Abstract
Background:
The majority of new cases of cystic fibrosis (CF) are diagnosed before age 2 years. Diagnoses in older individuals have increased because of improved genetic testing and increased awareness of the disease. A comprehensive description of clinical, genetic, and microbiologic characteristics of adult-age presentation of CF does not exist. We compare newly diagnosed CF in adults with newly diagnosed CF in children and adolescents in the United States.
Methods:
This is a cross-sectional study of new CF diagnoses from the Cystic Fibrosis Foundation Patient Registry between 1995 and 2005. Diagnostic, microbiologic, and clinical features during year of diagnosis were analyzed for subjects by age group. Descriptive statistics were calculated for variables on characteristics by age group.
Results:
A total of 9,766 new diagnoses of CF were reported to the Registry between 1995 and 2005. The proportion of adult diagnoses increased significantly in the years 2001 to 2005 as compared with 1995 to 2000 (9.0% vs 7.7%, P = .012). FEV1% predicted decreased with increasing age at diagnosis (P < .001). Infection with Pseudomonas aeruginosa was most common in adults (P < .001). Both the number of positive sweat chloride tests and prevalence of ΔF508 mutation, the most common mutation in the United States, decreased significantly with older age at diagnosis (P < .001).
Conclusions:
Between 1995 and 2005, the proportion of new diagnoses of CF in adults in the United States increased significantly. Adults present with commonly described CF respiratory disease (Pseudomonas aeruginosa infection and reduced lung function), but have lower sweat chloride values and lower frequency of ΔF508 mutation. Knowledge of clinical characteristics and diagnostic limitations of adult patients presenting with CF will hopefully lead to earlier recognition and intervention.
Cystic fibrosis (CF), a multiorgan genetic disease resulting in recurrent sinopulmonary infection, chronic airways obstruction, and exocrine pancreatic insufficiency was once a disease associated with early childhood death. However, currently 44.6% of the approximately 24,000 patients cared for in the Cystic Fibrosis Foundation (CFF)-accredited Care Center Network in the United States are older than 18 years of age, compared with 33.8% in 1993.1,2 Individuals now survive well into adulthood; predicted survival has increased from 18 years in 1980 to the most recent 2007 estimate of 37.9 years.2 Although median age at diagnosis remains at 6 months, the rate of new diagnoses in individuals older than 18 years has been increasing.3,4
Quantitative pilocarpine iontophoresis for elevated sweat chloride levels remains the primary diagnostic test for CF; however, the commercial availability of extensive genetic testing for mutations in the CF gene (the cystic fibrosis transmembrane conductance regulator [CFTR]) has broadened the diagnostic spectrum of the CF phenotype, allowing diagnosis in individuals with normal or indeterminate sweat test results. This has led to detection of atypical disease with more advanced age at diagnosis. Studies from the United Kingdom, Denmark, Canada, and the United States have shown that patients with CF diagnosed later in life represent a distinct population genetically and clinically.4-7 A recent study by Rodman et al7 described a cohort of 27 patients diagnosed in adulthood with higher lung function, less pancreatic insufficiency, and lower prevalence of Pseudomonas aeruginosa in their sputum, suggesting that adult onset is milder.
To date, a large cohort of patients with CF diagnosed during adulthood has not yet been described genotypically and phenotypically, as all prior publications were single-center studies. The CFF has maintained a national patient registry for >4 decades, tracking demographic, diagnostic, and clinical information of all patients with CF in the United States who receive care at CFF-accredited centers. We wished to characterize adult-diagnosis CF in this large cohort of patients and hypothesized that adults present atypically, with mild respiratory disease, fewer classic CF pathogens in the sputum, higher rates of normal sweat test results, and lower prevalence of ΔF508, the most common mutation overall. We provide here a detailed analysis of patients diagnosed with CF after age 18 years and compare them with those diagnosed as children and adolescents.
