Abstract
Metabolic engineering of plant carotenoids in food crops has been a recent focus for improving human health. Pathway manipulation is predicated on comprehensive knowledge of this biosynthetic pathway, which has been extensively studied. However, there existed the possibility of an additional biosynthetic step thought to be dispensable because it could be compensated for by light. This step, mediated by a putative Z-ISO, was predicted to occur in the sequence of redox reactions that are coupled to an electron transport chain and convert the colorless 15-cis-phytoene to the red-colored all-trans-lycopene. The enigma of carotenogenesis in the absence of light (e.g. in endosperm, a target for improving nutritional content) argued for Z-ISO as a pathway requirement. Therefore, understanding of plant carotenoid biosynthesis was obviously incomplete. To prove the existence of Z-ISO, maize (Zea mays) and Arabidopsis (Arabidopsis thaliana) mutants were isolated and the gene identified. Functional testing of the gene product in Escherichia coli showed isomerization of the 15-cis double bond in 9,15,9′-tri-cis-ζ-carotene, proving that Z-ISO encoded the missing step. Z-ISO was found to be important for both light-exposed and “dark” tissues. Comparative genomics illuminated the origin of Z-ISO found throughout higher and lower plants, algae, diatoms, and cyanobacteria. Z-ISO evolved from an ancestor related to the NnrU (for nitrite and nitric oxide reductase U) gene required for bacterial denitrification, a pathway that produces nitrogen oxides as alternate electron acceptors for anaerobic growth. Therefore, plant carotenogenesis evolved by recruitment of genes from noncarotenogenic bacteria.
Carotenoids are a structurally diverse class of isoprenoids synthesized in plants, bacteria, algae, and fungi (Britton et al., 2004). In plants, carotenoids serve as accessory pigments in photosynthesis and protect against photooxidative stress (Niyogi, 2000). Carotenoids are also precursors of apocarotenoids such as plant hormones that facilitate plant responses to abiotic stress and that control branching and rhizosphere signaling (Nambara and Marion-Poll, 2005; Akiyama and Hayashi, 2006; Gomez-Roldan et al., 2008; Umehara et al., 2008).
Nucleus-encoded enzymes mediate the plastid-localized biosynthesis of plant carotenoids (Matthews and Wurtzel, 2007). Phytoene synthase (PSY) catalyzes the committed step to carotenoids, drawing isoprenoid precursors generated from the upstream methylerythritol phosphate pathway and geranylgeranyl pyrophosphate synthase (GGPPS; Beyer et al., 1985; Dogbo et al., 1988; Misawa et al., 1994; Rodriguez-Concepcion and Boronat, 2002). The PSY product, 15-cis-phytoene, is then desaturated and isomerized to form all-trans-lycopene, having an extended conjugated double bond system. The desaturation steps are coupled to an electron transport chain with oxygen being the final acceptor (Beyer et al., 1989; Mayer et al., 1990, 1992). The downstream steps require that lycopene be in the all-trans-lycopene configuration for conversion to carotenes and xanthophylls.
Bacteria and plants differ in conversion of 15-cis-phytoene to all-trans-lycopene (for review, see Sandmann, 2009). Bacteria use a single enzyme, CrtI with FAD cofactor serving as a hydrogen acceptor, to catalyze isomerization and four desaturation reactions via the intermediate all-trans-ζ-carotene (Linden et al., 1991; Fraser et al., 1992). Plants and evolutionarily related cyanobacteria require two phylogenetically related desaturases and two isomerases, only one of which has been identified to date (Breitenbach and Sandmann, 2005; Li et al., 2007). In place of FAD, plants use oxidized plastoquinones as electron acceptors in the desaturation sequence (Mayer et al., 1990; Norris et al., 1995; Breitenbach et al., 1999). The plastoquinones are regenerated through photosynthetic electron transfer in photosynthetic tissue or via an alternative oxidase that functions as a plastoquinol-oxygen oxidoreductase in nonphotosynthetic tissue (for review, see Sandmann, 2009). The first desaturase, phytoene desaturase (PDS), removes two hydrogens and introduces trans-double bonds at 11 and 11′, along with a cis-bond at the 9 and 9′ double bond positions, producing 9,15,9′-tri-cis-ζ-carotene. It is thought that a bound flavin plays a role in electron transfer (Hugueney et al., 1992).
The PDS enzyme product 9,15,9′-tri-cis-ζ-carotene must be isomerized at the 15-cis-double bond to form 9,9′-di-cis-ζ-carotene, the substrate for the second desaturase ζ-carotene desaturase (ZDS; Beyer et al., 1989; Bartley et al., 1999; Matthews et al., 2003; Breitenbach and Sandmann, 2005; Fig. 1). This isomerization can be mediated by light. However, carotenogenesis in “dark” tissues such as roots and etiolated leaves would suggest that other components must be involved. Biochemical characterization of the maize (Zea mays) pale yellow9 (y9) locus along with a Euglena mutant provided evidence that there was a genetic locus required for isomerization of the 15-cis-bond in 9,15,9′-tri-cis-ζ-carotene. Recessive alleles caused the accumulation of 9,15,9′-tri-cis-ζ-carotene in dark tissues, whereas light photoisomerized the 15-cis-bond in photosynthetic tissue (but not in other tissues exposed to light) and thereby released the pathway block. The putative enzyme affected by the genetic lesions was termed Z-ISO (for 15-cis-ζ-carotene isomerase; Li et al., 2007).
Figure 1.
Carotenoid biosynthesis in plants showing 15-cis-double bond isomerization catalyzed by putative Z-ISO enzyme or alternatively photoisomerized by light. GGPP, Geranylgeranyl pyrophosphate. [See online article for color version of this figure.]
After 15-cis-ζ-carotene isomerization, the second desaturase, ZDS, removes two hydrogens and introduces cis-double bonds at the 7 and 7′ positions of 9,15,9′-tri-cis-ζ-carotene, leading to the formation of conjugated cis-double bonds at 7,9 and 7′,9′. The second required plant isomerase, CrtISO, has been proven to isomerize the cis-double bonds at 7,9 and 7′,9′ and does not act on the single 15,15′ cis-double bond substrate of the putative Z-ISO (Isaacson et al., 2004).
Available evidence supported the need of Z-ISO for plant carotenoid biosynthesis (Li et al., 2007). However, the Z-ISO enzyme and function of the maize Y9 gene product remained elusive. To identify Z-ISO, test its function, and demonstrate that it exists throughout the plant kingdom, additional Z-ISO mutants were assembled for maize and Arabidopsis (Arabidopsis thaliana). Phylogenetic analysis revealed the evolutionary origin of Z-ISO, and functional complementation established the role of Z-ISO in isomerization of the 15-cis-bond present in the PDS product, 9,15,9′-tri-cis-ζ-carotene, to form the ZDS substrate 9,9′-di-cis-ζ-carotene.
RESULTS
Isolation of an Allelic Series of Z-ISO Mutants in Arabidopsis and Maize
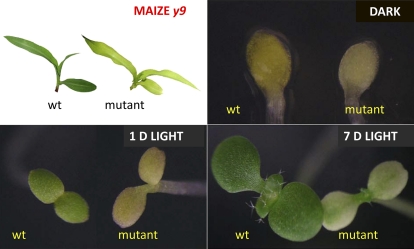
To clone the Z-ISO gene, 12 additional mutants, 10 of which were known to be allelic to maize y9, were requested from the Maize Genetic Stock Center. These mutants all accumulated 9,15,9′-tri-cis-ζ-carotene (data not shown) as reported previously (Li et al., 2007). The fact that 10 mutations were allelic suggested that Z-ISO activity is governed by a single locus. Many of the mutations were derived from Mutator (Mu) transposon lines, which could potentially facilitate gene isolation. However, the high Mu copy number as generally found in Mu lines made gene isolation problematic. Therefore, we chose to also screen for mutants from Arabidopsis to more rapidly isolate the gene. Based on the biochemical phenotype of the maize y9 mutants that accumulated 9,15,9′-tri-cis-ζ-carotene in the dark, we set up a simple screening method. Seeds from approximately 30,000 T-DNA insertion lines were germinated in the dark and then exposed to light. Z-ISO mutants would be predicted to lack carotenoids in the dark (appearing lighter yellow than the wild type) and have delayed greening when exposed to light. Two such recessive mutants were isolated and termed zic1-1 and zic1-2(for Z-ISO of carotenoid synthesis). The typical delayed greening phenotype is shown in Figure 2.
