Abstract
The maize (Zea mays) Miniature1 (Mn1) locus encodes the cell wall invertase INCW2, which is localized predominantly in the basal endosperm transfer layer of developing kernels and catalyzes the conversion of sucrose into glucose and fructose. Mutations in Mn1 result in pleiotropic changes, including a reduction in kernel mass and a recently reported decrease in indole-3-acetic acid (IAA) levels throughout kernel development. Here, we show that mn1-1 basal kernel regions (pedicels and basal endosperm transfer layer) accumulate higher levels of sucrose and lower levels of glucose and fructose between 8 and 28 d after pollination when compared with the wild type, whereas upper regions of mn1 accumulate similar or increased concentrations of sugars. To determine the cause of the reduction in IAA accumulation, we investigated transcript levels of several potential IAA biosynthetic enzymes. We demonstrate that reduced IAA levels most closely correspond to reduced transcript levels of ZmYUCCA (ZmYUC), a newly identified homolog of the Arabidopsis (Arabidopsis thaliana) gene YUCCA. We further demonstrate that ZmYUC catalyzes the N-hydroxylation of tryptamine and that sugar levels regulate transcript levels of ZmYUC, both in in vitro-cultured kernels and in a promoter-reporter fusion in Arabidopsis. These results indicate that developing seeds may modulate growth by altering auxin biosynthesis in response to sugar concentrations.
Establishment of sink strength in plants is dependent on several factors, including cell number, cell size, and metabolic activity in the sink tissue. We have recently shown that cell wall invertase-deficient miniature1 (mn1) kernels have greatly reduced levels of the auxin indole-3-acetic acid (IAA) throughout kernel development (LeClere et al., 2008). Cell wall invertases (INCWs) catalyze the conversion of Suc into Glc and Fru and play a critical role in establishing sink strength (Zhang et al., 1996; Weber et al., 1997; Lara et al., 2004; Roitsch and Gonzalez, 2004). Maize (Zea mays) INCW2, the primary invertase in immature maize kernels, is encoded by the Mn1 locus, and mn1 mutants result from point mutations in this locus that destabilize the INCW2 transcript or protein (Miller and Chourey, 1992; Cheng et al., 1996), resulting in a small-kernel phenotype. The mn1-1 allele is the result of a spontaneous mutation that causes a complete loss of INCW2 expression (Taliercio et al., 1999). The mn1-1 seed mutant shows a loss of 98% of the total invertase activity in kernels 12 d after pollination (DAP) and a reduction of nearly 70% in mature seed weight (Cheng et al., 1996; Carlson et al., 2000). INCW2 has been shown by immunohistochemistry to accumulate in the basal endosperm transfer layer (BETL), and it has been hypothesized that the inability of mn1 mutants to cleave incoming Suc results in a deficiency in free apoplastic hexose in the BETL. This deficiency is expected to result in decreased sugar uptake, thus leading to the decreased seed size phenotype of mn1. Our most recent data show that the Mn1-encoded INCW2 is in fact essential for the normal development of the BETL in developing endosperm (Kang et al., 2009).
Currently, there are hypothesized to be at least two pathways for the de novo biosynthesis of IAA: the Trp-dependent and the Trp-independent pathways. Evidence for the existence of a Trp-dependent pathway comes from young seedling tissues (Bialek et al., 1992; Koshiba et al., 1995), wounded tissues (Sztein et al., 2002), and maize kernels (Glawischnig et al., 2000). This pathway uses Trp as a precursor and proceeds through Trp decarboxylase to tryptamine (the TAM pathway), which is converted into N-hydroxytryptamine by the flavin monooxygenase YUCCA (YUC), further metabolized into indole-3-acetaldoxime and indole-3-acetaldehyde (IAAld), and oxidized to yield IAA (for review, see Bartel et al., 2001; Woodward and Bartel, 2005). Overexpression of AtYUC in Arabidopsis (Arabidopsis thaliana; Zhao et al., 2001) and the YUC-like gene OsYUC1 in rice (Oryza sativa; Yamamoto et al., 2007) results in high endogenous IAA levels, indicating that it catalyzes a rate-limiting step in Trp-dependent IAA biosynthesis in plants. A tomato (Solanum lycopersicum) YUC homolog has also been shown to catalyze the N-hydroxylation of tryptamine (Expósito-Rodríguez et al., 2007). Recently, another branch of the Trp-dependent pathway has been demonstrated in plants. This pathway utilizes the Trp aminotransferase TAA1 to catalyze the conversion of Trp into indole-3-pyruvic acid (the IPA pathway), which is further metabolized into IAA (Stepanova et al., 2008; Tao et al., 2008). The Trp-independent pathway was identified in the maize Trp auxotroph orange pericarp and the Arabidopsis Trp synthase mutants trp2 and trp3 (Wright et al., 1991; Normanly et al., 1993). This pathway is believed to use indole or indole-3-glycerol phosphate as a precursor for IAA biosynthesis, but no enzymes or intermediates in the Trp-independent pathway have yet been identified (Woodward and Bartel, 2005). Precursor labeling studies, however, have provided evidence for the existence of this pathway in intact Lemna gibba plants, carrot (Daucus carota) embryos, and bean (Phaseolus vulgaris) seedlings (Baldi et al., 1991; Michalczuk et al., 1992; Sztein et al., 2002). Indole could be a precursor to both pathways, as it is known to be part of the Trp biosynthetic pathway, and unlike Trp, it has auxin-like activity in Avena coleoptiles, suggesting that it can be converted to IAA independently from Trp (Winter, 1966). In addition to de novo synthesis, IAA can also be converted to and from storage forms such as sugar and amide conjugates (Bartel et al., 2001; Staswick et al., 2002, 2005).
To further investigate the observed IAA deficit in developing mn1 kernels, we investigated the expression levels of previously identified maize auxin biosynthetic genes and identified new maize homologs of known auxin biosynthetic enzymes from other plant species based on sequence homology. Most importantly, we identified a maize homolog of the Arabidopsis YUC gene, which we designated ZmYUC. ZmYUC is expressed highly in developing maize kernels, and this expression is dramatically reduced in mn1-1 mutants. In addition, we show that ZmYUC expression is regulated by sugars.
RESULTS
Suc, Glc, and Fru Accumulation Is Altered in mn1 Kernels
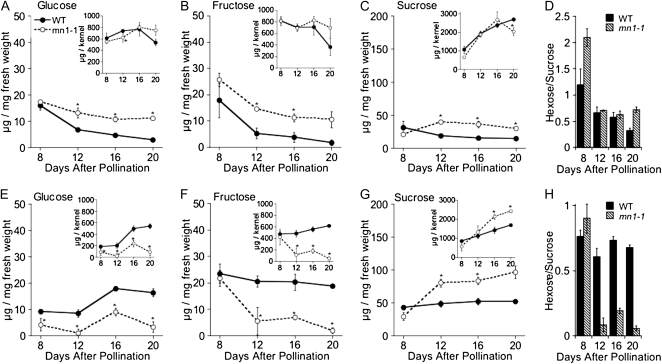
Kernels deficient in INCW2 are expected to display reduced conversion of Suc to hexose; thus, we first sought to measure the sugar content in the functionally distinct storage (upper) and uptake (basal) regions of developing kernels. Knowledge of sugar content and distribution is necessary to determine if Suc accumulates at the base of the kernel as hypothesized, and if so, how this affects sugar content in storage regions. Therefore, we measured the levels of Suc, Glc, and Fru in wild-type and mn1-1 basal regions and upper regions from developing kernels. As shown in Figure 1, basal sections of kernels, which include pedicel, BETL, and closely associated endosperm tissues, show increased levels of Suc (Fig. 1G) and decreased levels of Glc and Fru in mn1-1 at 12, 16, and 20 DAP (Fig. 1, E and F) when expressed either as μg per mg fresh weight or μg per kernel (Fig. 1, E–G, insets), indicating that the loss of INCW2 leads to an accumulation of Suc at the base of the kernel and an alteration in the Suc-to-hexose ratio in these tissues as predicted (Fig. 1H). Unexpectedly, upper kernel regions of the wild type and mn1-1, which include endosperm, aleurone, and pericarp tissues, display increased concentrations of Glc, Fru, and Suc per mg fresh weight at 12, 16, and 20 DAP (Fig. 1, A–C). In addition, when expressed on a per kernel basis, upper kernel regions from the mutant accumulate similar total amounts of Glc and Fru as the wild type and have only a slight decrease in Suc at 20 DAP (Fig. 1, A–C, insets). Thus, despite the loss of cell wall invertase activity in the BETL, the kernel maintains normal to increased levels of Suc and hexose in storage tissues.