Materials and Methods
Study Sample
The CFF Patient Registry was reviewed for individuals in whom CF was initially diagnosed between 1995 and 2005. This time frame was chosen so as to obtain a decade of data from a time when genetic testing was becoming readily available. All subjects or their guardians provided informed consent, approved by their local Institutional Review Board, to allow their data to be submitted to the Registry. Data were obtained from the first entry into the Registry database, which in the majority of individuals reflects the year of diagnosis. Specifically, FEV1% predicted (Hankinson et al8), height, weight, sputum microbiology, pancreatic enzyme use, CFTR genotype, sweat chloride values, transepithelial potential difference testing, and presenting signs/symptoms at diagnosis were collected. Patients with self-report of white were classified into the ethnic group of white. Otherwise patients were grouped as nonwhite, the definition of which encompasses black, Asian/Pacific Islander, Aleut/Eskimo/American Indian, other, and unknown. The study was approved by the Institutional Review Board of Columbia University and the CFF.
Statistical Analysis
χ2 Test and Kruskal-Wallis test were used for group differences in categorical and quantitative variables. Linear models were applied to assess the differences among the age groups at diagnosis in FEV1% measures, with and without control for race, sex, and the year of diagnosis. Spirometry data were compared only for individuals older than 6 years of age, as a very small number of individuals diagnosed before this age had pulmonary function data available. A marginal linear model with repeated measures was applied, controlling for gender, ethnic group, and baseline BMI, to examine the difference in FEV1% predicted change over time after diagnosis. These data are reported as mean and standard error of the mean. Cochran-Armitage tests detected linear trends in proportion of positive sputum cultures by age group. Logistic models were used to examine the association between age at diagnosis and binary outcome (any ΔF508 mutations detected), with and without control for race, sex, and the year of diagnosis. Multinomial logistic models were used for association between categorical outcomes (sweat chloride, zygosity of ΔF508 mutations) and age at diagnosis, with and without control for race, sex, and the year of diagnosis. Analyses were done for three distinct age groups (< 12 years, 12-18 years, and > 18 years). We chose these age bands to purposefully compare the adult age group with adolescents and children. All statistical analyses were performed using SAS 9.1.3 (SAS Institute Inc.; Cary, NC).
Results
Demographics
A total of 9,766 new cases of CF were reported to the CFF Registry between 1995 and 2005, with a mean of 888 new diagnoses per year (range 802-1,000). Age at diagnosis ranged from 0 to 81 years. Of all new diagnoses, 85.6% were in individuals age < 12 years, 6.1% were in individuals aged 12 to 18 years, and 8.3% were in adults (age > 18 years). Adults constituted 7.7% of new diagnoses between 1995 and 2000, and 9.0% of new diagnoses between 2001 and 2005 (P = .012). Median age of adult diagnoses increased from 32 years of age during the period 1995 to 2000 to 34 years of age in years 2001 to 2005. Distribution of gender and race was similar over time; proportion of male cases varied between 49.0% and 53.9%, (P = .459) and proportion of white diagnoses ranged from 89.5% to 93.6%, (P = .058).
Respiratory Disease
Microbiology: Sputum microbiology or nasopharyngeal swab results during the year of diagnosis were available for 8,796 (90%) patients (Table 1). Pseudomonas aeruginosa, specifically the mucoid variant, increased in prevalence as age at diagnosis increased, as did isolation of nontuberculous mycobacteria (NTM) and other less common CF pathogens, such as Aspergillus. Isolation of Staphylococcus aureus was most frequent in those diagnosed between the ages of 12 and 18 years. Prevalence of methicillin-resistant S aureus and Stenotrophomonas maltophilia remained constant across age groups. There was no difference in prevalence of mucoid or nonmucoid P aeruginosa infection in male vs female patients across all age groups (P = .556 and P = .782, respectively).
Table 1.
