Abstract
Electrolytic lesions of the medial prefrontal cortex (PFCX) were examined using fear conditioning to assess the recall of fear extinction and performance in the Y-maze, open field, and object location/recognition in male and female Sprague–Dawley rats. Rats were conditioned to seven tone/footshocks, followed by extinction after 1-h and 24-h delays, revealing PFCX effects and sex differences during all phases of fear conditioning. In male rats, PFCX impaired 24-h recall of fear extinction to tone, which required the 1-h delay extinction and was not attributed to nonassociative factors. In contrast, sham and PFCX females increased freezing to tone following a 24-h delay, whether or not 1-h delay tone extinction was presented. Moreover, PFCX females failed to extinguish to tone, contrasting to the robust extinction to tone that was observed for sham females, PFCX, and sham males. Also, sex differences were found during acquisition, with sham females acquiring fear conditioning slower than PFCX females. By the last tone-shock presentation, sham and PFCX females showed a slight but significant reduction in freezing to tone relative to those of sham and PFCX males. Of the other behavioral measures, PFCX females maintained exploration of a novel object during object recognition when sham females habituated. PFCX did not influence other behaviors in the remaining tasks. These findings show important sex differences in PFC function, with the PFC influencing the recall of fear extinction in males and contributing to the acquisition and maintenance of fear extinction memory in females, perhaps through altering perseveration.
The prefrontal cortex (PFC) regulates a number of higher cognitive functions, such as behavioral inhibition, attention, working memory, and planning (Squire et al. 2003). Overt lesions to the ventromedial PFC (PFCX) by electrolytic procedures increase perseveration (for review, see Kolb 1984), impair extinction of fear responses (Morgan et al. 1993), and attenuate the recall of fear extinction (Quirk et al. 2000). PFC alterations without obvious cell loss also disrupt PFC function. For instance, chronic stress or glucocorticoid exposure that is sufficient to compromise PFC neurons (Wellman 2001; Silva-Gómez et al. 2003; Cook and Wellman 2004; Cerqueira et al. 2005; Radley et al. 2005, 2006; Izquierdo et al. 2006; Liston et al. 2006) also impairs working memory (Cerqueira et al. 2005), attention set-shifting (Liston et al. 2006), or the recall of fear extinction (Izquierdo et al. 2006; Miracle et al. 2006; Garcia et al. 2008; Baran et al. 2009), as well as enhancing perseveration on recognition memory (McLaughlin et al. 2009). Therefore, PFC cell loss or chronic stress/glucocorticoid-induced dendritic restructuring contributes to altered PFC function.
The connection between the PFC and function is well established in males, but few studies examine the role of the PFC in females, and these reports show mixed findings. Working memory tasks and delayed spatial recognition often improve following treatment with estrogens in rodents (Gibbs and Johnson 2008), aged nonhuman primates (Lacreuse et al. 2002), and menopausal women (Duff and Hampson 2000), but not always. For example, estradiol impairs PFC function in some cases (Wang et al. 2008), which may involve perseverative tendencies (McLaughlin et al. 2009). Other studies found that females were unable to discriminate between relevant and irrelevant information (Toufexis et al. 2007; Nofrey et al. 2008). In our recent study, control females were resistant to fear-conditioning extinction under conditions that extinction occurred in males (Baran et al. 2009). Moreover, sex differences were also observed following chronic stress, with chronic stress impairing recall of fear extinction in males and recall of fear acquisition in females (Baran et al. 2009). A potential variable in this outcome is that females may have difficulty changing their behavior when information appears to interfere with subsequent behavior, as illustrated by latent inhibition (Nofrey et al. 2008). Behaving toward appropriate cues may be confounded in females because they are unable to inhibit fear responses on discrimination tasks (Toufexis et al. 2007). Consequently, PFC function may differ in females and males.
The current study sought to determine the role of the PFC in female rats during acquisition, extinction, and recall of extinguished conditioned fear, using a task that was similar to that used in our previous study (Baran et al. 2009) and used to assess PFCX outcomes in males (Quirk et al. 2000). To investigate sex differences in PFC-mediated behavior, male and female rats underwent either PFCX or sham surgery. PFCX male rats provided a comparison for previous findings seen with this treatment (Quirk et al. 2000). We hypothesized that PFCX and sex will influence acquisition, extinction, and recall of extinction in fear conditioning. Given the dearth of studies using females and to further characterize the effects of PFCX on behavior, we then tested sham and PFCX male and female rats on several other tasks that have been used in past studies following chronic stress and may be influenced, in part, by PFC function, including the Y-maze (Conrad et al. 2004; McLaughlin et al. 2007), object location (Beck and Luine 2002; McLaughlin et al. 2008), and object recognition (Beck and Luine 2002).
Results
Lesion placement
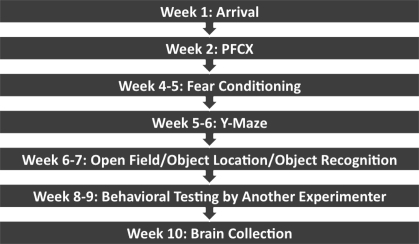
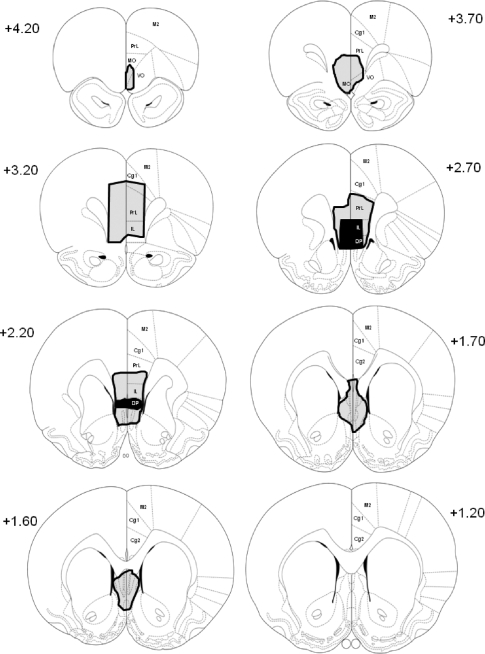
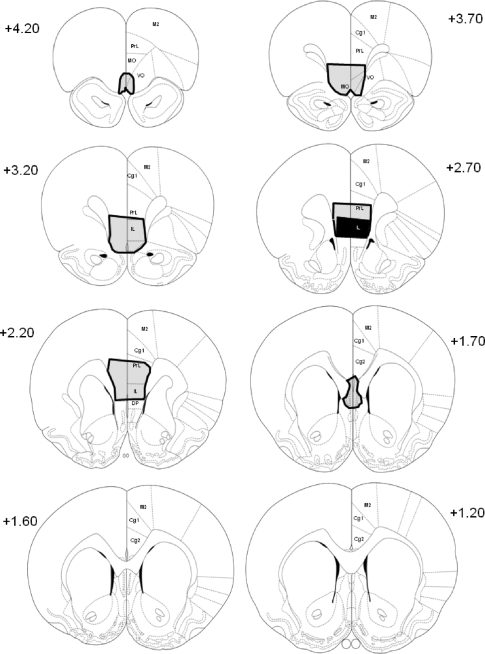
Figure 1 illustrates the time line leading up to brain processing. The ventral part of the medial prefrontal cortex was targeted (Quirk et al. 2000). These lesions included the ventral prelimbic cortex and the infralimbic cortex. Rats with incomplete or misplaced lesions were removed. The final number of female rats in each group after validating PFCX placement was: PFCX (n = 11), sham (n = 8), sham-unpaired (n = 7), and sham-extinction naïve (n = 8). The final number of male rats in each group after validating PFCX placement was: PFCX (n = 12), sham (n = 8), sham-unpaired (n = 5), and sham-extinction naïve (n = 8). The Cavalieri principle (Gundersen et al. 1988; Schmitz and Hof 2005) was used to estimate the total size of the lesions to determine whether sex differences existed in the relative size of the lesions. Lesion size was converted to a ratio of the total size of the region (infralimbic and prefrontal cortices), and one-way ANOVA was performed for sex (male, female) on infralimbic and prelimbic cortices. No significant differences in lesion size were found for either the infralimbic or prelimbic cortices. The average percentage of the infralimbic cortex lesion was 71.5±4.33% in males and 72.9±4.00% in females, and for the prelimbic cortex was 25.9±4.72% in males and 15.7±4.97% in females. The extent of the PFC damage for rats included in the final analysis is shown in Figures 2 and 3 for females and males, respectively.
Figure 1.
Time line of PFCX, behavioral tasks, and brain collection.
Figure 2.
