Plants are frequently exposed to natural and synthetic toxins such as heavy metals, allelochemicals, organic pollutants, and pesticides. Consequently, plants must mount specific and coordinated defense mechanisms for survival under adverse growing conditions (Zhang et al., 2007). One such mechanism is the capability for metabolizing organic compounds from abiotic origin (xenobiotics). Extensive biotransformation is part of a strategy for coping with the potentially negative impacts of xenobiotics on plant growth and development. This is particularly evident in the capability for metabolic detoxification of herbicides; plants are able to detoxify herbicides by complex multistep processes that exhibit extraordinary diversity among species (Kreuz et al., 1996). The differential ability of plant species to metabolize a particular herbicide is widely exploited in modern agriculture by the use of selective herbicides that are safe to the crop but effectively control associated weeds.
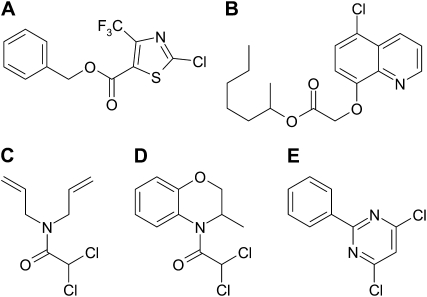
Another interesting feature of the herbicide detoxification system of some monocotyledonous crop species is inducibility by certain synthetic compounds collectively named “herbicide safeners.” Safeners are a group of chemically diverse compounds with the unique ability to protect grass crops from herbicide injury without reducing herbicide activity in target weed species (Davies and Caseley, 1999; Hatzios and Burgos, 2004). Safener protection from herbicide injury is accomplished by increasing the expression of genes encoding herbicide-metabolizing enzymes, such as the glutathione S-transferases (GSTs), cytochrome P450 monooxygenases (P450s), and several others (Hatzios, 1989; Farago et al., 1994; Riechers et al., 2005). Various safener chemical classes have been developed to enhance herbicide tolerance in maize (Zea mays), grain sorghum (Sorghum bicolor), rice (Oryza sativa), and small-grain cereals (Fig. 1). However, despite the widespread agronomic use of safeners for decades and ample information about their effects on increasing the activity of detoxification enzymes, there is virtually no knowledge of the molecular mechanisms for safener induction of their corresponding genes or signaling pathways being recruited. An exception is a safener-binding protein that was identified in maize, where its binding activity was characterized in maize seedlings (Walton and Casida, 1995) along with its gene expression patterns (Scott-Craig et al., 1998), although the precise role of this protein in the safener-mediated signaling pathway has yet to be established.
Figure 1.
Chemical structures of several herbicide safeners and their uses in arable crops. A, Flurazole (seed applied for grain sorghum). B, Cloquintocet-mexyl (foliar applied in small-grain cereals). C, Dichlormid (soil applied for maize). D, Benoxacor (soil applied for maize). E, Fenclorim (soil applied for rice).
Safeners induce the expression of genes involved in plant defense and detoxification, such as GSTs and P450s, yet they are not toxic to the target plant and confer protection from herbicide injury. This suggests that safeners are tapping into an unidentified, preexisting signaling pathway for detoxification of endogenous toxins or xenobiotics (Riechers et al., 2005). A new hypothesis resulting from our most recent research is that safeners may be utilizing an oxidized lipid-mediated (oxylipins; Mosblech et al., 2009) or cyclopentenone-mediated signaling pathway, which subsequently leads to the expression of GSTs and other proteins involved in detoxification and plant defense (Weber, 2002; Loeffler et al., 2005; Mueller et al., 2008; Mueller and Berger, 2009). The goal of this Update on detoxification is to briefly review herbicide safener mechanism of action in light of the many recent findings related to oxylipins and their roles in signaling, induction of defense genes, and activation of detoxification responses in plants (Mueller et al., 2008; Mueller and Berger, 2009).
ENZYMES INVOLVED IN HERBICIDE AND SAFENER METABOLISM
The metabolism of herbicides in plants generally requires the sequential action of several enzymes (Kreuz et al., 1996; Bounds and Hutson, 2000). Metabolic reactions and enzymes most commonly involved are oxidations by P450s and hydrolyses by carboxylesterases (phase I reactions); conjugations to endogenous molecules such as glutathione (GSH) or Glc, catalyzed by GSTs or UDP-dependent glycosyltransferases (UGTs), respectively (phase II); transport of conjugates into the vacuole by carriers of the multidrug resistance-associated protein (MRP) family or by other transport mechanisms (phase III); and processing of conjugates involving reactions such as partial degradation, secondary conjugations, and incorporation as “bound residue” into cell wall constituents (phase IV). Among the less frequently observed biotransformations of herbicides are, to name but a few, oxidations at nitrogen or sulfur atoms; reductions of nitro groups, aldehydes, ketones, and α,β-unsaturated carbonyl compounds; and isomerizations (Bounds and Hutson, 2000). The enzymes involved in these less common reactions in plants have not always been identified.
The initial step in the metabolism of a particular herbicide may not always lead to a complete detoxification or can even constitute an activation step (e.g. hydrolysis of an esterified “proherbicide” precursor). However, the formation of herbicide conjugates with GSH or Glc fundamentally alters their physicochemical properties, rendering the compounds more polar and unable to freely diffuse across cellular compartments or translocate throughout the symplast. Furthermore, such conjugates are usually amenable to further metabolism and can be accepted by energized carriers for their transport out of the cytosol. Plants thus possess an integrated multistep system for the detoxification of potentially harmful compounds such as herbicides (Kreuz et al., 1996) in addition to systems that enable plants to cope with the consequences of xenobiotic-induced stresses, such as antioxidative and radical-scavenging components. A unique and intriguing feature of safeners is their apparent ability to coordinately induce a whole array of critical components that constitute herbicide detoxification pathways in plants (Zhang et al., 2007). These aspects of safener action have attracted considerable interest from both industry and academic researchers since the discovery of the first herbicide safeners approximately 50 years ago (Hatzios, 1989; Davies and Caseley, 1999).
PHASE I AND PHASE II HERBICIDE METABOLISM
One of the most important reactions for herbicide metabolism is catalyzed by P450 enzymes, which typically introduce a reactive functional group suitable for further metabolism and detoxification. The most common P450-mediated reactions are hydroxylations of aromatic rings or alkyl groups and dealkylations resulting from the formal hydroxylation of a carbon adjacent to an oxygen or nitrogen atom (Kreuz et al., 1996; Barrett, 2000). Plant P450 proteins are encoded by very large and diverse multigene families consisting of approximately 350 genes in rice and 250 genes in Arabidopsis (Arabidopsis thaliana), whose protein products are involved in numerous processes such as secondary product biosyntheses, fatty acid metabolism, and hormone homeostasis (Nelson et al., 2004). The primary metabolism of herbicides from several important chemical classes is mediated by P450 activities, often leading to crop selectivity (Barrett et al., 1997), and the relationships between the physiological and detoxicative roles of individual P450 isoforms and herbicide selectivity in rice and maize are becoming more clear (Barrett, 2000; Persans et al., 2001; Pan et al., 2006; Nordby et al., 2008). There is ample circumstantial evidence from in vivo metabolism studies and experiments with isolated microsomes that P450 activities are induced by safeners. However, supporting evidence for safener-regulated expression of specific P450s at the transcriptional level is comparatively scarce (Persans et al., 2001), and information is lacking at the posttranscriptional or protein abundance level.
The products of phase I carboxylesterase- or P450-mediated reactions often undergo glycosylation to yield the corresponding O- or N-glucosides or Glc esters. The UGTs for conjugating lipophilic small molecules are encoded in plant genomes by large multigene families, with over 120 members in Arabidopsis (Bowles et al., 2006; Osmani et al., 2009). UGTs of this family perform crucial biological roles in plant secondary product biosynthesis, hormone homeostasis, and detoxification of toxins produced by pathogens. There are some reports demonstrating that safeners enhance the glycosylation of herbicides in protected plants (Kreuz et al., 1991; Hatzios and Burgos, 2004). Interestingly, the wheat (Triticum aestivum) safener cloquintocet-mexyl (Fig. 1), which enhanced the glycosylation of products formed in the metabolism of the herbicide clodinafop-propargyl (Kreuz et al., 1991), has also been shown to shift the metabolic profile of endogenous phenolics in wheat by increasing the activity of a glucosyltransferase and an O-methyltransferase toward flavonoid substrates (Cummins et al., 2006).