—Sputum Microbiology in Year of Diagnosis
| Pathogen | Age<12 y | Age 12-18 y | Age>18 y | P Valuea |
| Pseudomonas aeruginosa | 27.1 | 35.4 | 50.7 | <.0001 |
| Mucoid (n = 2,592) | 16.6 | 46.0 | 50.1 | <.0001 |
| Nonmucoid (n = 2,592) | 66.1 | 52.9 | 41.8 | <.0001 |
| Staphylococcus aureus | 43.5 | 63.7 | 43.3 | .002 |
| Aspergillus (any species) | 1.1 | 8.6 | 9.6 | <.001 |
| Stenotrophomonas maltophilia | 5.4 | 6.0 | 5.8 | .554 |
| MRSA | 3.0 | 3.0 | 4.8 | .017 |
| NTM | 0.1 | 1.5 | 4.6 | <.0001 |
| Alcaligenes xylosoxidans | 0.9 | 1.9 | 2.3 | <.0001 |
| Burkholderia cepacia complex | 0.4 | 1.5 | 1.2 | .0001 |
N = 8,796. Sputum microbiology data given as %. MRSA = methicillin-resistant Staphylococcus aureus; NTM = nontuberculous Mycobacterium.
Analysis by Cochran-Armitage test.
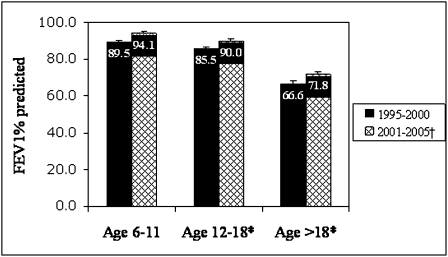
Lung Function: Lung function data were analyzed only for those individuals older than 6 years of age, because only a very small proportion of children younger than 6 years had spirometry measured. Data were available for 2,087 (91%) patients older than age 6 at time of diagnosis. Using linear regression models, initial FEV1% predicted measured during the year of diagnosis decreased as age of diagnosis increased, from a mean of 91.8% predicted in ages 6 to 11 years to 89.6% in ages 12 to 18 years to 69.2% in ages > 18 (P < .001). To determine if year of diagnosis influenced lung function at presentation, we compared FEV1% predicted in those diagnosed between 1995 and 2000 to those diagnosed between 2001 and 2005, and found there was a significant increase in lung function in all age groups over time (Fig 1). There was no significant difference in FEV1% predicted between newly diagnosed male and female patients (P = .575); however, there was a difference between whites and nonwhites (83.2% vs 78.9%, respectively, P = .01).
Figure 1.
FEV1% predicted by age group in years 1995 to 2000 vs 2001 to 2005. *P <.001 for decreasing lung function with increasing age. †P=.003 for higher lung function in latter years. Data remained significant after adjustment for race, gender, and BMI.
Pancreatic Status
A total of 83.4% of all new diagnoses were taking pancreatic enzyme supplementation (and were thus considered to be pancreatic insufficient) at time of diagnosis. Pancreatic insufficiency decreased with age at diagnosis: 88.5% of those aged < 12 years, 63.7% of those aged 12 to 18 years, and 45.2% of those diagnosed at age > 18 (P < .001).
Diagnostic Criteria for CF
Sweat Chloride Values: A total of 8,273 patients (85%) had sweat chloride values reported in the Registry (Table 2). Of those individuals, the proportion with positive chloride values (≥ 60 mmol/L) decreased significantly with increasing age at diagnosis (P < .001). Indeterminate (40-59 mmol/L) and normal tests (< 40 mmol/L) were more likely in patients with older age at diagnosis.
Table 2.
—Positive, Indeterminate, and Normal Sweat Tests by Age Group
| Sweat Test Result | Age <12 y % (No.) | Age 12-18 y % (No.) | Age >18 y% (No.) |
| CF (≥60 mmol/L) | 92.7 (6,548) | 85.5 (470) | 75.8 (498) |
| Indeterminate (40-59 mmol/L) | 3.2 (229) | 10.6 (58) | 13.6 (89) |
| Normal (<40 mmol/L) | 4.1 (289) | 4.0 (22) | 10.7 (70) |
| Unknown | n = 1,291 | n = 45 | n = 157 |
* P <.001 for differences in sweat chloride levels across age groups. CF = cystic fibrosis.