Figure 1.
Sugar levels in upper and basal kernel sections of the wild type (WT) and mn1-1. Glc (A and E), Fru (B and F), and Suc (C and G) were measured in upper sections (A–C) and basal sections (E–G) of maize kernels at 8, 12, 16, and 20 DAP. Data are presented as μg of sugar per mg fresh weight, with insets showing results expressed as μg per kernel. Error bars are sd (n = 3). Asterisks denote data points that are significantly different from the wild type (P ≤ 0.05). D and H show the hexose-to-Suc ratios in basal and upper kernel sections as calculated from the data in A to C and E to G.
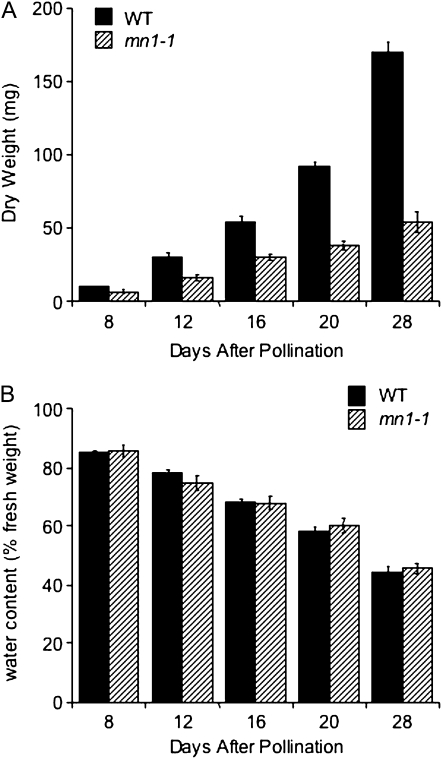
One theory is that mn1-1 has a reduced osmotic potential due to the invertase defect, which results in decreased water uptake and thus a decreased kernel mass, thereby accounting for the increased concentration of sugars on a per mg basis. To explore this possibility, we measured the fresh and dry weights of mn1-1 and wild-type kernels at 8, 12, 16, 20, and 28 DAP. We found that although the overall kernel mass of mn1-1 is reduced on both a fresh and dry weight basis, the percentage water content is identical to that of the wild type (Fig. 2). The increase in concentration of free sugars, therefore, is not an artifact of decreased osmotic potential but rather likely represents differences in downstream utilization pathways, such as starch biosynthesis, in the mutant.
Figure 2.
Water content in the wild type (WT) and mn1-1. A, Fresh weights of kernels were recorded, then kernels were dried and dry weights were measured. B, Percentage water was calculated as 100% dry weight/fresh weight.
Kernels of mn1-1 Have Altered Transcript Levels of Auxin Metabolic Genes
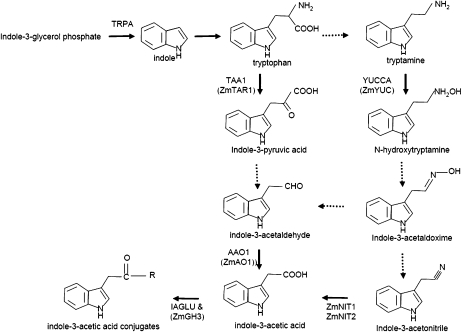
To explore the molecular basis for the large decrease in IAA levels in mn1-1 (LeClere et al., 2008), we measured expression levels of several known and putative IAA biosynthetic genes. The IAA pathway and enzymes are illustrated in Figure 3. PCR primers were designed from sequences annotated in GenBank as Trp synthase α-subunit (TRPA; Kramer and Koziel, 1995) and aldehyde oxidase (ZmAO1; Sekimoto et al., 1997). Because it is also possible that the decreased IAA levels could be due to increased conjugation or degradation of IAA, we also designed primers for IAGLU, an enzyme shown to conjugate IAA to Glc in maize (Szerszen et al., 1994). To identify additional maize genes possibly involved in IAA metabolism, we performed homology searches using BLAST analysis (www.ncbi.nlm.nih.gov/BLAST) against all translated maize sequences (TBLASTN) with known proteins from Arabidopsis. These homology searches uncovered one annotated but unpublished EST (CV985267) encoding a predicted protein with 53% amino acid identity to GH3.6 (At5g54510), which has been shown to conjugate IAA to certain amino acids (Staswick et al., 2002). We have designated this homolog as ZmGH3 (Supplemental Fig. S1). We also identified one nonannotated EST (BT016604) predicted to encode a protein with 40% amino acid identity to the tryptamine-hydroxylating enzyme AtYUC (Zhao et al., 2001), which we designated ZmYUC (Supplemental Fig. S2).
Figure 3.
Trp-dependent IAA biosynthesis. Solid arrows indicate known steps. Dashed arrows indicate steps where enzymes have not yet been identified. Newly identified homologs are shown in parentheses under the known enzymes.
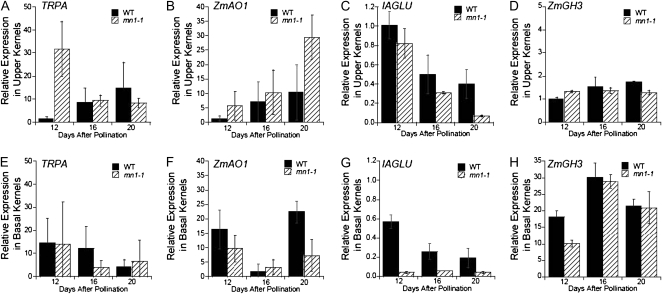
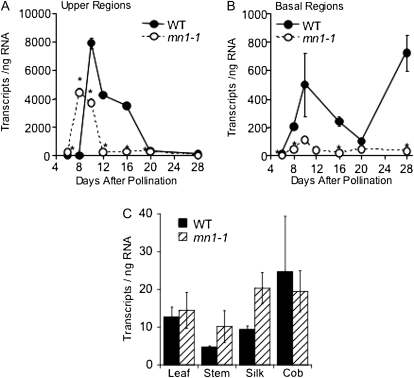
Relative expression levels of the above genes were measured by quantitative PCR (qPCR) in upper and basal regions of 8-, 12-, 16-, 20-, and 28-DAP kernels (Fig. 4). Six-DAP kernels were also included for ZmYUC (Fig. 5, A and B). Of the transcripts investigated, the only one that was consistently and significantly reduced in mn1-1 was ZmYUC (Figs. 4 and 5). In mn1-1, ZmYUC levels were reduced in basal kernels as early as 8 DAP and were consistently lower than the wild type in both upper and basal regions at 12, 16, and 20 DAP (Fig. 5, A and B). Interestingly, at 8 DAP, ZmYUC levels were transiently higher than in the wild type, and this was likewise the only time point at which we observed a lower Suc-Glc ratio in mn1-1 as compared with the wild type (Fig. 1, D and H). At 8 DAP, IAA levels begin to increase rapidly in the kernel but are not yet different between the wild type and mn1-1 (LeClere et al., 2008). To better understand the function of ZmYUC in maize, we looked at expression by qPCR in several other tissues as well. As shown in Figure 5C, whereas ZmYUC is expressed highly in kernels, it is expressed at low levels in other tissues tested.
Figure 4.
Relative expression of putative IAA biosynthetic genes in developing kernels. Relative expression levels (2−ΔΔCt) from real-time PCR analysis of TRPA (A and E), ZmAO1 (B and F), IAGLU (C and G), and ZmGH3 (D and H) in upper sections (A–D) and basal sections (E–H) are shown. Expression is normalized, with the expression level in wild-type (WT) 12-DAP upper sections being defined as 1. Error bars are sd (P < 0.05; n = 3).