Coronal diagrams of the rat brain showing the extent of the PFCX in female rats that were included in the final analysis. The largest lesion is outlined in black and the smallest lesion is filled in solid black at each level. The infralimbic region was targeted. M2 = secondary motor cortex, Cg1 = anterior cingulate cortex, PrL = prelimbic cortex, MO = medial orbital cortex, VO = ventral orbital cortex, IL = infralimbic cortex, and DP = dorsal peduncular nucleus. The numbers in each diagram represent the distance anterior to bregma in millimeters.
Figure 3.
Coronal diagrams of the rat brain showing the extent of the PFCX in male rats. The largest lesion is outlined in black and the smallest lesion is filled in solid black at each level. The infralimbic region was targeted. M2 = secondary motor cortex, Cg1 = anterior cingulate cortex, PrL = prelimbic cortex, MO = medial orbital cortex, VO = ventral orbital cortex, IL = infralimbic cortex, and DP = dorsal peduncular nucleus. The numbers on the perimeter of each diagram represent the distance anterior to bregma in millimeters.
Sex and PFCX differences in fear conditioning: Habituation and acquisition
Vaginal swabs confirmed that all of the female rats were showing consistent estrous cycles. However, the number of rats in each phase of the estrous cycle on a given test day was too small to use in further analyses. Proestrus and estrus were of particular interest because they represent the period of time when estrogen levels are at their peak and nadir of the cycle, respectively, and are most likely to result in the most behavioral variability (for review, see Korol 2004; Daniel 2006). The number of rats in these two key estrous phases on day 1 of training on fear conditioning were as follows: sham-proestrus (n = 1); PFCX-proestrus (n = 9); sham-estrus (n = 0); PFCX-estrus (n = 2). This imbalance between PFCX and sham females prevents the use of estrous phase as a variable in this study.
During habituation, males and females displayed similar and low levels of freezing to tone. A mixed-factor ANOVA for sex (male, female) and lesion (sham, PFCX) across the five habituation trials revealed no significant main effects or interactions, with freezing to tone near 0%. Thus, males and females responded similarly to tone presentation during habituation (Fig. 4A,D).
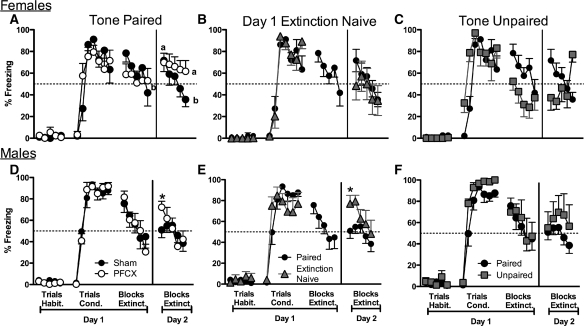
Figure 4.
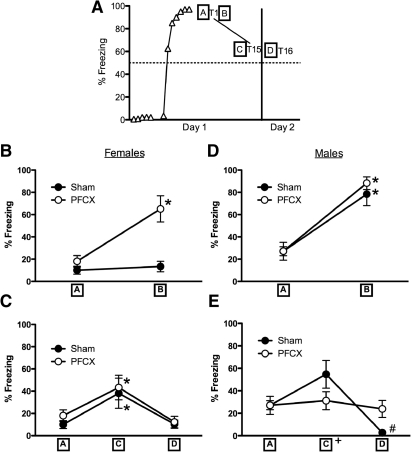
Percentage of freezing to tone during fear conditioning and extinction in female and males rats. (A) Comparison of sham and PFCX females in the tone paired with footshock condition. During conditioning, PFCX females acquired freezing to tone faster than did sham rats. Sham and PFCX females froze more to the tone during the first block of extinction on day 2 than they did during the last block of extinction on day 1. During both extinction sessions, sham females decreased freezing to tone while PFCX females failed to extinguish. (B) Comparison of sham females with and without extinction experience on day 1. Sham females with and without an extinction experience on day 1 froze similarly to tone during extinction day 2. (C) Comparison of sham females in the paired condition to the sham females in the unpaired condition. Sham females in the unpaired condition froze less to tone than did the females in the paired condition during extinction on days 1 and 2. (D) Comparison of sham and PFCX males in the tone paired with footshock condition. PFCX male rats froze significantly more to the tone during the first block of extinction on day 2 than did sham males, despite similar freezing to tone during fear conditioning and extinction on day 1. (E) Comparison of the sham males with and without extinction experience on day 1. Day 1 extinction naïve males froze more to the tone during extinction on day 2 than did the sham males given the paired tones. (F) Comparison of sham males in the paired condition to the sham males in the unpaired condition. Unpaired males froze more to tone than did paired males during acquisition of fear conditioning and also showed a nonsignificant tendency to freeze more to tone than did paired males during extinction on day 2. Data for extinction trials was averaged into five blocks of three trials each for analyses and figures. Habit. = habituation trials; Cond. = conditioning trials; Extinct. = extinction trials. Means with different letters represent statistical significance. *P < 0.05. Data represent means±SEM.
During acquisition of fear conditioning, sex differences and PFCX effects were found. A mixed-factor ANOVA for sex (males, females) and lesion (sham, PFCX) across the seven conditioning trials revealed a significant effect across trials, F(6,210) = 109.74, P < 0.001, and a significant interaction for sex and lesion across trials, F(6,210) = 2.96, P < 0.01, with no other main effects or interactions. Post-hoc tests revealed that all groups increased freezing to tone as trials progressed, but that females (sham, PFCX) showed a slight, yet significant reduction in freezing to tone during the last three trials compared with males (P < 0.05). In addition, post-hoc tests showed that PFCX females froze more to tone than did sham females in the second trial (P = 0.01): Sham and PFCX males displayed statistically similar levels of freezing to the second tone compared with each other and to both groups of females. Therefore, PFCX and sex differences occurred during the initial and final stages of acquisition.
Sex and PFCX differences in fear conditioning: 1-h and 24-h delay extinction
Extinction to tone following the 1-h delay continued to reveal sex and PFCX differences: PFCX and sham males, as well as the sham females reduced freezing to tone, whereas PFCX females maintained freezing to tone during extinction. Data from the 15 extinction trials were averaged into five blocks of three trials for analyses. A mixed-factor ANOVA for sex (male, female) and lesion (sham, PFCX) across the five extinction blocks on day 1 revealed significant effects across blocks, F(4,140) = 19.66, P < 0.001, significant interaction between sex and lesion, F(4,140) = 3.11, P < 0.05, and significant interaction between sex and lesion across blocks, F(4,140) = 3.35, P < 0.05. Post-hoc tests confirmed that PFCX females failed to extinguish by maintaining similar levels of freezing to tone across all five blocks of extinction, while sham females, sham males, and PFCX males decreased freezing to tone (P < 0.01, Fig. 4A,D). Therefore, PFCX females failed to acquire extinction to tone during the first extinction session when the sham females, sham males, and PFCX males exhibited extinction.
Previous research showed significant impairments in recall of fear extinction after a 24-h delay (Quirk et al. 2000; Miracle et al. 2006; Baran et al. 2009), and so freezing to tone in the last block of extinction on day 1 was compared with the first block of extinction on day 2. A mixed-factor ANOVA for sex (male, female) and lesion (sham, PFCX) revealed a significant effect across blocks, F(1,35) = 18.09, P < 0.001, and a significant interaction between sex and lesion across blocks, F(1,35) = 5.23, P < 0.05. Both sham and PFCX females froze significantly more to tone after a 24-h delay (P < 0.05) and at statistically similar levels, despite lower levels of freezing to tone on the previous day (Fig. 4A). In contrast, PFCX male rats froze significantly more to the tone during the first block of extinction on day 2, reaching nearly 75% of freezing to tone during testing than did the sham males (P < 0.05, reaching approximately 50% freezing to tone), despite both conditions (sham, PFCX) showing low (40%), yet similar freezing to tone during the last extinction trial on day 1 (Fig. 4D). Therefore, sex differences were found for how PFCX influenced the recall of fear extinction.
Following a 24-h delay, PFCX females maintained high freezing to tone throughout the second extinction session, while sham females and PFCX males decreased freezing to tone during the same period. A mixed-factor ANOVA for sex (male, female) and lesion (sham, PFCX) across the five extinction blocks on day 2 revealed a significant effect across blocks, F(4,140) = 11.25, P < 0.001, and a significant interaction for sex and lesion across blocks, F(4,140) = 3.95, P < 0.01. Post-hoc tests revealed that sham females and PFCX males exhibited high and similar levels of freezing to tone during the initial presentations, but that these freezing levels significantly declined with repeated tone presentations to levels displayed by the sham males (P < 0.01). In contrast, PFCX females exhibited high freezing to initial tone presentations that was comparable to that shown by sham females and PFCX males, but PFCX females' freezing to tone stayed elevated throughout the repeated tone presentations. Therefore, sex differences were again found, showing that PFCX females failed to extinguish to tone during a second extinction session on day 2, while the three other conditions (sham female, PFCX male, and sham male) exhibited extinction.