Genotype: A total of 85.8% (8,378) of the 9,766 newly diagnosed cases in the 11-year period had genetic mutations reported. The presence of ΔF508 mutation did not differ by gender (P = .87), but did differ by race (88.7% of whites vs 71.3% of nonwhites, P < .001). Prevalence of the ΔF508 mutation decreased with increasing age at diagnosis. Mutation status is listed in Table 3.
Table 3.
—ΔF508 Mutation Status by Age Group
| ΔF508 Mutation Status | Age<12 | Age 12-18 | Age>18 | Total |
| (n = 7,297) | (n = 426) | (n = 655) | (N = 8,378) | |
| Any ΔF508 mutationa | 89.45 (6,527) | 75.59 (322) | 74.05 (485) | 87.54 (7,334) |
| No ΔF508 mutation | 7.24 (528) | 14.79 (63) | 17.86 (117) | 8.45 (708) |
| Uncertain statusb | 3.32 (242) | 9.62 (41) | 8.09 (53) | 4.01 (336) |
| (n=6,367) | (n=298) | (n=471) | (N=7,136) | |
| ΔF508 Homozygotesa | 58.14 (3,702) | 27.85 (83) | 16.77 (79) | 54.15 (3,864) |
| ΔF508 Heterozygotesa | 33.56 (2,137) | 51.01 (152) | 58.39 (275) | 35.93 (2,564) |
Data presented as % (No.).
Analysis by Cochran-Armitage test, P <.0001.
Uncertain = unknown or not reported.
A total of 73% (7,136) of all newly diagnosed cases were fully genotyped (had two mutations reported). Of these, 54.2% were homozygous for ΔF508, 35.9% were heterozygous for ΔF508, and 9.9% had no ΔF508 mutation on either gene. The distribution of ΔF508 homozygotes differed by ethnic group (55.3% of whites vs 35.7% of nonwhites, P < .001) but not by sex (P = .139). The proportion of ΔF508 heterozygotes increased and the proportion of ΔF508 homozygotes decreased with older age at diagnosis. Seventeen percent of diagnoses after age 18 were identified as ΔF508 homozygotes (Table 3).
Specific non-ΔF508 mutations were more common in adult diagnoses. We list the 10 most commonly reported non-ΔF508 mutations, as the prevalence of the remaining were extremely low (Table 4). All except two (W1282× and L206W) are class IV or V mutations. Class IV and V mutations are less severe mutations. Class I and II mutations are associated with no functional CFTR in the cell membrane, compared with class IV and V, which are typically associated with reduced but detectable and partially functional CFTR in the cell membrane.9 Because of the low number of these rarer mutations, data are reported as only adult vs nonadult age groups.
Table 4.
—Cystic Fibrosis Transmembrane Conductance Regulator Mutation Frequency
| Mutation | Diagnosis ≤ 18 y (n = 7,723) | Diagnosis ≤ 18 y (n = 655) | P Value |
| ΔF508 | 88.7 (6,849) | 74.1 (485) | <.001 |
| R117H | 2.6 (199) | 15.6 (102) | <.001 |
| 3849+10kbC →T | 1.6 (127) | 7.8 (51) | <.001 |
| D1152H | 0.2 (18) | 6.0 (39) | <.001 |
| W1282X | 2.2 (168) | 4.6 (30) | <.001 |
| 2789+5G→A | 0.9 (66) | 3.5 (23) | <.001 |
| R334W | 0.4 (32) | 3.2 (21) | <.001 |
| A455E | 0.5 (37) | 2.4 (16) | <.001 |
| L206W | 0.2 (12) | 1.1 (7) | <.001 |
| I148T | 2.6 (20) | 0.9 (6) | <.013 |
| R347H | 0.2 (12) | 0.9 (6) | <.002 |
Data in first two columns given as % (No.).