Figure 2.
Greening phenotype of Z-ISO mutants in maize (y9) and Arabidopsis (zic1-1) compared with normal (wild type [wt]) counterparts. Maize greenhouse-grown plants are shown at top left, and Arabidopsis plants are shown in the remaining panels. Arabidopsis seeds were germinated in the dark and/or exposed to long-day growth (16 h of light/8 h of dark) for 1 or 7 d as noted.
Next, these Arabidopsis mutants were tested for accumulation of 9,15,9′-tri-cis-ζ-carotene to verify that they were biochemically similar to the maize y9 mutants. The isomers 9,15,9′-tri-cis-ζ-carotene and 9,9′-di-cis-ζ-carotene were identified by improving the separation method from that described earlier (Li et al., 2007). As shown in Figure 3 and quantified in Table I, extracts for standards were prepared both from maize y9 etiolated leaves and from “EBP” bacteria (Escherichia coli expressing bacterial GGPPS and PSY and maize PDS), where the predominant product is the PDS product 9,15,9′-tri-cis-ζ-carotene (Li et al., 2007). The isomer 9,15,9′-tri-cis-ζ-carotene was identified by exposure of extracts to light and demonstrating its conversion to 9,9′-di-cis-ζ-carotene, as evidenced by change in retention time and spectral properties, as we demonstrated before (Li et al., 2007). As shown in Figure 4, etiolated leaves of the Arabidopsis mutant zic1-1 (and Arabidopsis zic1-2; data not shown) accumulated 9,15,9′-tri-cis-ζ-carotene as found for maize y9 etiolated leaves. The Arabidopsis mutants also accumulated some 9,9′-di-cis-ζ-carotene. In contrast, the wild-type Columbia (Col-0) plants did not accumulate any ζ-carotene. As shown for homozygous seedlings of both zic1-1and zic1-2 mutants grown in the light for 6 d, plants contained approximately 20% to 30% reduced levels each of total carotenoids and chlorophyll compared with the wild type (Table II; Supplemental Fig. S1). The individual carotenoids were reduced in the mutants except for β-carotene, which was significantly elevated compared with the wild type. However, β-carotene remained as a minor component of the leaf carotenoids in the zic1 mutants.
Figure 3.
HPLC scans of carotenoid extracts showing light-mediated isomerization of 9,15,9′-tri-cis-ζ-carotene (Tri) to 9,9′-di-cis-ζ-carotene (Di). Top, extracts prepared from etiolated leaves of y9 (left) and dark-cultured E. coli producing 9,15,9′-tri-cis-ζ-carotene (right). Bottom, carotenoid extracts as in the top panels were exposed to 12 h of light. Insets show spectra of major peaks in the panels. Additional spectra can be found in Figure 4. Peak ζ2, unknown, similar spectrum to 9,15,9′-tri-cis-ζ-carotene; peak ζ3, unknown, similar spectrum to 9,15,9′-tri-cis-ζ-carotene; peak ζ5, unknown, similar spectrum to 9,9′-di-cis-ζ-carotene; peak ζ6, unknown, similar spectrum to 9,9′-di-cis-ζ-carotene. AU, Absorbance units. [See online article for color version of this figure.]
Table I. Geometric isomers accumulating in y9 etiolated leaves and dark-grown E. coli.
Nd, Not detected. Numbers in y9 and EBP columns correspond to percentage of total ζ-carotene based on peak area relative to total peak area of six ζ-carotene peaks.
| Carotene |
y9 |
EBP |
λmaxa | Percentage III/IIb | ||
| Etiolated Leaves | 12-h Light Treatment | Dark | 12-h Light Treatment | |||
| nm | ||||||
| Tri-cis-ζ-carotene | 92.05 ± 1.51 | 2.62 ± 2.20 | 34.77 ± 1.53 | 0.31 ± 0.12 | (296) 378 399 423 | 75 |
| ζ-2 | Nd | Nd | 2.28 ± 0.90 | 0.74 ± 0.10 | (296) 374 396 419 | 87 |
| ζ-3 | Nd | Nd | 1.60 ± 0.56 | 0.67 ± 0.10 | (296) 374 396 419 | 93 |
| Di-cis-ζ-carotene | 6.39 ± 0.77 | 88.06 ± 1.18 | 32.85 ± 1.05 | 53.27 ± 3.38 | 380 400 425 | 103 |
| ζ-5 | 1.55 ± 0.76 | 1.28 ± 1.69 | 23.16 ± 0.61 | 31.86 ± 1.63 | 380 400 425 | 102 |
| ζ-6 | Nd | Nd | 5.33 ± 0.50 | 9.51 ± 1.08 | 378 400 425 | 100 |
Absorbance spectra taken during HPLC separation. The underlined peak is the highest peak (II); parentheses indicate an additional cis-peak.
Fine structure of the absorption spectra expressed as the relative heights of the longest wavelength peak (III) to the middle peak (II).
Figure 4.
HPLC analysis of ζ-carotene extracted from etiolated leaves of maize y9 and Arabidopsis zic1-1. Left, HPLC scans. Right, Peak spectra. Peaks are labeled as in Figure 3. AU, Absorbance units; Di, 9,9′-di-cis-ζ-carotene; Tri, 9,15,9′-tri-cis-ζ-carotene. [See online article for color version of this figure.]
Table II. Changes in carotenoid and chlorophyll composition of Arabidopsis zic1-1 and zic1-2 compared with the wild type.
Values shown are averages ± sdfor three biological replicates. A representative HPLC scan for each genotype is shown in Supplemental Figure S1.
| Carotenoid and Chlorophyll | zic1-1 | zic1-2 |
| % of wild type | ||
| Neoxanthin | 76.11 ± 7.39 | 67.32 ± 3.79 |
| Violaxanthin | 69.25 ± 6.41 | 60.19 ± 3.05 |
| Lutein | 82.33 ± 7.28 | 72.13 ± 4.89 |
| β-Carotene | 150.03 ± 2.33 | 121.76 ± 18.76 |
| Total carotenoid | 81.65 ± 6.81 | 71.09 ± 4.94 |
| Chlorophyll | 76.78 ± 6.93 | 66.74 ± 3.95 |
Mapping and Identification of the Z-ISO Locus in Arabidopsis
To locate the affected locus, zic1 was fine-mapped (see “Materials and Methods” and Supplemental Table S1) to chromosome 1 between markers T16B5 and T19P16M1-2, and each respectively gave two and five recombinants out of 826 F2 plants (Fig. 5). In a region of approximately 69 kb, sequence comparison for 12 candidate genes pointed to only one mutated gene, At1g10830. Moreover, only this gene showed highly correlated coexpression with other carotenoid genes as compared with the background of all other Arabidopsis transcripts assayed over approximately 300 microarray experiments using the Arabidopsis Coexpression Data Mining Tool (http://www.arabidopsis.leeds.ac.uk/act/coexpanalyser.php). The highest coexpression correlation was found between Z-ISO and PDS (At4g14210), with an r value of 0.857, followed by PSY (AT5g17230) and ZDS (AT3g04870), with r values of 0.854 and 0.853, respectively.
Figure 5.
Fine-mapping of the Z-ISO locus, map-based cloning, and transcript expression pattern of the Arabidopsis Z-ISO gene (AtZ-ISO). The Z-ISO locus was mapped to chromosome 1 (Chr1) between markers T16B5 and T19P16M2. The Z-ISO locus cosegregated with marker T19D16M1. The numbers at the bottom indicate the number of recombinants identified from F2 plants. BACs refer to bacterial artificial chromosome clones in the region with associated markers identified. The round spot denotes the centromere. cM, Centimorgan.