Female fear conditioning: Additional control manipulations and measures
Another cohort of sham female rats that received extinction to tone on the second day only (i.e., day 1 extinction naïve) was tested to determine whether extinction experience on day 1 was necessary to observe the level of freezing to tone on day 2. Mixed-factor ANOVAs revealed no significant differences between sham female rats given tone–footshock pairings combined with day 1 extinction to tone (sham) and sham female rats given tone–footshock pairings but without day 1 extinction to tone (day 1 extinction naïve), P > 0.05 (Fig. 4B). Similar levels of freezing to tone during extinction on day 2 were observed whether or not sham female rats were given extinction to tone trials on day 1, revealing that levels of freezing to tone during the first block on day 2 could occur without the experience of the extinction to tone trials on day 1.
To determine whether nonassociative factors influenced conditioning or extinction, we compared freezing to tone in the sham-paired and sham-unpaired females. A mixed-factor ANOVA for test type (paired, unpaired) across the five habituation trials revealed no significant main effects or interactions, P > 0.05. Consequently, tone presentation did not evoke robust freezing during habituation. However, subsequent analyses revealed that sham females in the paired and unpaired conditions froze to tone differently during conditioning: extinction on day 1 and extinction on day 2. A mixed-factor ANOVA for test type (sham, unpaired) across the seven conditioning trials revealed a significant effect for trials, F(6,78) = 17.81, P < 0.001, and a significant interaction for test type across trials, F(6,78) = 3.71, P < 0.01 (Fig. 4C). Post-hoc tests showed the unpaired female rats froze significantly more to tone than did the paired female rats in the first two trials of conditioning (P < 0.05), but then freezing to tone was high and similar by trials three through seven. Moreover, these differences in freezing to tone between the females in the paired and unpaired groups persisted during extinction. A mixed-factor ANOVA for test type across the five extinction blocks on day 1 revealed a significant main effect of test type, F(1,13) = 4.61, P < 0.05, and a significant effect across blocks, F(4,52) = 6.97, P < 0.01, but no significant interaction. During extinction on day 1, unpaired females froze less to tone than did paired females. Moreover, both paired and unpaired females decreased freezing to tone across trials (Fig. 4C).
Lower freezing to tone by the unpaired females extended into the first block of extinction 24 h later. A mixed-factor ANOVA for test type across blocks during extinction on day 2 revealed a significant effect across blocks, F(4,52) = 2.54, P = 0.05, and a significant interaction for test type across blocks, F(4,52) = 2.57, P < 0.05. Notably, the unpaired females froze significantly less to tone in the first block than did the paired females. Interestingly, the unpaired females froze more to tone than did the sham females by the last block (Fig. 4C). Taken together, these results suggest that the freezing to tone by the paired females was due to associative effects between the tone and footshock, as opposed to nonassociative processes.
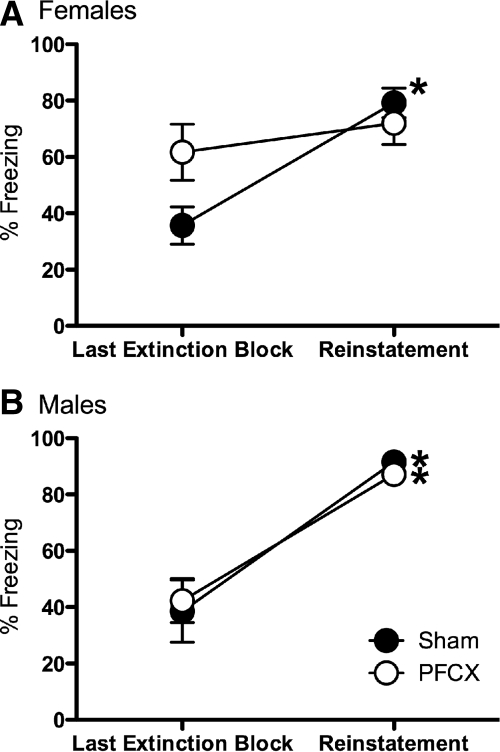
Another test was performed to determine whether freezing to tone reflected associative effects. If extinguished responses were caused by nonassociative factors, then unsignaled footshocks would be ineffective at reinstating freezing to tone. Female rats in the paired groups were given two unsignaled footshocks immediately after the last extinction trial on day 2. A mixed-factor ANOVA for lesion (sham, PFCX) across the last block of extinction on day 2 and the first block following the two unsignaled footshocks revealed a significant effect across blocks, F(1,17) = 19.17, P < 0.001, and a significant interaction for lesion across blocks, F(1,17) = 7.33, P < 0.05. As seen in Figure 5A, freezing to tone increased in the sham females after the unsignaled footshocks. PFCX females showed high freezing in the last extinction block on day 2, which provided very little room to increase further with this reinstatement paradigm.
Figure 5.
Percentage of freezing to tone following two unsignaled footshocks. (A) Sham females significantly increased freezing to tone following the two unsignaled footshocks. Changes in freezing in PFCX females were minimal, in part, because PFCX females maintained high freezing during extinction. (B) Male rats (sham and PFCX) significantly increased freezing to tone following the two unsignaled footshocks. Data represented as mean±SEM. *P < 0.05 compared with the last extinction trial of the same condition.
Freezing to context was measured at various points to examine whether females were showing generalized sensitization effects. A diagram outlining the time points during which contextual freezing was quantified is illustrated in Figure 6A. These time points were selected to capture freezing levels to the context without tone presentation, but in close proximity to the first and last tone presentation during extinction as indicated. First, freezing to context was assessed 30 sec before and 30 sec immediately after the first tone presentation during extinction on day 1. A mixed-factor ANOVA for lesion (sham, PFCX) for contextual freezing before and after the first extinction trial (context represented by A and B) on day 1 revealed a significant effect for context trial, F(1,18) = 88.17, P < 0.001, and a significant interaction for lesion across context trials, F(1,18) = 5.39, P < 0.03 (Fig. 6B). As seen in Figure 6B, PFCX females froze more to context than did sham females immediately after the presentation of the first tone of the extinction trial on day 1, P < 0.05. In contrast, sham females did not show any significant differences in freezing to context prior to and after the first tone presentation in extinction on day 1. Therefore, PFCX females discriminated between the context that was less likely to have a footshock (see point A in Fig. 6A) compared with the context that was most likely to have a footshock (see point B in Fig. 6A), while sham females did not appear to discriminate between these two time points.
Figure 6.
Percentage of freezing to context at designated 30-sec periods during extinction to tone. (A) Diagram outlining the different time points sampled for freezing to context. Triangles represent arbitrary data points of tone presentation. The boxed letter “A” represents the 30 sec before the first tone (T1) presented during extinction on day 1. The boxed letter “B” represents the 30 sec immediately following T1. The boxed letter “C” represents the 30 sec before the last tone presentation (T15) during extinction on day 1. The boxed letter “D” represents the 30 sec before the first tone presentation (T16) on extinction day 2. (B) PFCX females froze significantly more to context than did the sham females after the first tone presentation during extinction (compare context B with context A). Sham and PFCX females froze similarly to context before the first tone presentation during extinction (see context A). (C) As extinction trials progressed on day 1, sham and PFCX females significantly increased freezing to context (see context C), but freezing to context on the second day was low for both groups (see context D). (D) Sham and PFCX males significantly increased freezing to context after the first extinction tone (T1) was presented (compare context B with context A). (E) As extinction trials progressed on day 1, sham males significantly increased freezing to context, while freezing to context was stable for PFCX males (see context C). However, freezing to context on the second day significantly decreased in sham males and remained stable and low for the PFCX males (see context D). Data represent means±SEM. *P < 0.05 compared with context A of the same treatment group. +P < 0.05 compared with context A (collapsed across both sham and PFCX). #P < 0.05 compared with context C of the same treatment group.
We next investigated whether females reduced freezing to context during extinction. Freezing was measured for 30 sec immediately prior to the presentation of the first and last tones of extinction on day 1. A mixed-factor ANOVA for lesion (sham, PFCX) for contextual freezing prior to the first and last extinction trials on day 1 (context represented by A and C in Fig. 6A) revealed a significant effect of context trial, F(1,18) = 5.12, P < 0.05. The notable finding here is that neither sham nor PFCX females extinguished freezing to context on day 1 and instead, significantly increased freezing from ∼18% to ∼40% by the end of extinction on day 1 (compare context A and C in Fig. 6C).
