Abstract
Fruit softening in apple (Malus × domestica) is associated with an increase in the ripening hormone ethylene. Here, we show that in cv Royal Gala apples that have the ethylene biosynthetic gene ACC OXIDASE1 suppressed, a cold treatment preconditions the apples to soften independently of added ethylene. When a cold treatment is followed by an ethylene treatment, a more rapid softening occurs than in apples that have not had a cold treatment. Apple fruit softening has been associated with the increase in the expression of cell wall hydrolase genes. One such gene, POLYGALACTURONASE1 (PG1), increases in expression both with ethylene and following a cold treatment. Transcriptional regulation of PG1 through the ethylene pathway is likely to be through an ETHYLENE-INSENSITIVE3-like transcription factor, which increases in expression during apple fruit development and transactivates the PG1 promoter in transient assays in the presence of ethylene. A cold-related gene that resembles a COLD BINDING FACTOR (CBF) class of gene also transactivates the PG1 promoter. The transactivation by the CBF-like gene is greatly enhanced by the addition of exogenous ethylene. These observations give a possible molecular mechanism for the cold- and ethylene-regulated control of fruit softening and suggest that either these two pathways act independently and synergistically with each other or cold enhances the ethylene response such that background levels of ethylene in the ethylene-suppressed apples is sufficient to induce fruit softening in apples.
Apple (Malus × domestica) fruit softening is likely to be controlled by a complex interaction between developmental and environmental factors. The importance of ethylene as a developmental driver for ripening is well known, but the role of environmental stimuli such as cold temperatures is yet to be understood. While flesh softening in apples is highly dependent on ethylene (Johnston et al., 2009; Wang et al., 2009), softening can also partially occur in the absence of ethylene. There is evidence for a strong cold requirement to initiate ethylene-related ripening in some apple and pear cultivars such as Granny Smith and Passe-Crassane, while other apple cultivars such as Royal Gala produce ethylene without prolonged cold exposure (Larrigaudiere et al., 1997; El-Sharkawy et al., 2004). However, it is not known if exposure to cold can initiate ripening independently from ethylene.
Loss of flesh firmness in fleshy fruit is achieved by a suite of cell wall-related enzymes (Goulao and Oliveira, 2008). Reduction in the levels of a single enzyme often has only minor effects on the maintenance of fruit firmness (Sheehy et al., 1988; Smith et al., 1990). Softening in apples is associated with an increase in the expression of a number of cell wall-related genes such as POLYGALACTURONASE1 (PG1), B-GALACTOSIDASE, and XYLOGLUCAN ENDOTRANSGLYCOSYLASE1 (Atkinson, 1994; Goulao and Oliveira, 2007). The best characterized of these genes is PG1. Down-regulation of PG1 expression in tomato (Solanum lycopersicum) had little effect on fruit firmness (Sheehy et al., 1988; Smith et al., 1990), while in strawberry (Fragaria species), suppression of PG led to firmer fruit (Quesada et al., 2009). In apple, PG1 expression levels have been associated with softening patterns in a range of cultivars (Wakasa et al., 2006). Transgenic apple plants overexpressing PG1 have reduced cell-to-cell adhesion in the leaves (Atkinson et al., 2002), and suppression of PG1 results in firmer fruit (Atkinson et al., 2008). While these PG-suppressed apples were firmer than the controls, they were significantly softer than the ACC OXIDASE1 (ACO1)-suppressed apples, suggesting that also in apples a suite of enzymes is required for fruit softening. Fusions of the PG1 promoter to the GUS reporter gene were cloned into tomato, and the first 1.6 kb was found to have an expression pattern corresponding to tomato ethylene fruit ripening, while a larger 2.6-kb promoter did not and was hypothesized to contain an element that caused inhibition of expression (Atkinson et al., 1998).
While the transcription factors that regulate the expression of various apple fruit-ripening events are largely unknown, there is a considerable amount known about both the transcriptional regulation of the ethylene response pathway and the cold response pathway from the model plants Arabidopsis (Arabidopsis thaliana) and tomato (Alonso and Stepanova, 2004; Chen et al., 2005). In these systems, it has been shown that the ethylene signal cascade ultimately leads to stabilization of the transcription factor ETHYLENE INSENSITIVE3 (EIN3; Solano et al., 1998), which has been shown to bind and activate other transcription factors such as ETHYLENE RESPONSE FACTOR1 (ERF1; Solano et al., 1998). In Arabidopsis, the AP2/ERF-like genes belong to a large transcription factor family of 147 genes (Feng et al., 2005; Nakano et al., 2006), several of which are up-regulated by ethylene (Alonso and Stepanova, 2004). This family also includes the key cold induction genes COLD-BINDING FACTOR1 (CBF1) to CBF4, which are known to bind to and activate a number of cold response genes (Stockinger et al., 1997).
While fruit softening in apples has been previously tightly linked to ethylene, there is currently little understanding of the role of cold in this process. This study used the previously published ACO1-suppressed apples (Schaffer et al., 2007; Johnston et al., 2009), which produce levels of ethylene that are insufficient to cause a ripening response in apples (Johnston et al., 2009), to determine the role of cold in fruit ripening in the absence of exogenously added ethylene. Using the ripening-induced cell wall hydrolase, PG1, as a marker for fruit softening, we investigated the transcriptional control of this gene by the EIN3-like (EIL) and AP2 domain-containing transcription factors in response to both cold and ethylene.
RESULTS
Cold Alone Is Sufficient to Induce Apple Fruit Softening
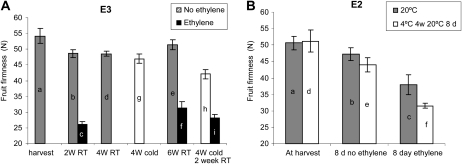
To test the effect of cold on fruit softening, ACO1-suppressed apples (A03 lines described by Schaffer et al., 2007) were treated with combinations of cold and ethylene treatments over three independent harvests (Fig. 1). In all cases, ethylene treatment of the ACO1-suppressed apples induced the greatest change in firmness, irrespective of storage time or temperature. Apples harvested in 2009 (E3; Fig. 1C) were either left at 20°C for a 4-week period or cold stored (0.5°C) for 4 weeks. After this time, the apples that had been cold stored showed no significant difference in firmness compared with the apples stored at room temperature (Fig. 2A, bars d and g). However, cold-treated apples transferred to 20°C for a further 2 weeks softened by a further 7.6 n (36% of softening observed with an ethylene treatment) compared with apples of the same age that had not had a cold treatment (Fig. 2A, bars e and h). This suggests that cold treatment alters the ACO1-suppressed apples in such a way that subsequent storage at 20°C is sufficient to cause fruit softening. Apples that had a 2-week ethylene treatment followed by storage at 0.5°C for 4 weeks were of a similar firmness to fruit that had a 2-week ethylene treatment followed by 4 weeks at 20°C (Fig. 2A, bars f and i).