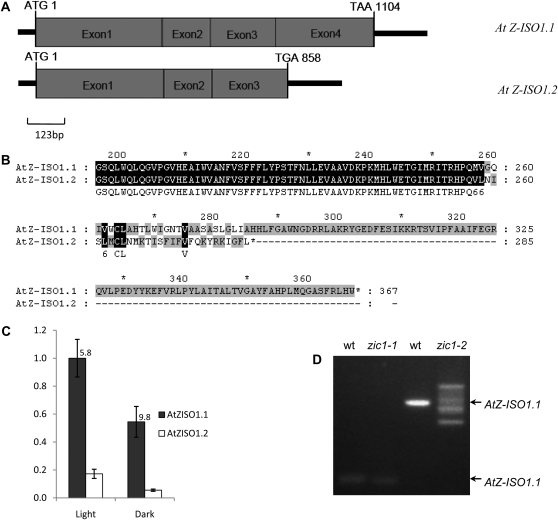
The Arabidopsis Z-ISO gene, At1g10830, shown in Figure 6 contains four exons and three introns. Mutations associated with the two Z-ISO mutants identified in this study (zic1-1 and zic1-2) and T-DNA insertion sites for four other T-DNA insertion mutants of At1g10830 (named zic1-3 to zic1-6), obtained from The Arabidopsis Information Resource (TAIR), are noted in Figures 6 and 7. The T-DNA insertion mutants share a similar phenotype with zic1-1 for cotyledon color and delayed leaf greening (data not shown).
Figure 6.
Gene structure of Arabidopsis At1g10830 (AtZ-ISO1.1) and the location of Z-ISO mutations. Exons are shown as gray boxes and introns are shown as white boxes. 5′ and 3′ untranslated regions are indicated by black lines. The start codon (ATG) and the stop codon (TAA) are indicated. The mutated sites of the six Z-ISO alleles are shown. zic1-1 has a 10-bp deletion in the fourth exon, creating a stop codon; zic1-2 has a 20-bp deletion at the end of intron 1, resulting in abnormal splicing. zic1-3 to zic1-6 are T-DNA lines obtained from TAIR, and the insertion sites are as shown.
Figure 7.
The structures of the AtZ-ISO1.1 gene transcript from the wild type (W.T.) and two Z-ISO alleles, zic1-1 and zic1-2. A, Structure of wild-type AtZ-ISO1.1 gene transcript. Numbers below indicate sites of primers 2590/2591 and 2567/2589 used for amplification of mutated AtZ-ISO1.1 transcripts in zic1-1 and zic1-2, respectively. B, Structure of AtZ-ISO1.1 transcript in zic1-1; the 10-bp deletion in the fourth exon creates a new stop codon. C, Structures of AtZ-ISO1.1 transcripts in zic1-2; the 20-bp deletion in the first intron causes alternative splicing resulting in the multiple transcripts shown in Figure 8D, including a trace amount of wild-type transcript (1,104 nucleotides).
The Z-ISO gene was found to be associated with two alternate transcripts, a longer Z-ISO1.1 and a shorter Z-ISO1.2 (Fig. 8, A and B), predicted to encode a truncated peptide. Both transcripts are expressed as detected by reverse transcription (RT)-PCR, with the longer Z-ISO1.1 transcript being predominant in both etiolated and green leaves (Fig. 8C). The zic1-1 mutant gene has a 10-nucleotide deletion in exon 4, creating a premature stop codon (Figs. 6 and 7), although the transcript level was comparable to the wild type (Fig. 8D). The zic1-2 mutant gene has a 20-nucleotide deletion at the end of intron 1, which caused abnormal splicing. Four transcripts were amplified, only one of which was identical to the wild type (Figs. 7C and 8D).
Figure 8.
Structures and expression levels of AtZ-ISO transcripts. A, The structures of two types of AtZ-ISO gene transcripts, AtZ-ISO1.1 and AtZ-ISO1.2. B, Sequence alignment of the C termini of AtZ-ISO1.1 and AtZ-ISO1.2. AtZ-ISO1.2 has an additional 89-bp fragment from the third intron and the fourth exon is not included, resulting in a premature stop codon and encoding a protein with the variant C terminus shown. C, mRNA levels of AtZ-ISO1.1 and AtZ-ISO1.2 in green (“light”) and etiolated (“dark”) seedlings of the wild type were measured by quantitative RT-PCR. Primers 2686/2687, used for AtZ-ISO1.1, are located in the third and fourth exons, respectively; primers 2684/2685, used for AtZ-ISO1.2, are located in the end of the third exon. For each set of amplifications, the levels were normalized to actin and presented relative to the level of AtZ-ISO1.1 in light-grown plants. The fold differences between AtZ-ISO1.1 and AtZ-ISO1.2 transcripts are indicated above the bars. Error bars represent the sdof three biological replicates. D, AtZ-ISO transcripts produced in the two zic1 alleles compared with the wild type (wt). For amplification of transcripts in each mutant, primers on both sides of the mutation site were used for cDNA synthesis from leaves of 3-week-old plants of Col-0 (wt), zic1-1, and zic1-2.The zic1-1 gene has a 10-bp deletion and produces a transcript close in size to the wild type; the mutant zic1-2 produces multiple transcripts as a result of abnormal splicing, as verified by sequence analysis.
Isolation and Mapping of the Maize Z-ISO Gene
Using the Arabidopsis sequence as a query, the maize Z-ISO gene (BT036679) was identified and mapped to chromosome 10, the location of y9 (Fig. 9). Sequence analysis revealed that maize Z-ISO shared a similar exon-intron structure with that of the Arabidopsis homolog. We confirmed that Z-ISO was encoded by y9 by sequencing the 12 mutant alleles found to accumulate 9,15,9′-tri-cis-ζ-carotene. All of the alleles contained either a Mu7 or Mu8 insertion in exon 1. For example, the y9 allele 5705B had a Mu7 insertion that caused reduced Z-ISO transcripts, as shown in etiolated homozygous mutant leaves (Fig. 10A).
Figure 9.
Chromosome mapping of maize Z-ISO (ZmZ-ISO), gene structure of wild-type and mutant alleles, and mutant transcript analysis. Z-ISO was mapped to chromosome (Chr.) 10, contig ctg394, the locus of y9 (from the Maize Genetics and Genomics Database). The ZmZ-ISO gene has four exons and three introns, as indicated by gray and white boxes, respectively. Z-ISO genotyping of y9 alleles showed that all were caused by insertion of a Mu transposon in exon 1. Alleles carrying a 2,199-bp Mu7 insertion were as follows: X34D, X34E, X34F, X34G, X34H, X34I, X34K, X07CC, 5705B, and 5705E. Alleles carrying a Mu8 insertion were as follows: X34L and X07CB. Both Mu insertions also caused insertion site duplication. BAC, Bacterial artificial chromosome.
Figure 10.
Maize Z-ISO transcript levels in the wild type and mutants. A, Expression level of ZmZ-ISO in etiolated leaves from the wild type (B73) and the y9 allele (5705B) as shown by quantitative RT-PCR. A 117-bp fragment of ZmZ-ISO cDNA located in the second and third exons was amplified. Transcript levels were normalized for actin levels and presented relative to the corresponding wild-type level. Data represent averages and SD of three biological replicates. B, Expression levels of ZmZ-ISO in different maize tissues as measured by quantitative RT-PCR. Transcript levels were normalized for actin levels and presented relative to levels in leaves. Etiolated leaves (L), roots (R), embryo at 20 d after pollination (Em), and endosperm at 20 d after pollination (En) of wild-type B73 were used. Data represent averages and SD of three biological replicates.