Figure 5.
qPCR determination of ZmYUC transcript levels in wild-type (WT) and mn1 alleles. A and B, Transcript abundance of ZmYUC in upper (A) and basal (B) sections of 6-, 8-, 10-, 12-, 16-, 20-, and 28-DAP wild-type and mn1-1 kernels was determined by real-time PCR. C, ZmYUC transcript abundance in adult stem, leaf, silk, and cob tissues. For all qPCRs, a dilution series of ZmYUC-containing plasmid was used to calculate the number of transcripts per pg of total RNA. Error bars are sd, and asterisks denote data points significantly different from the wild type (P < 0.05; n = 3).
In addition to ZmYUC, levels of IAGLU and ZmGH3 transcripts were also decreased at one or more time points (Fig. 4). We previously reported that ester conjugates of IAA were decreased in mn1-1 kernels and amide conjugates were below detectable levels (LeClere et al., 2008). Whereas measurement of the levels of conjugates could not prove if conjugates were synthesized and rapidly degraded or simply not synthesized, we wanted to look at levels of conjugate synthase transcripts to gain insight into the auxin pathway regulation occurring in mn1-1. These qPCR data support the role of IAGLU in the synthesis of IAA-ester conjugates in developing endosperm (Szerszen et al., 1994), as the levels of esterified IAA conjugates are reduced similarly to IAGLU in mn1-1 (LeClere et al., 2008). GH3 genes are known to be regulated by auxin in Arabidopsis (Hagen and Guilfoyle, 1985); thus, it is not surprising to see decreased levels of these transcripts in auxin-deficient kernels. Taken together, these data show that both IAA biosynthesis and IAA conjugation are down-regulated in mn1-1.
Recombinant ZmYUC Catalyzes the N-Hydroxylation of Tryptamine in Vitro
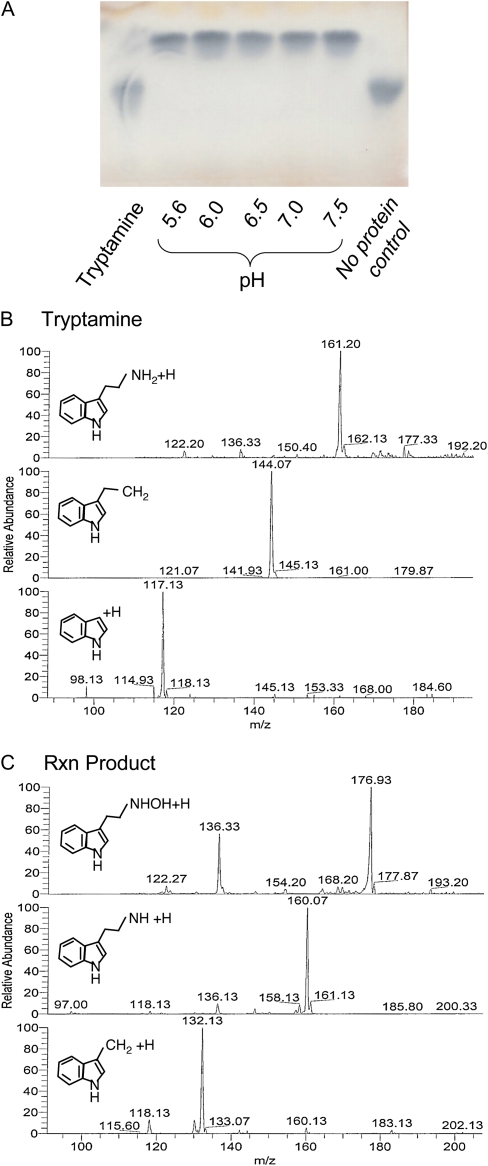
The predicted ZmYUC protein is 40% identical to an Arabidopsis enzyme shown to catalyze the N-hydroxylation of tryptamine, an apparent rate-limiting step in IAA biosynthesis. To determine whether the ZmYUC protein actually functions in the biosynthesis of IAA by N-hydroxylating tryptamine, we cloned the full-length cDNA, expressed the protein in Escherichia coli, and measured the activity of purified ZmYUC on tryptamine (Fig. 6). ZmYUC N-hydroxylates tryptamine in vitro to form N-hydroxytryptamine, as indicated by change in retention time by thin-layer chromatography (TLC; Fig. 6A) and change in Mr as indicated by mass spectroscopy (Fig. 6, B and C). The Mr of tryptamine is 160 and yields a [M+H]+ mass ion of 161 as expected. TLC-isolated reaction product produces a [M+H]+ mass ion of 177, the mass expected for hydroxytryptamine, which has Mr of 176. In addition, tandem mass spectrometry (MS2) data reveal a mass ion of 160 as expected for the loss of a hydroxyl group, and the major MS3 ion [M+H]+ of 132 indicates the further loss of CH2NH, as illustrated in Figure 6C. This demonstrates that ZmYUC catalyzes the N-hydroxylation of tryptamine, similar to its Arabidopsis and tomato homologs, consistent with a key role in Trp-dependent IAA biosynthesis (Zhao et al., 2001; Expósito-Rodríguez et al., 2007).
Figure 6.
Analysis of ZmYUC activity. A, Reaction products from bacterially expressed ZmYUC on tryptamine substrate analyzed by TLC. B and C, Mass spectra of tryptamine (B) and the TLC-purified reaction product, N-hydroxytryptamine (C). Top traces show the MS data from each compound, middle traces show MS2 data of the major peak from MS, and bottom panels show MS3 data for the major peak from MS2. Predicted structures for the major ions are shown in each trace. [See online article for color version of this figure.]
Metabolite Profiling
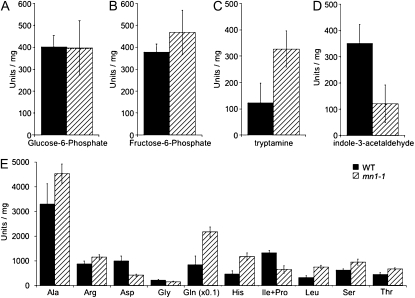
To further define any other metabolic changes in mn1-1 developing kernels, we used a gas chromatography (GC)-based approach to measure a number of sugar phosphates, amino acids, and auxin metabolites in whole 20-DAP kernels of both the wild type and mn1-1. As shown in Figure 7, we found no difference in the concentrations of Glc-6-P or Fru-6-P between the wild type and mn1-1. There was a difference in amino acid profiles, particularly with Gln, which was more abundant in mn1-1 than in the wild type. In addition, we found an increase in tryptamine and a decrease in IAAld, which is consistent with a decrease in tryptamine hydroxylation by ZmYUC.
Figure 7.
Metabolites in wild-type (WT) and mn1-1 20-DAP kernels. Metabolites from endosperm extracts of 20-DAP kernels were analyzed by GC. Shown are Glc-6-P (A), Fru-6-P (B), tryptamine (C), IAAld (D), and detectable amino acids (E). Note that Gln levels are scaled down 10-fold to allow illustration on the same graph.