We also investigated whether females froze less to context following the 24-h delay. Freezing was measured for the 30 sec immediately prior to the last tone of extinction on day 1 and the first tone of extinction on day 2 (see points C and D in Fig. 6A). A mixed-factor ANOVA for lesion (sham, PFCX) for contextual freezing (context represented by C and D in Fig. 6C) revealed a significant effect of context trial, F(1,18) = 7.67, P < 0.05. Both sham and PFCX females decreased freezing to context on day 2 compared with the last extinction trial on day 1.
Male fear conditioning: Additional control manipulations and measures
Similar to the females, a cohort of day 1 extinction naïve males were included to determine whether extinction exposure on day 1 was necessary to obtain the level of freezing to tone on day 2. The analysis shows significant differences in freezing to tone between sham males and extinction naïve males. A mixed-factor ANOVA for test type (paired, day 1 extinction naïve) across the blocks of extinction on day 2 revealed a significant main effect of test type, F(1,14) = 4.53, P = 0.05 (Fig. 4E). Day 1 extinction naïve male rats froze more to the tone during extinction on day 2 than did the sham male rats given extinction to tone on day 1. Indeed, freezing levels to the first tone presentation during extinction on day 2 by the day 1 extinction naïve males was greater than the freezing levels to the first tone presentation during extinction on day 2 in the paired males (compare 77% and 51%, respectively). These results indicate that extinction exposure during day 1 was necessary to cause the low freezing levels to tone on day 2.
A cohort of sham males with tone and footshock unpaired was used to determine whether nonassociative effects could have influenced performance. During habituation trials, sham and unpaired male rats froze to tone similarly across the habituation trials. A mixed-factor ANOVA for test type (paired, unpaired) across the five habituation trials revealed no significant main effects or interactions, P > 0.3. Consequently, tone presentations during habituation did not elicit robust freezing in either group. However, the sham male rats in the paired and unpaired conditions showed differences in freezing to tone during the acquisition of fear conditioning. A mixed-factor ANOVA for test type (paired, unpaired) across the seven conditioning trials indicated a significant effect across trials, F(6,66) = 83.87, P < 0.01, and a nearly significant main effect of test type, F(1,11) = 4.12, P = 0.06 (Fig. 4F). Overall, both paired and unpaired groups froze less to tone in the first two trials of conditioning than in trials three through seven. In addition, the paired males froze less to tone than did the unpaired males.
Paired and unpaired males showed similar freezing to tone during extinction on day 1 and 2. A mixed-factor ANOVA for test type (paired, unpaired) across the five extinction blocks on day 1 revealed a significant effect of block, F(4,44) = 4.65, P < 0.01. Post-hoc tests showed that freezing to tone decreased across the extinction blocks on day 1 (Fig. 4F). A mixed-factor ANOVA for test type (paired, unpaired) across the five extinction blocks on day 2 revealed no significant main effects or interactions (P > 0.2), although Figure 4 shows a nonsignificant tendency for unpaired males to show an increase in freezing to tone compared with paired males.
Two unsignaled footshocks following extinction on day 2 were given to determine whether freezing to tone reflected associative or nonassociative factors. A mixed-factor ANOVA for lesion (sham, PFCX) for freezing to tone across the last block of extinction on day 2 and the first block following the two unsignaled footshocks revealed a significant effect of block, F(1,18) = 86.55, P < 0.001. As seen in Figure 5B, sham and PFCX males reinstated freezing to the tone similarly following two unsignaled footshocks, demonstrating that freezing to tone was via associative factors.
As in females, freezing to context was measured at various points to examine whether males were showing generalized sensitization effects. First, freezing to context was assessed 30 sec before and 30 sec immediately after the first tone presentation during extinction on day 1. A mixed-factor ANOVA for lesion (sham, PFCX) for freezing to context before and after the first extinction trial (context represented by A and B) on day 1 revealed a significant effect of context trial, F(1,18) = 122.3, P < 0.001. As seen in Figure 6D, both sham and PFCX males froze more to the context immediately after the termination of the first extinction tone on day 1 than to context prior to the onset of this tone onset. These results indicate that sham and PFCX males discriminated between the contexts that had a higher probability of coinciding with a footshock than the contexts that had a lower probability (compare freezing to context B with context A in Fig. 6D).
We next investigated whether males extinguished freezing to context during extinction. A mixed-factor ANOVA for lesion (sham, PFCX) for contextual freezing prior to the presentation of the first and last tones of extinction on day 1 (context represented by A and C in Fig. 6A) revealed a significant effect of context trial, F(1,18) = 4.47, P < 0.05, but no other significant main effects or interactions (Fig. 6E). As seen in Figure 6E, the effect was carried mostly by the sham males that froze slightly more to context after the extinction experience on day 1 than prior to the first extinction trial.
We also investigated whether males froze less to context after the 24-h delay. A mixed-factor ANOVA for lesion (sham, PFCX) for contextual freezing (context represented by C and D in Fig. 6A) revealed a significant effect of context trial, F(1,18) = 11.62, P < 0.05, and a significant interaction for lesion across context trials, F(1,18) = 6.59, P < 0.05. The most notable finding was that sham males decreased freezing to context from the last trial of extinction on day 1 to the first trial of extinction on day 2, while PFCX males maintained similar and low levels of freezing to context across the 24-h delay (Fig. 6E).
Y-maze
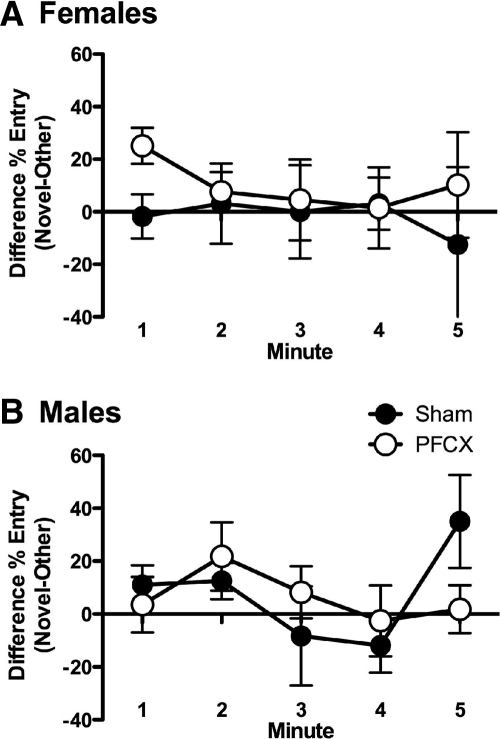
Sham and PFCX females showed similar preference for the novel arm in the Y-maze. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–5 of testing revealed no significant effects, P > 0.1. Extended entries into the novel arm were not observed (Fig. 7A). Wilcoxon nonparametric tests showed that PFCX females entered the novel arm more than the other arm in minute 1, but this effect was not observed in minute 2. Sham rats showed no significant differences between entries into the novel arm over the other arm in either minute 1 or 2.
Figure 7.
Y-maze performance following PFCX. (A) Difference scores during minutes 1 and 2 of Y-maze testing in PFCX and sham female rats were statistically similar. Extended preference for the novel arm was not seen. (B) Difference scores on the Y-maze were statistically similar for sham and PFCX male rats. Extended preference for the novel arm was not seen. Data represent means±SEM.
Sham and PFCX male rats showed similar performance on the Y-maze. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–5 of testing revealed no significant effects, P > 0.1. Extended entries into the novel arm were not observed, suggesting that the PFC does not influence novel arm preference in the Y-maze (Fig. 7B). Wilcoxon nonparametric tests revealed that no significant difference between entries into the novel arm over the other arm was observed in either minute 1 or 2 in sham or PFCX.
Open field
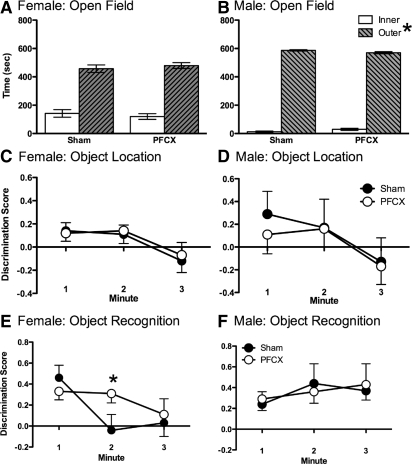
Sham and PFCX female rats spent more time in the outer zone than the inner zone of the open field. A mixed-factor ANOVA for lesion (sham, PFCX) on time spent in each zone of the open field (inner, outer) revealed a significant effect of zone, F(1,17) = 106.76, P < 0.01 (Fig. 8A). No other significant main effects or interactions were found.
Figure 8.