Transcript Analysis of Z-ISO
Microarray data analyzed using Genevestigator (https://www.genevestigator.com/gv/index.jsp) showed that the Arabidopsis gene had the highest transcript levels in leaf compared with other tissues such as roots, which were approximately 4-fold lower. Arabidopsis seedling transcript levels were induced by light and reduced by night extension. Published Northern data confirm that Z-ISO is a light-induced gene (Ishikawa et al., 2009). As shown in Figure 10B, maize Z-ISO transcripts were most prevalent in leaves compared with other tissues of nonmutant plants. National Center for Biotechnology Information EST data also indicate that rice (Oryza sativa) Z-ISO (Os12g0405200) transcripts are most abundant in green leaves compared with other tissues. The alternate transcript found in Arabidopsis is likely unique to this species, since we saw little evidence for alternate transcripts in maize and rice.
Z-ISO Gene Product
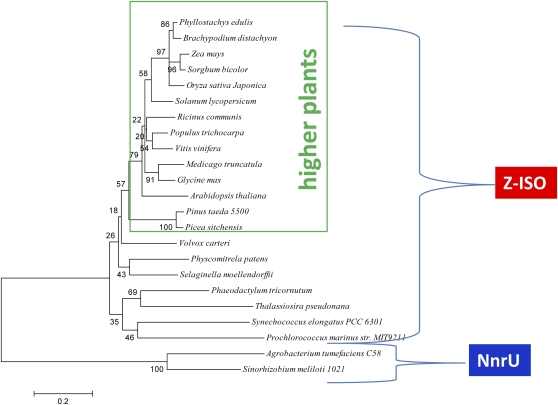
Z-ISO is a chloroplast-localized protein as verified through large-scale proteomics and GFP fusion experiments (Zybailov et al., 2008; Ishikawa et al., 2009). A transit peptide cleavage site is predicted to be at residue 58 and 46 for Arabidopsis and maize Z-ISO, respectively. Z-ISO is predicted by the TMHMM Server version 2.0 to be an integral membrane protein with five membrane-spanning domains. The shorter Arabidopsis Z-ISO transcript (Z-ISO1.2) encodes a protein with one fewer membrane-spanning domains. Previously, it was thought that Z-ISO might be related to other proteins from carotenogenic bacteria, as in the case of the relationship between plant CrtISO and bacterial CrtI (for review, see Sandmann, 2009). However, it was a surprise to find that the protein was related to nitrite and nitric oxide reductase U (NnrU), a transmembrane protein first described for Rhodobacter sphaeroides 2.4.3 as being required for bacterial denitrification (Bartnikas et al., 1997). Phylogenetic analysis shows that Z-ISO sequences in higher and lower plants, algae, diatoms, and cyanobacteria form a monophyletic group having evolved from a common progenitor of the NnrU gene found in denitrifying bacteria such as Agrobacterium tumefaciens C58 and Sinorhizobium meliloti 1021 (Fig. 11). Z-ISO amino acid sequence alignments show that they are highly conserved among higher plants, represented by angiosperms (monocots and dicots) and a conifer and mosses, algae, diatoms, and cyanobacteria (Supplemental Fig. S2A). Inclusion of denitrifying bacteria NnrU sequences shows the homology with Z-ISO but also reveals regions that distinguish Z-ISO from NnrU (Supplemental Fig. S2B).
Figure 11.
Phylogenetic tree showing that Z-ISO in plants is evolved from a common ancestor with bacterial NnrU sequences. The neighbor-joining tree was made using amino acid sequences analyzed by MEGA3.1. The confidence in the tree was determined by analyzing 1,000 bootstrap replicates, and bootstrap values are shown for each group. Branch lengths are drawn to the scale shown, indicating 0.2 amino acid substitutions per site. GenBank accessions are listed in Supplemental Table S2. [See online article for color version of this figure.]
Gene Clusters
Evidence that the Z-ISO gene evolved from an NnrU progenitor and acquired new function could be suggested by bacterial genomic location or “gene neighborhood” (Fig. 12). That is, in bacterial genomes, genes involved in related processes are often clustered. Early work on isolation of the carotenoid genes from cyanobacteria took advantage of this fact to isolate the PSY gene as an open reading frame adjacent to the PDS gene in Synechococcus elongatus PCC 7942 (Chamovitz et al., 1992). To examine where in the cyanobacterial genome Z-ISO homology was clustered, we searched for the Z-ISO/NnrU-like gene using the SEED viewer, where sequenced bacterial genomes have been compiled (http://theseed.uchicago.edu/FIG/index.cgi).
Figure 12.
Gene clustering in bacteria implies function. Genome organization was taken from the SEED database (http://theseed.uchicago.edu/FIG/index.cgi). [See online article for color version of this figure.]
In cyanobacteria utilizing plant-type desaturases for carotenoid biosynthesis, NnrU was usually clustered near other carotenoid genes and genes linked to redox activities (Fig. 12; Table III). For example, in S. elongatus PCC 7942 and S. elongatus PCC 6301, the Z-ISO/NnrU-like gene is three genes away from PDS and PSY, one of which is annotated as a “putative subunit of NAD(P)H:quinone oxidoreductase.” On the other side of the Z-ISO/NnrU-like gene, it is adjacent to genes encoding thioredoxin, NAD(P)H-quinone oxidoreductase chain L (EC 1.6.5.2), and NAD(P)H-quinone oxidoreductase chain M (EC 1.6.5.2; Fig. 12). Similarly, in the cyanobacterium Prochlorococcus marinus marinus CCMP1375, the Z-ISO/NnrU-like gene is flanked on one side by PSY, PDS, and the putative subunit of NAD(P)H:quinone oxidoreductase; on the other side are two genes annotated as “NADH dehydrogenase subunit, involved in CO2 fixation.”
Table III. Mapping of NnrU/Z-ISO homolog in cyanobacterial genomes.
This table is modified from Maresca et al. (2008). Five Prochlorococcus species strains are condensed to one row because they are identical in enzyme distribution. These include P. marinus MIT 9211, P. marinus MIT 9312, P. marinus NATL2A, P. marinus marinus CCMP1375, and P. marinus pastoris CCMP1986. To map Z-ISO/NnrU homologs, we searched for the NnrU-like gene using the SEED viewer, where sequenced bacterial and plant genomes have been compiled (http://theseed.uchicago.edu/FIG/index.cgi). The cluster analysis was done for available genomes for which the physical organization was available . +, Enzyme present; −, enzyme absent. Homologs of plant genes are as follows: CrtH(CrtISO), CrtP(PDS), CrtQ(ZDS). CrtI is the bacterial-type 4 step desaturase not found in plants.
| Cyanobacterial Species | CrtH | CrtI | CrtP | CrtQ | NnrU | NnrU Located Near Oxidoreductases | NnrU Located Near Carotenoid Genes |
| Anabaenavariabilis ATCC 29413 | + | − | + | + | + | + | − |
| Crocosphaera watsonii WH 8501 | + | − | + | + | + | − | − |
| Gloeobacter violaceus PCC 7421 | − | + | − | − | − | − | − |
| Nostoc punctiforme PCC 73102 | + | − | + | + | + | + | − |
| Nostoc species PCC 7120 | + | − | + | + | + | + | − |
| Prochlorococcus species (5) | + | − | + | + | + | + | + |
| Synechococcus elongatus PCC 6301 | + | − | + | + | + | + | + |
| Synechococcus elongatus PCC 7942 | + | − | + | + | + | + | + |
| Synechococcus species CC9311 | + | − | + | + | + | + | + |
| Synechococcus species CC9605 | + | − | + | + | + | + | + |
| Synechococcus species CC9902 | + | − | + | + | + | + | + |
| Synechococcus species RS9917 | + | − | + | + | + | + | + |
| Synechococcus species WH 5701 | + | − | + | + | + | + | + |
| Synechococcus species WH 7805 | + | − | + | + | + | + | + |
| Synechococcus species WH 8102 | + | − | + | + | + | + | + |
| Synechocystis species PCC 6803 | + | − | + | + | + | − | − |
| Thermosynechococcus elongatus BP-1 | − | − | + | + | − | − | − |
| Trichodesmium erythraeum IMS101 | + | − | + | + | + | + | − |
In denitrifying bacteria, the cluster containing the NnrU gene was related to the denitrification process and not to carotenogenesis. For example, in genomes of the purple sulfur bacterium R. sphaeroides 2.4.1, A. tumefaciens C58, and S. meliloti 1021, adjacent genes to NnrU are related to expression or formation of nitric oxide reductase (EC 1.7.99.7; Fig. 12). Although R. sphaeroides can produce carotenoids, the pathway does not use the partnered plant-type desaturases (Sandmann, 2009) and, therefore, was not expected to have the NnrU gene clustered near carotenoid pathway genes. As expected, nondenitrifier/nonphotosynthetic bacteria such as E. coli and the plant pathogen Pseudomonas syringae pv tomato DC3000 lacked an NnrU gene.