Of the 1,493 patients with unknown sweat chloride levels, 1,359 (91%) had two genes identified to make the diagnosis of CF. Only 23 patients had transepithelial nasal potential differences reported (this test is not widely available outside of research centers), of which 14 were done in those without positive sweat tests and without two known mutations. We presume the remaining patients were diagnosed on clinical grounds.
Symptoms Leading to Diagnosis
The CFF registry form provides a list of 13 CF-related diagnoses at the time of presentation. Adults were more likely to present with respiratory symptoms and less likely to present with gastrointestinal symptoms as compared with children. Table 5 lists the five most common presenting symptoms. Unfortunately, we are unable to report frequencies of idiopathic pancreatitis and male infertility, common symptoms leading to diagnosis in adults, as these categories are not specifically captured in the database and would be included in the “other” category.
Table 5.
—Clinical Presentation at Time of Diagnosis
| Clinical Presentation | Age < 12 y | Age 12-18 y | Age < 18 y | P Value |
| Acute respiratory symptoms | 42.4 | 64.2 | 70.6 | <.001 |
| Othera | 3.7 | 11.4 | 18.1 | <.001 |
| Nasal polyps/sinus disease | 2.6 | 19.3 | 16.1 | <.001 |
| Family history | 16.0 | 13.1 | 15.7 | <.18 |
| Steatorrhea/abnormal stools | 24.7 | 23.9 | 14.6 | <.001 |
Data given as %.
This category includes idiopathic pancreatitis, male infertility.
Discussion
Patient Registry data spanning the years 1995 to 2005 demonstrate that patients with CF diagnosed as adults present with typical respiratory features. They have lower lung function and more P aeruginosa infection than individuals diagnosed with CF at a younger age. Additionally, in the years 1995 to 2005 we found a statistically significant increase in adult diagnoses of CF over time, suggesting physicians are broadening their differential when presented with an adult with chronic lung disease. However, patients diagnosed as adults are less likely to be identified by sweat testing or limited genetic analysis, as they are more likely to harbor milder, less common mutations. Patients diagnosed with CF as adults are significantly less likely to have gastrointestinal symptoms and pancreatic insufficiency, diagnoses that are associated with classic CF.
When one compares lung function impairment in newly diagnosed adults (FEV1% predicted of 69.2%) to the general US cohort of adult patients with CF, it is milder; the mean FEV1 reported for all patients with CF ≥ 18 years of age in 2007 was 63.4%. Newly diagnosed adolescents similarly had a higher presenting FEV1% (89.6% vs 88.3% national average), whereas children aged 6 to 11 years had lower lung function at presentation (91.8% vs 95.4% national average).10
The mean FEV1% predicted at time of diagnosis was higher in all age groups during the latter half of the 11-year time period. This perhaps reflects increased clinical acumen with earlier detection of disease in more recent years, with some contribution from earlier diagnosis through newborn screening in the younger patients.