Open field, object location, and object recognition. (A) Sham and PFCX females spent more time in the outer zone than in the inner zone of the open field. Since sham and PFCX were statistically similar, then PFCX did not alter anxiety levels. (B) PFCX and sham females spent similar amounts of time with the moved object across the 3 min of testing. Although there was no statistical difference between groups, the discrimination index, preference for the novel object, was positive for both groups in minutes 1 and 2 and reached chance by minute 3. (C) PFCX females explored the novel object more than the familiar object in minutes 1 and 2, while sham females explored the novel object more than the familiar object in minute 1 only. (D) Sham and PFCX males spent more time in the outer zone than in the inner zone of the open field. Since sham and PFCX were statistically similar, then PFCX did not alter anxiety levels. (E) Sham and PFCX males spent similar amounts of time with the moved object across the minutes of testing. (F) Sham and PFCX male rats spent similar amounts of time with the novel object across the minutes of testing. Although there was no statistical difference between groups, the discrimination index, preference for the novel object, was positive for both groups across all 3 min. Data represent means±SEM. *P < 0.05.
Sham and PFCX male rats spent more time in the outer zone than the inner zone of the open field. A mixed-factor ANOVA for lesion (sham, PFCX) on time spent in each zone of the open field (inner, outer) revealed a significant effect of zone, F(1,18) = 3452.63, P < 0.001 (Fig. 8B). No other significant main effects or interactions were found.
Object location
PFCX and sham female rats spent a similar amount of time with the moved object across the minutes of testing. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–3 on discrimination scores (time with moved object − time with static object/total time with both objects) revealed no significant main effects or interactions (Fig. 8C). Although there was no statistical difference between groups, the discrimination index was positive for both sham and PFCX females in minutes 1 and 2 and then reached chance by minute 3, suggesting that more time was spent with the novel object over the other object during the first 2 min of testing.
Sham and PFCX male rats spent a similar amount of time with the moved object across the minutes of testing. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–3 on discrimination scores revealed no significant main effects or interactions (Fig. 8D). Although the means were in the positive range for minutes 1 and 2, the errors included levels around chance, indicating that interest in the moved object was statistically similar as the static object.
Object recognition
PFCX female rats explored the novel object for a longer duration than did the sham female rats. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–3 revealed a significant effect of minute, F(2,32) = 3.61, P < 0.05 (Fig. 8E). A previous study showed that chronically stressed female rats perseverate on the novel arm of the Y-maze in the first 2 min of testing (McLaughlin et al. 2009). So, a second ANOVA for lesion (sham, PFCX) across minutes 1 and 2 showed a significant interaction between minute and lesion, F(1,16) = 5.53, P < 0.05. PFCX female rats continued exploring the novel object in minute 2 as they did in minute 1, while sham female rats explored the novel object more than the familiar object in minute 1, and then explored both objects similarly in minute 2.
Sham and PFCX male rats spent a similar amount of time with the novel object across the minutes of testing. A mixed-factor ANOVA for lesion (sham, PFCX) across minutes 1–3 revealed no significant main effects or interactions (Fig. 8F). Although there was no statistical difference between groups, the discrimination index, preference for the novel object, was positive for both groups across all 3 min. These data fail to show that PFCX male rats perseverated on the novel object within this time frame.
Discussion
This study is the first to characterize the effect of PFCX in female rats on a fear extinction paradigm and on a battery of behavioral tests. Novel data are presented showing that PFCX females may have difficulty changing their behavior with competing demands, based upon extinction in fear conditioning and the object recognition task. In the fear conditioning paradigm, PFCX females failed to extinguish freezing to tone in both extinction sessions. Moreover, both sham and PFCX female rats froze significantly more to tone during extinction on day 2 than they did to the last tone of extinction on day 1. Additional control groups and testing conditions, such as the object recognition task, helped form the interpretation that females resisted changing their behavior when competing information was present, although this was expressed differently for sham and PFCX females, and is discussed more in the following sections. For the male rats, we corroborate past research showing that PFCX impaired the recall of conditioned fear extinction (Quirk et al. 2000). A reviewer asked whether lesion size in the current study may differ than in prior reports (Quirk et al. 2000; Lebron et al. 2004). While the lesions in the current study appear to extend across the same regions as the previous reports, the disruptions in male PFCX performance are consistent across studies. Moreover, the current study quantified the size of the lesion to show that damage to the PFC was similar between the sexes. Therefore, the PFCX males provide a consistent baseline for comparisons with previous studies and with the females of the current study. Since PFC lesions in the current study were focused within the infralimbic cortex of the PFC and were similar in size within males and females, then these findings support the interpretation that sex differences exist in PFC function, with the PFC contributing to the acquisition and maintenance of fear extinction memory in females through altering perseveration, while influencing the recall of extinction in males.
Impaired recall of fear extinction in females
Both the sham and PFCX females failed to recall the prior extinction session from day 1; however, the PFCX females continued to maintain high freezing levels during day 2 when the sham females reacquired extinction. To understand these results, additional groups and controls were used. The day 1 extinction naïve control group demonstrated that the extinction experience on day 1 was unnecessary, as freezing to tone on day 2 was similar whether or not extinction to tone was given on day 1. Consequently, freezing to tone on day 2 reflected learning related to the acquisition of fear conditioning and not the extinction trials. The control studies also reveal that freezing to tone was not likely caused by nonassociative effects as the unpaired sham females froze to tone significantly less during extinction than the paired sham females, and the sham females increased freezing to tone following the unsignaled footshock. In contrast, the PFCX females did not increase freezing to tone following unsignaled footshock, in part, because freezing levels to the tone by the end of extinction were relatively high, providing little room for freezing to increase further. Indeed, the freezing level of the PFCX females following the unsignaled footshocks was statistically similar to the potentiated freezing level of the sham females following unsignaled footshocks. To interpret these data, freezing to context was measured prior to and immediately after the first tone presentation during extinction: PFCX females froze more to context following a tone than they did to the context preceding the same tone, demonstrating that the PFCX females learned the association between tone and footshock. However, the sham females showed low freezing to context prior to and immediately after the first extinction tone presentation. Overall, sufficient evidence supports the interpretation that freezing to tone represented an associative process in females, with unsignaled footshocks and the unpaired tone-footshock group providing support that associative processes were involved in sham females and the temporal parameters of freezing to context support associative processes in PFCX females.
A possible confound was that disproportionately more females of PFCX were in proestrus (n = 9) at the start of training than were observed for the sham females (n = 1). Given the literature that estrous cycle phase could significantly influence cognition (Warren and Juraska 1997; Conrad et al. 2004; Korol et al. 2004; Luine et al. 2007), then perhaps estrous phase contributed to the current findings for females. We have begun to investigate this question further by using ovariectomized females injected repeatedly with estradiol in order to time the injections with the start of training, but failed to find an effect of estradiol on tone fear conditioning for these parameters (Hoffman et al. 2009). Other reports have also found negligible effects for estrous phase influences on learning (Berry et al. 1997; Stackman et al. 1997). While we acknowledge that the estrous phase may affect cognition, our subsequent study reveals that estradiol fails to influence cued fear conditioning under these behavioral testing parameters, and hence, downplays the influence of the estrous cycle.
PFCX impairs fear extinction recall in males
PFCX in male rats impaired recall of extinction, which corroborates previous research (Quirk et al. 2000). Moreover, our control group that received no extinction training to tone on day 1 supports past studies showing that the low freezing during extinction to tone on day 2 requires a prior extinction experience (Quirk et al. 2000; Baran et al. 2009). To determine whether nonassociative factors influenced performance, a cohort of sham rats was exposed to unpaired tones and footshocks during conditioning. Paired males froze significantly less to tone than unpaired males during acquisition of conditioning, but statistically significant differences were not observed during both extinction sessions. In another measure, however, both sham and PFCX males reinstated freezing to tone similarly after the presentation of two unsignaled footshocks. As a final measure, freezing to context was determined just prior to and immediately after the first tone during extinction to determine whether rats had appropriate temporal freezing. Indeed, both groups of males (sham and PFCX) froze minimally to context before the first extinction tone was presented, and then increased freezing immediately after the first extinction tone finished, indicating that sham and PFCX male rats recognized the association between tone and footshock. Consequently, nonassociative factors were unlikely to have caused the differences between sham and PFCX males, and the data in its entirety support the interpretation that freezing to tone in males was caused by learning the association between the tone and footshock. Thus, these data indicate that the PFC is responsible for recall of fear extinction in males.