It was surprising that there was no evidence for a Z-ISO gene in the complete genome of Chlorobium tepidum, a green sulfur bacterium that grows anaerobically and produces carotenoids via the plant-type enzymes, PDS and ZDS (Frigaard et al., 2004); a CrtISO homolog has also been identified. C. tepidum also lacks plant-type lycopene cyclases (Maresca et al., 2008), suggesting that carotenogenesis in this organism may not really be plant like in entirety. Given that this bacterium requires a minimum of light to survive, such light might be sufficient to photoisomerize the PDS product for the downstream ZDS, as seen in the biochemical phenotype of leaves from light-exposed Z-ISO mutant plants. Z-ISO sequences were previously found to be conserved in all oxygenic autotrophs analyzed in one study but not in nonoxygenic organisms (Ishikawa et al., 2009). The difference in oxygen utilization may be reflected in a mechanistic difference in isomerization for an anaerobic organism such as C. tepidum.
Functional Complementation of Z-ISO
To demonstrate the function of Z-ISO, a well-established E. coli complementation platform was utilized. Using this system, the 9,15,9′-tri-cis-ζ-carotene product of PDS was converted to 9,9′-di-cis-ζ-carotene only in the presence of either Arabidopsis or maize Z-ISO, AtZ-ISO1.1 or ZmZ-ISO, respectively (Fig. 13; Table IV). No function was detected for the Arabidopsis zic1-1 mutant gene product (AtZ-ISO1.1M) having an altered C terminus, the product of the shorter Arabidopsis transcript (AtZ-ISO1.2), or NnrU from a denitrifying bacterium, S. meliloti 1021. These results confirm that the maize y9 gene product is Z-ISO and that Z-ISO from maize and Arabidopsis function to isomerize the 15-cis-bond in 9,15,9′-tri-cis-ζ-carotene produced by PDS.
Figure 13.
Functional analysis of Z-ISO in E. coli. E. coli encoding bacterial GGPPS, PSY, and maize PDS accumulate 9,15,9′-tri-cis-ζ-carotene and were additionally transformed with the genes denoted or empty vector (pCOLADuet-1) as a control, and carotenoids were extracted and analyzed by HPLC. Under dark culturing conditions, Z-ISO from Arabidopsis (AtZ-ISO1.1) and maize (ZmZ-ISO1.1) convert most of the 9,15,9′-tri-cis-ζ-carotene (Tri) to 9,9′-di-cis-ζ-carotene (Di). Panels on the right show nonfunctioning proteins: truncated versions of Z-ISO missing the C terminus, such as the product of the short Arabidopsis transcript (AtZ-ISO1.2), transcript from the zic 1-1 mutant (AtZ-ISO1.1M), and NnrU from a denitrifying bacterium, S. meliloti 1021. Peaks are labeled as in Figure 3. [See online article for color version of this figure.]
Table IV. Functional testing of Z-ISO in E. coli accumulating 9,15,9′-tri-cis-ζ-carotene.
E. coli carrying bacterial crtE (encoding GGPPS), crtB (encoding PSY), and maize PDS accumulate 9,15,9′-tri-cis-ζ-carotene and were additionally transformed with the genes denoted or empty vector (pCOLADuet-1) as a control. Numbers correspond to percentage of total ζ-carotene based on peak area relative to total peak area of six ζ-carotene peaks.
| Added Genes | 9,15,9′-Tri-cis-ζ-carotene | 9,9′-Di-cis-ζ-carotene |
| Empty vector | 34.40 ± 1.05 | 33.02 ± 0.64 |
| AtZ-ISO1.1 | 8.91 ± 2.44 | 52.81 ± 3.95 |
| ZmZ-ISO | 6.09 ± 1.26 | 67.57 ± 3.01 |
| AtZ-ISO1.1M | 33.53 ± 2.85 | 36.64 ± 4.60 |
| AtZ-ISO1.2 | 33.14 ± 2.26 | 32.08 ± 3.07 |
| Bacterial NnrU | 36.83 ± 3.75 | 33.38 ± 1.60 |
DISCUSSION
We previously predicted that the two isomerases Z-ISO and CrtISO would differ structurally because of their different substrate specificities (Li et al., 2007). The Z-ISO substrate is one double bond as compared with the conjugated double bond substrate of CrtISO. The possibility that Z-ISO might be a paralog of CrtISO was tested, but gene products never showed evidence of Z-ISO activity (data not shown). The plant CrtISO product retains isomerase activity of an evolutionary progenitor related to bacterial CrtI, a carotenogenic enzyme combining desaturase and isomerase activities (for review, see Sandmann, 2009). In contrast, the plant Z-ISO gene appears to have evolved from an ancestor of the NnrU gene, which is ubiquitous in denitrifying bacteria.
Denitrification is widespread in bacteria ranging from plant symbionts to human pathogens and some fungi (for review, see Zumft, 1997; Shapleigh, 2009). Denitrification produces nitrogen oxides for use as electron acceptors for respiration to support growth under anaerobic or microaerobic conditions. For example, denitrification ensures the survival of nitrogen-fixing S. meliloti that must endure oxygen-limiting conditions in plant root nodules or when free living in soil. Four reductases catalyze reduction of nitrate or nitrite to N2 via nitric oxide and nitrous oxide. In gram-negative bacteria, the periplasmic nitrite reductase (NIR) converts nitrite to nitric oxide, and then the membrane-bound nitric oxide reductase (NOR) converts nitric oxide to nitrous oxide (for review, see Zumft, 1997). NnrU is part of the NOR operon and encodes a six-membrane-spanning domain protein that is required for both NIR and NOR activity, although its exact role is unclear (Bartnikas et al., 1997).
Phylogenetic analysis and genomic clustering of physiologically related genes linked Z-ISO (an NnrU homolog) in cyanobacteria to carotenogenesis, while NnrU genes in denitrifying proteobacteria were linked to denitrification. Cyanobacteria may have recruited the NnrU gene from the process of denitrification control for use in new reactions requiring redox components, such as carotenogenesis. Protein structure predictions using the METASERVER Web site (Ginalski et al., 2003) to model Z-ISO on known protein crystal structures revealed structural similarity with oxidoreductases. This finding suggests that Z-ISO may belong to the class of oxidoreductases that have intramolecular oxidoreductase activity and transpose C=C bonds (GO: 0016863; http://www.yeastrc.org/pdr/pages/front.jsp). Z-ISO may mediate isomerization by utilizing aspects of electron transfer that may be inherent in NnrU. Unfortunately, it is not known exactly how NnrU functions in denitrifying bacteria. Further study of Z-ISO may help shed light on the role of NnrU in denitrification, an important survival process found in organisms that range from soil bacteria to human pathogens.