Sugar Levels Regulate Expression of ZmYUC
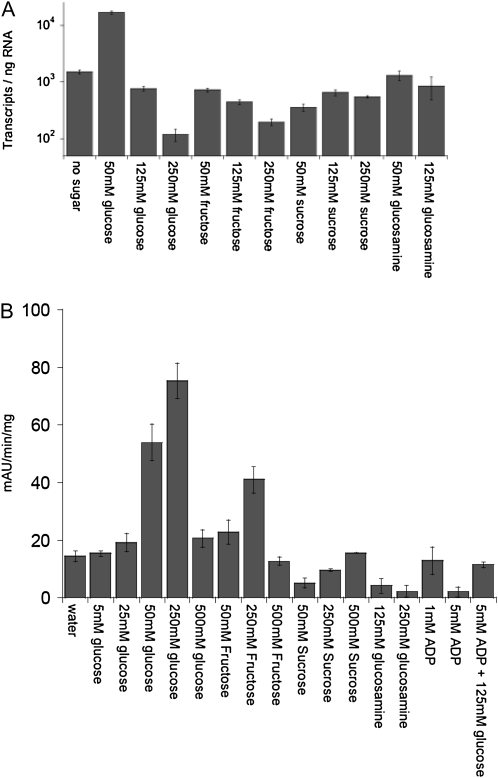
To dissect the molecular mechanisms underlying the change in ZmYUC expression, we explored the effects of sugars on levels of the transcript in cultured maize kernels under various sugar concentrations as well as on the expression of a ZmYUC promoter:GUS fusion in a heterologous Arabidopsis system. Sugar concentrations measured in Figure 1 indicate that the endogenous concentration of Glc is approximately 50 mm in 8- and 12-DAP basal kernel sections, and Suc levels climb to more than 250 mm in upper sections of mn1-1 kernels; therefore, we chose to culture kernels on 50, 125, and 250 mm sugars to try to reproduce physiological levels. In addition, we also cultured kernels on glucosamine, which can be taken up similarly to Glc and Fru but cannot be phosphorylated by hexokinase. By including glucosamine, we hoped to gain an understanding of whether hexokinase activity might be involved in the signaling pathway controlling ZmYUC expression. In wild-type maize kernels cultured on sugars from 8 to 12 DAP, we found that ZmYUC expression was enhanced by 50 mm Glc but was repressed by higher concentrations of Glc (Fig. 8A). Other sugars did not enhance ZmYUC expression, and higher concentrations of Fru also caused repression. To verify changes in sugars in cultured kernels, we also measured Glc, Fru, and Suc from these cultured kernel samples as well as from intact kernels from an ear pollinated at the same time for comparison; the results are shown in Supplemental Figure S3. We found that increasing exogenous Glc increased endogenous Glc and Suc, increasing exogenous Fru increased endogenous Fru and Suc, and increasing exogenous Suc increased endogenous Suc, as expected. Overall, it appears that the ratio of Glc to Suc correlates most closely with relative ZmYUC expression.
Figure 8.
Sugar effects on ZmYUC transcript abundance in maize and ZmYUC promoter:GUS activity in Arabidopsis. A, Cultured Mn1 maize kernels in the presence of sugars or inhibitors. B, Transgenic Arabidopsis seedlings containing a ZmYUC promoter:GUS fusion were treated overnight with the indicated sugar concentrations, then β-glucosidase activity was measured. Bars show the average of three replicates of three seedlings each, and error bars are sd.
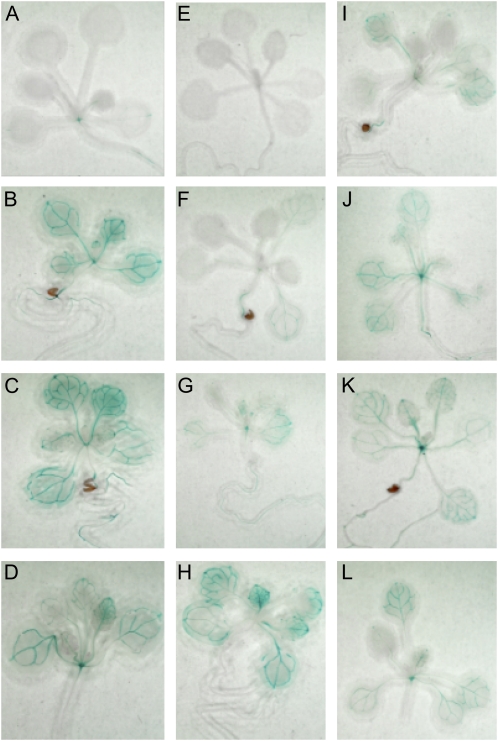
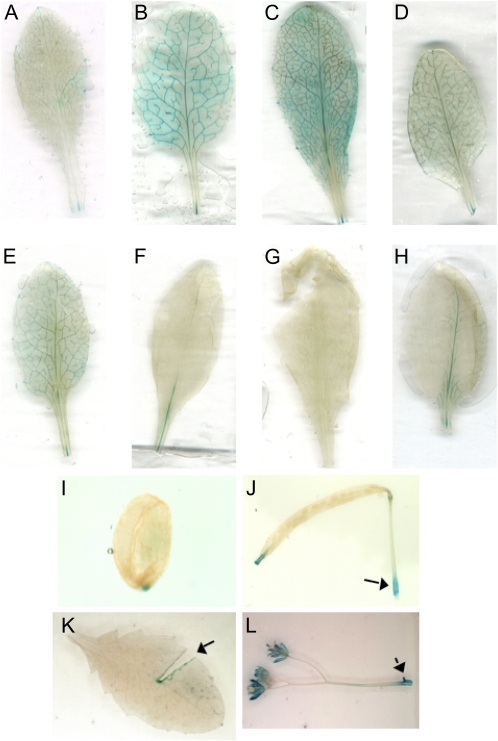
In the heterologous Arabidopsis reporter system, GUS activity as measured by p-nitrophenyl-β-d-glucopyranoside (PNPG) assay was enhanced by both 50 and 250 mm Glc (Fig. 8B). In addition, 250 mm Fru also enhanced activity. In this assay, Suc did not appear to enhance GUS activity, whereas glucosamine and 5 mm ADP, both inhibitors of noncytosolic hexokinases (Rezende et al., 2006), repressed GUS activity. The repression by ADP was partially relieved by the addition of 125 mm Glc. GUS expression was also observed by staining, both to identify expression patterns and to compare the effects of sugars on these patterns. In both seedlings and mature plants, GUS was expressed in the vascular tissue of developing leaves (Figs. 9 and 10), even in the absence of exogenous sugars. When exogenous sugars were added, GUS staining was enhanced, especially by 0.5% and 2.5% Glc (approximately 25 and 125 mm Glc) in seedlings (Fig. 9) and by 1% Glc in adult leaves (Fig. 10). Staining was also enhanced by 5% Fru and 0.5% and 2.5% Suc in seedlings and by 1% Fru, Suc, and mannitol in adult leaves. The enhancement by Suc and mannitol was not as robust as that seen with either Glc or Fru. Although Suc enhancement was not seen in the GUS activity assay, the expression is highly localized and may well have been below the limits of detection in that assay. Interestingly, glucosamine (Fig. 9E) was similar to the control, except that the presence of glucosamine seemed to reduce the level of GUS stain relative to Glc alone (Fig. 9, B and F), indicating that Glc induction may be reduced or blocked by glucosamine. Glucosamine was not tested on adult leaves, but Glc-6-P and Fru-6-P, reaction products of hexokinase, both decreased GUS staining, whereas tryptamine, the substrate for ZmYUC, had no effect (Fig. 10, F–H). In adult tissues, GUS staining was also apparent at wound sites in leaves and stems, in vascular tissue in flowers, near the tips of developing siliques, and near the BETL region in developing seeds (Fig. 10, I–L).
Figure 9.
ZmYUC promoter:GUS expression in Arabidopsis seedlings. Transgenic Arabidopsis seedlings containing a ZmYUC promoter:GUS fusion were treated overnight with the indicated sugar concentrations and then stained to visualize GUS expression. Treatments were as follows: A, water; B, 0.5% Glc; C, 2.5% Glc; D, 5% Glc; E, 2.5% glucosamine; F, 0.5% Fru; G, 2.5% Fru; H, 5% Fru; I, 2.5% glucosamine + 2.5% Glc; J, 0.5% Suc; K, 2.5% Suc; L, 5% Suc. [See online article for color version of this figure.]
Figure 10.
ZmYUC promoter:GUS expression in mature Arabidopsis tissues. Youngest fully expanded rosette leaves from transgenic Arabidopsis plants containing a ZmYUC promoter:GUS fusion were excised and treated overnight with the indicated sugar concentrations and then stained to visualize GUS expression. Leaves were treated as follows: A, water; B, 1% Glc; C, 1% Fru; D, 1% Suc; E, 1% mannitol; F, 1% Glc-6-P; G, 1% Fru-6-P; H, 1 mm tryptamine. Expression was also investigated in intact developing seeds (I), siliques (J), leaves (K), and flowers (L). Rapid induction of expression was also seen after wounding, as denoted by arrows in J, K, and L. [See online article for color version of this figure.]