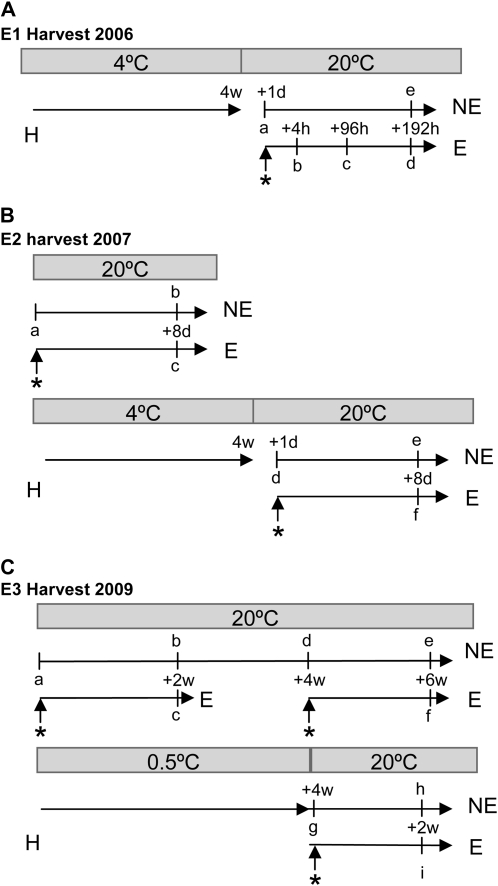
Figure 1.
Postharvest regimes for ACO1-suppressed apples. Asterisks mark the point of 100 μL L−1 ethylene treatment. NE, No ethylene treatment; E, ethylene treatment; H, harvest; w, weeks. Lowercase letters represent sampling points. A, For the 2005 harvest (E1), apples were harvested and stored at 4°C for 1 month before they were warmed to 20°C for 24 h, and half were treated with 100 μL L−1 ethylene. Apple samplings are labeled a to e as follows: a, just before ethylene treatment; b, 4 h after ethylene treatment; c, 4 d (96 h) after ethylene treatment; d, 8 d (192 h) after ethylene treatment; e, 8-d no-ethylene control. Six apples were sampled at a time. B, For the 2007 harvest (E2), apples were sampled either immediately following ethylene treatment or after being store in the cold for 4 weeks. Sample times are labeled a to f as follows, with six apples in each group: a, sampled immediately; b, stored in an ethylene-free environment for 8 d before being sampled; c, sampled after being treated with 100 μL L−1 ethylene for 8 d; d to f, stored at 4°C for 4 weeks and then transferred to 20°C for 1 d as follows: d, sampled immediately; e and f, sampled following an 8-d treatment either with or without 100 μL L−1 ethylene. C, For the 2009 harvest (E3), apples were sampled following a room temperature treatment or a 0.5°C treatment. Sampling times are labeled a to i, with eight apples sampled at each time, as follows: a, sampled immediately; b, d, and e, stored in an ethylene-free environment at 20°C and sampled after 2 weeks of storage (b), after 4 weeks of storage (d), and after 6 weeks of storage (e); c, treated with 100 μL L−1 ethylene for 2 weeks; f, treated with 100 μL L−1 ethylene for 2 weeks after 4 weeks of storage at 20°C; g, h, and i, stored at 0.5°C in an ethylene-free environment for 4 weeks, then g was sampled immediately and h and i were transferred to 20°C and sampled after 2 weeks either with or without treatment with 100 μL L−1 ethylene.
Figure 2.
Flesh firmness (n) of ACO1-suppressed apples following different treatments. A, Harvest E3. Letters represent sampling times, gray bars represent a no-ethylene treatment at 20°C, white bars represent a no-ethylene treatment after a 0.5°C treatment, and black bars represent apples treated with 100 μL L−1 ethylene for 2 weeks (W). RT, Room temperature. B, Harvest E2. Apples were either treated at 20°C (gray bars) or stored at 4°C for 4 weeks before being transferred to 20°C (white bars). Firmness was assessed either immediately or following an 8-d treatment either with or without 100 μL L−1 ethylene. Error bars represent se (n = 6).
Analysis of the firmness of cold-treated ACO1-suppressed apples from the 2007 harvest (E2; Fig. 1B) showed that with a shorter ripening time (8 d instead of 2 weeks), apples prestored at 4°C softened faster with ethylene than ethylene-treated apples that had not been cold stored (Fig. 2B, bars c and f). This suggests, first, that both the E3 ethylene-treated apples and E3 cold- and ethylene-treated apples had reached maximum amounts of softening after 2 weeks, and second, that cold and ethylene have an additive effect on apple fruit softening.
Apple PG1 Is Regulated Late in Fruit Development by Both Ethylene and Cold
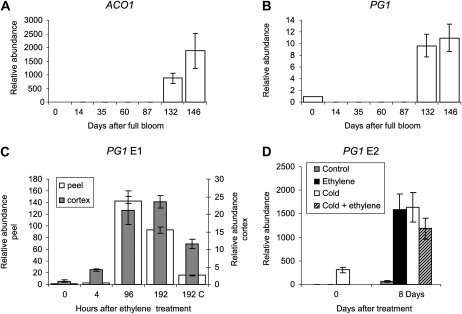
Expression patterns of the cell wall gene PG1 were measured over Royal Gala fruit development (Janssen et al., 2008) using quantitative reverse transcription-PCR (qPCR). There was a small peak of PG1 expression at full bloom, then there was no detectable expression until late in fruit development, 132 d after full bloom (DAFB), that coincided with the up-regulation of ACO1 (Fig. 3, A and B). To assess the effect of ethylene and cold on PG1 expression, PG1 levels were measured in tissue from ACO1-suppressed apple treated as shown for apple harvested in 2005 (E1) and E2 (Fig. 3, C and D). In the E1 harvest, there was a large increase in PG1 expression in both the peel and cortex of ethylene-treated apple and a smaller increase in the 192-h non-ethylene-treated tissue. This suggests that while ethylene is a dominant activator of PG expression, a 4-week, 4°C treatment followed by storage at 20°C is sufficient to increase the level of PG1 expression. Interestingly, although the pattern of expression was similar in the two tissues tested, there was considerably higher expression in the peel tissue than the cortex tissue. PG1 expression in the E2 harvest confirmed that a 4°C cold treatment followed by an 8-d, 20°C treatment in the absence of added ethylene was sufficient to induce or up-regulate transcription of the PG1 gene. The combination of ethylene and cold did not have an additive effect on PG1 expression levels at this time point (Fig. 3D).