Z-ISO was found to be a single-copy, highly conserved gene with NnrU homologs present throughout oxygenic autotrophs (Ishikawa et al., 2009). Like NnrU, Z-ISO is predicted to be a transmembrane protein. Using systems tools, we found that Z-ISO transcript levels were highly correlated with mRNAs of other genes encoding carotenoid biosynthetic enzymes compared with the background of approximately 30,000 Arabidopsis genes. The most highly correlated gene was PDS, encoding the enzyme that produces the substrate for Z-ISO. Large-scale proteomics experiments have previously verified the localization of Z-ISO to chloroplasts, the site of carotenoid biosynthesis (Zybailov et al., 2008; Ishikawa et al., 2009). Using a simple heterologous platform, we were able to demonstrate that Z-ISO from both a monocot and a eudicot mediate isomerization of the 9,15,9′-tri-cis-ζ-carotene product of PDS to 9,9′-di-cis-ζ-carotene, the substrate required of the downstream enzyme, ZDS. Although Z-ISO is related to bacterial NnrU, the NnrU peptide did not have isomerase activity, suggesting that sequence changes allowed Z-ISO to acquire a new function for carotenogenesis. Proteins encoded by the short alternative Arabidopsis transcript 1.2 and the zic1-1 truncated mutant Z-ISO revealed that the C-terminal region were important for Z-ISO enzyme activity.
In endosperm and other tissues not exposed to light, biosynthesis of carotenoids is dependent on Z-ISO. Without Z-ISO, carotenoid biosynthesis is blocked and 9,15,9′-tri-cis-ζ-carotene accumulates. It was thought that photosynthetic tissue did not require Z-ISO because light could mediate nonenzymatic photoisomerization of the 15-cis-bond in 9,15,9′-tri-cis-ζ-carotene. Therefore, it was unexpected to find that among the various tissues sampled in several species, Z-ISO was expressed at highest levels in green leaf tissue. The possibility that Z-ISO was actually important in photosynthetic tissue was suggested by the mutant phenotypes in Arabidopsis and maize. Arabidopsis Z-ISO mutant plants exhibited a delayed greening phenotype. When grown in the light, Arabidopsis mutant plants contained lower levels of carotenoids and chlorophylls as compared with the wild type. The effect of the Z-ISO mutations on chlorophyll content is likely to be a secondary effect, since variations in leaf carotenoids are known to be associated with altered chlorophyll content and chloroplast ultrastructure (Robertson and Anderson, 1961; Bachmann et al., 1973). Maize y9 plants also had reduced carotenoids and chlorophyll and were less vigorous and lighter green than nonmutant siblings, the phenotype being influenced by environmental factors (Robertson, 1975; Janick-Buckner et al., 2001). Exacerbation of this phenotype was observed upon exposure to a fluctuating temperature regime (Janick-Buckner et al., 2001). These results suggest that Z-ISO is needed in green tissue even under nonstressed conditions. Z-ISO becomes even more important in response to stress and, therefore, Z-ISO may play a crucial role in adaptation to environmental stress. These results suggest that light-mediated isomerization is insufficient in photosynthetic tissue and that Z-ISO is necessary both in light-exposed and in light-limited tissues (e.g. endosperm and roots).
Since the discovery that the PDS enzymatic product was a different geometrical isomer than the substrate needed for ZDS, it has been an enigma how these consecutive enzymes might function, especially in the absence of light. Discovery of Z-ISO provides the missing link. Further analysis of Z-ISO will contribute to better understanding of the complexity of carotenoid desaturation in oxygenic autotrophs, which evidently requires four enzymes compared with one in bacteria. The importance of Z-ISO is not limited to engineering plant carotenoids for nutritional benefit, but its further study will help define the mechanisms allowing plants, diatoms, algae, and cyanobacteria to adapt to environmental changes or, in the case of plants, to make the carotenoid-derived signals needed to mediate developmental processes.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana Col-0) wild-type and T-DNA lines in the Col-0 background were obtained from TAIR, contributed by J. Alonso and D. Weigel. To obtain etiolated seedlings, seeds were cold treated at 4°C for 3 to 4 d and then grown on half-strength Murashige and Skoog basal salt medium with 1% Suc for another 6 d at 25°C in the dark. T-DNA insertion lines for the Z-ISO gene requested from TAIR (SALK_136385, CS859876, SALK_057053, and SALK_057915C) were named zic1-3 to zic1-6, respectively. Maize (Zea mays) inbred B73 (Y9) and 12 y9 alleles (Z-ISO gene mutant; X34D, X34E, X34F, X34G, X34H, X34I, X34K, X34L, X07CB, X07CC, 5705B, and 5705E) were obtained from the Maize Genetic Stock Center. 5705B and 5705E were the only maize pale yellow endosperm mutants not yet tested for allelism to y9 prior to our genomic DNA analyses, which later confirmed them to be y9 alleles. For etiolated leaves and roots, seeds were germinated and grown in the dark at 25°C for 10 to 14 d. Green leaves were collected from field-grown plants.
Screening for Arabidopsis Z-ISO Mutants
Seeds of Arabidopsis (30,000 T-DNA insertion lines) were germinated in the dark on sterile plant growth medium. In the dark, wild-type plants develop yellow cotyledons; we screened for those that appeared lighter yellow, due to putative blocks in carotenoid biosynthesis. The plants were then exposed to light, where if they were blocked in a nonisomerase structural gene, the plants would become albino and not survive to maturity; isomerase mutants were expected to be light green because light photoisomerization would overcome the mutant lesion. The delayed greening phenotype was also expected, since light photoisomerization is less efficient than Z-ISO activity. The plants were then grown to maturity, and seeds were collected, germinated, and self-pollinated to confirm the Z-ISO morphological and biochemical phenotypes. Plant pigments were extracted and analyzed by HPLC, with the expectation that a true Z-ISO mutant should accumulate 9,15,9′- tri-cis-ζ-carotene. From these, we obtained two confirmed mutants, which we named zic1-1 and zic1-2. The zic mutants appeared light yellow when germinated in the dark and exhibited delayed greening when shifted to the light; etiolated homozygous plants accumulated 9,15,9′- tri-cis-ζ-carotene similar to maize y9 plants.
Map-Based Cloning of Arabidopsis Z-ISO
The zic1-1 mutant was selected for map-based cloning, and genetic mapping was performed according to Lukowitz et al. (2000). zic1-1 was crossed to the ecotype Landsberg erecta, and F2 seedlings were selected for the zic1 mutant phenotype. Genomic DNAs were extracted from 826 F2 plants with the zic1 phenotype, and simple sequence length polymorphism markers were used for rough mapping, locating the Z-ISO gene to the north end of chromosome 1, between two markers, F17L22 and ciw12. Primers are listed in Supplemental Table S1. Fine-mapping eventually mapped the Z-ISO gene to a region of 69 kb. Subsequently, multiple fragments of open reading frames for 12 candidate genes included in this region were amplified by PCR using primers listed in Supplemental Table S1 and then sequenced to determine where the mutation was located.
Carotenoid Extraction for Analysis of ζ-Carotene
The carotenoid extraction procedure for plants was based on Vallabhaneni et al. (2009) with some modification. Five hundred milligrams of maize or Arabidopsis tissues was ground in liquid nitrogen and then incubated with 6 mL of extraction buffer (ethanol with 1% butylated hydroxytoluene [BHT]; Sigma) at 85°C for 6 min, followed by 10 min of saponification with 120 μL (1 g mL−1) of KOH (Sigma). Samples were then vortexed and cooled on ice with the addition of 4 mL of water and 3 mL of 2:1 petroleum ether:diethyl ether (v/v), vortexed, and centrifuged for 10 min at 3,500 rpm. The upper layer was collected, and petroleum ether:diethyl ether extraction of the lower layer was repeated twice. The combined upper layer fractions were dried with nitrogen gas, and carotenoids were dissolved in 400 μL of methanol with 1% BHT. The Escherichia coli carotenoid extraction was performed as described previously (Li et al., 2007) except for the addition of 1% BHT in methanol in the extraction buffer. For photoisomerization analysis, carotenoid extracts were exposed to light for 12 h at room temperature, and controls were put under the same temperature except under dark conditions.