DISCUSSION
Sugar Accumulation Is Altered in mn1 Developing Kernels
The maize mn1-1 mutant has been previously characterized and shown to have reduced cell wall invertase activity due to loss of INCW2, reduced endosperm mass, and reduced cell number in developing kernels (Cheng and Chourey, 1999). Because Suc is the main phloem transport sugar in developing seeds of maize (Shannon, 1972; Miller and Chourey, 1992), a loss of INCW2 function is expected to alter the ability of the BETL to convert incoming apoplastic Suc into Glc and Fru. This conversion is thought to be necessary to drive phloem unloading and uptake of sugars into the endosperm (Shannon, 1972; Miller and Chourey, 1992). Therefore, it was hypothesized that loss of INCW2 would lead to increased accumulation of Suc in the pedicel region and decreased accumulation of Glc and Fru.
We found that mn1-1 basal kernel sections comprising the pedicel, BETL, and closely associated tissues have increased amounts of Suc and decreased Fru and Glc as compared with the wild type. This finding indicates that basal kernel tissues fail to convert Suc into hexose; therefore, Suc accumulates in or near the pedicel region as expected. Interestingly, however, mn1-1 and wild-type kernels accumulate similar amounts of these sugars in upper storage regions of the kernel and in fact have a higher amount of sugars relative to total mass (Fig. 1). These results suggest that although sugar homeostasis is altered in basal regions of the kernel, endosperm in the upper kernel region apparently compensates for this defect either by up-regulating other enzymes that metabolize or transport Suc or by altering other metabolic pathways to maintain optimal Suc-to-hexose ratios. These data are consistent with the flux analyses, using 13C-NMR and GC-MS approaches, that the distribution of carbohydrate fluxes is stable and not determined by sink strength in developing maize kernels, including the mn1 seed mutant (Spielbauer et al., 2006).
At 8 DAP, both wild-type and mn1-1 caryopses are composed predominantly (greater than 80%) of maternal nucellar tissue and are expected to be metabolically similar because the Mn1 expression is spatially restricted to the filial endosperm tissue, an extremely small mass relative to the total caryopsis. At 12 DAP and thereafter, higher Suc levels in upper sections of the mn1-1 may be due to passive symplastic transport from the basal regions that accumulate higher than normal levels of Suc. It is worth noting that although a higher hexose-Suc ratio is known to stimulate cell divisions (Weber et al., 1997), the upper kernel sections of mn1-1 showed reduced cell size and cell number relative to the wild type (Vilhar et al., 2002), although the hexose-Suc ratio of upper sections of mn1-1 is similar to or higher than that of the wild type (Fig. 1, G and H). The facts that cell number and cell size are decreased in mn1-1 and that hexose-Suc ratios are decreased in basal regions but not upper regions of 12- to 20-DAP kernels suggest that some signaling mechanism exists in basal kernel regions that controls cell division and enlargement throughout the endosperm.
Whereas we observed differences in Glc and Fru between mn1-1 and the wild type, metabolite profiling by GC indicated that there were no differences in Glc-6-P or Fru-6-P between 20-DAP whole kernels of mn1-1 and the wild type (Fig. 7). However, we cannot rule out differences in these metabolites in localized regions or cellular compartments within the kernels. We did observe changes in amino acid levels, particularly that of Gln, between mn1-1 and wild-type kernels, which could be a response to perceived sugar starvation (Yu, 1999) or could represent differences in protein synthesis in the mutant. In addition, we observed an accumulation of tryptamine in the GC-based metabolite profiles, which is consistent with a decrease in ZmYUC levels resulting in a decrease in tryptamine hydroxylation. Despite repeated attempts, we were unable to measure N-hydroxytryptamine by our GC analyses, similar to the recent report by Quittenden et al. (2009) in pea (Pisum sativum). Undetectability of N-hydroxytryptamine using definitive techniques of GC-MS in various IAA-rich tissues in pea to quantify IAA precursors of the TAM pathway is attributed to its high instability due to rapid turnover and/or in levels below the detection limits (Quittenden et al., 2009). It is of significant interest that the mutant showed a decrease in the levels of IAAld relative to the wild-type kernels (Fig. 7). IAAld is a common intermediate to both the TAM and IPA pathways of Trp-dependent IAA biosynthesis. We have recently cloned and characterized the ZmTAR1 (for Aminotransferase-related1) gene, an ortholog of the Arabidopsis TAA gene (Stepanova et al., 2008; Tao et al., 2008) that is critical to the IPA pathway. A comparative qPCR analysis for ZmTAR1 RNA levels has shown nearly 50% reduction in these transcripts in the mn1 mutant against the wild type in 20-DAP kernels (P.S. Chourey, Q.-B. Li, and D. Kumar, unpublished data). The reduced levels of IAAld are compatible with the reduced level of ZmTAR1 expression.
Transcript Levels of a Key Trp-Dependent IAA Biosynthetic Enzyme Are Decreased in mn1-1
To investigate the possible molecular mechanisms behind the IAA deficiency in mn1-1 and to understand potential cross talk between sugar and auxin pathways in this mutant, we measured the transcript abundance of several putative IAA metabolic and conjugate synthase genes in the wild type and mn1-1 using real-time PCR. Of the transcripts examined, only one, ZmYUC, showed alterations in mRNA levels consistent with the observed changes in IAA levels. ZmYUC is 40% identical at the amino acid level to the AtYUC flavin monooxygenase protein from Arabidopsis (Supplemental Fig. S2). In vitro analysis of bacterially expressed ZmYUC shows that this enzyme catalyzes the conversion of tryptamine to N-hydroxytryptamine in the presence of NADPH, similar to the Arabidopsis and tomato enzymes. We used qPCR to measure transcript levels of ZmYUC in several tissues and found that it is highly expressed in developing kernels. These results are in agreement with stable isotope labeling studies showing that the Trp-dependent pathway is the primary route of IAA biosynthesis in developing maize kernels (Glawischnig et al., 2000). These results also indicate that expression of ZmYUC is very tissue specific, and when considered with recent findings by Gallavotti and others (2008), they suggest that different YUC homologs have distinct spatiotemporal expression patterns giving them distinct functions in development. It is interesting that the maize homolog SPI1 was not expressed in roots or endosperm, whereas ZmYUC is highly expressed in endosperm but not in other tissues tested. There are seven other members of the YUC family of proteins in maize (Gallavotti et al., 2008), although their expression patterns have not been characterized. It is also of interest that ZmYUC promoter:GUS fusions showed rapid wound-induced expression in a heterologous system. This finding supports the hypothesis that the Trp pathway can be rapidly induced to provide high levels of IAA to wounded tissues (Sztein et al., 2002).
Sugar and Phytohormones Cross Talk in Developing Kernels
It has become evident from recent reports that there is a great deal of cross talk between sugar and hormone signaling pathways (León and Sheen, 2003; Moore et al., 2003; Zanor et al., 2009). Results from this work indicate that sugar influx into sink tissues may influence sink size and strength by regulating auxin levels. Auxin and cytokinin have been previously reported to regulate invertases (Roitsch et al., 2003; Lara et al., 2004), and a recent report indicates that Glc can affect the transcription of many auxin-responsive genes including the YUC genes (Mishra et al., 2009). Consistent with our data here (Fig. 8) in transgenic Arabidopsis seedlings containing a ZmYUC promoter:GUS fusion that showed greatly increased GUS activity with higher levels of Glc are results from Mishra et al. (2009) that show up-regulation of the YUC2 gene in Arabidopsis roots of young seedlings with exogenous supplement of Glc in the medium. Similarly, reduced levels of endogenous hexoses were also associated with reduced levels of the ZmYuc1 RNA in the mn1 endosperm. In addition, the Arabidopsis hexokinase mutant glucose insensitive2 is less sensitive to exogenous auxin but has no defect in endogenous IAA levels (Moore et al., 2003). Other correlations between changes in sugar content and hormone homeostasis have also been uncovered. For example, ethylene and abscisic acid have been shown to cross talk with sugar pathways (Zhou et al., 1998; Yanagisawa et al., 2003), and in tobacco (Nicotiana tabacum) leaves, cytokinin has been shown to mediate delayed senescence by increasing cell wall invertase activity and Glc levels. To explore the effects of sugars on ZmYUC expression, we used an in vitro kernel culture system to manipulate sugar levels in developing wild-type kernels to examine the effects on ZmYUC transcript levels by qPCR. We found that the addition of 50 mm Glc, a level similar to that found to be present in basal regions of developing kernels (Fig. 1), enhanced ZmYUC expression nearly 10-fold above no-sugar controls, whereas other sugar treatments either had no effect or had a negative effect. Interestingly, high levels of Glc actually repressed expression in cultured kernels. Similar results were seen for Glc with the ZmYUC promoter:GUS reporter assays, with enhancement at 50 and 250 mm Glc and loss of enhancement at 500 mm Glc. In contrast to the kernel culture system, increased GUS activity was also seen with the addition of Fru as well as Glc in the Arabidopsis reporter lines. While there were some differences between the endogenous maize system and the Arabidopsis reporter system, both systems showed clear enhancement of expression upon the addition of low to moderate levels of exogenous Glc, with a loss of enhancement at high concentrations of exogenous Glc. The manipulation of sugar levels in developing maize kernels is technically challenging due to the many pathways controlling carbohydrate fluxes. This is evidenced by similar sugar-labeling patterns regardless of Glc or Suc feeding during kernel development (Cheng and Chourey, 1999; Spielbauer et al., 2006). The use of the Arabidopsis reporter system, however, allows easier manipulations. In addition, potential differences in downstream storage and utilization systems and sensitivity to sugar concentrations may uncover potential regulation that was not observed in the endogenous system. These data show that Glc and Fru can both regulate the expression of ZmYUC and point to the ratio of hexose to Suc as being an important factor in this regulation. Additionally, inhibitors of hexokinase activity such as ADP and glucosamine, as well as the hexokinase reaction products Glc-6-P and Fru-6-P, all inhibit expression of the ZmYUC promoter:GUS reporter in Arabidopsis. These findings raise the possibility that hexose sugars modulate the expression of ZmYUC through a hexokinase-dependent pathway.
