Abstract
2-Cyano-3,10-dioxooleana-1,9(11)-dien-28-oic acid anhydride (CDDO anhydride) has been synthesized, which is the first example of an oleanane triterpenoid anhydride. CDDO anhydride shows potency similar to or higher than the corresponding acid (CDDO) in various in vitro and in vivo assays related to inflammation and carcinogenesis. Notably, preliminary phamacokinetics studies show that CDDO anhydride levels are higher than CDDO levels in mouse tissues and blood. Further evaluation of CDDO anhydride is in progress.
Keywords: Triterpene, Oleanolic acid, Acid anhydride, Inhibitors of nitric oxide production, Inducers of heme oxygenase-1
Over the past decade, we have been engaged in the improvement of anti-inflammatory and antiproliferative activity of oleanolic acid, a naturally occurring triterpenoid. This led to the discovery of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO, bardoxolone).1,2 Its methyl ester (CDDO-Me, bardoxolone methyl) is presently being developed in late Phase II clinical trials for the treatment of severe chronic kidney disease in type 2 diabetes mellitus patients. Although CDDO was also evaluated in Phase I clinical trials for the treatment of leukemia and solid cancer,3 due to its low bioavailability, the evaluation has been suspended. To increase the bioavailability, in particular through oral administration (p.o.), we designed some possible pro-drugs, but unfortunately they were not successful as pro-drugs for the following reasons: 1) Some analogues did not generate CDDO because the linkage between CDDO and the leaving groups was too robust. 2) Some analogues did not increase the bioavailabilty through p.o. because of low polarity.
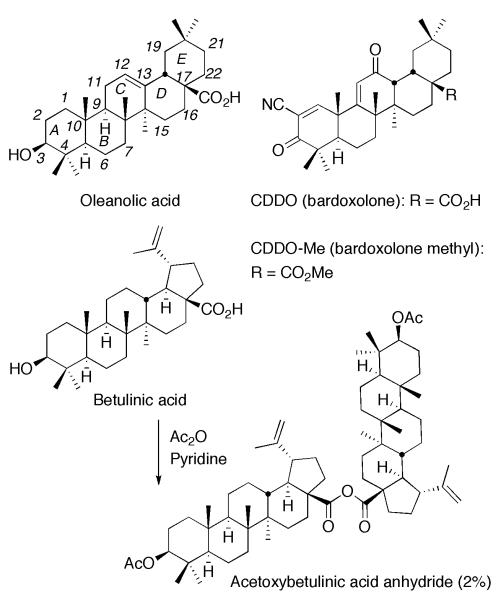
After several attempts, we have envisioned that CDDO anhydride 1 would have better bioavailability through p.o.4 than CDDO because an anhydride is generally less polar than the corresponding acid. Thus, we have decided to investigate properties of CDDO anhydride including the possibility that CDDO anhydride would work as a pro-drug of CDDO. Our literature survey has disclosed that oleanane and ursane triterpenoid anhydrides have not been reported. Although only acetoxybetulinic acid anhydride with a lupane triterpenoid skeleton was reported,5 it was produced as a by-product (2% yield) during acetylation of betulinic acid (Figure 1). Herein we report the first synthesis of oleanane triterpenoid anhydrides and the promising biological results for CDDO anhydride 1.
Figure 1.