Figure 3.
Patterns of gene expression measured by qPCR of either ACO1 or PG1. A and B, ACO1 expression (A) and PG1 expression (B) during fruit development of Royal Gala apples, from open flowers (0 DAFB) to eating ripe (146 DAFB). C, Expression of PG1 in Royal Gala ACO1-suppressed mutants from the E1 harvest at 0, 4, 96, and 192 h of 100 μL L−1 ethylene treatment or 192 h of no-ethylene treatment. White bars represent expression in peel tissue, and gray bars represent expression in cortex tissue. D, Expression analysis of PG1 from the E2 harvest. The gray bar represents apples stored at 20°C, the white bars represent apples stored at 4°C for 4 weeks followed by a 20°C no-ethylene treatment, the black bar represents ethylene-treated apples stored at 20°C, and the hatched bar represents apples treated at 4°C followed by a 100 μL L−1 ethylene treatment. Error bars represent se (n = 4).
Characterization of Ethylene- and Cold-Related Transcription Factors in Apple
To identify potential regulators of PG1, we examined two classes of transcription factors, the EIL genes and the AP2/ERF class of genes. The Arabidopsis EIN3 and EIL protein sequences were compared with six frame translations of nonredundant (NR) contiguous sequence Malus ESTs (Newcomb et al., 2006; Wisniewski et al., 2008). Three NR sequences showed high homology to the EIN3 protein sequence from Arabidopsis, and the clones from the most 5′ EST of each of these were sequenced and translated into the longest open reading frame and compared with the Arabidopsis protein sequences using cluster analysis. These apple EILs showed higher similarity to AtEIN3/AtEIL1 than the other AtEILs, suggesting a gene duplication in apple (Fig. 4A). These were labeled EIL1 to EIL3. A similar method was used to select a second class of transcription factors containing an AP2/ERF domain. This class of transcription factor was chosen because it includes family members involved in both ethylene signal transduction and cold response (Kim et al., 2006; Nakano et al., 2006). Sixty independent apple AP2/ERF genes (named APETELA2 DOMAIN [AP2D] hereafter) were identified, and the DNA-binding domains from each of these AP2D genes were aligned with the DNA-binding domains from the 147 Arabidopsis AP2/ERFs (Supplemental Table S1). The 12 Arabidopsis subgroups described by Nakano et al. (2006) were clearly represented, except subgroup VI, which was separated into two distinct clades (Fig. 4B; Supplemental Fig. S1). Each full-length apple gene was assigned a name and a subgroup based on the clade it was separated into (Table I).
Figure 4.
Phylogenetic clustering. A, Phylogenetic relationship among the six Arabidopsis EIL proteins and three apple EIL proteins. B, Phylogenetic relationship among the 147 Arabidopsis AP2 domain-containing proteins and 60 apple AP2 domain-containing proteins. The previously published Arabidopsis cluster groups are shown. For the full cluster and the number of apple and Arabidopsis genes per group, see Supplemental Figure S1 and Supplemental Table S1.
Table I. Selected genes containing an AP2 binding domain.
| Apple Gene | Subgroup | GenBank Accession No. | Gene Expression Measured | Assayed Transiently |
| AP2D22 | Ib | GU732446 | Yes | Yes |
| AP2D28 | Ib | GU732452 | Yes | Yes |
| AP2D34 | Ib | GU732458 | ||
| AP2D51 | Ib | GU732475 | ||
| AP2D42 | IIa | GU732466 | ||
| AP2D45 | IIa | GU732469 | ||
| AP2D6 | IIb | GU732430 | Yes | Yes |
| AP2D56 | IIb | GU732480 | ||
| AP2D23 | IIIa | GU732447 | Yes | Yes |
| AP2D33 | IIIa | GU732457 | Yes | Yes |
| AP2D16 | IIIb | GU732440 | Yes | Yes |
| AP2D54 | IIIb | GU732478 | ||
| AP2D7/CBF2 | IIIc | GU732431 | Yes | Yes |
| AP2D30 | IIIc | GU732454 | Yes | Yes |
| CBF1 | IIIc | DQ074478 | Yes | |
| AP2D9 | IIIe | GU732433 | Yes | Yes |
| AP2D39 | IIIe | GU732463 | Yes | Yes |
| AP2D44 | IIIe | GU732468 | ||
| AP2D48 | IIIe | GU732472 | ||
| AP2D49 | IIIe | GU732473 | ||
| AP2D38 | IVa | GU732462 | Yes | |
| AP2D21 | Va | GU732445 | Yes | Yes |
| AP2D35 | Va | GU732459 | Yes | Yes |
| AP2D13 | VI | GU732437 | Yes | Yes |
| AP2D20 | VI | GU732444 | Yes | Yes |
| AP2D1 | VIIa | BAF43419 | Yes | Yes |
| AP2D11 | VIIa | GU732435 | Yes | Yes |
| AP2D15 | VIIa | GU732439 | Yes | |
| AP2D24 | VIIa | GU732448 | Yes | |
| AP2D50 | VIIa | GU732474 | ||
| AP2D55 | VIIa | GU732479 | ||
| AP2D5 | VIIIa | GU732429 | Yes | Yes |
| AP2D10 | VIIIa | GU732434 | Yes | Yes |
| AP2D18 | VIIIa | GU732442 | Yes | Yes |
| AP2D25 | VIIIa | GU732449 | Yes | Yes |
| AP2D37 | VIIIa | GU732461 | Yes | Yes |
| AP2D43 | VIIIa | GU732467 | ||
| AP2D52 | VIIIa | GU732476 | ||
| AP2D57 | VIIIa | GU732481 | ||
| AP2D2 | IXa | ACT79399 | Yes | Yes |
| AP2D27 | IXa | GU732451 | Yes | Yes |
| AP2D41 | IXa | GU732465 | ||
| AP2D4 | IXb | GU732428 | Yes | Yes |
| AP2D8 | IXb | GU732432 | Yes | Yes |
| AP2D19 | IXb | GU732443 | Yes | Yes |
| AP2D47 | IXb | GU732471 | ||
| AP2D60 | IXb | GU732483 | ||
| AP2D29 | IXc | GU732453 | Yes | Yes |
| AP2D31 | IXc | GU732455 | Yes | Yes |
| AP2D26 | Xa | GU732450 | Yes | Yes |
| AP2D32 | Xa | GU732456 | Yes | Yes |
| AP2D46 | Xa | GU732470 | ||
| AP2D17 | Xb | GU732441 | Yes | Yes |
| AP2D58 | Xb | GU732482 | ||
| AP2D3 | VI-L | GU732427 | Yes | Yes |
| AP2D14 | VI-L | GU732438 | Yes | Yes |
| AP2D53 | VI-L | GU732477 | ||
| AP2D36 | RAV | GU732460 | Yes | Yes |
| AP2D12 | AP2 | GU732436 | Yes | Yes |
| AP2D40 | AP2 | GU732464 |
EIL and AP2D Expression Analysis
To assess whether these transcription factors were expressed at the same time as PG1, PCR primers that were able to discriminate the three EIL genes from each other were designed and their expression patterns determined during apple fruit development series. EIL1 and EIL3 showed no change in expression during fruit development (Fig. 5A). EIL2, however, showed a greater than 10-fold induction of expression over fruit development (Fig. 5A).