It is intriguing to consider that sugar availability may alter the transcription of key phytohormone biosynthetic enzymes and thus regulate cell division and sink size based on these signals. Previous reports have indicated a link between hexokinase and auxin pathways (Moore et al., 2003). Here, we have shown further connections between sugar signals and auxin biosynthetic pathways. We have demonstrated linkages between cell wall invertase-mediated sugar metabolism and Trp-dependent IAA biosynthesis in developing maize kernels. We have shown that decreased cell wall invertase activity in the mn1-1 mutant results in decreased hexose-Suc ratios in basal kernel tissues as well as reductions in IAA biosynthesis via a decrease in expression of ZmYUC, a kernel-specific maize homolog of the flavin monooxygenase YUC. We have further shown that sugars, particularly Glc, can regulate this rate-limiting step in IAA biosynthesis by regulating the expression of ZmYUC through an apparent hexokinase-mediated pathway.
MATERIALS AND METHODS
Plant Materials
Mutant mn1-1 is from the genetic background represented by the maize (Zea mays) W22 inbred line. All plants were grown in the field in summer of either 2005 or 2006 and self-pollinated by hand. Ears were harvested at 6, 8, 10, 12, 16, 20, 24, or 28 DAP. Kernels were individually removed with a paring knife to include the undamaged base (pedicel) of each kernel, which was immediately frozen in liquid nitrogen. Upper and basal regions were dissected as described previously (LeClere et al., 2008). Frozen samples were stored at –80°C until analysis.
Kernel Culture
Greenhouse-grown plants were self-pollinated by hand and ears were harvested 8 DAP. Kernel culture was based on methods described by Gengenbach (1977) and Glawischnig et al. (2000). Individual kernels were carefully removed with a paring knife to include the undamaged base (pedicel) of each kernel. Kernels were harvested into sterile water, and bleach was added to 10% to surface sterilize kernels. Kernels were swirled for 60 s in the bleach solution and then rinsed several times in distilled water. Sugar solutions in kernel culture media (Gengenbach, 1977) were added to sterile floral foam squares approximately 2 cm thick in magenta boxes until foam was saturated (generally this was approximately 20 mL of solution), then 12 kernels were placed, pedicels down, into the foam. Kernels were incubated in darkness at 28°C for 4 d, then harvested and rinsed briefly in sterile water to remove culture solutions. For subsequent qPCR and sugar analysis, three samples of three kernels each were analyzed.
Determination of Sugar Levels
For analysis of upper and basal kernels, kernels were divided with a razor blade just above the pedicel and midway through the embryo. Each sample contained upper or basal parts of three kernels. All experiments were replicated at least three times with similar results each time. Similar results were obtained for samples from both 2005 and 2006 growing seasons. Colorimetric assays to measure Glc, Fru, and Suc were performed using established methods (Hendrix, 1993; Tarpley et al., 1993). Briefly, kernel samples were homogenized in a FastPrep machine (Q-Biogene) for 60 s in a 2-mL centrifuge tube containing Zirmil (Zirpro) cubic zirconium beads and 1 mL of 80% ethanol. Samples were transferred to 5-mL tubes and diluted to a final volume of 4 mL. Samples were heated to 85°C for 15 min to solubilize all sugars and then centrifuged briefly to pellet insoluble debris. Five microliters of supernatant was analyzed in triplicate for each sample. Reaction volumes were 100 μL, and assays were performed on 96-well flat-bottom polystyrene plates (Fisher) and read with a Benchmark microplate reader (Bio-Rad). Colorimetric assay buffers contained 20 mm HEPES, 47 μm MgSO4, 952 μm ATP, 0.125 mg mL−1 NAD, 1.25 units mL−1 hexokinase, 0.4 units mL−1 Glc-6-P dehydrogenase, 0.4 mg mL−1 p-iodonitrotetrazolium, 0.1 mg mL−1 phenazine methosulfate, and 0.6% BRIJ 35 (polyoxethylene 23-lauryl ether) for Glc assays and included 4 units mL−1 phosphoglucose isomerase for Fru assays plus 75 units mL−1 invertase for Suc assays. All samples were quantified by comparison with a standard curve included on the same microplate. Statistical comparisons were done using Student's t test.
RNA Extraction
Tissues were dissected as described above, placed in 2-mL centrifuge tubes containing Zirmil cubic zirconium beads, and weighed to the nearest milligram. A total of 500 μL of RNA extraction buffer (100 mm LiCl, 100 mm Tris-HCl, pH 8.0, 1% SDS, and 10 mm EDTA) and 500 μL of phenol were added to each tube, and samples were homogenized using a FastPrep machine for 60 s. A total of 500 μL of chloroform was added to each extract, and tubes were sealed and vortexed. Samples were spun at 16.1 relative centrifugal force for 1 min to separate phases, and the aqueous layer was moved to a fresh tube and extracted again with 500 μL of chloroform. The aqueous phase was added to a 1.5-mL tube containing 150 μL of 8 m LiCl and placed at 4°C for 2 h to allow RNA to precipitate. RNA was pelleted by centrifugation at 16.1 relative centrifugal force for 5 min, resuspended in 200 μL of water, and reprecipitated by adding 20 μL of 5 m NaCl and 800 μL of ethanol. RNA samples were resuspended in water to a final concentration of 500 ng μL−1.
Identification of Putative IAA Metabolic Genes
Enzymes potentially involved in IAA biosynthesis were identified from available maize sequences in GenBank (www.ncbi.nlm.nih.gov/GenBank) based on annotation for TRPA (Kramer and Koziel, 1995), AO1 (Sekimoto et al., 1997), and IAGLU (Szerszen et al., 1994) or by BLAST (www.ncbi.nlm.nih.gov/BLAST/) analysis with known sequences for GH3 (Staswick et al., 2002) and AtYUC (Zhao et al., 2001).
qPCR
One microgram of RNA was used for first-strand cDNA synthesis using the SuperScript First-Strand Synthesis kit from Invitrogen. Equal amounts of first-strand reaction were used for qPCR using the DyNAmo HS SYBR Green qPCR Kit (New England Biolabs) with primers 5′-CTGGAGATGCTGAGGGAGGTGAC-3′ and 5′-TTCACGTGCTCGGGCTTGGATATG-3′ for TRPA, 5′-GCTACTTGAACTACGGAGCTGG-3′ and 5′-TCCTTGACGACAGGCATCGTC-3′ for AO1, 5′-CGTCGTTCGTGTTCGACCATG-3′ and 5′-CTAGCCACTTGGTGCACGCATC-3′ for IAGLU, 5′-GCTGCTGTACAGCCTCCAGATG-3′ and 5′-TCTTGTAGTAGCTCGTGAGGAC-3′ for ZmGH3, and 5′-CGGTCCTAGACGTTGGCACAAC-3′ and 5′-CACGAGAGATGCCAGCCAGTCC-3′ for ZmYUC. SUS3 was amplified with primers 5′-GCCATGTCTGCGCCGAAGCTGGACCGC-3′ and 5′-CGAACGGCGGCAGCACGATCGCCTCC-3′ and used as the internal standard to calculate ΔCt (cycle threshold). 2−ΔΔCt was calculated using wild-type 12-DAP upper kernels as the calibrator. For calculation of transcript number in subsequent ZmYUC qPCR, the PCR product was cloned into TOPO vector (Invitrogen) and quantified based on absorbance. A dilution series was made, real-time PCR was performed, and a standard curve was plotted. The slope of this line was used to calculate transcript number for PCR performed under identical conditions and expressed as transcripts per ng of total RNA.