The best-studied group of plant enzymes involved in herbicide metabolism is unquestionably the GSTs, which catalyze the conjugation of the major cellular thiol, GSH, to an electrophilic site of lipophilic substrates. The catalytic mechanism involves an addition of the GSH thiolate anion to the substrate with concomitant rearrangement or liberation of a leaving group (e.g. a halogen or phenolic moiety) from the substrate. Plant GSTs form a large and diverse group, with 54 GST genes encoded by the Arabidopsis genome, and have been classified based on sequence similarity, genomic organization, and functions into several distinct subclasses (Dixon et al., 2009), with the ϕ and τ classes being most prominent in plants (Edwards et al., 2000; Dixon et al., 2002, 2010). GSTs are abundant proteins that are predominantly expressed in the cytosol under nonstressful conditions but have also displayed diverse subcellular localizations when expressed as GFP fusions in tobacco (Nicotiana tabacum; Dixon et al., 2009). Additionally, GST proteins have been localized to the vacuoles of subepidermal cells upon exposure to safeners in wheat (Riechers et al., 2003). In addition to their well-described activity as transferases involved in xenobiotic metabolism, several diverse endogenous roles for plant GSTs have recently been described, including functions as ligandins in flavonoid sequestration and hormone homeostasis, as isomerases in Tyr catabolism, GSH conjugation and transport of reactive oxylipins, and as GSH peroxidases (Edwards et al., 2000; Dixon and Edwards, 2009; Dixon et al., 2010). The latter activity is of particular significance for the prevention of oxidative injury; certain GSTs catalyze the GSH-dependent reduction of fatty acid hydroperoxides to the corresponding alcohols, thereby preventing their degradation to cytotoxic aldehydes. Stress-inducible GSTs of the θ, ϕ, and τ classes display GSH peroxidase activity (Cummins et al., 2009). Plant GSTs respond to a wide range of agents (pathogens, heavy metals, hydrogen peroxide, heat shock, hormones) in which production of reactive oxygen species (ROS) is a common factor (Theodoulou et al., 2003; Sappl et al., 2009). Moreover, recent studies have also implicated GSTs as components of UV light-inducible cell signaling pathways and as potential regulators of apoptosis (Dixon et al., 2002). The first evidence for safener regulation of GST expression at the transcriptional level was demonstrated in a study with the safener flurazole on the induction of a GST transcript in maize (Wiegand et al., 1986). In addition to up-regulating GST expression, safeners enhance the activity of enzymes involved in sulfate assimilation and Cys or GSH biosynthesis (Table I), thereby triggering an elevation of GSH levels (Farago et al., 1994), which is in accord with the requirement for GSH as a cosubstrate for GST-catalyzed detoxification reactions.
Table I. Parallels between safener-regulated and RES oxylipin-regulated expression patterns.
Compilation of OPDA and phytoprostane-regulated gene expression is based on transcript profiling studies in Arabidopsis (Loeffler et al., 2005; Taki et al., 2005; Mueller et al., 2008), whereas safener regulation of gene expression is based on results determined by the induction of transcripts, proteins, and/or enzyme activity in several plant species (Riechers et al., 2003; Theodoulou et al., 2003; Rishi et al., 2004; Zhang and Riechers, 2004; Baerson et al., 2005; Zhang et al., 2007). Only up-regulated genes or proteins are included for comparison. A, Enzyme activity; P, protein abundance; T, transcript.
| Function | Gene/Protein | OPDA | Phytoprostanes | Safeners |
| Detoxification/secondary metabolism | P450s | T | T | T, A |
| GSTs | T, Aa | T, Aa | T, P, A | |
| UGTs | T | T | T, A | |
| Vacuolar transport | ABC transporter family proteins | T | T | T |
| GSH-conjugate ABC transporters (MRPs) | T | T | T, A | |
| Amino acid/GSH metabolism/sulfate assimilation | β-Cyano-Ala synthase | P | ||
| Cys synthase | T, A | |||
| ATP sulfurylase | Ab | |||
| 5′-Adenylylsulfate sulfotransferase | Ab | |||
| 5′-Adenylylsulfate reductase | T | |||
| Trp synthase (β-chain 1 precursor) | T | |||
| γ-Glutamyl transpeptidase | P | |||
| Hormones/signaling | OPRs | T, Ac | T, Ac | T, P |
| Auxin-induced protein family | T | T, P | ||
| Stress/other/unknown | In2.1-like protein | T | T | T, P |
| GSH peroxidase | T | Ad | ||
| Monodehydroascorbate reductase | P | |||
| Aldo/keto-reductase family | T | T | T, P | |
| Fru bisP aldolase | T | P | ||
| Mannitol dehydrogenase | T | T | P | |
| Cinnamyl alcohol dehydrogenase | T | T | T |
OPDA- and phytoprostane-induced GST activity (toward conjugation of OPDA and A1-phytoprostanes with GSH) was shown by Mueller et al. (2008).
Extractable activities of ATP sulfurylase and 5′-adenylylsulfate sulfotransferase were strongly induced by the safeners dichlormid and benoxacor in etiolated maize seedlings (Farago et al., 1994).
OPDA-induced OPR activity (toward reduction of OPDA and A1-phytoprostanes) and phytoprostane-induced OPR activity (toward reduction of A1-phytoprostanes) was shown by Mueller et al. (2008).
Some safener-induced GSTs also display GSH peroxidase activity (Dixon et al., 2002, 2010).
SUBCELLULAR FATE OF HERBICIDE CONJUGATES
Several studies have shown that herbicide-GSH and herbicide-Glc conjugates are transported into the vacuole of plant cells by MRP transporters and that safeners enhance this phase III transport activity. The first evidence for the presence of MRP activity in plants was the observation that the GSH conjugate of the herbicide metolachlor and other compounds was actively transported into isolated barley (Hordeum vulgare) vacuoles by ATP-energized transporters residing in the tonoplast membrane (Martinoia et al., 1993). Subsequently, genes belonging to the MRP family, a subfamily of the ubiquitous ATP-binding cassette (ABC) transporters, have been identified in many plant species (for review, see Klein et al., 2006). For example, the genomes of Arabidopsis and rice contain 15 and 17 MRP genes, respectively. However, few plant MRPs have been investigated with respect to their substrates and transport characteristics. The sequestration mechanism for herbicide-Glc conjugates in the vacuole is not fully understood and may depend on the particular species and chemical nature of the glucoside conjugate (Klein et al., 2006). For example, glucosylated chlorsulfuron (a sulfonylurea herbicide) is transported via a proton-antiport mechanism into sugar beet (Beta vulgaris) tonoplast vesicles. In contrast, hydroxyprimisulfuron-glucoside, the detoxification product of the sulfonylurea herbicide primisulfuron, is transported into barley vacuoles via an ABC-type transport mechanism (Klein et al., 2006).
Treatment with the safener cloquintocet-mexyl (Fig. 1) increased the activity of a MRP transporter for metolachlor-GSH or hydroxyprimisulfuron-glucoside conjugates in isolated mesophyll vacuoles from barley (Gaillard et al., 1994). In studies conducted in wheat, transcripts encoding five GSTs (belonging to three different subclasses) and a MRP transporter were induced by cloquintocet-mexyl (Theodoulou et al., 2003), and two MRP transcripts were differently regulated in response to cloquintocet-mexyl in various wheat seedling tissues (Zhang et al., 2007). The functional significance for the elimination of phase II conjugates from the cytosol by vacuolar deposition may be to protect GSH-dependent enzymes from product inhibition (Grzam et al., 2007) or to avoid possible reactivation of metabolites via catabolism of phase II conjugates in the cytosol (Brazier-Hicks et al., 2008).
