Abstract
The inhibitors of monoamine oxidase B (MAO B) are effectively used as therapeutic drugs for neuropsychiatric and neurodegenerative diseases. However, their mechanism of action is not clear, since the neuroprotective effect of MAO B inhibitors is associated with the blockage of glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-death cascade, rather than the inhibition of MAO B. Here, we provide evidence that GAPDH potentiates the ethanol-induced activity of MAO B and brain cell toxicity. The levels of nuclear GAPDH and MAO B activity are significantly increased in brain-derived cell lines upon 75 mM ethanol-induced cell death. Over-expression of GAPDH in cells enhances ethanol-induced cell death, and also increases the ethanol-induced activation of MAO B. In contrast, the MAO B inhibitors rasagiline and selegiline (0.25 nM) and the rasagiline metabolite, 1-R-aminoindan (1 μM) decreases the ethanol-induced MAO B, prevents nuclear translocation of GAPDH and reduces cell death. In addition, GAPDH interacts with transforming growth factor-beta-inducible early gene (TIEG2), a transcriptional activator for MAO B, and this interaction is increased in the nucleus by ethanol but reduced by MAO B inhibitors and 1-R-aminoindan. Furthermore, silencing TIEG2 using RNAi significantly reduces GAPDH-induced MAO B upregulation and neurotoxicity. In summary, ethanol-induced cell death, attenuated by MAO B inhibitors, may result from disrupting the movement of GAPDH with the transcriptional activator into the nucleus and secondly inhibit MAO B gene expression. Thus, the neuroprotective effects of rasagiline or 1-R-aminoindan on ethanol-induced cell death mediated by a novel GAPDH-MAO B pathway may provide a new insight in the treatment of neurobiological diseases including alcohol-use disorders.
Keywords: Monoamine oxidase B, Glyceraldehyde-3-phosphate dehydrogenase, Apoptosis, MAO B inhibitors, The metabolite of rasagiline, Transforming growth factor-beta-inducible early gene 2, Brain cell lines, Alcohol-use disorders
Introduction
The anti-Parkinson drugs, deprenyl (selegiline) and Azilect (rasagiline), are also effectively used for the treatment of several neuropsychiatric and neurodegenerative diseases, including Parkinson's disease (Fernandez and Chen 2007) and senile dementia (Tariot et al. 1987; Youdim 2006). The mechanism for these benefits is thought to depend on MAO B inhibition. However, a large body of research has now shown that selegiline and rasagiline can also increase neuronal survival independently of MAO B inhibition, because MAO B inhibitors, such as selegiline and rasagiline, were shown to protect neuronal cells from apoptosis at a low concentration (Tatton et al. 1994; Hara et al. 2006) which would not inhibit MAO B activity. In addition, the optical isomer of rasagiline, TVP102 which is devoid of MAO inhibitory activity, has similar neuroprotective property (Youdim et al. 2001). MAO B inhibitors, selegiline and rasagiline, have been further reported to interfere with apoptosis signaling pathways mediated by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Tatton et al. 2003).
GAPDH is an important enzyme in the glycolysis and gluconeogenesis pathways. Recently, it has been shown that GAPDH is not only a traditional metabolic enzyme involved in energy production, but also plays multiple roles in numerous intracellular activities including the initiation of cell death by translocating into the nucleus as a transcription factor (Hara et al. 2005). However, whether GAPDH is involved in alcohol-induced brain cell death has not been documented.
MAO B is an enzyme that degrades a number of biogenic amines (such as phenylethylamine tyramine, tryptamine, and dopamine) and generates inert hydrogen peroxide (H2O2) which can interact with iron initiating Fentons reaction to produce reactive hydroxyl radicals that cause toxicity to cells and neurons (Gerlach et al. 2006). Therefore aberrant increase in MAO B activity in the elderly has been implicated in neurodegenerative diseases. The 5′-flanking sequence (the promoter) of the MAO B gene contains a maximal activity region which is located between −246 to −99 bp (a core promoter region) (Shih et al. 1999; Wong et al. 2001). This core promoter region consists of two clusters of overlapping Sp1-binding sites. The members of the Sp1 family, such as transforming growth factor-beta-inducible early gene 2 (TIEG2), can bind to Sp1-binding sites in this region and activate MAO B promoter activity (Zhang et al. 2001; Ou et al. 2004).