Protein Expression and Enzyme Assays
ZmYUC was expressed as a glutathione S-transferase fusion (GST-ZmYUC) using the pET system (Novagen). Recombinant GST-ZmYUC was purified using the GST·Bind kit (Novagen), and GST·Resin-bound protein was cleaved with 1 mg mL−1 enterokinase in 50 mm sodium phosphate buffer, pH 5.6, with 2 mm CaCl2 to remove GST and release ZmYUC. Protein was frozen and stored at –80°C and thawed immediately before use. Assays were performed at 30°C at pH 5.6 to 8.0 as indicated. The 100-μL reaction volumes contained final concentrations of 9 μg μL−1 purified ZmYUC, 2 mm tryptamine, and 2 mm NADPH in 50 mm phosphate buffer at pH 5.6, 6.0, 6.5, 7.0, and 7.5. Reactions were incubated for 20 h at 30°C. Control reactions contained GST in enterokinase cleavage buffer instead of ZmYUC. Five microliters was spotted for TLC with a solvent system containing 30% methanol in chloroform. Plates were stained with Van Urk-Salkowski reagent (Ehmann, 1977) to visualize indolic compounds. For TLC isolation of reaction product, the RF of the product was calculated from a stained reference plate, and the corresponding regions were removed from an unstained plate run at the same time in the same solvent. Product was eluted from the TLC using methanol and directly injected into the mass spectrometer for analysis.
Binary Vector Construction
The ZmYUC promoter:GUS fusion was constructed in pCAMBIA vector 1381Z. Primers 5′-GGATCCATTTGTACTAGAAGCTAG-3′ and 5′-AAGCTTAACTGCTGCTAGCTGGATG-3′ were used to amplify the ZmYUC promoter from maize genomic DNA from the W22 inbred and to introduce BamHI and HindIII sites at the beginning and end, respectively, of the promoter. The PCR product was cloned into pCRII TOPO (Invitrogen) and excised with BamHI and HindIII, then cloned into pCAMBIA1381Z (GenBank accession no. AF234306) cut with the same enzymes. This vector was transformed into Arabidopsis (Arabidopsis thaliana) using the Agrobacterium tumefaciens floral dip method (Clough and Bent, 1998).
PNPG Assays
Arabidopsis seedlings were grown on PN medium (Haughn and Sommerville, 1986) for 14 d under white light at 22°C. Seedlings were removed from plates with sterile forceps and placed in 1 mL of sugar solution. Three replicates of three seedlings each were assayed for each treatment. Seedlings were incubated overnight in sugar solutions at 22°C under white light, rinsed briefly with water to remove sugars, and placed immediately in preweighed tubes on dry ice. Samples were weighed and 500 μL of extraction buffer (50 mm sodium phosphate, pH 7.0, 10 mm 2-mercaptoethanol, 10 mm EDTA, 0.1% Triton X-100, 0.1% SDS, and 5% polyvinylpolypyrrolidone) was added to each tube. Samples were homogenized in a FastPrep machine for twice for 30 s and placed immediately on ice. Samples were spun down, supernatant was transferred to a new tube, and 1 volume of extraction buffer was added. Samples were spun again to remove any remaining insoluble material. A total of 100 μL of supernatant was placed on a 96-well plate, and 100 μL of 2 mg mL−1 PNPG (Sigma-Aldrich) in extraction buffer was added. Samples of 50 μL were removed at 0 and 70 min and stopped by the addition of 1 volume of 1 m Na2CO3. Color development was read at 405 nm. Protein content was determined by measurement of A280 for each sample and used to calculate activity as ΔmAU (change in milli absorption unit) min−1 mg−1 protein.
GUS Staining
Seedlings were grown and treated with sugars as described above for PNPG assays. For adult leaf sugar treatments, the youngest fully expanded rosette leaves from 35-d-old soil-grown plants were treated overnight in sugar solution as described above. For wounding, plant parts from 42-d-old mature plants were either wounded and harvested 5 min later or were harvested without wounding and placed immediately in staining solution: 0.5 mm potassium ferricyanate, 0.5 mm potassium ferrocyanate, 200 mm NaPO4, pH 7.0, 2 mm EDTA, 0.01% Triton X-100, 20% dimethylformamide, and 0.05% 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (Martinez-Zapater and Salinas, 1998). Samples were placed under vacuum for 15 min and then stained in darkness at 37°C for 48 h.
Metabolite Analysis
Endosperms from three replicates of two kernels per sample were weighed and homogenized in 80% ethanol in a FastPrep machine as described above. Homogenized samples were incubated at 70°C for 20 min to solubilize metabolites, then cell debris was spun down at 12,000 rpm for 5 min, 800 μL of supernatant was moved to a new tube, and 300 μL of 80% ethanol was added to the pellet to reextract. Samples were spun down, and 200 μL of supernatant from the second extraction was combined with the 800 μL from the first extraction, 3 mL of 5% acetic acid in water was added to the combined supernatant, and 200 μL was placed in a glass vial and dried down at 65°C. Dried samples were resuspended in 50 μL of 20 mg mL−1 methoxamine HCl in pyridine and heated at 70°C for 20 min. A total of 50 μL of bis-trimethylsilyl-trifluoroacetamide was added, samples were heated for another 20 min at 70°C, and 1 μL was analyzed by GC.
Exogenous IAA Treatment of Intact Kernels
Greenhouse-grown plants were self-pollinated by hand. At 6 DAP, several cobs from both the wild type and mn1-1 were selected. Husks were peeled back, 1-cm-thick slabs of 0.6% agar or 0.6% agar plus 100 μm IAA were placed on opposite sides of the same cob, wrapped in Saran Wrap to seal, and recovered. Husks were covered with pollination bags to protect the ears. After 48 d, kernels were removed with a paring knife and weighed individually. Average fresh weight per kernel was calculated.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Protein alignment of GH3-like protein from maize (ZmGH3) with homologs from Arabidopsis.
Supplemental Figure S2. Protein alignment of ZmYUC from maize with homologs from Arabidopsis (AtYUC), Petunia (FZY), tomato (LeYUC), and rice (OsYUC7).
Supplemental Figure S3. Sugar content of Mn1 cultured kernels.
Supplementary Material
Acknowledgments
We thank Daryl Pring and Marina Dermastia for critical comments on the manuscript. We also thank Harry Klee for Arabidopsis seed for transformations, Hans Alborn for assistance in interpreting the liquid chromatography-MS data, and Jeff Browning and Q.-B. Li for excellent technical assistance.