Once transported into the vacuole, processing of GSH conjugates is initiated by the concerted action of vacuolar enzymes such as carboxypeptidase and γ-glutamyl transpeptidase to yield their corresponding Cys conjugates (Wolf et al., 1996; Grzam et al., 2007). Studies in Arabidopsis indicate the presence of two distinct processing pathways to yield Cys conjugates, one functioning in the vacuole and another in the cytosol (for summary, see Brazier-Hicks et al., 2008). In either case, the resulting herbicide-Cys conjugates are usually further processed, e.g. by secondary conjugation to malonate, or by the action of Cys conjugate β-lyase and S-methyltransferase activity to yield the well-known S-methylthio derivatives (Kreuz et al., 1996; Bounds and Hutson, 2000). However, the effect of safeners on these phase IV activities or the functional significance in promoting herbicide tolerance in cereal crops has rarely been addressed.
METABOLIC FATE OF SAFENERS IN PLANTS
There is a large body of evidence demonstrating that safeners enhance the metabolic activities of P450s, UGTs, GSTs, and MRPs involved in the detoxicative processes for herbicides described above. It has been proposed that safeners are metabolized in plants by the same enzyme systems as herbicides and induce their own metabolism as well as the metabolism of other xenobiotics. Accordingly, many safeners (such as flurazole, benoxacor, and fenclorim; Fig. 1) form GSH conjugates in plants (Breaux et al., 1989; Miller et al., 1996; Brazier-Hicks et al., 2008). For example, mono- and di-GSH conjugates of the dichloroacetamide safener benoxacor were identified in maize cell suspension cultures (Miller et al., 1996), and two consecutive rounds of GSH conjugation of the rice safener fenclorim were demonstrated in Arabidopsis cell cultures (Brazier-Hicks et al., 2008), indicating that these safeners are metabolized via conjugation with GSH. It has recently been reported that the S-methylthio derivative of fenclorim [4-chloro-6-(methylthio)-2-phenylpyrimidine] is formed via the GSH conjugation pathway in Arabidopsis and that this metabolite induces GST activity when added exogenously to rice and Arabidopsis cell cultures (Brazier-Hicks et al., 2008). Moreover, 4-chloro-6-(methylthio)-2-phenylpyrimidine displayed a safening effect to rice plants against injury from the herbicide pretilachlor. This biological activity shown for a hydrophobic safener metabolite, however, was concluded to be the result of a “metabolic reactivation” of safening activity rather than an essential bioactivation step (Brazier-Hicks et al., 2008). In contrast with these safeners, cloquintocet-mexyl effectively induces GST activity in wheat seedlings (Riechers et al., 2003, 2005) but reportedly is not metabolized via GSH conjugation in wheat foliage or in rats (Roberts, 1998).
In conclusion, these studies on safener metabolism with intact plants or cell cultures did not provide direct clues to the nature or components of the signaling pathway involved in safener mechanism of action, although multiple rounds of GSH conjugation of fenclorim suggest the involvement of substrate-induced regulation of the GSH-mediated detoxification pathway (Brazier-Hicks et al., 2008; Fig. 2). The multiplicity of safener effects on the expression and activity of diverse proteins involved in detoxification calls for integrated transcriptomic, metabolomic, and proteomic approaches in a safener-responsive cereal crop, conducted in parallel with safener fate and metabolism studies, to fully elucidate the mechanism of action and signaling pathways utilized by safeners.
Figure 2.
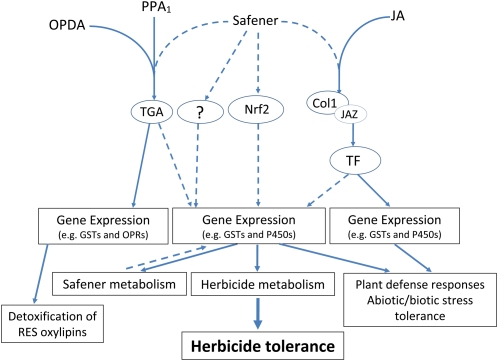
Possible safener-mediated signaling pathways for the regulation of defense genes and activation of detoxification pathways in plants. Safeners may tap into a RES oxylipin-mediated signaling pathway (e.g. OPDA and PPA1) and up-regulate TGA transcription factors (for review, see Mueller and Berger, 2009), an Nrf2-Keap1-mediated signaling pathway (Nguyen et al., 2009), and/or a JA-mediated signaling pathway (Schaller and Stintzi, 2009; Zander et al., 2010) to induce the expression of proteins involved in herbicide detoxification in plants. It is also possible that safeners may utilize an unknown (?) signaling pathway to trigger plant defense gene expression. Dashed lines indicate possible but unproven signaling pathways, and solid lines indicate known signaling pathways for the regulation of plant gene expression. Note that the three distinct “Gene Expression” boxes indicate that only a subset of GST enzymes can metabolize herbicides or safeners as substrates, leading to detoxification and herbicide tolerance in cereal crops. CoI1, Coronatine-insensitive protein1; JAZ, transcriptional repressor protein; TF, transcription factor/activator. [See online article for color version of this figure.]
TISSUE-SPECIFIC EFFECTS OF SAFENERS ON HERBICIDE DETOXIFICATION
The first safeners introduced into agricultural practice were developed for herbicides of the thiocarbamate and chloroacetamide classes for maize, grain sorghum, and wet-sown rice (Hatzios, 1989). These herbicides are primarily detoxified in tolerant plants through GSH conjugation. Lay et al. (1975) demonstrated that dichlormid (Fig. 1) and related safeners of the dichloroacetamide class elevate the levels of both GSH and extractable GST activity toward the bioactivated herbicide substrate, EPTC-sulfoxide, in maize seedlings.
Thiocarbamates and chloroacetamides are typically applied together with their safeners to the soil at or before planting in maize or grain sorghum. The predominant site of herbicide uptake is the coleoptile, while the most vulnerable sites are the shoot meristems and developing leaves, where elongases of very long-chain fatty acid biosynthesis are inhibited (Trenkamp et al., 2004). Benoxacor (Fig. 1), a safener developed for the chloroacetamide herbicide metolachlor in maize, increased GSH-mediated metabolism of metolachlor mainly in maize coleoptiles (Kreuz et al., 1989). A similar conclusion was drawn using the safener BAS 145-138 to investigate the tolerance of maize seedlings to the chloroacetamide herbicide metazachlor (Fuerst et al., 1991) and was corroborated at the GST transcript, protein, and enzyme activity levels in the diploid wheat Triticum tauschii (Riechers et al., 2003). Additionally, the chemically unrelated safeners cloquintocet-mexyl and fluxofenim dramatically increased the level of immunodetectable GST protein specifically in the outer cell layers of the coleoptile (Riechers et al., 2003). As a result, safeners for thiocarbamates and chloroacetamides exert their main effect on herbicide metabolism by inducing GSTs in the coleoptile that rapidly detoxify the herbicide and prevent it from reaching the new leaves as the shoot emerges from the soil (Kreuz et al., 1989; Fuerst et al., 1991; Riechers et al., 2003).
Collectively, these studies on the role of GSTs in herbicide metabolism and safener-increased crop tolerance to herbicides have established that the expression of many different GST subunits and isoforms is organ or tissue specific, developmentally regulated, and that GST genes differentially respond to safeners. However, it is important to note that many GSTs also respond to a wide range of stress-inducing agents in Arabidopsis (Sappl et al., 2009) and provide biotic and abiotic stress tolerance in monocot and dicot plants (Dixon et al., 2002), apparently without concomitant cross-protection from herbicide injury (as depicted in Fig. 2). For example, Arabidopsis responds to safener treatment (as measured by induction of specific GST and MRP transcripts and proteins), yet Arabidopsis and other dicots are not protected from herbicide injury by safeners (DeRidder et al., 2002; DeRidder and Goldsbrough, 2006), implying that some other distinctive mechanism or aspect of the detoxification pathway must be integral in conferring safener protection in cereal crops.