Structures of oleanolic acid, CDDO, CDDO-Me, betulinic acid and acetoxybetulinic acid anhydride
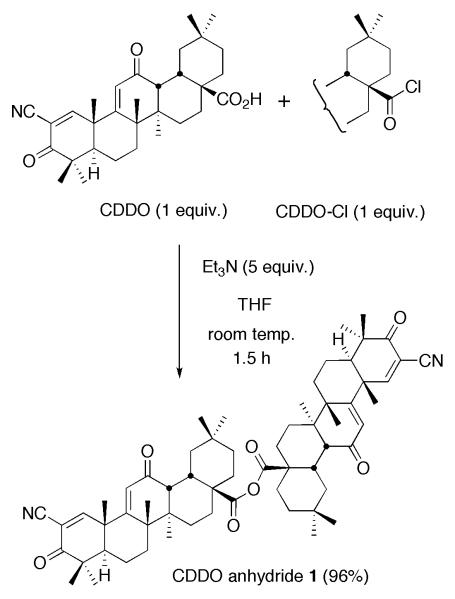
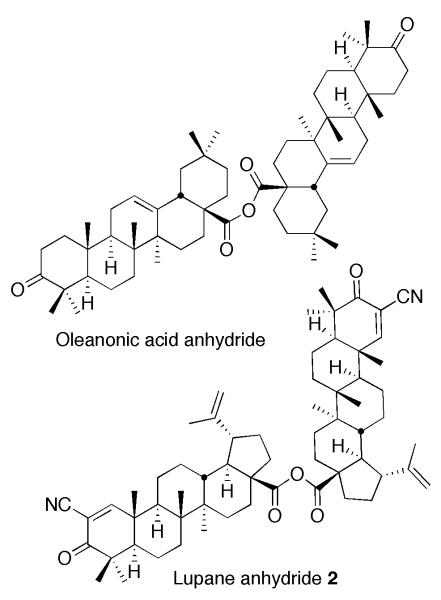
Since a carboxyl group at C17 of oleanolic acid is very hindered, we anticipated that the anhydride synthesis would be difficult. However, classical conditions (Et3N, THF, room temp.) gave the desired anhydride6 from CDDO and CDDO-Cl7 in 96% yield (Scheme 1). Because a naturally occurring oleanane triterpenoid anhydride has not been reported as we described above, we have applied the same conditions for the synthesis of oleanonic acid anhydride6,8 (Figure 2). The conditions gave the anhydride in 85% yield from oleanonic acid9 and oleanonyl chloride.9 We have also tried to synthesize a new lupane triterpenoid anhydride, 2-cyano-3-oxolupa-1,20(29)-dien-28-oic acid anhydride (lupane anhydride 2, Figure 2)6 to compare biological properties of 1 with those of 2. However, these reaction conditions gave 2 in low yield (14%) from 2-cyano-3-oxolupa-1,20(29)-dien-28-oic acid (3)10 and its acyl chloride.10 These results suggest that a carboxyl group at C17 of lupane skeleton is more hindered than that of oleanane skeleton.
Scheme 1.
Synthesis of CDDO anhydride
Figure 2.
Structures of oleanonic acid anhydride and lupane anhydride 2
We have evaluated the potency of new anhydrides 1 and 2 in comparison with their corresponding acids, for inhibition of NO production in RAW 264.7 cells stimulated with interferon-γ (iNOS assay, Table 1). The IC50 values are shown in Table 1. Notably, CDDO anhydride 1 is about 2 times more potent than the corresponding acid, CDDO, but lupane anhydride 2 is inactive whilst the corresponding acid 3 shows moderate potency at 200 nM. We have also evaluated 1 and 2 for induction of the anti-inflammatory and cytoprotective enzyme, heme oxygenase-1 (HO-1) in RAW cells (HO-1 assay, Figure 3). There is major interest in stimulating HO-1 as a protective enzyme in many chronic disease conditions in which inflammation and oxidative stress play a key role.11 CDDO anhydride 1 shows the same potency as CDDO. To the contrary, 2 is inactive while the corresponding acid 3 shows moderate potency.
Table 1.
Inhibition of NO production in RAW cells stimulated with interferon-γ by new anhydrides 1 and 2.
RAW 264.7 cells were treated with various concentrations of compounds and interferon-γ (10 ng/mL) for 24 hours. Supernatants were analyzed for NO by the Griess reaction. IC50 values are an average of three experiments.
This data was previously reported in reference 10.
Figure 3.