Figure 5.
Expression analysis of EILs and AP2D genes over fruit development in Royal Gala apples, from open flowers (0 DAFB) to eating ripe (146 DAFB). A, EIL1, EIL2, and EIL3. B, Relative expression patterns of 38 apple AP2 domain genes comparing 35 with 132 DAFB. A high ratio represents high expression late in fruit development. The letter a represents undetectable qPCR expression, y represents genes for which the error was too great to provide meaningful information, and n represents genes that were not assayed for this time point. The bar underneath represents the cluster groups for each of the AP2D genes; on this bar, a represents AP2 class genes, b represents RAV class genes, and c represents the VI-L class genes. Error bars represent se (n = 4).
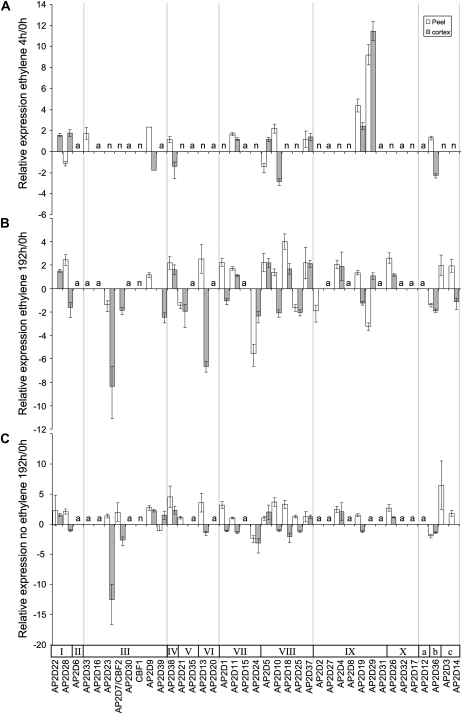
The expression patterns of 38 apple AP2D transcription factors were assessed during fruit development and in response to ethylene treatment. Two time points from fruit development were selected: (1) 35 DAFB, when PG1 expression was very low; and (2) 132 DAFB, when PG1 was first detected (Fig. 2B). Seventeen were predominantly expressed at 35 DAFB compared with 132 DAFB (Fig. 5B), and two were predominantly expressed at 132 DAFB. The remaining genes were either undetectable or showed less than a 2-fold change in expression between these time points. Three or four time points (depending on the gene) were selected to screen for expression changes in the E1 harvest: 0 h, 4 h, 192 h, and 192 h no-ethylene control. These time points were selected as they represent low PG1 (0 h) and high PG1 (192 h, ethylene treatment); the 4-h time point was chosen to identify genes that rapidly respond to ethylene treatment (Fig. 6).
Figure 6.
Relative expression analysis of apple AP2 genes in the E1 harvest of ACO1-suppressed apples that have been induced with ethylene. Values represent expression relative to the 0-h sample. A, Expression after a 4-h, 100 μL L−1 ethylene treatment. B, Expression after a 192-h, 100 μL L−1 ethylene treatment. C, Expression after 192 h in an ethylene-free environment. The genes are arranged in order of cluster groupings. Other features are as described in Figure 5.
For the ethylene-induced experiment (E1), the largest increase in gene expression at 4 h was in subgroup IX, which has been previously associated with increasing expression in response to ethylene such as AtERF1 (AP2D29 and AP2D19; Fig. 6A). After 192 h of ethylene treatment, genes from many of the subgroups had altered expression profiles (Fig. 6, B and C). Most of the changes observed were similar in both the ethylene-treated and the ethylene-untreated tissue, suggesting a predominantly ethylene-independent effect.
Transient Assays
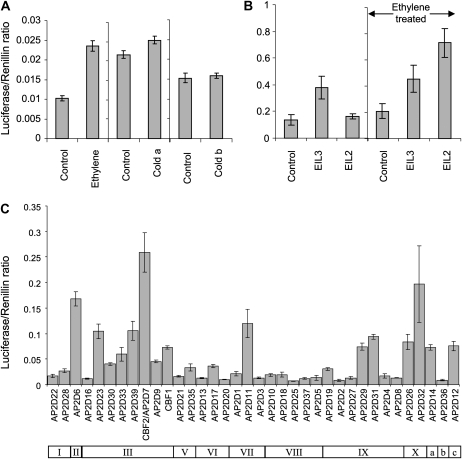
To test if these transcription factors were involved in the regulation of PG1, a transient assay (Hellens et al., 2005) was used to measure the levels of transactivation each transcription factor had on the 2.8-kb PG1 promoter (Atkinson et al., 1998). A fragment of the PG1 promoter, including the ATG start codon and extending 2.8 kb upstream, was amplified from genomic DNA from cv Granny Smith apples, sequenced, and cloned as an ATG fusion into the pLUC 0800 transient assay cassette (Hellens et al., 2005) and named PG1-Luc. The sequence information showed a very high level of similarity with only six polymorphisms in the 2.8-kb fragment between the Granny Smith and previously published Royal Gala PG1 promoter sequences (Supplemental Fig. S2). To ascertain the levels of autoactivation of this promoter, PG1-Luc was infiltrated into the leaves of tobacco (Nicotiana benthamiana) and exposed to either 100 μL L−1 ethylene for 24 h or cold (4°C) for 3 d. Both these treatments showed only a weak transactivation of the luciferase reporter gene (Fig. 7A). Tobacco plants infiltrated with PG1-Luc and subjected to cold for 24 h followed by 2 d at 21°C also showed no significant activation (Fig. 7A).