Sputum microbiology has important consequences in CF as some infections, specifically P aeruginosa, are associated with more severe disease and a more accelerated decline in lung function. Gan et al,6 Gilljam et al,4 and Rodman et al7 found decreased incidence of P aeruginosa in patients with a delayed diagnosis of CF in their adult CF centers. In the US cohort described here, P aeruginosa, specifically the mucoid variant, was found at a striking prevalence rate of 50% in adult sputum cultures. This finding highlights the importance of diagnostic testing for CF in patients with unexplained bronchiectasis and growth of P aeruginosa in the airways. The disparity between our finding and those of previous studies may lie in the method of data collection. The requirement of Gan et al6 for chronic infection with P aeruginosa was consecutive positive sputum cultures for 6 months, whereas Gilljam and colleagues4 included data on only two-thirds of their adult patients with CF. In addition to the smaller population studied, a high prevalence of NTM in Rodman’s cohort (their center being an NTM referral center) may explain the lower prevalence of P aeruginosa infection as NTM culture-positive patients with CF have been found to have lower frequency of P aeruginosa.11
Both absence of and heterozygosity for the ΔF508 mutation have been correlated with delayed onset of symptoms, older age of diagnosis, and lower sweat chloride levels.12,13 However, the relationship between disease severity and genotype is not direct, suggesting an influence of extrinsic and intrinsic factors on disease progression.14,15 Homozygosity for ΔF508, a genotype associated with severe lung disease, pancreatic insufficiency, and median survival of 24 years,16 was found in an unexpectedly high number (17%) of patients diagnosed as adults. Recently described non-CF genes that modify phenotype in CF, such as transforming growth factor-β1, may explain how patients with severe class I and II mutations (absent CFTR function) can present later in life with milder lung function.17-19 Patients diagnosed as adults have a higher proportion of the milder class IV or V CFTR mutations, which are associated with present, but decreased, CFTR function. Our findings support earlier findings that presence of these milder mutations corresponds to advanced age at diagnosis.5,15,20,21 and is correlated with normal or indeterminate sweat chloride tests.15,20,22 We surmise that the higher incidence of rare mutations in patients diagnosed as adults may contribute to the delay in diagnosis. In our study, 11% of patients diagnosed as adults with sweat tests reported had normal sweat chloride levels and 13.6% had intermediate levels, thus falling into the previously termed “nonclassic” CF definition.23 We therefore conclude that sweat testing may be inadequate for adult patients in whom there is a suspicion for CF, emphasizing recent guidelines stating that DNA analysis for CFTR mutations be performed for individuals with clinical suspicion of CF but indeterminate sweat chloride results.24 One constraint of the Registry database is the absence of available data regarding duration of symptoms or details regarding previous CF testing. However the severity of disease in adults at time of diagnosis does suggest prolonged symptoms prior to diagnosis.
Our limited analysis of gender differences at time of diagnosis demonstrated no difference between male and female patients in lung function, microbiology, or genotype.25-28 Although CF is mainly a disease of whites, there are important ethnic differences in CF in the United States; nonwhites present with lower lung function at time of diagnosis and are less likely to carry the ΔF508 mutation.
Limitations of this study include use of a multicenter-generated database. Registry information is subject to misclassifications, incomplete records, and data altered to fit into preset categories. Unspecified presenting symptoms, such as male infertility and recurrent unexplained pancreatitis, are likely to be common in adult presentations and lead to diagnoses, although design of the Registry does not allow for capture of these diagnoses. Furthermore, although we assume this sample to be representative of patients with CF in the United States, there remain patients cared for outside of the CF care network. We do not believe the advent of newborn screening programs in the United States affect our data to a great degree, as the majority of states did not implement screening until after 2005. Indeed only about 10% of all new diagnoses in children between 1995 and 2005 were made, at least in part, by newborn screening. Newborn screening is thus most likely to affect those patients presently under the age of 6 years.
In summary, to our knowledge, this is the largest study to date to describe the clinical, microbiologic, and genotypic features of newly diagnosed adults with CF. Often classified as being atypical (normal sweat chloride tests, mild pulmonary involvement, single-organ disease), we found that other than later onset of symptoms, adults commonly present with typical respiratory symptoms, significant lung dysfunction, and infection with mucoid P aeruginosa. Adults are less likely to be pancreatic insufficient, though almost half require enzyme replacement. Twenty-four percent of adults have indeterminate or normal sweat chloride levels and are more likely to be heterozygous than homozygous for ΔF508, although a substantial proportion (17%) carry two ΔF508 mutations.
Investigation into radiographic findings at presentation, progression of lung disease, and mortality rates will give more insight into identification and prognosis of the population of patients diagnosed as adults. The recent institution of newborn screening in the majority of states in the United States may translate to fewer patients remaining undiagnosed through childhood and adolescence, as even individuals with pancreatic sufficiency (and presumably milder disease) may be detected by newborn screening.29 However, there will likely remain a cohort of patients with CF, including those with normal sweat chloride tests, who will escape diagnosis until adulthood. Heightened awareness about the presenting signs and symptoms in adults with CF and knowledge of the limitations of diagnostic testing can lead to more timely diagnosis and ultimately improved health outcomes for these patients.