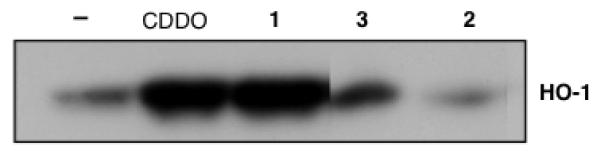
CDDO and CDDO anhydride 1 induce HO-1 in RAW 264.7 cells. Cells were incubated with compounds (100 nM) for 6 hours. Total cell lysates were analyzed by SDS-PAGE, probed with an HO-1 antibody, and developed by ECL. The HO-1 in the negative control lane is basal expression of HO-1.
If CDDO anhydride 1 is immediately converted to CDDO in the RAW cell growth medium (RMPI supplemented with 10% fetal bovine serum) at 37 °C, because the cells take in CDDO, we have merely evaluated the potency of CDDO instead of the potency of 1 in two assays above. Thus, we have evaluated the lifetime of CDDO anhydride 1 in the cell growth medium. The results are shown in Figure 4. Even after 24 hours, the percentage of material remaining of 1 is much higher than that of CDDO. Therefore we have concluded that the cells take in 1 in the two assays above.
Figure 4.
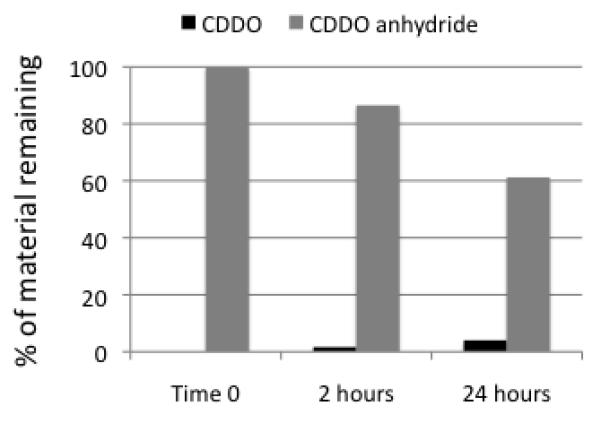
Lifetime of CDDO anhydride in the cell culture medium
Subsequent to the in vitro assay, we have evaluated the potency of CDDO anhydride 1 for induction of HO-1 in the liver (in vivo, i.p. injection4). CDDO anhydride 1 is as potent as CDDO in the liver at 2 μmol dosage (Figure 5).
Figure 5.
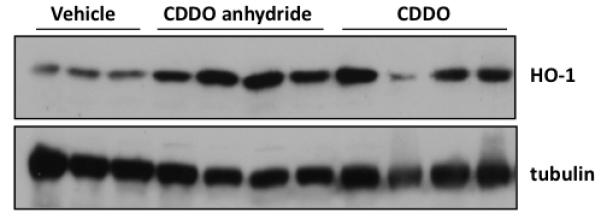
CDDO anhydride 1 and CDDO induce HO-1 in the liver. CD-1 mice (4 per group) were injected i.p. with 2 μmol of CDDO anhydride or CDDO. Six hours later, livers were harvested and homogenized. Lysates were separated by SDS-PAGE, probed with HO-1 antibodies, and developed by ECL. The tubulin blot is a loading control.
To compare the bioavailability of CDDO anhydride and CDDO, we have performed pharmacokinetics studies using mice (i.p. injection4, Table 2). Notably, CDDO anhydride levels in mouse tissues and blood are higher than CDDO levels. Particularly, CDDO anhydride level in plasma is much higher than CDDO level. These data demonstrate that bioavailability of CDDO anhydride is definitely better than CDDO. CDDO was not detected in the tissues and blood of mice that were injected with CDDO anhydride.
Table 2.
Preliminary pharmacokinetics of CDDO anhydride and CDDO in mouse tissues and blood
| CDDO anhydride | CDDO | |
|---|---|---|
| Liver (μmol/kg) | 0.045±0.013 | 0.041±0.035 |
| Lung (μmol/kg) | 0.031±0.009 | 0.018±0.022 |
| Whole blood (μM) | 52±45 | 19±12 |
| Plasma (μM) | 43±29 | 2±1 |
Female CD-1 mice were injected i.p. with 2 μmol of compounds in DMSO–cremophor–PBS (1:1:8). Six hours later, the mice were sacrificed and blood and tissues were collected. Levels were quantified by HPLC/MS using compound added to control blood and tissues for standard.