CYCLOPENTENONES, CYCLOPENTANONES, AND OXYLIPINS
Biological Activities of Jasmonates and Oxidized Fatty Acids
The octadecanoid pathway in plants, which leads to the biosynthesis of jasmonic acid (JA) in both dicot and monocot species, is a critical signaling pathway involved in mediating plant responses to a wide range of biotic (such as insect or fungal attack) and abiotic (such as mechanical wounding and drought) stresses (Fig. 2). JA and structurally related compounds (collectively termed jasmonates) modulate various physiological events, including defense responses to pathogen and insect attack, root growth, fruit ripening, and the regulation of many other plant developmental processes (Creelman and Mullet, 1997; Schaller and Stintzi, 2009). Jasmonates modulate the expression of numerous genes and influence plant growth, development, and stress responses through intracellular and intercellular signaling pathways (Creelman and Mullet, 1997; Reymond and Farmer, 1998). JA is synthesized via a series of steps, starting with the oxygenation of α-linolenic acid from membrane lipids via lipoxygenase activity and subsequent conversion to 12-oxo-phytodienoic acid (OPDA) in reactions catalyzed by allene oxide synthase and allene oxide cyclase (Schaller and Stintzi, 2009). The end product of the pathway, JA, is synthesized from OPDA through reduction by NADPH-dependent 12-oxo-phytodienoic acid reductase (OPR) activity followed by three cycles of β-oxidation. JA can be further converted to methyl jasmonate and various conjugates that may also have biological activities (Creelman and Mullet, 1997; Schaller, 2001).
Until recently, JA has been viewed as the end product of the pathway and as the predominant bioactive hormone in plant defense-related signaling processes. However, it has become increasingly clear that biological activity may differ among the various JA metabolites and conjugates as well as its biosynthetic precursors (for review, see Schaller and Stintzi, 2009). For example, other jasmonates and cyclopentenones (i.e. OPDA and the phytoprostanes) as well as keto-, hydroxy-, and hydroperoxy-fatty acids may also possess significant and unique biological activities, including initiation of cell senescence as well as the expression of stress-related plant defense and detoxification genes (Weber, 2002; Mueller and Berger, 2009; Schaller and Stintzi, 2009). It has also become clear that oxidized fatty acids and lipids can give rise to a diverse array of bioactive signaling compounds through either enzymatic or nonenzymatic transformation (Mosblech et al., 2009; Schaller and Stintzi, 2009). In spite of their diverse structures and biosynthetic origins, these “oxylipin” signaling molecules often display common, overlapping effects on gene expression patterns yet retain some specificity in their regulation of gene expression (Loeffler et al., 2005; Taki et al., 2005; Mueller et al., 2008; Mueller and Berger, 2009).
Synthesis and Properties of Oxylipins
Oxylipins are structurally diverse metabolites derived from fatty acid oxidation and can be formed through either nonenzymatic or enzymatic reactions. Nonenzymatically generated oxylipins are formed via free radical-catalyzed reactions in or near cell membranes, where polyunsaturated fatty acids serve as precursors for their synthesis, and include different types of phytoprostanes (A1 and B1), hydroxy fatty acids, malondialdehyde, and 4-hydroxy-2E-nonenal (Mosblech et al., 2009). These unstable, reactive oxylipins have historically been used as biochemical markers for monitoring oxidative stress and membrane damage in plant and animal systems (Mueller and Berger, 2009). Enzymatically produced oxylipins include JA (and its conjugates) and OPDA, which are part of the jasmonate biosynthetic pathway in plants; this pathway and its enzymes have been well studied due to the hormonal activity of jasmonates and defense gene activation in response to stress (Mosblech et al., 2009; Schaller and Stintzi, 2009). Oxylipins differ not only in their origin, synthesis, and structures but also in their electrophilicity. For example, strong reactive electrophilic species (RES) include the A1-type phytoprostanes and OPDA, and weak RES include the B1-type phytoprostanes and JA (Farmer and Davoine, 2007). Regardless of their origin or route for biosynthesis, the various types of oxylipins possess biological activities as signaling molecules in response to oxidative stresses (Mueller and Berger, 2009).
Reactive electrophilic oxylipins (RES oxylipins) share several common properties, including a lipophilic nature that aids in binding to hydrophobic pockets or active sites of proteins, thiol reactivity due to an electrophilic site, and the ability to modify proteins and strongly induce genes and enzymes involved in detoxification (Mueller and Berger, 2009). Safeners are also lipophilic, typically contain electrophilic sites (and may consequently react with cellular thiols such as GSH), and are potent inducers of detoxification enzymes in plants (Zhang et al., 2007). However, while several chemical classes of safeners have been developed for different monocot crops, a unifying structural motif across these classes is not readily apparent (Fig. 1). Furthermore, published information on determining structure-activity relationships for safener action in plants is limited (Komives and Hatzios, 1991). Many safeners, however, contain electrophilic sites that react under physiological conditions with GSH via displacement of chlorine (Breaux et al., 1989; Miller et al., 1996; Brazier-Hicks et al., 2008). Other safeners could perhaps be “activated” to a reactive electrophile by a preceding metabolic step in the cell.
INDUCTION OF PLANT DEFENSE GENE EXPRESSION
Parallels between Oxylipin- and Safener-Regulated Expression Patterns
Following the rapid formation of ROS and RES in response to severe oxidative stress is the accumulation of oxidized lipids, or RES oxylipins (Mueller and Berger, 2009). RES oxylipins (most notably OPDA and phytoprostanes) cause a massive induction of genes involved in secondary metabolism as well as detoxification and stress responses and defense genes (Mueller and Berger, 2009; Table I). Induction of secondary metabolism, however, is not specific to RES oxylipins (Mueller and Berger, 2009), since jasmonates and non-RES oxylipins also trigger the accumulation of secondary metabolites and defense or stress-related genes. This presumably occurs in a reactive manner in response to the massive accumulation of ROS, RES, and subsequent cellular toxicity. However, safeners and RES oxylipins have a similar and unique “proactive” effect on the massive, coordinated induction of detoxification genes and enzymes (Table I; Fig. 2). Such genes and enzymes include P450s, oxidoreductases (e.g. OPRs), GSH peroxidases, GSTs, UGTs, and vacuolar ABC transporters, all of which are known to participate in the different sequential phases for cellular detoxification of xenobiotics (Kreuz et al., 1996; Baerson et al., 2005). In addition to the proteins listed above, safeners also induced the expression of an enzyme involved in Cys and GSH biosynthesis (β-cyano-Ala synthase; Zhang et al., 2007).
Significantly, exogenous OPDA treatment induces OPR enzymes that reduce the cyclopentenone substrate OPDA (Mueller et al., 2008) and GSTs that conjugate GSH to the cyclopentenone ring of OPDA (Mueller et al., 2008; Dixon and Edwards, 2009; Fig. 2). Safeners also coordinately induce OPRs and several GST proteins (Zhang et al., 2007), which conjugate GSH to many herbicide substrates (Kreuz et al., 1996) and possibly to safeners as well (Breaux et al., 1989; Miller et al., 1996; Brazier-Hicks et al., 2008). These findings suggest a link between substrate induction and plant defense and detoxification mechanisms (Fig. 2). However, many herbicides are also effective substrates for GSTs but are not effective inducers of GSTs or other detoxification enzymes (Zhang et al., 2007), possibly because they rapidly exert their phytotoxic effects on sensitive plants. All herbicides have a specific target site or a protein that binds to or interacts with the herbicide. For example, atrazine binds to the QB binding site of the D1 protein in PSII and blocks photosynthetic electron transport, leading to the rapid generation of ROS and subsequent lipid peroxidation (Hess, 2000). In contrast with herbicides, safeners are not known to interact with a specific target site that leads to cellular damage or phytotoxicity, yet they are recognized by the plant's defense signaling system and effectively up-regulate genes and enzymes involved in detoxification (Zhang et al., 2007).