Figure 7.
Tobacco transient assay of plants infiltrated with PG1-Luc. Transactivation of the promoter is measured as a ratio of the luciferase signal to the renillin signal. A, PG1-Luc-infiltrated plants with or without 100 μL L−1 ethylene for 24 h prior to assay. PG1-Luc-infiltrated plants were kept at either 20°C or 4°C for 24 h prior to assay (cold a) or for 4°C for 24 h followed by 20°C for 2 d (cold b). B, Coinfiltration of PG1-Luc with EIL1 or EIL2 with or without a 24-h, 100 μL L−1 ethylene treatment. C, Coinfiltration of PG1-Luc with 37 different apple AP2 domain-containing genes. The bar underneath represents the cluster groups as defined in Figure 5. Error bars represent se (n = 4).
Two EIL and 36 AP2D transcription factors were cloned, as a fusion to the cauliflower mosaic virus 35S promoter, into a binary vector (Hellens et al., 2005) and coinfiltrated into tobacco with PG1-Luc. EIL3 showed a small but significant transactivation of the PG1 promoter (Fig. 7B). As Arabidopsis EIL proteins are stabilized by ethylene, the transient assay was repeated, but 3 d after agroinfiltration the plants were treated with 100 μL L−1 ethylene. Under these conditions, EIL2 strongly transactivated the PG1 promoter (Fig. 7B). The highest activator of expression was a CBF-like transcription factor (AP2/ERF group III), AP2D7. Neither the closely related CBF1 gene, nor any of the group IX transcription factors, transactivated the PG1 promoter (Fig. 7C).
Cold Regulation of AP2D7/CBF2 in Cell Culture
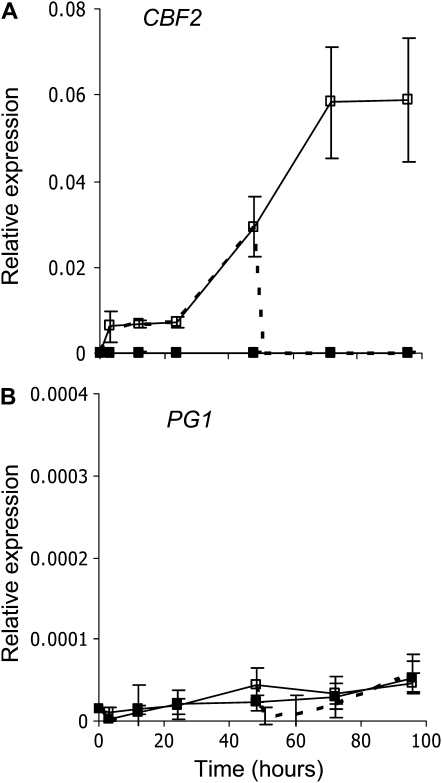
The AP2D7 gene was not up-regulated late in fruit development or by ethylene (Figs. 5 and 6). As AP2D7 was grouped with the Arabidopsis CBF-like proteins, it suggested that this gene might be regulated by cold. To establish whether AP2D7 is transcriptionally regulated by cold, apple cell cultures (Wang et al., 2001) were treated with cold (1°C) or cold followed by a 25°C treatment. Expression analysis revealed that AP2D7 was strongly regulated by cold (Fig. 8). Upon the transfer from cold to 25°C, the transcripts rapidly dropped to background levels within 1 h of transfer (Fig. 8A). PG1 did not increase in expression over this time period, suggesting that other control mechanisms are regulating PG1 (Fig. 8B). Because of the phylogenic proximity to the CBF genes and the rapid increase in expression with cold, we have subsequently named AP2D7 as CBF2.
Figure 8.
qPCR measuring gene expression in apple culture cells grown at 20°C (solid squares), 1°C (open squares), or 1°C for 2 d followed by 20°C (dashed lines). A, CBF-like gene (AP2D7- CBF2). B, PG1. Error bars represent se (n = 4).
There Is a Synergistic Effect of CBF2 and Ethylene
To establish whether EIL2 and CBF2 were acting in the same pathway, transient assays were used to measure the transactivation effect of a combination of the two transcription factors with PG1-Luc. These were performed with and without exogenous ethylene added. It was found that the combination of ethylene and/or EIL2 with CBF2 showed a synergistic transactivation of the PG promoter, suggesting that the CBF2 and the ethylene responses are acting on different parts of the promoter (Fig. 9).
Figure 9.
Tobacco transient assays of PG1-Luc coinfiltrated with a combination of empty vector control (pHex) and CBF2 with and without a 24-h, 100 μL L−1 ethylene treatment. Error bars represent se (n = 4).
DISCUSSION
Previous attempts at quantifying the importance of cold in apple fruit softening have been complicated by the presence of increasing endogenous ethylene production during ripening, making it difficult to determine if ripening responses were due to ethylene, cold, or a combination of these factors. This study circumvented this problem by using ACO1-suppressed transgenic apples that produce no detectable ethylene-related ripening (Johnston et al., 2009). This system allowed the controlled addition of cold to an apple system devoid of ethylene-related ripening. The results from this study demonstrate the role of cold in modulating fruit softening. However, while cold is a contributing factor to fruit softening, it produces significantly less softening than ethylene.
From this research, there are three models that could explain the action of cold in relation to fruit ripening: model 1 would hypothesize that cold is acting independently of ethylene; model 2 that cold is enhancing the ethylene response to such an extent that the fruit is responding to much lower levels of ethylene that may still be present in the ACO1-suppressed apples; and model 3 that cold increases the concentrations of ethylene (El-Sharkawy et al., 2004). Using transient assays, we have shown that the promoter of PG1 transactivates the promoter of both genes homologous to ethylene signal transduction transcription factors (EIN3) and a cold-regulating transcription factor that clusters in the CBF family (Stockinger et al., 1997). Because of the nature of the transient assays, it is possible that overexpressing a key regulatory transcription factor activates an endogenous signaling pathway in the host plant. Thus, transactivation does not show direct binding but can point to the role of upstream transcription factors that are involved in the regulation of a gene. For example, there is a possibility that CBF2 is acting through the up-regulation of an endogenous tobacco EIL that then transactivates PG1. However, when CBF2 is coinfiltrated with the PG1 promoter followed by an ethylene treatment, there is an enhanced transactivation (Fig. 9), suggesting that EIL and CBF2 are acting independently of each other, consistent with model 1. However, it is possible that the other two models are contributing to fruit softening. With recent technical advances, it is now possible to detect ethylene below 1 nL L−1. These studies suggest that very low levels of ethylene may play an important role in plant development (Thain et al., 2004). Using a less sensitive detection system, only background levels of ethylene have been detected in the ACO1-suppressed apples. While the independent regulation of softening model best fits the molecular data, there is the possibility that the apples become more sensitive to very low levels of ethylene during a cold treatment and the softening observed is due to the response of fruit to a basal level of ethylene production, or that there is a slight increase in ethylene production through an ACO that is independent of ACO1. However, it has been shown that cold activation of ethylene in cv Braeburn apples is mediated at least in part through an increase in both ACS and ACO1 expression (Tian et al., 2002), making it likely that these two genes are contributing to the cold-induced ethylene effect seen in apples and therefore are not contributing to the softening responses observed in this study.