Acknowledgments
Author contributions: Dr Keating: contributed to the conception and design of the study; acquisition, analysis, and interpretation of the data; and statistical analysis and drafting of the manuscript.
Dr Liu: contributed to acquisition, analysis, and interpretation of the data; and statistical analysis and drafting of the manuscript.
Dr DiMango: contributed to the conception and design of the study; acquisition, analysis, and interpretation of the data; and critical revision of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Other contributions: We thank the Cystic Fibrosis Foundation for providing Patient Registry data, especially Monica Brooks for her kind support. We also thank Dr Lynne Quittell and Dr David Lederer for careful review of the manuscript.
Abbreviations
- CF
cystic fibrosis
- CFF
Cystic Fibrosis Foundation
- CFTR
cystic fibrosis transmembrane conductance regulator
- NTM
nontuberculous mycobacteria
Footnotes
Funding/Support: This work was supported in part by the Cystic Fibrosis Foundation Clinical Research Facilitation Award [C079-CRF06A] and the National Institute of Environmental Health Sciences Center [Grant P30ES09089].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Cystic Fibrosis Foundation Patient Registry 2006 Annual Report. Bethesda, MD: Cystic Fibrosis Foundation; 2008. [Google Scholar]
- 2.Cystic Fibrosis Foundation Patient Registry 2003 Annual Report to the Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2004. [Google Scholar]
- 3.Widerman E, Millner L, Sexauer W, Fiel S. Health status and sociodemographic characteristics of adults receiving a cystic fibrosis diagnosis after age 18 years. Chest. 2000;118(2):427–433. doi: 10.1378/chest.118.2.427. [DOI] [PubMed] [Google Scholar]
- 4.Gilljam M, Ellis L, Corey M, Zielenski J, Durie P, Tullis DE. Clinical manifestations of cystic fibrosis among patients with diagnosis in adulthood. Chest. 2004;126(4):1215–1224. doi: 10.1378/chest.126.4.1215. [DOI] [PubMed] [Google Scholar]
- 5.McCloskey M, Redmond AO, Hill A, Elborn JS. Clinical features associated with a delayed diagnosis of cystic fibrosis. Respiration. 2000;67(4):402–407. doi: 10.1159/000029538. [DOI] [PubMed] [Google Scholar]
- 6.Gan KH, Geus WP, Bakker W, Lamers CB, Heijerman HG. Genetic and clinical features of patients with cystic fibrosis diagnosed after the age of 16 years. Thorax. 1995;50(12):1301–1304. doi: 10.1136/thx.50.12.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodman DM, Polis JM, Heltshe SL, et al. Late diagnosis defines a unique population of long-term survivors of cystic fibrosis. Am J Respir Crit Care Med. 2005;171(6):621–626. doi: 10.1164/rccm.200403-404OC. [DOI] [PubMed] [Google Scholar]
- 8.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 9.Choo-Kang LR, Zeitlin PL, Type I. Type I, II, III, IV, and V cystic fibrosis transmembrane conductance regulator defects and opportunities for therapy. Curr Opin Pulm Med. 2000;6(6):521–529. doi: 10.1097/00063198-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 10.2007 National Patient Cystic Fibrosis Foundation Registry Report to Center Directors. Bethesda, MD: Cystic Fibrosis Foundation; 2008. [Google Scholar]
- 11.Olivier KN, Weber DJ, Wallace RJ, Jr, et al. Nontuberculous Mycobacteria in Cystic Fibrosis Study Group Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–834. doi: 10.1164/rccm.200207-678OC. [DOI] [PubMed] [Google Scholar]
- 12.Johansen HK, Nir M, Høiby N, Koch C, Schwartz M. Severity of cystic fibrosis in patients homozygous and heterozygous for delta F508 mutation. Lancet. 1991;337(8742):631–634. doi: 10.1016/0140-6736(91)92449-c. [DOI] [PubMed] [Google Scholar]