PFCX increased perseveration during object recognition
The rats were tested on several tasks including the Y-maze, object location, object recognition, and open field, but PFCX only altered object recognition in females. Specifically, PFCX extended the exploration of a novel object in females, while sham and PFCX male rats showed novel object discrimination similar as each other. These findings suggest that PFCX in females increases perserverative tendencies toward novel objects and corroborates previous research showing that perirhinal cortex lesions, but not PFCX, impair novel object recognition (Barker et al. 2007). No differences were detected in the other behavioral measures. While PFCX was predicted to increase perseveration on the novel arm of the Y-maze in a manner similar to chronically stressed females (McLaughlin et al. 2009), sham females failed to show a novel arm preference, which makes further speculation difficult. These behavioral measures were unlikely attributed to differences in the anxiety profile because the proportion of time spent in the center arena of the open field was unaltered by PFCX.
Sex, the PFC, and extinction
Both males and females with PFCX acquired fear conditioning to tone, but differed in how extinction to tone was processed. The PFC is involved in the recall of fear extinction in males, and appears to be involved in processing competing demands in females. In males, PFCX impairs recall of fear extinction memory (Quirk et al. 2000; this study), which indicates that the PFC processes the recall of fear extinction. A recent study corroborating this assertion showed that activation of the prelimbic region of the PFC modulates expression of extinction memory in male rats, possibly by integrating information from other limbic regions, such as the hippocampus and amygdala (Burgos-Robles et al. 2009). In support of this hypothesis, the PFC is known to regulate competing information that may interfere with memory formation (Costanzi et al. 2009). While disrupting the PFC impairs immediate recall of extinction memory, reacquisition of fear extinction occurs rapidly in males, as shown by the male rats in the current study and others (Quirk et al. 2000; Lebron et al. 2004). The rapid reacquisition of fear extinction in males suggests that the memory for fear extinction remains intact, which is supported by studies that extended extinction training across days (Lebron et al. 2004). In contrast to males, PFCX females fail to acquire fear extinction following a 1-h and 24-h delay, suggesting that the PFC contributes to extinction memory in females. In addition, the slight but significant reduction of fear conditioning that occurred by the last acquisition trial suggests an unstable fear-conditioning process in both sham and PFCX females. This is the first study to examine the impact of PFCX on recall of fear extinction in females, and the data suggest a sex difference in PFC influence over fear extinction memory: The PFC influences the recall of fear extinction in males while affecting the formation/maintenance of fear extinction in females.
An anomalous finding from this study was that sham females showed a different pattern of behavior during fear extinction than the control females from our previous study (Baran et al. 2009). In the present study, sham females acquired extinction on day 1, while the control females in our previous study were resistant to extinction on day 1. The difference between the studies may be attributed to handling procedures. The sham females in the current study underwent daily vaginal swabbing in the hopes of using the phase of the estrous cycle as an independent factor in our dependent variables, whereas the control females in the previous study remained unhandled until behavioral testing. A review of the literature reveals that females show conditioned place preference for vaginal lavage (Walker et al. 2002) and handling could influence radial arm maze performance (Bohacek and Daniel 2007). These studies suggest that vaginal swabbing may have inadvertently influenced fear conditioning to produce differences between control females in current and previous work (Baran et al. 2009). Nonetheless, both of the females were handled in the current study so that handling effects should have occurred similarly in both sham and PFCX females.
In summary, this study demonstrates that the PFC regulates the recall of fear extinction in males, and contributes to the acquisition and maintenance of fear extinction memory in females. These findings corroborate previous work showing that PFCX impairs recall of fear extinction in males (Quirk et al. 2000; Lebron et al. 2004), and are among the first to examine the role of the PFC in the recall of fear extinction in females. The PFC mediates a number of functions and PFCX in males also disrupts fear avoidance in a shuttle box and increases working memory errors on the radial arm maze (Fritts et al. 1998). In females, PFCX slows acquisition of fear extinction, which is not resolved during a second extinction session, and extends exploration of a novel object in the object recognition task. It is notable that in females, PFCX increased preservative behaviors in tone extinction and place recognition, both of which involve learning that does not require the hippocampus (Phillips and LeDoux 1992; Ennaceur et al. 1997). In contrast, PFCX in females did not alter performance in tasks that require hippocampal function, such as contextual conditioning (Phillips and LeDoux 1992), the Y-maze (Conrad et al. 1996), and object placement (Ennaceur et al. 1997). Future studies will need to determine whether hippocampal function is less perturbed by PFC lesions in females. Another consideration is that PFC lesions interfere with the function of brain regions involved in the neuroendocrine and autonomic responses to stress (Gerrits et al. 2003) and/or reversal learning (Churchwell et al. 2009). Together, these findings suggest that the PFC interacts with sex to influence fear extinction and perseverative behaviors, perhaps through nonhippocampal-dependent memory.
Materials and Methods
Subjects
Female and male Sprague–Dawley rats (Charles River Laboratories, Hollister, CA) were housed in light and sound-attenuating chambers on a 12:12 light cycle (lights off at 6 a.m.) according to conditions specified by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Science, National Research Council, 1996). Females weighed approximately 275 g upon arrival with male's age matched to females, but since lesions were performed on rats as they approached 300 g to improve accuracy, the behavioral tasks were performed separately on males and females. Food and water were administered ad libitum. All females were swabbed daily to measure the phase of the estrous cycle to evaluate whether estrous cycle influenced performance (Conrad et al. 2004). However, given the number of days of testing and the tasks being used, we decided against controlling for estrous phase during testing, and instead, hoped that a sufficient number of rats in the same phase of the estrous cycle permitted statistical analysis of fear conditioning. Vaginal swabbing started 5 d after surgery and continued throughout testing. A timeline of the behavioral tasks is shown in Figure 1.
Vaginal swabbing
Vaginal smears were obtained daily (weekends off unless testing occurred on a weekend) throughout the experiment by dipping a sterile swab (0.6-mm diameter, 0.025 in, Fisher Scientific) in sterile saline and then gently swabbing the vaginal lumen. The swabs were smeared onto labeled glass slides that were cleaned with 95% ethanol prior to swabbing. The vaginal lumen cells were fixed with 95% ethanol and then air dried for 15 min before staining with a hematoxylin and eosin stain. The vaginal smears were categorized using an Olympus microscope (40×) according to Evans and Long (1922). Rats in proestrus with the highest estrogen levels show nucleated epithelial cells, while rats in estrus show cornified cells that lack nuclei. While all rats were cycling, the number of rats in each phase on a given testing day was too small to use in further analyses and so estrous phase was not used as a variable in this study.
PFC lesions (PFCX)
After 1 wk of acclimation to the facilities at Arizona State University, rats underwent bilateral PFC electrolytic lesions (PFCX) or sham surgery. Rats were anesthetized with a 1 mL/kg (i.p.) of a cocktail of ketamine (50 mL/kg), xylazine (5 mg/kg), acepromazine (1 mg/kg) in sodium chloride. The head was shaved, disinfected with chlorhexidine surgical scrub (Fort Dodge Animal Health), and secured in a stereotaxic frame (Kopf Instruments). The skull was exposed and the head leveled. Small holes were bilaterally drilled in the skull +2.7 mm anterior, ±0.5 mm lateral, and −5.2 mm ventral relative to bregma (Paxinos and Watson 1998), and a 125-µm Teflon-insulated stainless-steel wire electrode (California Fine Wire) with a 0.5-mm exposed tip was lowered into the infralimbic cortex of the ventromedial PFC. An electrolytic lesion was made by passing a 1.0-mA anodal current through the electrode for 12 sec using a DC stimulator with constant current output (model #83297, Ugo Bastile). For sham-operated rats, the electrode was lowered, but without passing current. The electrode was then removed and bone wax used to fill the holes in the skull. The skin was sutured and an antibiotic ointment was applied. The rats were placed under a heat lamp on clean paper towels until they awoke. After surgery, rats were returned to the housing chamber and were single housed for 2–3 wk of recovery prior to behavioral testing.
Fear conditioning
Apparatus
Rodent fear-conditioning chambers (25 mm d × 29 mm h × 26 mm w: Coulbourn Instruments, E10-18TC) were contained in sound-attenuating cubicles (Coulbourn, E10-23, white). A PC interface card (Coulbourn, L18-16S/C), a universal link (Coulbourn, L91-04S), and Winlinc software (v 1.1, Coulbourn, D91-04) controlled the stimulus presentation. A frequency generator (Coulbourn, E12-01) produced a tone (75 dB, ∼3.0 kHz) through a speaker located in the side panel of the conditioning chamber. The shock (500 msec, 0.5 mA, Coulbourn Animal Shock Generator, E13-14) was a current, equally distributed through a metal grid floor (Coulbourn, E10-18RF). Behavior was videotaped for analysis using a camera (Coulbourn, E27-01) mounted on the ceiling. Infrared lights (Coulbourn, E27-91) located on the side panels of the chamber denoted the onset and offset of the tone, since there was no audio on the videotaped recordings. The infrared lights were undetectable to the rats. A house light (Coulbourn, E11-01) mounted in the side panel illuminated the chamber. The fear conditioning chambers were cleaned with 95% ethanol each time a rat was removed from the chamber.