Carotenoid Analysis by HPLC
HPLC separation was performed with a Waters HPLC system equipped with a 2695 Alliance separation module, Empower I software (Waters), and a 996 photodiode array detector (Waters). Separation was conducted with a C30 Develosil 5u RPAQUEOUS (250 × 4.6 mm) column (Phenomenex). Isocratic separation was performed using a mobile phase of 4 parts water, 66 parts methanol, and 30 parts methyl-t-butyl-ether at a constant flow rate of 1 mL min−1 for 80 min. Column and sample temperatures were kept at 20°C. Identification of ζ-carotene was based on comparison with spectra and elution times of ζ-carotene isomers produced with expression of pACCRT-EBP (Matthews et al., 2003) and from published data (Li et al., 2007). Quantification was performed by measuring peak areas using the Empower I software (Waters).
Changes in Carotenoid and Chlorophyll Composition of Arabidopsis zic1 Mutants Compared with the Wild Type
Seedlings were grown for 6 d on half-strength Murashige and Skoog medium plus 1% Suc in the light at 25°C. Carotenoids and chlorophyll were extracted as described (Pogson et al., 1996) and separated by HPLC (Muller-Moule et al., 2002), and peaks were identified by comparison of spectra with previously published data (Schiedt and Liaaen-Jensen, 1995). The changes in carotenoid and chlorophyll amounts were calculated by normalizing peak areas for sample fresh weight and then comparing with that of the wild type. For total carotenoid and chlorophyll, the peak area data for the four carotenoid peaks (neoxanthin, violaxanthin, lutein, and β-carotene) and two chlorophyll peaks (chlorophyll a and chlorophyll b) were summed and processed as described above. Values given in Table II are averages and SD for three biological replicates. A representative HPLC scan for each genotype is shown in Supplemental Figure S1.
Expression of Z-ISO in E. coli
Full-length coding sequences of AtZ-ISO1.1 and AtZ-ISO1.2 were amplified using cDNA derived from Col-0 leaves with primers 2567/2578 (Supplemental Table S1) and 2567/2577, respectively; mutated AtZ-ISO1.1 coding sequence amplified using cDNA derived from zic1-1 leaves and primers 2567/2578 was referred to as AtZ-ISO1.1M. ZmZ-ISO coding sequence was amplified using cDNA from maize leaves with primers 2586/2587. The NnrU gene was amplified using genomic DNA from Sinorhizobium meliloti1021 with primers 2595/2596. The Z-ISO genes and NnrU gene were then inserted into BamHI and SalI sites of expression vector pCOLADuet-1 (Invitrogen) to produce plasmids pColAtZ-ISO1.1, pColAtZ-ISO1.2, pColAtZ-ISO1.1M, pColZmZ-ISO, and pColNnrU, respectively. The plasmids were further transformed into E. coli strain XL1-Blue carrying plasmid pACCRT-EBP, which carries crtE and crtB genes from Pantoea ananatis (formerly Erwinia uredovora) and the PDS gene from maize and confers accumulation of ζ-carotenes in E. coli (Matthews et al., 2003). For testing accumulating carotenoids, 2-mL overnight cultures were inoculated into 100 mL of Luria-Bertani broth in a 500-mL flask with appropriate antibiotics: chlorophenicol (34 mg L−1), ampicillin (50 mg L−1), and kanamycin (50 mg L−1). Cells were cultured for 8 h at 37°C in the dark and then induced with 10 mM isopropylthio-β-galactoside, followed by 40 h of slow shaking (100 rpm) at room temperature and an additional 2 d without shaking prior to carotenoid extraction. For each construct, three individual colonies were cultured and sd was calculated based on three replicates each.
Semiquantitative and Quantitative RT-PCR
To assess transcript patterns for Z-ISO in zic1-1 and zic1-2 using semiquantitative RT-PCR, cDNAs were derived from total RNAs of leaves of 3-week-old plants of two zic1 alleles and the wild type using primers flanking the corresponding mutation sites. Amplification products were gel purified, cloned into the pGEM-T Easy vector (Promega), and sequenced. Primers 2590/2591 were used for analysis of zic1-1, and a 181-bp product was amplified from zic1-1 (which carried a 10-bp deletion), compared with 191 bp from the wild type. For zic1-2 analysis, primers 2567/2589 were used. Multiple products of 491, 628, 688, and 789 bp were amplified in zic1-2, compared with 628 bp in the wild type.
For quantifying transcript levels of Z-ISO, total RNA was extracted from different tissues of Arabidopsis and maize. Arabidopsis seedlings were produced from seeds stratified in the cold for 4 d prior to germination on plates for 6 d in a growth chamber as described above (plant growth). Etiolated leaves and roots were obtained from maize grown in the dark for 10 d after soaking seeds in water. Field-grown plants were used to collect green leaves, and endosperm and embryo were dissected from ears harvested 20 d after pollination. Quantitative RT-PCR was performed using gene-specific primers: 2686/2687 for AtZ-ISO1.1, 2684/2685 for AtZ-ISO1.2, and 2582/2583 for ZmZ-ISO. Primer sequence and amplification conditions are listed in Supplemental Table S1. Results were normalized to actin. Values were expressed as means of three replicates with sd as before (Vallabhaneni and Wurtzel, 2009).
Chromosome Mapping and Sequence Analysis
The location of the y9 locus was obtained from the Maize Genetics and Genomics Database (www.maizegdb.org). The Arabidopsis Z-ISO amino acid sequence was used to BLAST (Altschul et al., 1997) the maize genome and locate its ortholog ZmZ-ISO at the y9 locus. Specific primer combinations 2593/2594, 2575/2576, 2613/2614, 2615/2616, and 2572/2574, which cover the promoter and open reading frame, were used to amplify ZmZ-ISO from DNAs of B73 and 12 y9 alleles. Amplification products were gel purified and cloned into the pGEM-T Easy vector (Promega) for sequencing. The Z-ISO amino acid sequences from other plants were obtained by BLAST homology searching of the National Center for Biotechnology Information database (www.ncbi.nlm.nih.gov). NnrU amino acid sequences of cyanobacteria, Agrobacterium tumefaciensC58, and Sinorhizobium meliloti 1021 were obtained from the SEED database (http://theseed.uchicago.edu) by searching for NnrU domain proteins. The Arabidopsis Z-ISO was used as a query to identify by BLAST the homolog from the diatom Thalassiosira pseudonana (version 3.0; http://www.jgi.doe.gov/). Sequence analysis and phylogenetic tree construction were performed using MEGA3 and the neighbor-joining method (Kumar et al., 2008). GenBank accessions are listed in Supplemental Table S2. Prediction of transmembrane domains was made using TMHMM (http://www.cbs.dtu.dk/services/TMHMM/). Transit peptide prediction was made using ChloroP (http://www.cbs.dtu.dk/services/ChloroP/). Protein structure predictions were made using METASERVER (http://meta.bioinfo.pl/submit_wizard.pl; Ginalski et al., 2003).
Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession numbers listed in Supplemental Table S2.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Carotenoid and chlorophyll composition of Arabidopsis Z-ISO mutants compared with the wild type.
Supplemental Figure S2. Alignment of Z-ISO amino acid sequences.
Supplemental Table S1. Primers and amplification conditions.
Supplemental Table S2. GenBank accession numbers.
Supplementary Material
Acknowledgments
We thank Rena Quinlan for technical advice, Jesus Beltran for technical assistance, Dr. Maria Shumskaya for suggestions on homology modeling, and Dr. Hai-Ping Cheng for providing genomic DNA of S. meliloti1021. Maize y9 mutants and Arabidopsis plants (SALK lines and T-DNA collections) were obtained from the Maize Genetic Stock Center and the Arabidopsis Stock Center, respectively.