- 13.Kerem E, Corey M, Kerem BS, et al. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (delta F508) N Engl J Med. 1990;323(22):1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 14.Lester LA, Kraut J, Lloyd-Still J, et al. Delta F508 genotype does not predict disease severity in an ethnically diverse cystic fibrosis population. Pediatrics. 1994;93(1):114–118. [PubMed] [Google Scholar]
- 15.The Cystic Fibrosis Genotype-Phenotype Consortium Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med. 1993;329(18):1308–1313. doi: 10.1056/NEJM199310283291804. [DOI] [PubMed] [Google Scholar]
- 16.McKone EF, Goss CH, Aitken ML. CFTR genotype as a predictor of prognosis in cystic fibrosis. Chest. 2006;130(5):1441–1447. doi: 10.1378/chest.130.5.1441. [DOI] [PubMed] [Google Scholar]
- 17.Dorfman R, Sandford A, Taylor C, et al. Complex two-gene modulation of lung disease severity in children with cystic fibrosis. J Clin Invest. 2008;118(3):1040–1049. doi: 10.1172/JCI33754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drumm ML, Konstan MW, Schluchter MD, et al. Gene Modifier Study Group Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353(14):1443–1453. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 19.Arkwright PD, Pravica V, Geraghty PJ, et al. End-organ dysfunction in cystic fibrosis: association with angiotensin I converting enzyme and cytokine gene polymorphisms. Am J Respir Crit Care Med. 2003;167(3):384–389. doi: 10.1164/rccm.200204-364OC. [DOI] [PubMed] [Google Scholar]
- 20.Augarten A, Kerem BS, Yahav Y, et al. Mild cystic fibrosis and normal or borderline sweat test in patients with the 3849 + 10 kb C→T mutation. Lancet. 1993;342(8862):25–26. doi: 10.1016/0140-6736(93)91885-p. [DOI] [PubMed] [Google Scholar]
- 21.Mussaffi H, Prais D, Mei-Zahav M, Blau H. Cystic fibrosis mutations with widely variable phenotype: the D1152H example. Pediatr Pulmonol. 2006;41(3):250–254. doi: 10.1002/ppul.20343. [DOI] [PubMed] [Google Scholar]
- 22.Feldmann D, Couderc R, Audrezet MP, et al. CFTR genotypes in patients with normal or borderline sweat chloride levels. Hum Mutat. 2003;22(4):340. doi: 10.1002/humu.9183. [DOI] [PubMed] [Google Scholar]
- 23.Boyle MP. Nonclassic cystic fibrosis and CFTR-related diseases. Curr Opin Pulm Med. 2003;9(6):498–503. doi: 10.1097/00063198-200311000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Farrell PM, Rosenstein BJ, White TB, et al. Cystic Fibrosis Foundation Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153(2):S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48(8):1041–1049. doi: 10.1016/0895-4356(94)00230-n. [DOI] [PubMed] [Google Scholar]
- 26.Levy H, Kalish LA, Cannon CL, et al. Predictors of mucoid Pseudomonas colonization in cystic fibrosis patients. Pediatr Pulmonol. 2008;43(5):463–471. doi: 10.1002/ppul.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenfeld M, Davis R, FitzSimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145(9):794–803. doi: 10.1093/oxfordjournals.aje.a009172. [DOI] [PubMed] [Google Scholar]
- 28.Verma N, Bush A, Buchdahl R. Is there still a gender gap in cystic fibrosis? Chest. 2005;128(4):2824–2834. doi: 10.1378/chest.128.4.2824. [DOI] [PubMed] [Google Scholar]
- 29.Waters DL, Dorney SF, Gaskin KJ, Gruca MA, O’Halloran M, Wilcken B. Pancreatic function in infants identified as having cystic fibrosis in a neonatal screening program. N Engl J Med. 1990;322(5):303–308. doi: 10.1056/NEJM199002013220505. [DOI] [PubMed] [Google Scholar]