Distinct gene expression profiles are observed when comparing OPDA or phytoprostane (cyclopentenones) treatment with JA or methyl jasmonate (cyclopentanones) treatment in Arabidopsis, yet some overlapping sets of genes are observed as well (for review, see Mueller and Berger, 2009). One interesting question to investigate further is if distinct biological activities (i.e. disease resistance or abiotic stress tolerance) can be correlated with these unique gene expression profiles. Additionally, it may be possible that additive or synergistic effects on defense gene expression and the resulting plant phenotypes (enhanced stress tolerance or rates of xenobiotic detoxification) are observed in response to treatments containing combinations of diverse RES oxylipins and/or cyclopentanones. Since most analytical or molecular-genetic studies to date have focused on a specific type or class of oxylipin signaling molecule in response to a stress, it has been postulated that the diversity and total accumulation of RES oxylipins have likely been underestimated in vivo and that a much larger array of different RES oxylipins with overlapping biological activities may actually contribute to defense gene expression and the resulting whole-plant stress responses (Mueller and Berger, 2009).
A Common Thread among Inducers of Plant Defense Gene Expression
Safeners and RES oxylipins have a common biological activity in that they both strongly induce the expression of defense genes and activate detoxification responses in plants (Zhang et al., 2007; Mueller and Berger, 2009). As mentioned previously, it is well known that safeners induce the expression of enzymes involved in an entire detoxification pathway (phases I–III; Kreuz et al., 1996; Theodoulou et al., 2003; Zhang et al., 2007). Consistent with this notion, a pioneering study showed that B1-phytoprostanes, the jasmonate-like products formed nonenzymatically from α-linolenic acid, dramatically increased the expression of genes involved in plant defense and detoxification pathways, including several P450s, UGTs, GSTs, and GSH-conjugate ABC transporters (Loeffler et al., 2005; Table I). These results indicate that B1-phytoprostanes, and possibly other oxylipins as well, may trigger plant defense and detoxification responses in a manner similar to safeners. Significantly, treatment of tobacco suspension cultures with B1-phytoprostanes protected the cells from subsequent exposure to copper sulfate (Loeffler et al., 2005), analogous to the safener response in protecting cereal crops from herbicide injury (Zhang et al., 2007). In accord with this hypothesis, it has been proposed in the literature that induction of detoxification genes appears to be most strongly stimulated by RES oxylipins (Mueller and Berger, 2009) and that this coordinated detoxification response in Arabidopsis resembles the sequential phase I, II, and III pathway for xenobiotic metabolism in plants (Kreuz et al., 1996; Ekman et al., 2003; Beynon et al., 2009). Collectively, these findings support the hypothesis that safeners may be tapping into a RES oxylipin-mediated signaling pathway, which subsequently induces the expression of proteins involved in a highly conserved detoxification pathway in plants (Table I; Fig. 2).
In addition to the synthesis and accumulation of RES oxylipins, TGA transcription factors appear to be involved in the signaling process for activation of detoxification responses in plants (Fode et al., 2008; Mueller et al., 2008; Mueller and Berger, 2009; Fig. 2). Treatment with xenobiotics or RES oxylipins results in the activation of defense- and stress-related genes that contain activation sequence-1 (as-1)-like cis-elements in their promoters (Xiang et al., 1996; Fode et al., 2008). These as-1-like sequences are recognized by basic domain/Leu zipper transcription factors of the TGA protein family (Singh et al., 2002). Therefore, coordinated induction of detoxification and plant defense genes in response to several diverse xenobiotic, phytohormone, or environmental stresses may be achieved through a common regulatory motif in the genes’ promoters (Xiang et al., 1996; Fode et al., 2008; Mueller et al., 2008; Zander et al., 2010). The coordinated induction of genes involved in plant defense and secondary metabolism, in particular the P450 gene family in Arabidopsis, has been demonstrated in response to diverse abiotic and biotic stresses (Glombitza et al., 2004; Naruska et al., 2004).
A similar mechanism for the coordinated induction of detoxification enzymes, which involves the Nrf2-Antioxidant Response Element (ARE) transcriptional pathway, has been well studied and characterized in mammalian systems (for review, see Nguyen et al., 2009). Under nonstressful basal conditions, the basic Leu zipper transcription factor Nrf2 is highly unstable due to constant degradation and turnover in the nucleus by a Keap1-mediated ubiquitination process. Upon exposure to inducers of oxidative stress (typically sulfhydryl-reactive electrophiles) that interact with the transcriptional repressor Keap1 in the cytoplasm, several key Cys residues on Keap1 become modified and degradation in the nucleus is impaired, thus stabilizing Nrf2 protein by release from repression (Nguyen et al., 2009). The free Nrf2 proteins bind with AREs of phase I and II detoxification genes such as GSTs or oxidoreductases to activate their transcription, resulting in protection against cytotoxicity (Nguyen et al., 2009).
It is possible that safeners may also utilize a similar signaling mechanism to the Nrf2-Keap1-ARE transcriptional activation system in mammals (Fig. 2). Safener-regulated signaling may occur in concert or in parallel with RES oxylipin-mediated signaling pathways (Farmer and Davoine, 2007; Mueller and Berger, 2009) to coordinately up-regulate the expression of enzymes involved in herbicide metabolism and detoxification (Zhang et al., 2007; Fig. 2), based on similarities in their expression profiles (Table I) and since most safeners contain sulfhydryl-reactive electrophilic sites (Fig. 1). In support of this theory, the promoter of the safener-inducible TtGSTU1 gene contains an ARE-like element (Xu et al., 2002), which may function similarly to the regulation of GST genes in mammalian systems (Nguyen et al., 2009). Another interesting parallel is that Nrf2 proteins belong to the same group of basic Leu zipper transcription factors that also constitute the plant TGA transcription factors (described above) that are involved in RES oxylipin-mediated signaling of GSTs and OPRs in Arabidopsis (Mueller et al., 2008; Mueller and Berger, 2009).
A Possible Link between OPR Expression and Safener-Induced Detoxification
Information is limited regarding the biochemical and molecular events that occur between initial safener treatment and the end result (e.g. increased GST activity and enhanced herbicide metabolism; Fig. 2). A few possible mechanisms for safener-regulated signaling have been proposed in the literature. Safeners may modulate the activity or abundance of transcriptional activators (or repressors) that interact with regulatory elements in the promoters of plant defense genes and therefore transcriptionally regulate the expression of genes encoding enzymes involved in herbicide detoxification (Hatzios, 1989; Davies and Caseley, 1999; Hatzios and Burgos, 2004). Our previous research utilized proteomic and targeted transcriptomic approaches to describe the molecular components of the herbicide detoxification pathway in wheat seedling tissues (Zhang et al., 2007). A novel finding was that three OPR isoforms were highly induced by safener treatment and were differentially expressed in etiolated tissues of T. tauschii seedlings. OPRs belong to a group of flavin-dependent oxidoreductases as part of a multigene family common to all plants (Schaller, 2001; Strassner et al., 2002); they are well known for their involvement in JA biosynthesis and responses to numerous biotic and abiotic stimuli (Schaller and Stintzi, 2009). For example, plant OPR genes are induced by a variety of stimuli including touch, wounding, UV light, phytohormones, xenobiotics, and pathogen attack (Stintzi et al., 2001; Strassner et al., 2002; Beynon et al., 2009; for review, see Schaller and Stintzi, 2009). Several OPR proteins (Zhang et al., 2007) and transcripts (Q. Zhang and D.E. Riechers, unpublished data) are strongly induced by safener treatment in T. tauschii seedling tissues. Consequently, it is possible that safeners may be tapping into a RES oxylipin-mediated signaling pathway (Loeffler et al., 2005; Taki et al., 2005), as evidenced by the induction of OPR expression (Fig. 2), which subsequently leads to the expression of GSTs and other proteins involved in detoxification and plant defense in a tissue-specific manner (Zhang et al., 2007). Accordingly, another study using the model dicot Populus to analyze safener-inducible ESTs (via cDNA microarray) showed that one OPR transcript was induced 5-fold by safeners, which was further confirmed by reverse transcription-PCR (Rishi et al., 2004). In Arabidopsis seedlings, it is interesting that many of the detoxification/defense genes listed in Table I that are induced by RES oxylipins and safeners are also induced by treatment with the allelochemical benzoxazolin-2(3H)-one (including the gene encoding AtOPR2; Baerson et al., 2005).