References
- Akiyama K, Hayashi H. (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot (Lond) 97: 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann MD, Robertson DS, Bowen CC, Anderson IC. (1973) Chloroplast ultrastructure in pigment-deficient mutants of Zea mays under reduced light. J Ultrastruct Res 45: 384–406 [DOI] [PubMed] [Google Scholar]
- Bartley GE, Scolnik PA, Beyer P. (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259: 396–403 [DOI] [PubMed] [Google Scholar]
- Bartnikas TB, Tosques IE, Laratta WP, Shi J, Shapleigh JP. (1997) Characterization of the nitric oxide reductase-encoding region in Rhodobacter sphaeroides 2.4.3. J Bacteriol 179: 3534–3540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer P, Mayer M, Kleinig K. (1989) Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem 184: 141–150 [DOI] [PubMed] [Google Scholar]
- Beyer P, Weiss G, Kleinig H. (1985) Solubilization and reconstitution of the membrane bound carotenogenic enzymes from daffodil chromoplasts. Eur J Biochem 153: 341–346 [DOI] [PubMed] [Google Scholar]
- Breitenbach J, Kuntz M, Takaichi S, Sandmann G. (1999) Catalytic properties of an expressed and purified higher plant type zeta-carotene desaturase from Capsicum annuum. Eur J Biochem 265: 376–383 [DOI] [PubMed] [Google Scholar]
- Breitenbach J, Sandmann G. (2005) ζ-Carotene cis isomers as products and substrates in the plant poly-cis carotenoid biosynthetic pathway to lycopene. Planta 220: 785–793 [DOI] [PubMed] [Google Scholar]
- Britton G, Liaaen-Jensen S, Pfander H, (2004) Carotenoids Handbook. Birkhäuser Verlag, Basel [Google Scholar]
- Chamovitz D, Misawa N, Sandmann G, Hirschberg J. (1992) Molecular cloning and expression in Escherichia coli of a cyanobacterial gene coding for phytoene synthase, a carotenoid biosynthesis enzyme. FEBS Lett 296: 305–310 [DOI] [PubMed] [Google Scholar]
- Dogbo O, Laferrière A, D'Harlingue A, Camara B. (1988) Carotenoid biosynthesis: isolation and characterization of a bifunctional enzyme catalyzing the synthesis of phytoene. Proc Natl Acad Sci USA 85: 7054–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Misawa N, Linden H, Yamano S, Kobayashi K, Sandmann G. (1992) Expression in Escherichia coli, purification, and reactivation of the recombinant Erwinia uredovora phytoene desaturase. J Biol Chem 267: 19891–19895 [PubMed] [Google Scholar]
- Frigaard NU, Maresca JA, Yunker CE, Jones AD, Bryant DA. (2004) Genetic manipulation of carotenoid biosynthesis in the green sulfur bacterium Chlorobium tepidum. J Bacteriol 186: 5210–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginalski KEA, Fischer D, Rychlewski L. (2003) 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics 19: 1015–1018 [DOI] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Hugueney P, Römer S, Kuntz M, Camara B. (1992) Characterization and molecular cloning of a flavoprotein catalyzing the synthesis of phytofluene and zeta-carotene in Capsicum chromoplasts. Eur J Biochem 209: 399–407 [DOI] [PubMed] [Google Scholar]
- Isaacson T, Ohad I, Beyer P, Hirschberg J. (2004) Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol 136: 4246–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Fujiwara M, Sonoike K, Sato N. (2009) Orthogenomics of photosynthetic organisms: bioinformatic and experimental analysis of chloroplast proteins of endosymbiont origin in Arabidopsis and their counterparts in Synechocystis. Plant Cell Physiol 50: 773–788 [DOI] [PubMed] [Google Scholar]
- Janick-Buckner D, O'Neal J, Joyce E, Buckner B. (2001) Genetic and biochemical analysis of the y9 gene of maize, a carotenoid biosynthetic gene. Maydica 46: 41–46 [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. (2008) MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform 9: 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Murillo C, Wurtzel ET. (2007) Maize Y9 encodes a product essential for 15-cis zetacarotene isomerization. Plant Physiol 144: 1181–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, Misawa N, Chamovitz D, Pecker I, Hirschberg J, Sandmann G. (1991) Functional complementation in Escherichia coli of different phytoene desaturase genes and analysis of accumulated carotenoids. Z Naturforsch [C] 46: 1045–1051 [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible W-R. (2000) Positional cloning in Arabidopsis: Why it feels good to have a genome initiative working for you. Plant Physiol 123: 795–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresca JA, Graham JE, Bryant DA. (2008) The biochemical basis for structural diversity in the carotenoids of chlorophototrophic bacteria. Photosynth Res 97: 121–140 [DOI] [PubMed] [Google Scholar]
- Matthews PD, Luo R, Wurtzel ET. (2003) Maize phytoene desaturase and zetacarotene desaturase catalyze a poly-Z desaturation pathway: implications for genetic engineering of carotenoid content among cereal crops. J Exp Bot 54: 2215–2230 [DOI] [PubMed] [Google Scholar]
- Matthews PD, Wurtzel ET. (2007) Biotechnology of food colorant production. Socaciu C, , Food Colorants: Chemical and Functional Properties. CRC Press, Boca Raton, FL, pp 347–398 [Google Scholar]
- Mayer MP, Beyer P, Kleinig K. (1990) Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem 191: 359–363 [DOI] [PubMed] [Google Scholar]
- Mayer MP, Nievelstein V, Beyer P. (1992) Purification and characterization of a NADPH dependent oxidoreductase from chromoplasts of Narcissus pseudonarcissus: a redox mediator possibly involved in carotene desaturation. Plant Physiol Biochem 30: 389–398 [Google Scholar]
- Misawa N, Truesdale MR, Sandmann G, Fraser PD, Bird C, Schuch W, Bramley PM. (1994) Expression of a tomato cDNA coding for phytoene synthase in Escherichia coli, phytoene formation in vivo and in vitro, and functional analysis of the various truncated gene products. J Biochem. 116: 980–985 [DOI] [PubMed] [Google Scholar]
- Muller-Moule P, Conklin PL, Niyogi KK. (2002) Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol 128: 970–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Niyogi KK. (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. (1995) Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell 7: 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B, McDonald K, Truong M, Britton G, DellaPenna D. (1996) Arabidopsis carotenoid mutants demonstrate lutein is not essential for photosynthesis in higher plants. Plant Cell 8: 1627–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DS. (1975) Phenotypic and genetic studies of a new mutant of yellow endosperm in maize. J Hered 66: 127–130 [Google Scholar]
- Robertson DS, Anderson IC. (1961) Temperature sensitive alleles of the y1 locus in maize. J Hered 52: 53–60 [Google Scholar]
- Rodriguez-Concepcion M, Boronat A. (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids: a metabolic milestone achieved through genomics. Plant Physiol 130: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmann G. (2009) Evolution of carotene desaturation: the complication of a simple pathway. Arch Biochem Biophys 483: 169–174 [DOI] [PubMed] [Google Scholar]
- Schiedt K, Liaaen-Jensen S. (1995) Isolation and analysis. Britton G, Liaaen-Jensen S, Pfander H, , Carotenoids: Isolation and Analysis, Vol 1A. Birkhäuser, Basel, pp 81–108 [Google Scholar]
- Shapleigh JP. (2009) Dissimilatory and assimilatory nitrate reduction in the purple photosynthetic bacteria. Hunter CN, Daldal F, Thurnauer MC, Beatty JT, , The Purple Phototrophic Bacteria, Vol 28. Springer, Dordrecht, The Netherlands, pp 623–642 [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Vallabhaneni R, Gallagher CE, Licciardello N, Cuttriss AJ, Quinlan RF, Wurtzel ET. (2009) Metabolite sorting of a germplasm collection reveals the Hydroxylase3 locus as a new target for maize provitamin A biofortification. Plant Physiol 151: 1635–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhaneni R, Wurtzel ET. (2009) Timing and biosynthetic potential for carotenoid accumulation in genetically diverse germplasm of maize. Plant Physiol 150: 562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft WG. (1997) Cell biology and molecular basis of denitrification. Microbiol Mol Biol Rev 61: 533–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B, Rutschow H, Friso G, Rudella A, Emanuelsson O, Sun Q, van Wijk KJ. (2008) Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One 3: e1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.