This study sought to determine whether GAPDH has a relation with MAO B using brain cell lines and also investigates whether MAO B inhibitors have neuroprotective activity in ethanol-induced brain cell death. The selective irreversible MAO B inhibitor drug, rasagiline, has recently been approved by the US Food and Drug Administration as an anti-Parkinson drug (Checkoway et al. 2002). Unlike selegiline, rasagiline is not metabolized to potentially toxic methamphetamine metabolites but rather to its major metabolite, 1-R-aminoindan which is not an MAO B inhibitor and has been shown to possess neuroprotective properties both in vivo (Speiser et al. 1998) and in vitro (Bar-Am et al. 2007); however, the neuroprotective mechanism of 1-R-aminoindan is unknown. Therefore, 1-R-aminoindan (1 μM) was studied as a neuroprotective agent and compared to selegiline (0.25 nM) and rasagiline (0.25 nM) in this study.
Materials and Methods
Cell Lines and Reagents
The human brain cell lines, glioblastoma U-118 MG and neuroblastoma SH-SY5Y, were purchased from The American Type Culture Collection (ATCC). The antibodies used in this study: mouse monoclonal antibodies for GAPDH (Santa Cruz; sc-32233) and TIEG2 (BD Transduction Laboratory; 611402); rabbit polyclonal antibody for GAPDH (for immunoprecipitation assay, Santa Cruz; sc-25778) and goat polyclonal antibodies for MAO B (Santa Cruz; sc-18401). Rasagiline was synthesized by a PhD student, Hailin Zheng, in the laboratory of Dr. Youdim's (Haifa, Israel). 1-R-aminoindan and selegiline (deprenyl) were purchased from Sigma-Aldrich, USA. 2′,7′-Dichlorofluorescin-diacetate (for measurement of the generation of H2O2) was purchased from Sigma (D6883). A TIEG2 small interfering RNA (siRNA, sc-38546) and the transfection kit were purchased from Santa Cruz and Ambion (Austin, TX, USA), respectively.
Treating Cells with Ethanol and 1-R-aminoindan
An aliquot of 75 mM of ethanol or 1 μM of 1-R-aminoindan (stock solution is 1 mM in autoclaved water) or both ethanol and 1-R-aminoindan were added into a medium of each well or dish directly. If needed, 0.25 nM of either rasagiline or selegiline (stock solution is 500 nM in autoclaved water for each) was used as indicated in the text.
Since ethanol is volatile, a closed chamber system is utilized to stabilize the ethanol concentration in the culture medium (Adickes et al. 1988; Pantazis et al. 1992; Luo and Miller 1996, 1997). With this system, ethanol concentrations are maintained at steady ethanol levels in medium. In brief, cell culture dishes or multi-well trays containing U-118 MG or SH-SY5Y cells were placed on a rack inside a plastic container that could be tightly sealed. A separate sealed container was used for controls. The bottom of each container served as a reservoir filled with 200 ml of an aqueous solution with 75 mM of ethanol. The alcohol in the bath evaporates into the air inside the sealed container establishing a stable vapor pressure so that there is no net loss of ethanol from the culture medium. The culture medium with ethanol was changed every 24 h. For the nonethanol control, a bath consisting of water alone was used. Noting that ethanol at 50–100 mM reflects blood ethanol levels in chronic alcoholics (Henriksen et al. 1997; Yao et al. 2001), the ethanol concentrations used in this study (75 mM) are within the levels that result in physiological effects observed in alcoholics.