Cyclopentenone levels (such as OPDA and dinor-OPDA) were increased several-fold in response to RES treatment (acrolein) in Arabidopsis, while JA levels displayed only a small increase (Alméras et al., 2003). Since cyclopentenones (i.e. JA precursors) and cyclopentanones (i.e. JA) differ in their biological activities (Stintzi et al., 2001; Taki et al., 2005), the reduction of the cyclopentenone ring (via OPR activity) may therefore be particularly important for regulation of the pathway, as it controls the relative levels of these two classes of signaling molecules (Schaller and Stintzi, 2009). Increased levels of cyclopentenones in response to acrolein may have arisen from RES-stimulated lipase activity on esterified cyclopentenone pools or from de novo biosynthesis (Alméras et al., 2003). Interestingly, OPR transcripts were also induced by RES treatment in this system. Since safeners increase OPR expression as well (Rishi et al., 2004; Zhang et al., 2007), are safeners triggering the massive accumulation of cyclopentenones, thereby leading to the induction of GST and OPR proteins that facilitate conjugation and reduction, respectively, of the electrophilic substrates OPDA and/or A1-phytoprostanes (Mueller et al., 2008)? To directly test this hypothesis, a metabolite profiling experiment could be performed in maize, wheat, or rice seedlings in response to safener treatment to quantify levels of cyclopentenones and JA (Alméras et al., 2003).
FUTURE PROSPECTS FOR HERBICIDE SAFENERS
Safeners are an intriguing group of chemicals that possess an unknown molecular mechanism for protecting cereal crops from herbicide injury. Safeners strongly induce the expression of plant defense and detoxification genes, such as GSTs, P450s, OPRs, UGTs, and ABC transporters, yet they are not toxic to plants. As described above, it is likely that safeners tap into preexisting signaling pathways for detoxification, possibly via a RES oxylipin-mediated signaling pathway (Mueller and Berger, 2009), which subsequently leads to the expression of GSTs and other proteins involved in herbicide detoxification and defense responses (Fig. 2). Several unique applications of safeners toward crop improvement and plant biology are discussed below, along with several areas of research that require further attention and detailed information regarding safener mechanism of action.
Crop Improvement and Precise Control of Transgene Expression in Plants
Although safeners do not improve herbicide tolerance in dicot plants such as Arabidopsis (DeRidder et al., 2002; DeRidder and Goldsbrough, 2006) or soybean (Glycine max; Hatzios, 1989; Davies and Caseley, 1999), it may be possible to utilize biotechnology to extend the safener response from monocot to dicot crops. Paradoxically, dicots do not respond to safeners at the whole-plant level despite significant increases in GST and MRP expression (DeRidder et al., 2002; DeRidder and Goldsbrough, 2006). Several hypotheses exist for the lack of dicot response to safeners toward improving herbicide tolerance, including lack of herbicide-metabolizing GST isozymes with appropriate substrate specificity, lack of the proper tissue-specific expression of herbicide-detoxifying enzymes, a defect in some other aspect of the overall herbicide detoxification pathway, or some combination of these components. If, however, the safener response could be engineered into dicot crops, this would offer new weed management options by improving the margin of herbicide selectivity between dicot crops and target weed species as well as providing new chemical control options for managing herbicide-resistant weeds.
Additionally, knowledge of critical regulatory elements in the promoters or untranslated regions of genes encoding detoxification enzymes, or a comprehensive understanding of how gene expression is up-regulated by safeners, might lead to the precise manipulation of transgene expression in plants. For example, GST gene promoters could be used to introduce novel safener-responsive traits into important cereal crops such as maize, grain sorghum, wheat, rice, and barley (Ward et al., 1993) or possibly perennial grasses grown for biofuels in the future, such as switchgrass (Panicum species) or Miscanthus. This new technology would also be extremely useful from the standpoint of developing new gene expression systems for basic plant biology applications. Defining the functions of candidate genes by introducing them into plants and chemically inducing gene expression through safener application to the foliage of a plant would allow researchers to observe the resulting phenotype at any given developmental or growth stage (Zuo and Chua, 2000; Padidam, 2003).
Future Research Areas to Explore
One area of great interest and relevance to understanding safener-mediated signaling is to determine whether safeners trigger an increase in levels of specific RES oxylipins (Mosblech et al., 2009), which could be accomplished via metabolite profiling techniques. Safeners affect plant secondary metabolism in wheat and the grass weed Alopecurus myosuroides (Cummins et al., 2006, 2009); as a result, in addition to providing insight into safener-regulated signaling mechanisms, it is important to determine how specific or precise the use of safeners might be for controlling transgene expression in plants (Ward et al., 1993).
Another important area of safener research that is currently lacking is to determine the metabolic fate of additional safeners in cereal crops. For example, is there a common structural motif shared among diverse safeners (Fig. 1) that is important in conferring safening activity in cereal crops, other than just being a strong electrophile (Farmer and Davoine, 2007)? Is mono- or di-GSH conjugate formation important (Miller et al., 1996), as indicated by the proposed “metabolic reactivation” theory with fenclorim (Brazier-Hicks et al., 2008) via further catabolic activities? Is P450 involvement (via oxidative metabolism or other phase I reactions) requisite for activation and further glutathionylation and GST induction by safeners (Fig. 2)? Accordingly, would treatment with a specific P450 inhibitor, such as tetcyclacis, attenuate the strong induction of GSTs by safeners?
Regarding biochemical and molecular aspects of safener mechanism of action pertaining to the regulation of cellular detoxification pathways, it is important to ascertain organ-, tissue-, and cell-specific patterns of gene expression (Xu et al., 2002; Riechers et al., 2003; Zhang et al., 2007), determine the subcellular locations of the encoded detoxification proteins before and after exposure to diverse stresses (Riechers et al., 2005), and further investigate whether posttranslational modification of detoxification proteins plays a role in safener-regulated herbicide metabolism (Zhang and Riechers, 2004; Brazier-Hicks et al., 2008). Additionally, temporal patterns of gene expression (as reported with different herbicides by Pasquer et al., 2006) in response to safeners or RES oxylipins would be extremely informative from the standpoint of understanding the time course of plant responses to a potent inducer of xenobiotic detoxification, in comparison with endogenous RES oxylipin signaling molecules. From a molecular standpoint, it would be of great interest to determine if regulation of OPR gene expression in response to various stresses occurs at the transcriptional, posttranscriptional, or posttranslational level. From a biochemical perspective, the cellular transport, fate, and metabolism of OPDA-GSH and phytoprostane-GSH conjugates merit further investigation in plants.