We have two possible scenarios that would account for the high potency of CDDO anhydride in the three assays (iNOS assay and HO-1 assay in vitro and in vivo). The first one is that CDDO anhydride itself is active. The other possibility is that CDDO anhydride works as a pro-drug of CDDO. The preliminary phamacokinetics studies did not give any evidence that CDDO anhydride is converted to CDDO in tissues and blood. This result would support the former scenario. Lupane anhydride 2 is inactive in two bioassays (iNOS and HO-1 in vitro) while its corresponding acid 3 is active in both. To the contrary, CDDO anhydride is more potent than CDDO in the iNOS assay and is similar to CDDO in the HO-1 assay. The difference between 1 and 2 in potency would support the latter scenario. As we described in the chemistry section, because the yield of 2 is much lower than that of 1 from each corresponding acid, a carboxyl group at C17 of lupane skeleton is considered to be more hindered than that of oleanane skeleton. This speculation would lead us to consider that the conversion of 2 to 3 is much slower than that of 1 to CDDO in the cells. Lupane anhydride 2 is deemed to be too large to affect protein targets. If this is the case, CDDO anhydride 1 is also too large to affect protein targets. However, since 1 is converted to CDDO in the cells and affects protein targets, 1 is seemingly potent. To the contrary, 2 is not converted to 3 in the cells, 2 is inactive. Further pharmacokinetics studies on CDDO anhydride are in progress. Through the studies, it would be clarified which scenario is correct.
In summary, we have found the following interesting features about CDDO anhydride. These features demonstrate that CDDO anhydride is more promising than CDDO.
CDDO anhydride is the first example of oleanane triterpenoid anhydride, which is easily synthesized in good yield from the corresponding acid and acyl chloride
CDDO anhydride is more potent than CDDO in the iNOS assay and is similar in potency to CDDO in the HO-1 assay in vitro. CDDO anhydride is stable in the RAW cell growth medium.
CDDO anhydride is slightly more potent than CDDO for induction of HO-1 in the liver (in vivo)
Notably, CDDO anhydride levels in mouse tissues and blood are higher than CDDO levels. As we expected, the bioavailability of CDDO anhydride is improved in comparison with CDDO.
We have not clarified whether CDDO anhydride works as a pro-drug of CDDO or whether CDDO anhydride itself is potent in the iNOS assay and the HO-1 assay (in vitro and in vivo).
Further preclinical evaluation of CDDO anhydride is in progress.
Acknowledgments
We thank Renee Risingsong and Darlene Royce (Dartmouth Medical School) for expert technical assistance. This investigation was supported by funds from NIH Grant R01-CA78814, from John Zabriskie ’61 Undergraduate Research Fellowship, and from Reata Pharmaceuticals. E.M.P. is a James O. Freedman Presidential Scholar at Dartmouth College. M.B.S. is Oscar M. Cohn Professor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Honda T, Gribble GW, Suh N, Finlay HJ, Rounds BV, Bore L, Favaloro FG, Jr., Wang Y, Sporn MB. J. Med. Chem. 2000;43:1866. doi: 10.1021/jm000008j. [DOI] [PubMed] [Google Scholar]
- 2.Honda T, Rounds BV, Bore L, Finlay HJ, Favaloro FG, Jr., Suh N, Wang Y, Sporn MB, Gribble GW. J. Med. Chem. 2000;43:4233. doi: 10.1021/jm0002230. [DOI] [PubMed] [Google Scholar]
- 3.CDDO was evaluated as an injection despite its low water solubility, because the bioavailability through oral administration is quite poor.
- 4.In preliminary stages, we used i.p. injection instead of p.o. administration because CDDO anhydride 1 is deemed to be readily converted to CDDO in the stomach. In advanced stages, we will use duodenum injection and/or we will administer 1 as an entric coated pill.