Procedure
This procedure was adapted from Quirk et al. (2000) and is consistent with our previous work (Baran et al. 2009). In the study by Quirk et al. (2000), both lever pressing and freezing to tone were utilized to assess fear extinction recall. Since both measures accurately represented recall of fear extinction, we incorporated a paradigm that measured freezing only, as done recently (Baran et al. 2009). Fear conditioning was conducted over 3 d, beginning approximately 3 wk after surgery. The transport of rats to the fear-conditioning chamber occurred in the rats’ home cages. The average intertrial interval (ITI) during each phase of fear conditioning was 4 min, with an ITI range of 2 min to 6 min.
On day 0 (acclimation), rats were placed in the fear-conditioning chamber for 10 min. Following acclimation, the rats were returned to the colony room.
On day 1 (habituation, conditioning, 1-h extinction), control and PFCX rats were placed in the fear-conditioning chambers and given five habituation trials consisting of 30-sec tones. Immediately following the habituation trials, seven conditioning trials occurred in which the completion of a 30-sec tone was immediately paired with a footshock. The habituation and conditioning trials lasted approximately 1 h. After the conditioning trials, the rats were transported to the colony room for 1 h before being returned to the chambers. Rats were then given 15 extinction trials consisting of 30-sec tones without footshock. The extinction trials lasted approximately 1 h.
On day 2 (24-h extinction), rats were given another 15 extinction trials. Immediately following these 15 extinction trials, two unsignaled footshocks were administered to reinstate extinguished conditioned responses, followed by another 15 extinction trials. Previous studies show that unsignaled shocks partially reinstate extinguished conditioned responses and that reinstatement quickly extinguishes, which suggests that reinstatement is due to a previously conditioned association instead of a sensitization effect (Rescorla and Heth 1975; Quirk et al. 2000). The trials on day 2 lasted approximately 2 h.
As done in previous work (Quirk et al. 2000), two additional control groups (unpaired and day 1 extinction naïve) received slightly different fear-conditioning procedures and served as controls for potential nonassociative and ceiling effects, respectively. For the unpaired group, the fear-conditioning procedure was the same as described previously for the paired group, except the footshock and tone were never explicitly paired during the conditioning trials. For the day 1 extinction-naïve rats, the habituation and conditioning procedures were the same as previously described for the paired group, but explicit extinction to tone was absent on day 1, although the day 1 extinction-naïve rats were placed in the chamber as was done to the paired and unpaired rats to keep the exposure to the environment consistent. Consequently, all groups spent the same amount of time in the fear-conditioning chambers and received the same number of tones and footshocks during conditioning.
Dependent variable
The dependent variable measured was the number of seconds freezing during each 30-sec tone presentation or freezing during the 30 sec prior to or immediately after tone presentations to measure freezing to context. For extinction trials, freezing to tone was averaged into blocks of three trials. Freezing was defined as the absence of all movements except those associated with respiration (Blanchard and Blanchard 1969; Conrad et al. 1999, 2001; Quirk et al. 2000; Baran et al. 2009). For graphical presentations, freezing duration was converted into a percentage of tone duration.
Y-maze
The Y-maze used in this experiment was similar to that developed by Dellu and colleagues (1992), and was validated as a spatial memory test that requires an intact hippocampus in rats for successful performance (Conrad et al. 1996; Conrad 2006; McLaughlin et al. 2007). The Y-maze was used in this study to determine whether PFCX influences novel arm perseveration in females.
Apparatus
The Y-maze had three identical arms (50 cm l × 16 cm w × 32 cm h), and was made of black Plexiglas. Each arm had slots at the entrance in order to block one arm during training with a piece of black Plexiglas. The bottom was filled with crushed corncob bedding that was mixed between training and testing to reduce the use of odor cues when navigating the maze. Two identical mazes were used that were swapped between the training period and the testing period to eliminate the potential use of inconspicuous visual cues within the maze. Conspicuous cues inside the maze were absent, but extramaze cues of different shapes, sizes, and colors surrounded the maze to help the rats orient themselves spatially. Both training and testing were videotaped for later analysis.
Procedure
During training, one arm of the maze was blocked and designated as the “novel” arm. The rat was randomly placed in one of the two arms that was accessible and allowed to explore both of these arms, named the “start” and “other” arms, for 15 min. At the end of training, the rats were placed back in their home cages. A 4-h interval separated training and testing on the Y-maze. During this interval, the two Y-mazes were swapped to reduce the use of intermaze cues. At the beginning of testing, the rat was placed into the original start location in the room. Spatial memory was assessed by allowing the rat access to all three arms of the maze for 5 min. A rat with intact spatial memory will recognize the novel arm as being previously unvisited and will enter that arm more often than the remaining arms, while rats with spatial memory impairments will visit all arms similarly.
Dependent variables
The dependent variables were the number of entries into each arm of the maze. Entry quantification began when the rat left the start arm. An entry was scored when both forepaws crossed into an arm. Difference scores were calculated by subtracting the percent entry into the novel arm by the percent entry into the other arm. A positive difference score indicates preference for the novel arm, while values near zero indicate chance performance.
Open field and object location/recognition
Object location and object recognition were used in this study to determine whether PFCX would extend the time rats spent with the moved object (object location) or the novel object (object recognition) compared with sham rats.
Apparatus
An open arena (roughly 88 × 88 cm with 60-cm high walls) made of black Plexiglas was used for open field, object location, and object recognition testing. The arena was cleaned with a paper towel and an odor reducer between each trial.
Procedure
The open field and object location/recognition procedures for this experiment were implemented as previously described (McLaughlin et al. 2008). For the open field, rats were placed in the arena and allowed to explore for 10 min. The arena was divided into two sections, the outer edge of the maze 15 cm from the walls (outer annulus) and the center square (inner annulus). Distance traveled in the annuli was measured using Ethovision software (Noldus Instruments).
Object location training and testing occurred 1 d after the open field. The rats were placed in the same open field arena and allowed to explore two similar objects placed equal distances from the north corners (NE or NW). After 3 min, the rats were placed back in their home cage for a 4 h ITI before being placed back in the apparatus for a second trial. The second trial consisted of another 3-min exploratory period with the same two objects; however, one of the objects was moved to one corner of the field (i.e., NW moved to SE or NE moved to SW). The location of the moved object was randomized between the groups. Rats were able to use extramaze cues outside of the open field to orient themselves and determine the new location of the object.
Object recognition testing and training occurred 48 h after object location testing. Preference for objects is not influenced by order of these two tasks, and the interval between the two tasks must be at least 24 h (K Beck, pers. comm.). The same open field arena was used in the same room; however, curtains were hung around the maze to provide new external-maze cues. The rats were placed in the open field arena and allowed to explore two identical, novel objects (different from those used during object location testing) that were placed equal distances from the north corners for 3 min. Then, rats were placed in their home cage for a 4 h ITI. One of the two objects was replaced by a novel object and the rats were allowed to explore the objects for 3 min. The object being replaced was randomized between the groups. Following object recognition, rats were used by another investigator for 2 wk and exposed to additional testing (not reported here) before being returned to us for histological verification.
Dependent variable
The trials were videotaped and the amount of time in seconds spent exploring each object was measured for both object location and object recognition. Exploration was defined as sniffing, touching, or facing the object (within 2 cm), but not when the rat was using the object to prop itself up during rearing or exploring the region around the object. Time spent with the novel or displaced object will be represented as a percentage of the total time investigating both objects in the test trial.
Tissue processing
Within 1 wk of the last day of behavioral testing, PFCX rats were given an overdose of Euthasol (100 mg/kg, sodium pentobarbital) until no longer responding to tail and foot pinch. The brains were removed and placed in 10% formalin for at least 1 wk prior to sectioning. At least 2 d prior to sectioning, the brains were placed in 30% sucrose for cryoprotection. Coronal sections (50 µm) were cut using a micron cryostat (HM 500 OM) at −23°C. The slices were mounted on 0.5% gelatin-chromium subbed slides. Sections were stained using cresyl violet. Damage was traced (13.8×) using a microprojector (2410, Bausch and Lomb) and locations identified using a rat brain atlas (Paxinos and Watson 1998).
A modified procedure from Vogels et al. (1995) was used to estimate the lesion volume in the infralimbic and prelimbic cortices. Sections from each brain were matched to corresponding Figures 8–10 from Paxinos and Watson (1998) that contained the infralimbic cortex in order to represent regions spanning the anterior, middle, and posterior ends. An image of each section was obtained using a Leica DM 4000B digital microscope at 400× magnification (Nuhsbaum). Then each image was overlaid with a grid of horizontal and vertical lines using Adobe Photoshop. The length of each side of the squares in the grid was 0.4 mm. The area of each square of the grid was 0.16 mm2. The volume of each region and lesion was calculated by multiplying the number of grid intersections that overlapped the region or lesion being measured by the area equivalent of each grid intersection (area of a grid square/magnification2) and then multiplying by the section thickness (50 µm). The volumes were then converted into percentages of lesion in each region.