References
- Alméras E, Stolz S, Vollenweider S, Reymond P, Mène-Saffrané L, Farmer EE. (2003) Reactive electrophile species activate defense gene expression in Arabidopsis. Plant J 34: 205–216 [DOI] [PubMed] [Google Scholar]
- Baerson SR, Sánchez-Moreiras A, Pedrol-Bonjoch N, Schulz M, Kagan IA, Agarwal AK, Reigosa MJ, Duke SO. (2005) Detoxification and transcriptome response in Arabidopsis seedlings exposed to the allelochemical benzoxazolin-2(3H)-one. J Biol Chem 280: 21867–21881 [DOI] [PubMed] [Google Scholar]
- Barrett M. (2000) The role of cytochrome P450 enzymes in herbicide metabolism. Cobb AH, Kirkwood RC, , Herbicides and Their Mechanisms of Action. CRC Press, Boca Raton, FL, pp 25–37 [Google Scholar]
- Barrett M, Polge N, Baerg R, Bradshaw R, Poneleit C. (1997) Role of cytochrome P450 in herbicide metabolism and selectivity and multiple herbicide metabolizing cytochrome P450 activities in maize. Hatzios KK, , Regulation of Enzymatic Systems Detoxifying Xenobiotics in Plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 35–50 [Google Scholar]
- Beynon ER, Symons ZC, Jackson RG, Lorenz A, Rylott EL, Bruce NC. (2009) The role of oxophytodienoate reductases in the detoxification of the explosive 2,4,6-trinitrotoluene by Arabidopsis. Plant Physiol 151: 253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounds SVJ, Hutson DH. (2000) The comparative metabolism of agrochemicals in plants and mammals. Roberts T, , Metabolism of Agrochemicals in Plants. John Wiley & Sons, Chichester, UK, pp 179–209 [Google Scholar]
- Bowles D, Lim EK, Poppenberger B, Vaistij FE. (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57: 567–597 [DOI] [PubMed] [Google Scholar]
- Brazier-Hicks M, Evans KM, Cunningham OD, Hodgson DRW, Steel P, Edwards R. (2008) Catabolism of glutathione conjugates in Arabidopsis thaliana: role in metabolic reactivation of the herbicide safener fenclorim. J Biol Chem 283: 21102–21112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaux EJ, Hoobler MA, Patanella JE, Leyes GA. (1989) Mechanisms of action of thiazole safeners. Hatzios KK, Hoagland RE, , Crop Safeners for Herbicides: Development, Uses, and Mechanisms of Action. Academic Press, San Diego, pp 163–175 [Google Scholar]
- Creelman RA, Mullet JE. (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355–381 [DOI] [PubMed] [Google Scholar]
- Cummins I, Brazier-Hicks M, Stobiecki M, Franski R, Edwards R. (2006) Selective disruption of wheat secondary metabolism by herbicide safeners. Phytochemistry 67: 1722–1730 [DOI] [PubMed] [Google Scholar]
- Cummins I, Bryant DN, Edwards R. (2009) Safener responsiveness and multiple herbicide resistance in the weed black-grass (Alopecurus myosuroides). Plant Biotechnol J 7: 807–820 [DOI] [PubMed] [Google Scholar]
- Davies J, Caseley JC. (1999) Herbicide safeners: a review. Pestic Sci 55: 1043–1058 [Google Scholar]
- DeRidder BP, Dixon DP, Beussman DJ, Edwards R, Goldsbrough PB. (2002) Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol 130: 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRidder BP, Goldsbrough PB. (2006) Organ-specific expression of glutathione S-transferases and the efficacy of herbicide safeners in Arabidopsis. Plant Physiol 140: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Edwards R. (2009) Selective binding of glutathione conjugates of fatty acid derivatives by plant glutathione transferases. J Biol Chem 284: 21249–21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R. (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60: 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Lapthorn A, Edwards R. (2002) Plant glutathione transferases. Genome Biol 3: 3004.1–3004.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon DP, Skipsey M, Edwards R. (2010) Roles for glutathione transferases in plant secondary metabolism. Phytochemistry 71: 338–350 [DOI] [PubMed] [Google Scholar]
- Edwards R, Dixon DP, Walbot V. (2000) Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Ekman DR, Lorenz WW, Przybyla AE, Wolfe NL, Dean JF. (2003) SAGE analysis of transcriptome responses in Arabidopsis roots exposed to 2,4,6-trinitrotoluene. Plant Physiol 133: 1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farago S, Brunold C, Kreuz K. (1994) Herbicide safeners and glutathione metabolism. Physiol Plant 91: 537–542 [Google Scholar]
- Farmer EE, Davoine C. (2007) Reactive electrophile species. Curr Opin Plant Biol 10: 380–386 [DOI] [PubMed] [Google Scholar]
- Fode B, Siemsen T, Thurow C, Weigel R, Gatz C. (2008) The Arabidopsis GRAS protein SCL14 interacts with class II TGA transcription factors and is essential for the activation of stress-inducible promoters. Plant Cell 20: 3122–3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuerst EP, Lamoureux GL, Ahrens WH. (1991) Mode of action of the dichloroacetamide antidote BAS 145-138 in corn. I. Growth responses and fate of metazachlor. Pestic Biochem Physiol 39: 138–148 [Google Scholar]
- Gaillard C, Dufaud A, Tommasini R, Kreuz K, Amrhein N, Martinoia E. (1994) A herbicide antidote (safener) induces the activity of both the herbicide-detoxifying enzyme and of a vacuolar transporter for the detoxified herbicide. FEBS Lett 352: 219–221 [DOI] [PubMed] [Google Scholar]
- Glombitza S, Dubuis PH, Thulke O, Welzl G, Bovet L, Gotz M, Affenzeller M, Geist B, Hehn A, Asnaghi C, et al. (2004) Crosstalk and differential response to abiotic and biotic stressors reflected at the transcriptional level of effector genes from secondary metabolism. Plant Mol Biol 54: 817–835 [DOI] [PubMed] [Google Scholar]
- Grzam A, Martin MN, Hell R, Meyer AJ. (2007) γ-Glutamyl transpeptidase GGT4 initiates vacuolar degradation of glutathione S-conjugates in Arabidopsis. FEBS Lett 581: 3131–3138 [DOI] [PubMed] [Google Scholar]
- Hatzios KK. (1989) Mechanisms of action of herbicide safeners: an overview. Hatzios KK, Hoagland RE, , Crop Safeners for Herbicides: Development, Uses, and Mechanisms of Action. Academic Press, San Diego, pp 65–101 [Google Scholar]
- Hatzios KK, Burgos N. (2004) Metabolism-based herbicide resistance: regulation by safeners. Weed Sci 52: 454–467 [Google Scholar]
- Hess FD. (2000) Light-dependent herbicides: an overview. Weed Sci 48: 160–170 [Google Scholar]
- Klein M, Burla B, Martinoia E. (2006) The multidrug resistance-associated protein (MRP/ABCC) subfamily of ATP-binding cassette transporters in plants. FEBS Lett 580: 1112–1122 [DOI] [PubMed] [Google Scholar]
- Komives T, Hatzios KK. (1991) Chemistry and structure-activity relationships of herbicide safeners. Z Naturforsch 46c: 798–804 [Google Scholar]
- Kreuz K, Gaudin J, Ebert E. (1989) Effects of the safeners CGA 154281, oxabetrinil and fenclorim on uptake and degradation of metolachlor in corn (Zea mays L.) seedlings. Weed Res 29: 399–405 [Google Scholar]
- Kreuz K, Gaudin J, Stingelin J, Ebert E. (1991) Metabolism of the aryloxyphenoxypropanoate herbicide, CGA 184927, in wheat, barley and maize: differential effects of the safener, CGA 185072. Z Naturforsch 46c: 901–905 [Google Scholar]
- Kreuz K, Tommasini R, Martinoia E. (1996) Old enzymes for a new job: herbicide detoxification in plants. Plant Physiol 111: 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay MM, Hubbell JP, Casida JE. (1975) Dichloroacetamide antidotes for thiocarbamate herbicides: mode of action. Science 189: 287–289 [DOI] [PubMed] [Google Scholar]
- Loeffler C, Berger S, Guy A, Durand T, Bringmann G, Dreyer M, von Rad U, Durner J, Mueller MJ. (2005) B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol 137: 328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N. (1993) An ATP-dependent glutathione S-conjugate “export” pump in the vacuolar membrane of plants. Nature 364: 247–249 [Google Scholar]
- Miller KD, Irzyk GP, Fuerst E, McFarland JE, Barringer M, Cruz S, Eberle WJ, Föry W. (1996) Identification of metabolites of the herbicide safener benoxacor isolated from suspension-cultured Zea mays cells 3 and 24 hours after treatment. J Agric Food Chem 44: 3335–3341 [Google Scholar]