In Arabidopsis, CBF2 expression is strongly cold regulated, with an increase in transcript detectable within 1 h of cold treatment and loss of transcript upon removal from cold treatment. In apple tissue culture cells, the up-regulation of CBF2 does not switch on PG1, suggesting that there may be other factors such as a developmentally regulated inhibitor of PG1 expression that also influences CBF2 action. This is consistent with the observation of an inhibition element in the promoter between −1,460 and −2,356 (Atkinson et al., 1998). While the data presented here show that expression of CBF2 is not sufficient for PG1 expression, in a transient system CBF2 can transactivate the PG1 promoter, and this transactivation is enhanced by ethylene.
During late fruit development, as the apple matures there is an increase in sensitivity to ethylene. Sensitivities to ethylene may be brought about by reducing the numbers of ethylene receptors (Chen et al., 2005) that negatively regulate the ethylene response. However, overexpression of EIN3 gives an enhanced triple response in Arabidopsis (Chao et al., 1997), suggesting that ethylene sensitivity itself is modulated through levels of EIN3 expression. Here, we find that, in apple, EIL2 increases in expression through fruit development, suggesting that this may contribute to ethylene sensitivity during ripening. This is unlike the EIL genes in tomato, Arabidopsis, and kiwifruit (Actinidia deliciosa), where EIN3 transcripts have a constant expression level (Chao et al., 1997; Tieman et al., 2001; Guo and Ecker, 2003). However, this is not unique; in banana (Musa acuminata), an EIL gene shows an increase in transcript accumulation during fruit development (Mbeguie-A-Mbeguie et al., 2008).
The EIL transactivation of the PG1 promoter suggests either a direct binding or binding through an ERF-independent pathway. When the PG1 promoter sequence is analyzed using the motif finder in PLACE (Higo et al., 1999), there are two putative PERE binding sites 538 and 903 bp from the ATG start; these are the minimal motifs thought to be necessary for regulation by EIL genes (Kosugi and Ohashi, 2000) rather than the longer inverted palindrome reported by Solano et al. (1998). If the transactivation by the EILs is acting through an endogenous tobacco intermediate, then it is unlikely to be a member of the group IX ERF-like genes. In our assay, none of the apple genes in this cluster transactivated the PG promoter, even though some of these genes were up-regulated by ethylene (Fig. 6).
During the late summer ripening period of apple, it is conceivable that apple relies on both internal and environmental cues, such as temperature, to time its developmental processes. While apple softening is highly dependent on ethylene, here we have shown that a cold effect can also modulate fruit softening in cv Royal Gala. By applying a molecular approach to identify how temperature may be modulating fruit softening, we have found that both ethylene- and cold-related transcription factors were able to transactivate the cell wall gene PG1. These results support a model where both cold and ethylene signals appear to coordinate the ripening process. The combination of these signals enhances ripening. Using both these signals would allow plants to shorten ripening time in colder autumns, allowing fully edible fruit to be produced before freezing temperatures arrive.
MATERIALS AND METHODS
Fruit Growth and Ripening Treatments
Transgenic apple (Malus × domestica) cv Royal Gala containing an antisense ACO1 construct (A03 lines; Schaffer et al., 2007) was grown in greenhouse conditions. For the fruit development analysis, tissue from orchard-grown Royal Gala apple was used as described by Janssen et al. (2008). Following harvest, apples were stored in 340-L bins containing Purafil (Multimix MM-1000; Circul-Aire) to absorb ethylene and lime to absorb CO2. The ambient ethylene concentrations were regularly tested by gas chromatography as described by Johnston et al. (2009). For ethylene treatment, 100 μL L−1 ethylene was injected into the fruit-ripening bins containing lime to absorb CO2, and air was continuously circulated. Ethylene concentrations were tested and adjusted as necessary. Fruit firmness was measured as described by Johnston et al. (2009).
Harvesting Regimes
ACO1-suppressed transgenic Royal Gala apples (Schaffer et al., 2007) from 3 years were assessed: 2005 harvest (E1), 2007 harvest (E2), and 2009 harvest (E3; Fig. 1). The E1 apples were harvested and stored in 4°C for 1 month before they were warmed to 20°C for 24 h, divided into five groups of six apples each, and all but one group (used as a no-ethylene, 8-d control) were treated with ethylene. These apples had RNA extracted 4 h, 4 d, and 8 d following treatment; for full details, see Schaffer et al. (2007). The E2 apples (Fig. 1B) were randomly divided into six batches of six apples labeled a to f; a was assessed for firmness at harvest, b was stored in an ethylene-free environment for 8 d before being measured for firmness, and c was treated with 100 μL L−1 ethylene for 8 d before being measured for firmness. Batches d to f were stored at 4°C for 4 weeks and then transferred to 20°C for 1 d; d was measured for firmness; e and f were either not treated or treated with 100 μL L−1 ethylene, respectively, and then assessed for firmness after 8 d of treatment. The E3 harvest (Fig. 1C) apples were randomly separated into nine batches of eight apples labeled a to i. Batch a was assessed for firmness at harvest, and b, d, and e were kept at 20°C in an ethylene-free environment containing Purafil (Multimix MM-1000; Circul-Aire); b was assessed after 2 weeks of storage, d after 4 weeks of storage, and e after 6 weeks. Batch c was immediately treated with 100 μL L−1 ethylene at 20°C for 2 weeks and assessed for firmness, and f was treated with 100 μL L−1 ethylene for 2 weeks after a 4-week, 20°C storage. Batches g, h, and i were stored at 0.5°C in an ethylene-free environment for 4 weeks, and g was assessed for firmness in the cold; h and i were transferred to 20°C, h was left at 20°C and i was treated with 100 μL L−1 ethylene, and both were assessed following a 2-week treatment period.