Data analysis
For all parametric tests, mixed-factor ANOVAs with lesion (sham, PFCX) as the independent variable were used. Newman–Keuls post-hoc tests were used when statistical significance was achieved. For nonparametric data in the Y-maze, Wilcoxon nonparametric tests were used. Data in the figures are represented as means±SEM.
Acknowledgments
This work was supported by NIMH 64727 (C.D.C.), a grant from the Institute for Mental Health Research (C.D.C.), and the Arizona Biomedical Research Commission (C.D.C.). Undergraduate support was provided by funds from ASU School of Life Sciences and the Howard Hughes Medical Institute through the Undergraduate Science Education Program (C.E.A. and D.C.N.). We gratefully acknowledge the following individuals: Katie McLaughlin, Michelle Sparks, Gillian Hamilton, Ryan Wright, and especially Drs. Heather Bimonte-Nelson, Janet Neisewander, Federico Sanabria, and Michelle “Lani” Shiota.
References
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD 2009. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem 91: 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Bird F, Alexander V, Warburton EC 2007. Recognition memory for objects, place, and temporal order: A disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27: 2948–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN 2002. Sex differences in behavioral and neurochemical profiles after chronic stress: Role of housing conditions. Physiol Behav 75: 661–673 [DOI] [PubMed] [Google Scholar]
- Berry B, McMahan R, Gallagher M 1997. Spatial learning and memory at defined points of the estrous cycle: Effects on performance of a hippocampal-dependent task. Behav Neurosci 111: 267–274 [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC 1969. Crouching as an index of fear. J Comp Physiol Psychol 67: 370–375 [DOI] [PubMed] [Google Scholar]
- Bohacek J, Daniel JM 2007. Increased daily handling of ovariectomized rats enhances performance on a radial-maze task and obscures effects of estradiol replacement. Horm Behav 52: 237–243 [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ 2009. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci 29: 8474–8482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OF, Sousa N 2005. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci 25: 7792–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP 2009. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci 123: 1185–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD 2006. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cog Neurosci 5: 41–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Galea LAM, Kuroda Y, McEwen BS 1996. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci 110: 1321–1334 [DOI] [PubMed] [Google Scholar]
- Conrad CD, Magariños AM, LeDoux JE, McEwen BS 1999. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113: 902–913 [DOI] [PubMed] [Google Scholar]
- Conrad CD, Mauldin-Jourdain ML, Hobbs RJ 2001. Metyrapone reveals that previous chronic stress differentially impairs hippocampal-dependent memory. Stress 4: 305–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wieczorek L, Baran SE, Harman JS, Wright RL, Korol DL 2004. Acute restraint stress impairs spatial memory in male but not female rats: Influence of estrous cycle. Pharmacol Biochem Behav 78: 569–579 [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL 2004. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol 60: 236–248 [DOI] [PubMed] [Google Scholar]
- Costanzi M, Saraulli D, Rossi-Arnaud C, Aceti M, Cestari V 2009. Memory impairment induced by an interfering task is reverted by pre-frontal cortex lesions: A possible role for an inhibitory process in memory suppression in mice. Neuroscience 158: 503–513 [DOI] [PubMed] [Google Scholar]
- Daniel JM 2006. Effects of oestrogen on cognition: What have we learned from basic research? J Neuroendocrinol 19: 787–795 [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Moal ML, Simon H 1992. A two-trial memory task with automated recording: Study in young and aged rats. Brain Res 588: 132–139 [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E 2000. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav 38: 262–276 [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP 1997. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113: 509–519 [DOI] [PubMed] [Google Scholar]
- Evans HM, Long JA 1922. Characteristic effects upon growth, oestrus and ovulation induced by the intraperitoneal administration of fresh anterior hypophyseal substance. Proc Natl Acad Sci 8: 38–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritts ME, Asbury ET, Horton JE, Isaac WL 1998. Medial prefrontal lesion deficits involving or sparing the prelimbic area in the rat. Physiol Behav 64: 373–380 [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau JL, Deschaux O 2008. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol Learn Mem 89: 560–566 [DOI] [PubMed] [Google Scholar]
- Gerrits M, Westenbroek C, Fokkema DS, Jongsma ME, Den Boer JA, Ter Horst GJ 2003. Increased stress vulnerability after a prefrontal cortex lesion in female rats. Brain Res Bull 61: 627–635 [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA 2008. Sex-specific effects of gonadectomy and hormone treatment on acquisition of a 12-arm radial maze task by Sprague Dawley rats. Endocrinology 149: 3176–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard B, Pakkenberg B, Sørensen FB, Vesterby A, et al. 1988. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96: 379–394 [DOI] [PubMed] [Google Scholar]
- Hoffman A, Armstrong CE, Dille R, Hanna JJ, Nelson C, Huynh T, Conrad CD 2009. Chronic stress facilitates acquisition of fear conditioning in ovariectomized female rats, regardless of 17β-estradiol treatment. Soc Neurosci Abstr 35: 777.13 [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A 2006. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26: 5733–5738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B 1984. Functions of the frontal cortex of the rat: A comparative review. Brain Res 320: 65–98 [DOI] [PubMed] [Google Scholar]
- Korol DL 2004. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem 82: 309–323 [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J 2004. Shifts in preferred strategy across the estrous cycle in female rats. Horm Behav 45: 330–338 [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG 2002. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging 23: 589–600 [DOI] [PubMed] [Google Scholar]
- Lebron K, Milad MR, Quirk GJ 2004. Delayed recall of fear extinction in rats with lesions of ventral medial prefrontal cortex. Learn Mem 11: 544–548 [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS 2006. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 26: 7870–7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Beck KD, Bowman RE, Frankfurt M, MacLusky NJ 2007. Chronic stress and neural function: Accounting for sex and age. J Neuroendocrinol 19: 743–751 [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD 2007. The effects of chronic stress on hippocampal morphology and function: An evaluation of chronic restraint paradigms. Brain Res 1161: 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Bimonte-Nelson HA, Neisewander JL, Conrad CD 2008. Assessment of estradiol influence on spatial tasks and hippocampal CA1 spines: Evidence that the duration of hormone deprivation after ovariectomy compromises 17β-estradiol effectiveness in altering CA1 spines. Horm Behav 54: 386–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD 2009. Sex-specific neuromorphological changes in limbic structures and their functional outcomes. Mol Neurobiol 40: 166–182 [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL 2006. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 85: 213–218 [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE 1993. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett 163: 109–113 [DOI] [PubMed] [Google Scholar]
- Nofrey BS, Ben-Shahar OM, Brake WG 2008. Estrogen abolishes latent inhibition in ovariectomized female rats. Brain Cogn 66: 156–160 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998. The rat brain in stereotaxic coordinates Academic Press, Orlando, FL: [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285 [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K 2000. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci 20: 6225–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WG, Hof PR, McEwen BS, Morrison JH 2005. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exp Neurol 196: 199–203 [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH 2006. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex 16: 313–320 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD 1975. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process 1: 88–96 [PubMed] [Google Scholar]
- Schmitz C, Hof PR 2005. Design-based stereology in neuroscience. Neuroscience 130: 813–831 [DOI] [PubMed] [Google Scholar]
- Silva-Gómez AB, Rojas D, Juarez I, Flores G 2003. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res 983: 128–136 [DOI] [PubMed] [Google Scholar]
- Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ 2003. Fundamental neuroscience Academic Press, San Diego, CA [Google Scholar]
- Stackman RW, Blasberg ME, Langan CJ, Clark AS 1997. Stability of spatial working memory across the estrous cycle of Long–Evans rats. Neurobiol Learn Mem 67: 167–171 [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M 2007. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor α (ERα) and ERβ. J Neurosci 27: 9729–9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogels OJM, Zijlmans JCM, van't Hof MA, Thijssen HOM, Horstink MWIM 1995. MR volume estimation of subcortical brain lesions and ventricular cerebrospinal fluid: A simple and accurate stereological method. Am J Neuroradiol 16: 1441–1445 [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM 2002. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav 73: 743–752 [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL 2008. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behav Neurosci 122: 794–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Juraska JM 1997. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci 111: 259–266 [DOI] [PubMed] [Google Scholar]
- Wellman CL 2001. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. Brain Res 828: 127–134 [DOI] [PubMed] [Google Scholar]