- Mosblech A, Feussner I, Heilmann I. (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47: 511–517 [DOI] [PubMed] [Google Scholar]
- Mueller MJ, Berger S. (2009) Reactive electrophilic oxylipins: pattern recognition and signalling. Phytochemistry 70: 1511–1521 [DOI] [PubMed] [Google Scholar]
- Mueller S, Hilbert B, Dueckershoff K, Roitsch T, Krischke M, Mueller MJ, Berger S. (2008) General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell 20: 768–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naruska Y, Naruska M, Seki M, Umezawa T, Ishida J, Nakajima M, Enju A, Shinozaki K. (2004) Crosstalk in the responses to abiotic and biotic stresses in Arabidopsis: analysis of gene expression in cytochrome P450 gene superfamily by cDNA microarray. Plant Mol Biol 55: 327–342 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Schuler MA, Paquette SM, Werck-Reichhart D, Bak S. (2004) Comparative genomics of rice and Arabidopsis: analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol 135: 756–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T, Nioi P, Pickett CB. (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordby JN, Williams MM, II, Pataky JK, Riechers DE, Lutz JD. (2008) A common genetic basis in sweet corn inbred Cr1 for cross sensitivity to multiple cytochrome P450-metabolized herbicides. Weed Sci 56: 376–382 [Google Scholar]
- Osmani SA, Bak S, Møller BL. (2009) Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry 70: 325–347 [DOI] [PubMed] [Google Scholar]
- Padidam M. (2003) Chemically regulated gene expression in plants. Curr Opin Plant Biol 6: 169–177 [DOI] [PubMed] [Google Scholar]
- Pan G, Zhang X, Liu K, Zhang J, Wu X, Zhu J, Tu J. (2006) Map-based cloning of a novel rice cytochrome P450 gene CYP81A6 that confers resistance to two different classes of herbicides. Plant Mol Biol 61: 933–943 [DOI] [PubMed] [Google Scholar]
- Pasquer F, Ochsner U, Zarn J, Keller B. (2006) Common and distinct gene expression patterns induced by the herbicides 2,4-dichlorophenoxyacetic acid, cinidon-ethyl and tribenuron-methyl in wheat. Pest Manag Sci 62: 1155–1167 [DOI] [PubMed] [Google Scholar]
- Persans MW, Wang J, Schuler MA. (2001) Characterization of maize cytochrome P450 monooxygenases induced in response to safeners and bacterial pathogens. Plant Physiol 125: 1126–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P, Farmer EE. (1998) Jasmonate and salicylate as global signals for defense gene expression. Curr Opin Plant Biol 1: 404–411 [DOI] [PubMed] [Google Scholar]
- Riechers DE, Vaughn KC, Molin WT. (2005) The role of plant glutathione S-transferases in herbicide metabolism. Clark JM, Ohkawa H, , Environmental Fate and Safety Management of Agrochemicals. ACS Symposium Series 899. American Chemical Society, Washington, DC, pp 216–232 [Google Scholar]
- Riechers DE, Zhang Q, Xu FX, Vaughn KC. (2003) Tissue-specific expression and localization of safener-induced glutathione S-transferase proteins in Triticum tauschii. Planta 217: 831–840 [DOI] [PubMed] [Google Scholar]
- Rishi A, Muni S, Kapur V, Nelson ND, Goyal A. (2004) Identification and analysis of safener-inducible expressed sequence tags in Populus using a cDNA microarray. Planta 220: 296–306 [DOI] [PubMed] [Google Scholar]
- Roberts TR. (1998) Cloquintocet-mexyl. Hutson DH Lee PW Nicholls PH Plimmer JR, , Metabolic Pathways of Agrochemicals. Part 1. Herbicides and Plant Growth Regulators. Royal Society of Chemistry, Cambridge, UK, pp 833–836 [Google Scholar]
- Sappl PG, Carroll AJ, Clifton R, Lister R, Whelan J, Millar AH, Singh KB. (2009) The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J 58: 53–68 [DOI] [PubMed] [Google Scholar]
- Schaller A, Stintzi A. (2009) Enzymes in jasmonate biosynthesis: structure, function, regulation. Phytochemistry 70: 1532–1538 [DOI] [PubMed] [Google Scholar]
- Schaller F. (2001) Enzymes of the biosynthesis of octadecanoid-derived signalling molecules. J Exp Bot 52: 11–23 [PubMed] [Google Scholar]
- Scott-Craig JS, Casida JE, Poduje L, Walton JD. (1998) Herbicide safener-binding protein of maize: purification, cloning, and expression of an encoding cDNA. Plant Physiol 116: 1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KB, Foley RC, Onate-Sanchez L. (2002) Transcription factors in plant defense and stress responses. Curr Opin Plant Biol 5: 430–436 [DOI] [PubMed] [Google Scholar]
- Stintzi A, Weber H, Reymond P, Browse J, Farmer EE. (2001) Plant defense in the absence of jasmonic acid: the role of cyclopentenones. Proc Natl Acad Sci USA 98: 12837–12842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassner J, Schaller F, Frick UB, Howe GA, Weiler EW, Amrhein N, Macheroux P, Schaller A. (2002) Characterization and cDNA-microarray expression analysis of 12-oxophytodienoate reductases reveals differential roles for octadecanoid biosynthesis in the local versus the systemic wound response. Plant J 32: 585–601 [DOI] [PubMed] [Google Scholar]
- Taki N, Sasaki-Sekimoto Y, Obayashi T, Kikuta A, Kobayashi K, Ainai T, Yagi K, Sakurai N, Suzuki H, Masuda T, et al. (2005) 12-Oxo-phytodienoic acid triggers expression of a distinct set of genes and plays a role in wound-induced gene expression in Arabidopsis. Plant Physiol 139: 1268–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoulou FL, Clark IM, He XL, Pallett KE, Cole DJ, Hallahan DL. (2003) Co-induction of glutathione S-transferases and multidrug resistance associated protein by xenobiotics in wheat. Pest Manag Sci 59: 202–214 [DOI] [PubMed] [Google Scholar]
- Trenkamp S, Martin W, Tietjen K. (2004) Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc Natl Acad Sci USA 101: 11903–11908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JD, Casida JE. (1995) Specific binding of a dichloroacetamide herbicide safener in maize at a site that also binds thiocarbamate and chloroacetanilide herbicides. Plant Physiol 109: 213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward ER, Ryals JA, Miflin BJ. (1993) Chemical regulation of transgene expression in plants. Plant Mol Biol 22: 361–366 [DOI] [PubMed] [Google Scholar]
- Weber H. (2002) Fatty-acid derived signals in plants. Trends Plant Sci 7: 217–224 [DOI] [PubMed] [Google Scholar]
- Wiegand RC, Shah DM, Mozer TJ, Harding EI, Diaz-Collier J, Saunders C, Jaworski EG, Tiemeier DC. (1986) Messenger RNA encoding a glutathione S-transferase responsible for herbicide tolerance in maize is induced in response to safener treatment. Plant Mol Biol 7: 235–243 [DOI] [PubMed] [Google Scholar]
- Wolf AE, Dietz KJ, Schröder P. (1996) Degradation of glutathione S-conjugates by a carboxypeptidase in the plant vacuole. FEBS Lett 384: 31–34 [DOI] [PubMed] [Google Scholar]
- Xiang C, Miao ZH, Lam E. (1996) Coordinated activation of as-1-type elements and a tobacco glutathione S-transferase gene by auxins, salicylic acid, methyl-jasmonate and hydrogen peroxide. Plant Mol Biol 32: 415–426 [DOI] [PubMed] [Google Scholar]
- Xu FX, Lagudah ES, Moose SP, Riechers DE. (2002) Tandemly duplicated safener-induced glutathione S-transferase genes from Triticum tauschii contribute to genome- and organ-specific expression in hexaploid wheat. Plant Physiol 130: 362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zander M, Camera SL, Lamotte O, Métraux JP, Gatz C. (2010) Arabidopsis thaliana class-II TGA transcription factors are essential activators of jasmonic acid/ethylene-induced defense responses. Plant J 61: 200–210 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Riechers DE. (2004) Proteomic characterization of herbicide safener-induced proteins in the coleoptile of Triticum tauschii seedlings. Proteomics 4: 2058–2071 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Xu FX, Lambert KN, Riechers DE. (2007) Safeners coordinately induce the expression of multiple proteins and MRP transcripts involved in herbicide metabolism and detoxification in Triticum tauschii seedling tissues. Proteomics 7: 1261–1278 [DOI] [PubMed] [Google Scholar]
- Zuo J, Chua NH. (2000) Chemical-inducible systems for regulated expression of plant genes. Curr Opin Biotechnol 11: 146–151 [DOI] [PubMed] [Google Scholar]