AP2/ERF Gene Selection
Apple AP2/ERF family members were identified by comparing representatives of the 12 Arabidopsis (Arabidopsis thaliana) AP2/ERF DNA-binding domain subgroups (Nakano et al., 2006) with apple NR sequences as well as the AP2-like genes and RAV-like genes (Feng et al., 2005; Kim et al., 2006; Nakano et al., 2006). In total, 127 apple NR sequences representing 1,370 ESTs were identified using BLASTX with a P value of less than E-05. Eighty-seven of these were represented by at least one EST (Newcomb et al., 2006). The most 5′ EST from this collection was identified, and the representative clone was fully sequenced. One clone was not recoverable, 10 were collapsed into existing NR sequences, and 12 were deemed not to be full length. Sixty-two full-length sequences were found to code for a Met (ATG) that was at a similar location to the start point of the closest Arabidopsis homolog. Two genes showed DNA sequence identity to other sequenced cDNAs at greater than 97% and were assumed to be allelic. The remaining full-length sequences were labeled AP2D1 to AP2D60. AP2D59 was shown to be homologous to the already submitted gene CBF1 and was changed to CBF1. AP2D1 and -2 were named after the previously published MdERF1 and -2, respectively (Wang et al., 2007).
qPCR
For expression analysis, both new and previously published cDNAs were used. cDNAs from a fruit development series (Janssen et al., 2008) and the E1 harvest regime (Schaffer et al., 2007) were used. For new cDNA, RNA was extracted and cDNA was synthesized as described by Schaffer et al. (2007). qPCR was conducted on a LightCycler 480 (Roche) using the LightCycler 480 SYBR Green 1 Master kit (Roche). Each time point was replicated four times, and a 10-μL reaction comprising 5 μL of Mastermix, 2 μL of 5 μm primers (forward and reverse), and 3 μL of cDNA was used. All expression was normalized to an apple actin gene described by Espley et al. (2007). qPCR primers used are shown in Supplemental Table S2.
Promoter Isolation
Genomic DNA was isolated from cv Granny Smith apple using the DNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. Primers PRM4 (5′-TGGTGTCCGTGTATGAAGGATAAGCCCTAG-3′) and RJS019 (5′-AAACTATTTGGACCATTCCGAGCAAGTCTATC-3′) were used to amplify the 2.8-kb PG1 promoter region from this genomic DNA. A 2.6-kb fragment, modified from the ATG start codon to incorporate a NcoI restriction site, was then amplified from this 2.8-kb fragment using RJS019 and the NcoI-modifying primer RJS018 (5′-CAACTGTGTTTTTAAAGCCATGGATGCTTTC-3′). PCR was performed using Platinum Taq (Invitrogen) according to the manufacturer's protocol with an annealing temperature of 55°C for 30 cycles. This was cloned first into pGEM T-Easy (Promega), then cut with NotI/NcoI and cloned into a pGreen 0800-LUC (Hellens et al., 2005), with the modified NcoI site producing the ATG start codon for the luciferase reporter gene. All constructs were confirmed by sequencing.
Construction of T-DNAs Overexpressing Apple Transcription Factors
cDNAs from expressed sequenced apple libraries (Newcomb et al., 2006) were cloned either using restriction enzymes into pSAK778 or using Gateway cloning (Invitrogen) into pHEX (Hellens et al., 2005).
Transient Assays
The PG1-Luc plasmid was inserted into Agrobacterium tumefaciens GV101 (MP) and tested for transactivation as described by Hellens et al. (2005). For ethylene induction, plants were injected with Agrobacterium containing PG1-Luc, left for 2 d, and then transferred to a 340-L container with a circulating fan and a final concentration of 100 μL L−1 ethylene for 24 h. Ethylene concentration was checked 1 and 20 h after application using a gas chromatograph to confirm that levels remained consistent. Leaf discs were analyzed for renillin and luciferase activity. For cold induction, instead of a container with ethylene, plants were transferred to a 4°C cold room for 24 h. In the second cold induction experiment, infiltrated plants were transferred into a cold room immediately after infiltration and then warmed to 20°C for 2 d before being assayed. With promoter transcription factor transactivation, one part PG1-Luc was combined with nine parts pHEX transcription factor, coinfiltrated in tobacco (Nicotiana benthamiana) leaves, and assayed as described by Hellens et al. (2005).
Phylogenetic Lineups
ESTs that contained an AP2 domain were selected by homology to the Arabidopsis AP2 domain-containing genes. Clones that represented the most 5′ EST (Newcomb et al., 2006) were isolated and fully sequenced. Sixty unique full-length AP2 domain-containing genes have been deposited in GenBank (accession nos. GU732427 to GU732483). The protein sequences of the AP2 domain from the 145 Arabidopsis genes were aligned with the AP2 domain from the 60 apple genes using ClustalW (using an opening penalty of 15 and an extension penalty of 0.3) using the AlignX software in Vector NTI 9.0. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 3.1 (Kumar et al., 2004) using a minimum evolution phylogeny test and 1,000 bootstrap replicates. The EIL genes (accession nos. GU732484 to GU732486) were compared similarly.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ACT79399, BAF43419, DQ074478, and GU732427 to GU732486.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Cluster of AP2D genes.
Supplemental Figure S2. Promoter region of the PG1 gene.
Supplemental Table S1. Class of AP2D proteins.
Supplemental Table S2. qPCR primers.
Supplementary Material
Acknowledgments
We thank Julie Nicholls for growing the tobacco plants for transient infiltration and Richard Espley and Charles A. Dwamena for critically reading the manuscript.
