embedding. Seven- to 8-µm-thick serial sections were collected and numbered in sequence from rostral to caudal. Light microscopic analysis after H&E staining was completed on each sample in three regions: anterior, middle, and posterior. Images were captured by Zeiss Axioplan. Serial sections were also evaluated for the expression of laminin. After blocking with 10% normal goat serum/PBS, the tissues were incubated in 1:30 anti-laminin polyclonal antibody (Sigma, St. Louis, MO) for 2 hr at room temperature. After rinsing, the primary antibody was detected by 1:100 fluorescein isothiocyanate–conjugated goat anti-rabbit antibody (Jackson ImmunoResearch, PA). After mounting, the images were viewed by using confocal microscopy and arranged with Adobe Photoshop and PageMaker programs.
Confocal Laser Scanning Microscopy
Carboxyfluorescein of whole-mount palatal tissues and laminin reaction were analyzed with the Leica upright TCS-SP1 or TCS-SP2 confocal microscopes, equipped with argon, krypton, and helium neon lasers with excitation wavelengths of 488, 543, and 633 µm, respectively. The tissues were analyzed with a 10× air lens. Confocal images from representative depths were analyzed, enhanced, and stored. Images were arranged with Adobe Photoshop and PageMaker programs.
Statistics
The MFS was obtained by multiplying the stage (1–5) by the number of embryos and dividing the sum by the total number of embryos in that group (Table 2; Fig. 2). The MFS was compared between groups by using Kruskal-Wallis test, a nonparametric test (distribution-free) used to compare three or more independent groups of sampled data. All P values of less than 0.01 were considered statistically significant.
ACKNOWLEDGMENTS
We thank Jesus Acevedo, Adrienne Douglas, Tamara Field, Jan Lalor, and Petra Moessner for their technical assistance.
Grant sponsor: Baylor Oral Health Foundation; Grant sponsor: NIH; Grant number: EY-08886; Grant sponsor: Tobacco Endowment Fund, Texas A&M University System.
Abbreviations
- CCFSE
5 (and 6) carboxy 2,7’ dichlorofluorescein diacetate succinimidyl ester
- DiI
1,1-dioctadecyl-3,3,3’-tetramethylindocarbocyanine perchlorate
- EMT
epithelial-mesenchymal transformation
- H&E
hematoxylin and eosin staining
- ILK
integrin linked kinase
- MEE
medial edge epithelia
- MFS
mean fusion score
- MMP
matrix metalloproteinase
- PDK
3-phosphoinostide-dependent protein kinase
- PI-3 kinase
phosphatidylinositol-3 kinase
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- TGFβ
transforming growth factor β
REFERENCES
- Attisano L, Wrana JL. Smads as transcriptional co-modulators. Curr Opin Cell Biol. 2000;12:235–243. doi: 10.1016/s0955-0674(99)00081-2. [DOI] [PubMed] [Google Scholar]
- Bakin AV, Tomlinson AK, Bhowmick NA, Moses HL, Arteaga CL. Phosphatidylinositol 3-kinase function is required for transforming growth factor beta -mediated epithelial to mesenchymal transition and cell migration. J Biol Chem. 2000;275:36803–36810. doi: 10.1074/jbc.M005912200. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui XM, Chai Y, Ito Y, Shuler CF. Expression of TbetaR-I and SMAD2 in embryonic palatal tissues. J Dent Res. 2000;79:416. [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989;131:455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Hay ED. Epithelial-mesenchymal transformation during palatal fusion: carboxyfluorescein traces cells at light and electron microscopic levels. Development. 1992;116:1087–1099. doi: 10.1242/dev.116.4.1087. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Bayani J, Weksberg R, Beatty B, Pandita A, Dedhar S, Squire J. Mapping of the gene encoding the integrin-linked kinase, ILK, to human chromosome 11p15.5-p15.4. Genomics. 1997;42:177–179. doi: 10.1006/geno.1997.4719. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat. 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Janji B, Melchior C, Gouon V, Vallar L, Kieffer N. Autocrine TGF-beta-regulated expression of adhesion receptors and integrin-linked kinase in HT-144 melanoma cells correlates with their metastatic phenotype. Int J Cancer. 1999;83:255–262. doi: 10.1002/(sici)1097-0215(19991008)83:2<255::aid-ijc18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Alvarez C, Tudela C, Perez-Miguelsanz J, O’Kane S, Puerta J, Ferguson MW. Medial edge epithelial cell fate during palatal fusion. Dev Biol. 2000;220:343–357. doi: 10.1006/dbio.2000.9644. [DOI] [PubMed] [Google Scholar]
- Metzner B, Barbisch M, Bachmann F, Czech W, Norgauer J. Evidence of the involvement of phosphatidylinositol 3-kinase in the migration, actin stress fiber formation, and alpha v beta 3-integrin-mediated adherence of human melanoma cells. J Invest Dermatol. 1996;107:597–602. doi: 10.1111/1523-1747.ep12583096. [DOI] [PubMed] [Google Scholar]
- Miyazono K. TGF-beta signaling by Smad proteins. Cytokine Growth Factor Rev. 2000;11:15–22. doi: 10.1016/s1359-6101(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell-cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem. 1999;274:19347–19351. doi: 10.1074/jbc.274.27.19347. [DOI] [PubMed] [Google Scholar]
- Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- Shuler CF, Halpern DE, Guo Y, Sank AC. Medial edge epithelium fate traced by cell lineage analysis during epithelial-mesenchymal transformation in vivo. Dev Biol. 1992;154:318–330. doi: 10.1016/0012-1606(92)90071-n. [DOI] [PubMed] [Google Scholar]
- Smiley GR, Mato M. A comparison of secondary palate development with different in vitro techniques. Ultrastructural changes in rat palatal epithelium after beta-aminopropionitrile. Anat Rec. 1975;181:711–723. doi: 10.1002/ar.1091810404. [DOI] [PubMed] [Google Scholar]
- Somasiri A, Wu C, Ellchuk T, Turley S, Roskelley CD. Phosphatidylinositol 3-kinase is required for adherens junction-dependent mammary epithelial cell spheroid formation. Differentiation. 2000;66:116–125. doi: 10.1046/j.1432-0436.2000.660206.x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F. Function of alpha3beta1-tetraspanin protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler MS. In vitro development of palatal tissues from embryonic mice. I. Differentiation of the secondary palate from 12-day mouse embryos. Anat Rec. 1975;182:297–301. doi: 10.1002/ar.1091820304. [DOI] [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Whitman M. Smads and early developmental signaling by the TGFbeta superfamily. Genes Dev. 1998;12:2445–2462. doi: 10.1101/gad.12.16.2445. [DOI] [PubMed] [Google Scholar]
- Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, McDonald JA, Dedhar S. Integrin-linked protein kinase regulates fibronectin matrix assembly, E- cadherin expression, and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- Zuk A, Hay ED. Expression of beta 1 integrins changes during transformation of avian lens epithelium to mesenchyme in collagen gels. Dev Dyn. 1994;201:378–393. doi: 10.1002/aja.1002010409. [DOI] [PubMed] [Google Scholar]