Cell Culture and Stable Cell Line Selection
The human glioblastoma and neuroblastoma cells were grown in Dulbecco's modified Eagle's medium supplemented with 100 units/ml penicillin, 10 mg/ml streptomycin, and 10% FBS. The SH-SY5Y cells contain only attached cells which are the high passage of SH-SY5Y (Ou et al. 1998, 2004). In generating the GAPDH-stable cell line, U-118 MG and SH-SY5Y cells were plated at a density of 5 × 9 106 cells in a 10-cm dish. Twenty-four hours later, the GAPDH-expression vector or pcDNA3.1 was transfected into cells with a superfect transfection reagent (Qiagen, Inc.). After 24 h, cells were re-plated onto 5-cm dishes, and Genectin (G418; 600 μg/ml) was added. Resistant clones were isolated into separate dishes after 6 days and cultured under continuous G418 selection (Ou et al. 2006b). Three independent GAPDH-stable cell lines were generated and used in each experiment. The over-expressed GAPDH protein was verified by Western blot analysis.
Quantitative Real-Time Reverse Transcription-Polymerase Chain reaction
In a 10-cm dish, 105 cells were grown for 24 h, and then ethanol (75 mM) or 1-R-aminoindan was added into medium of each dish daily for 2 days. Following incubation, total RNA was isolated from each group by using RNA isolation reagent (Invitrogen). The mRNAs were reverse-transcribed into cDNAs. Specific primers for the human MAO B were designed as follows: sense, 5–-GAC CATGTGGGAGGCAGGACTTAC-3′, antisense, 5′-CGC CCACAAATTTCCTCTCCTG-3′. The mRNA content for each group was analyzed by real-time reverse transcription-polymerase chain reaction (RT-PCR) using a Bio-Rad iCycler system. The RT-PCR was performed with a SYBR supermix kit (Bio-Rad), and 18S Ribosomal RNA primer was used as internal control in each plate to avoid sample variations. The average threshold cycle (CT) for the triplicate was used in all subsequent calculations. A ΔCT value was then calculated for each sample using the CT value minus the CT value obtained from the respective control sample. The ΔCT was converted to the fold induction required to reach the threshold amount of PCR by raising 2 to the ΔCT power. The relative differences between each treated group and control group were determined using the ΔCT method and presented as the fold control (taken as 1) (Ou et al. 2006a).
MAO B Catalytic Activity Assay
Ten thousands cells were plated in a 10-cm dish. After being treated with 75 mM of ethanol or/and 1-R-aminoindan (1 μM), rasagiline (0.25 nM) or selegiline (0.25 nM) daily for 3 days, cells were harvested in PBS (pH 7.4).
One hundred micrograms of total proteins were incubated with 10 μM 14C-labeled phenylethylamine (Amersham) in the assay buffer (50 mM sodium phosphate buffer, pH 7.4) at 37°C for 20 min and terminated by the addition of 100 μl 6 N HCl. The reaction products were then extracted with ethylacetate/toluene (1:1) and centrifuged at 4°C for 10 min. The organic phase containing the reaction product was extracted and its radioactivity was obtained by liquid scintillation spectroscopy (Geha et al. 2001; Ou et al. 2004).
Immunofluorescence
Cells were plated on a 4-well chamber slide (Nalge) on the day preceding the experiment, and treated with or without ethanol (75 mM) or with ethanol plus 1-R-aminoindan (1 μM) daily for 2 days. Then the cells were fixed in 4% paraformaldehyde in PBS for 20 min and immunostained by mouse anit-GAPDH antibody (1/1000) at 4°C overnight and visualized with Cy3-conjugated anti-mouse (red) secondary antibody. Stained slides were mounted by Vecta-shield (Vector Laboratories) in the presence of 4,6-diamino-2-phenylindole (DAPI, for nuclear staining) and examined under a fluorescence microscope (Ou et al. 2006a).