References
- Alonso JM, Stepanova AN. (2004) The ethylene signaling pathway. Science 306: 1513–1515 [DOI] [PubMed] [Google Scholar]
- Atkinson RG. (1994) A cDNA clone for ENDOPOLYGALACTURONASE from apple. Plant Physiol 105: 1437–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RG, Bolitho KM, Wright MA, Iturriagagoitia-Bueno T, Reid SJ, Ross GS. (1998) Apple ACC-OXIDASE and POLYGALACTURONASE: ripening-specific gene expression and promoter analysis in transgenic tomato. Plant Mol Biol 38: 449–460 [DOI] [PubMed] [Google Scholar]
- Atkinson RG, Schaffer RJ, Gunaseelan K, Schroder R, inventors. September 1, 2008. Methods and compositions for increasing storage-life of fruit. New Zealand Patent No. NZ570886 [Google Scholar]
- Atkinson RG, Schroder R, Hallett IC, Cohen D, MacRae EA. (2002) Overexpression of POLYGALACTURONASE in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol 129: 122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen YF, Etheridge N, Schaller GE. (2005) Ethylene signal transduction. Ann Bot (Lond) 95: 901–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy I, Jones B, Gentzbittel L, Lelievre JM, Pech JC, Latch A. (2004) Differential regulation of ACC SYNTHASE genes in cold-dependent and -independent ripening in pear fruit. Plant Cell Environ 27: 1197–1210 [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49: 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JX, Liu D, Pan Y, Gong W, Ma LG, Luo JC, Deng XW, Zhu YX. (2005) An annotation update via cDNA sequence analysis and comprehensive profiling of developmental, hormonal or environmental responsiveness of the Arabidopsis AP2/EREBP transcription factor gene family. Plant Mol Biol 59: 853–868 [DOI] [PubMed] [Google Scholar]
- Goulao LF, Oliveira CM. (2007) Molecular identification of novel differentially expressed mRNAs up-regulated during ripening of apples. Plant Sci 172: 306–318 [Google Scholar]
- Goulao LF, Oliveira CM. (2008) Cell wall modifications during fruit ripening: when a fruit is not the fruit. Trends Food Sci Technol 19: 4–25 [Google Scholar]
- Guo H, Ecker JR. (2003) Plant responses to ethylene gas are mediated by SCF(EBF1/EBF2)-dependent proteolysis of EIN3 transcription factor. Cell 115: 667–677 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. (2005) Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJ, Thodey K, Schaffer RJ, Alba R, Balakrishnan L, Bishop R, Bowen JH, Crowhurst RN, Gleave AP, Ledger S, et al. (2008) Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JW, Gunaseelan K, Pikakala P, Wang M, Schaffer RJ. (2009) Co-ordination of early and late ripening events in apples is regulated through differential sensitivities to ethylene. J Exp Bot 60: 2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Soltis PS, Wall K, Soltis DE. (2006) Phylogeny and domain evolution in the APETALA2-like gene family. Mol Biol Evol 23: 107–120 [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. (2000) Cloning and DNA-binding properties of a tobacco ETHYLENE-INSENSITIVE3 (EIN3) homolog. Nucleic Acids Res 28: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Tamura K, Nei M. (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5: 150–163 [DOI] [PubMed] [Google Scholar]
- Larrigaudiere C, Graell J, Salas J, Vendrell M. (1997) Cultivar differences in the influence of a short period of cold storage on ethylene biosynthesis in apples. Postharvest Biol Technol 10: 21–27 [Google Scholar]
- Mbeguie-A-Mbeguie D, Hubert O, Fils-Lycaon B, Chillet M, Baurens FC. (2008) EIN3-like gene expression during fruit ripening of Cavendish banana (Musa acuminata cv. Grande Naine). Physiol Plant 133: 435–448 [DOI] [PubMed] [Google Scholar]
- Nakano T, Suzuki K, Fujimura T, Shinshi H. (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb RD, Crowhurst RN, Gleave AP, Rikkerink EHA, Allan AC, Beuning LL, Bowen JH, Gera E, Jamieson KR, Janssen BJ, et al. (2006) Analyses of expressed sequence tags from apple. Plant Physiol 141: 147–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada MA, Blanco-Portales R, Pose S, Garcia-Gago JA, Jimenez-Bermudez S, Munoz-Serrano A, Caballero JL, Pliego-Alfaro F, Mercado JA, Munoz-Blanco J. (2009) Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for polygalacturonase in strawberry fruit softening. Plant Physiol 150: 1022–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer RJ, Friel EN, Souleyre EJ, Bolitho K, Thodey K, Ledger S, Bowen JH, Ma JH, Nain B, Cohen D, et al. (2007) A genomics approach reveals that aroma production in apple is controlled by ethylene predominantly at the final step in each biosynthetic pathway. Plant Physiol 144: 1899–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy RE, Kramer M, Hiatt WR. (1988) Reduction of POLYGALACTURONASE activity in tomato fruit by antisense RNA. Proc Natl Acad Sci USA 85: 8805–8809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJS, Watson CF, Morris PC, Bird CR, Seymour GB, Gray JE, Arnold C, Tucker GA, Schuch W, Harding S, et al. (1990) Inheritance and effect on ripening of antisense POLYGALACTURONASE genes in transgenic tomatoes. Plant Mol Biol 14: 369–379 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA 94: 1035–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thain SC, Vandenbussche F, Laarhoven LJJ, Dowson-Day MJ, Wang ZY, Tobin EM, Harren FJM, Millar AJ, Van Der Straeten D. (2004) Circadian rhythms of ethylene emission in Arabidopsis. Plant Physiol 136: 3751–3761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian MS, Prakash S, Zhang N, Ross GS. (2002) Chilling-induced ethylene biosynthesis in Braeburn apples. Plant Growth Regul 38: 249–257 [Google Scholar]
- Tieman DM, Ciardi JA, Taylor MG, Klee HJ. (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26: 47–58 [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Kudo H, Ishikawa R, Akada S, Senda M, Niizeki M, Harada T. (2006) Low expression of an ENDOPOLYGALACTURONASE gene in apple fruit with long-term storage potential. Postharvest Biol Technol 39: 193–198 [Google Scholar]
- Wang A, Tan D, Takahashi A, Zhong Li T, Harada T. (2007) MdERFs, two ethylene-response factors involved in apple fruit ripening. J Exp Bot 58: 3743–3748 [DOI] [PubMed] [Google Scholar]
- Wang A, Yamakake J, Kudo H, Wakasa Y, Hatsuyama Y, Igarashi M, Kasai A, Li TZ, Harada T. (2009) Null mutation of the MdACS3 gene, coding for a ripening-specific 1-aminocyclopropane-1-carboxylate synthase, leads to long shelf life in apple fruit. Plant Physiol 151: 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Bowen JH, Weir IE, Allan AC, Ferguson IB. (2001) Heat-induced protection against death of suspension-cultured apple fruit cells exposed to low temperature. Plant Cell Environ 24: 1199–1207 [Google Scholar]
- Wisniewski M, Bassett C, Norelli J, Macarisin D, Artlip T, Gasic K, Korban S. (2008) Expressed sequence tag analysis of the response of apple (Malus × domestica ‘Royal Gala’) to low temperature and water deficit. Physiol Plant 133: 298–317 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.