- 5.Urban M, Sarek J, Klinot J, Korinkova G, Hajduch M. J. Nat. Prod. 2004;67:1100–1105. doi: 10.1021/np049938m. [DOI] [PubMed] [Google Scholar]
- 6.All new anhydrides provided acceptable HRMS data (±5 ppm) and 1H NMR spectra that exhibit no discernible impurities. CDDO anhydride 1: 1H NMR (CDCl3) δ 8.04 (1H, s), 5.95 (1H, s), 2.88 (1H, s), 1.49 (3H, s), 1.37 (3H, s), 1.25 (3H, s), 1.17 (3H, s), 1.01 (3H, s), 0.99 (3H, s), 0.91 (3H, s); 13C NMR δ 198.3, 196.7, 174.5, 169.1, 165.9, 124.0, 114.8, 114.6, 49.9, 48.8, 47.9, 46.1, 45.2, 42.7, 42.3, 35.8, 34.3, 33.3, 32.0, 31.9, 31.8, 30.8, 27.9, 27.1, 26.8, 25.0, 23.03, 22.96, 21.8, 21.7, 18.4; MS (ESI+) m/z 966 [M+H]+; HRMS (ESI+) calculated for C62H80N2O7 + H 965.6044, found 965.6033. Oleanonic acid anhydride: 1H NMR (CDCl3) δ 5.34 (1H, s), 3.11 (1H, m), 2.84 (1H, m), 2.56 (1H, m), 2.36 (1H, m), 1.49 (3H, s), 1.42 (2H, s), 1.16 (3H, s), 1.09 (3H, s), 1.05 (3H, s), 1.04 (3H, s), 0.94 (3H, s), 0.92 (5H, s), 0.85 (2H, s); 13C NMR (CDCl3) δ 217.9, 173.1, 143.5, 123.0, 55.5, 48.6, 47.7, 47.1, 46.0, 45.9, 42.1, 41.5, 39.6, 39.4, 36.9, 34.4, 33.8, 33.2, 32.4, 31.6, 30.9, 27.7, 26.6, 26.0, 23.8, 23.7, 23.2, 21.7, 19.8, 17.3, 15.2, 8.8; MS (ESI+) m/z 892 [M+H]+; HRMS (ESI+) calculated for C60H90O5 + H 891.6867, found 891.6888. Lupane anhydride 2: 1H NMR (CDCl3) δ 7.82 (1H, s), 4.76 (1H, s), 4.66 (1H, s), 3.02 (1H, m), 2.37 (1H, m), 2.23 (1H, m), 2.00 (2H, m), 1.85 (1H, m), 1.71 (3H, s), 1.27 (3H, s), 1.19 (3H, s), 1.13 (3H, s), 1.12 (3H, s), 1.08 (3H, s), 1.00 (3H, s). MS (ESI+) m/z 938 [M+H]+; HRMS (ESI+) calculated for C62H84N2O5 + H 937.6458, found 937.6471.
- 7.Honda T, Honda Y, Favaloro FG, Jr., Gribble GW, Suh N, Place AE, Rendi MH, Sporn MB. Bioorg. Med. Chem. Lett. 2002;12:1027. doi: 10.1016/s0960-894x(02)00105-1. [DOI] [PubMed] [Google Scholar]
- 8.There are many publications, in which oleanonic acid was isolated from natural sources. For example, Ikuta A, Itokawa H. J. Nat. Prod. 1989;52:623–628.
- 9.Oleanonic acid was prepared in 95% yield from oleanolic acid by Jones oxidation. Oleanonyl chloride, was obtained in 87% yield by chlorination of oleanonic acid with oxalyl chloride in CH2Cl2.
- 10.Honda T, Liby KT, Su X, Sundararajan C, Honda Y, Suh N, Risingsong R, Williams CR, Royce DB, Sporn MB, Gribble GW. Bioorg. Med. Chem. Lett. 2006;16:6306. doi: 10.1016/j.bmcl.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryter SW, Alam J, Choi AM. Physiol. Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]