References
- Baldi BG, Maher BR, Slovin JP, Cohen JD. (1991) Stable isotope labeling in vivo of D and L tryptophan pools in Lemna gibba and the low incorporation of label into indole-3-acetic acid. Plant Physiol 95: 1203–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B, LeClere S, Magidin M, Zolman BK. (2001) Inputs to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-butyric acid β-oxidation. J Plant Growth Regul 20: 198–216 [Google Scholar]
- Bialek K, Michalczuk L, Cohen JD. (1992) Auxin biosynthesis during germination in Phaseolus vulgaris. Plant Physiol 100: 509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SJ, Shanker S, Chourey PS. (2000) A point mutation at the Miniature1 seed locus reduces levels of the encoded protein, but not its mRNA, in maize. Mol Gen Genet 263: 367–373 [DOI] [PubMed] [Google Scholar]
- Cheng WH, Chourey PS. (1999) Genetic evidence that invertase-mediated release of hexoses is critical for appropriate carbon partitioning and normal seed development in maize. Theor Appl Genet 98: 485–495 [Google Scholar]
- Cheng WH, Taliercio EW, Chourey PS. (1996) The miniature seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8: 971–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Ehmann A. (1977) The Van Urk-Salkowski reagent: a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr A 132: 267–276 [DOI] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Hernández M, Pérez JA. (2007) Cloning and biochemical characterization of ToFZY, a tomato gene encoding a flavin monooxygenase involved in a tryptophan-dependent auxin biosynthesis pathway. J Plant Growth Regul 26: 329–340 [Google Scholar]
- Gallavotti A, Barazesh S, Malcomber S, Hall D, Jackson D, Schmidt RJ, McSteen P. (2008) sparse inflorescence 1 encodes a monocot-specific YUCCA-like gene required for vegetative and reproductive development in maize. Proc Natl Acad Sci USA 105: 15196–15201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengenbach BG. (1977) Genotypic influences on in vitro fertilization and kernel development of maize. Crop Sci 17: 489–492 [Google Scholar]
- Glawischnig E, Tomas A, Eisenreich W, Spiteller P, Bacher A, Gierl A. (2000) Auxin biosynthesis in maize kernels. Plant Physiol 123: 1109–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Guilfoyle TJ. (1985) Rapid induction of selective transcription by auxins. Mol Cell Biol 5: 1197–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughn GW, Sommerville C. (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204: 430–434 [Google Scholar]
- Hendrix DL. (1993) Rapid extraction and analysis of non-structural carbohydrates in plant tissues. Crop Sci 33: 1306–1311 [Google Scholar]
- Kang BH, Xiong Y, Williams DS, Pozueta-Romero D, Chourey PS. (2009) Miniature1-encoded cell wall invertase is essential for assembly and function of wall-in-growth in the maize endosperm transfer cell. Plant Physiol 151: 1366–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T, Kamiya Y, Iino M. (1995) Biosynthesis of indole-3-acetic acid from L-tryptophan in coleoptile tips of maize (Zea mays L.). Plant Cell Physiol 36: 1503–1510 [Google Scholar]
- Kramer VC, Koziel MG. (1995) Structure of a maize tryptophan synthase alpha subunit gene with pith enhanced expression. Plant Mol Biol 27: 1183–1188 [DOI] [PubMed] [Google Scholar]
- Lara MEB, Garcia MCG, Fatima T, Ehneb R, Lee TK, Proels R, Tanner W, Roitsch T. (2004) Extracellular invertase is an essential component of cytokinin-mediated delay of senescence. Plant Cell 16: 1276–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClere S, Schmelz EA, Chourey PS. (2008) Cell wall invertase-deficient miniature1 kernels have altered phytohormone levels. Phytochemistry 69: 692–699 [DOI] [PubMed] [Google Scholar]
- León P, Sheen J. (2003) Sugar and hormone connections. Trends Plant Sci 8: 1360–1385 [DOI] [PubMed] [Google Scholar]
- Martinez-Zapater JM, Salinas J, (1998) Arabidopsis Protocols, Methods in Molecular Biology, Vol 82. Humana Press, Totowa, NJ, pp 397–407 [Google Scholar]
- Michalczuk L, Ribnicky DM, Cooke TJ, Cohen JD. (1992) Regulation of indole-3-acetic acid biosynthetic pathways in carrot cell cultures. Plant Physiol 100: 1346–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Chourey PS. (1992) The maize invertase-deficient miniature1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4: 297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BS, Singh M, Aggrawal P, Laxmi A. (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One 4: e4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J. (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300: 332–336 [DOI] [PubMed] [Google Scholar]
- Normanly J, Cohen JD, Fink GR. (1993) Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci USA 101: 8039–8044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quittenden LJ, Davies NW, Smith JA, Molesworth PP, Tivendale ND, Ross JJ. (2009) Auxin biosynthesis in pea: characterization of the tryptamine pathway. Plant Physiol 151: 1130–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende GL, Logullo C, Meyer L, Machado LB, Oliveira-Carvalho AL, Zingali RB, Cifuentes D, Galina A. (2006) Partial purification of tightly bound mitochondrial hexokinase from maize (Zea mays L.) root membranes. Braz J Med Biol Res 39: 1159–1169 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Balibrea ME, Hoffmann M, Proels R, Sinha AK. (2003) Extracellular invertase: key metabolic enzyme and PR protein. J Exp Bot 54: 513–524 [DOI] [PubMed] [Google Scholar]
- Roitsch T, Gonzalez MC. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Sekimoto H, Sea M, Dohmae N, Takio K, Kamiya Y, Koshiba T. (1997) Cloning and molecular characterization of plant aldehyde oxidase. J Biol Chem 272: 15280–15285 [DOI] [PubMed] [Google Scholar]
- Shannon JC. (1972) Movement of 14C-labeled assimilates into kernels of Zea mays L. I. Patterns and rates of sugar movement. Plant Physiol 49: 198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielbauer G, Margl L, Hannah CL, Romisch W, Ettenhuber C, Bacher A, Gierl A, Eisenreich W, Genschel U. (2006) Robustness of central carbohydrate metabolism in developing maize kernels. Phytochemistry 67: 1460–1475 [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe ML, Tiryaki I, Maldonando MT, Maldonado MC, Suza W. (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. (2002) Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, Dolezal K, Schlereth A, Jürgens G, Alonso JM. (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191 [DOI] [PubMed] [Google Scholar]
- Szerszen JB, Szczyglowski K, Bandurski RS. (1994) IAGLU, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science 265: 1699–1701 [DOI] [PubMed] [Google Scholar]
- Sztein AE, Ilic N, Cohen JD, Cooke TJ. (2002) Indole-3-acetic acid biosynthesis in isolated axes from germinating beans seeds: the effect of wounding on the biosynthetic pathway. Plant Growth Regul 36: 201–207 [Google Scholar]
- Taliercio EW, Kim JY, Mahe A, Shanker S, Choi J, Cheng WH, Prioul JL, Chourey PS. (1999) Isolation, characterization and expression analysis of two cell wall invertase genes in maize. J Plant Physiol 155: 197–204 [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Mreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarpley L, Dahlberg DMV, Miller FR. (1993) Batch anion exchange separation and quantification of [14C]hexose from [14C]sucrose. Crop Sci 33: 338–341 [Google Scholar]
- Vilhar B, Kladnik A, Blejec A, Chourey P, Dermastia M. (2002) Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol 129: 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Borisjuk L, Wobus U. (1997) Sugar import and metabolism during seed development. Trends Plant Sci 2: 169–174 [Google Scholar]
- Winter A. (1966) A hypothetical route for the biogenesis of IAA. Planta 71: 229–239 [DOI] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. (2005) Auxin: regulation, action, interaction. Ann Bot (Lond) 95: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AD, Sampson MB, Neuffer MG, Michalczuk L, Slovin JP, Cohen JD. (1991) Indole-3-acetic acid biosynthesis in the mutant maize orange pericarp, a tryptophan auxotroph. Science 254: 998–1000 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T. (2007) Auxin biosynthesis by the YUCCA gene in rice. Plant Physiol 143: 1362–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J. (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425: 521–525 [DOI] [PubMed] [Google Scholar]
- Yu SM. (1999) Cellular and genetic responses of plants to sugar starvation. Plant Physiol 121: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanor MI, Osorio S, Nunes-Nesi A, Carrari F, Lohse M, Usadel B, Kuhn C, Bleiss W, Giavalisco P, Willmitzer L, et al. (2009) RNA interference of LIN5 in tomato confirms its role in controlling Brix content, uncovers the influence of sugars on the level of fruit hormones, and demonstrates the importance of sucrose cleavage for normal fruit development and fertility. Plant Physiol 150: 1204–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cohn NS, Mitchell JP. (1996) Induction of a pea cell-wall invertase gene by wounding and its localized expression in phloem. Plant Physiol 112: 1111–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J. (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291: 306–309 [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang J-C, Jones TL, Sheen J. (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.