Nuclear Protein Extraction
In a 10-cm dish, 105 cells were grown for 24 h, and then ethanol (75 mM) and 1-R-aminoindan were added directly into the medium of each dish daily for 3 days. Then cells (in one dish) were harvested by scraping. The cell pellets were washed by PBS and then re-suspended in 20 μl of buffer A [10 mM KCl, 10 mM HEPES, 1.5 mM MgCl2; (0.5 mM DTT and 0.1% NP-40 were freshly added just before using)] and incubated on ice for 10 min and centrifuged at 4°C for 10 min (6000 rpm). The pellets (containing nucleus) were re-suspended in 15 μl of buffer C [20 mM HEPES (pH 7.9), 25% glycerol, 420 mM NaCl, 0.2 mM EDTA, 1.5 mM MgCl2 (0.5 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride were added freshly)] and incubated on ice for 15 min, and then centrifuged for 10 min (3000 rpm) at 4°C. The supernatant containing nuclear proteins was diluted with 75 μl of buffer D [20 mM HEPES (pH 7.9), 20% glycerol, 50 mM KCl, 0.2 mM EDTA (0.5 mM DTT and 0.5 mM phenylmethylsulfonyl fluoride were added freshly)] and stored at −80°C (if not used immediately) (Ou et al. 2004). Protein concentrations were determined by BCA protein Assay Kit (PIERCE).
Co-Immunoprecipitation
Nuclear proteins were extracted from U-118 MG or SH-SY5Y cells (1 × 107) which were treated with or without ethanol/1-R-aminoindan, as indicated in the text, daily for 3 days. Samples were adjusted to 200 μg/ml with ice-cold PBS. The nuclear protein was immunoprecipitated by incubating with anti-TIEG2 antibody (4 μl antibody in 1 ml PBS) with BioMag beads (Anti-Mouse, QIAGEN) overnight at 4°C with rotation. As a negative control, BioMag beads without antibody were used. Beads and bound proteins were pelleted by centrifugation, and washed thrice with 1 ml PBS. Proteins were eluted from beads by boiling them in SDS sample buffer (60 mM Tris–HCl, pH 6.8, 100 mg/ml sucrose, 2% SDS, 0.05 mg/ml bromophenol blue, and 100 mM DTT) for 5 min (Ou et al. 2006b). Proteins co-immunoprecipitated with TIEG2 were analyzed by Western blot with rabbit anti-GAPDH antibody.
Western Blot Analysis
An aliquot of 2.5 μm (for GAPDH assay) of total proteins was separated by 10.5% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After the transfer, membranes were blocked at room temperature for 2 h with 5% non-fat dry milk in TBS (10 mM Tris/HCl pH7.5 and 150 mM NaCl). The membranes were then incubated with anti-GAPDH antibody (1:2500) overnight at 4°C. After incubation with secondary antibody at room temperature for 2 h, the bands were visualized by horseradish peroxidase reaction using SuperSignal West Pico Chemiluminescent Substrate (PIERCE).
Measurement of Intracellular H2O2 Generation
The intracellular generation of H2O2 induced by ethanol was tested utilizing 2′,7′-dichlorofluorescin-diacetate. The presence of H2O2 can oxidize 2′,7′-dichlorofluorescin-diacetate into the highly fluorescent compound 2′,7′-dichlorofluorescin which can be measured by a fluorometer. In brief, cells were seeded into a 6-well plate. The following day, cells were treated with or without ethanol (75 mM) or ethanol/1-R-aminoindan (1 μM) daily for 3 days and then harvested by rubber policeman in 200 μl of PBS. An aliquot of 75 μl of cell suspension from each well (a 6-well plate) was transferred to a 96-well plate, and an equal volume of 2′,7′-dichlorofluorescin-diacetate (final concentration was 10 μg/ml) was added into each well (a 96-well plate). After 5 min, the generation of H2O2 was read using a fluorescence spectrophotometer (wavelength 485/535 nm) (Liu et al. 2003).
MTT Assay for Proliferation Rate/Cell Viability Evaluation
Cells were grown in a 24-well plate. After the treatment of cells with or without ethanol (75 mM), 1-R-aminoindan (1 μM) alone, or both ethanol and 1-R-aminoindan daily for 3 days, the medium in excess of 0.5 ml per well was removed, and 30 μl of MTT dye (5 mg/ml, Sigma) in sterile PBS was added to 300 μl of medium in each well (final concentration is 0.5 mg/ml). Plates were incubated for 4 h, during which time the mitochondria in living cells converted the soluble yellow dye into an insoluble purple crystal. Cells and dye were then solubilized by the addition of 700 μl of DMSO per well. Optical density of each well at 570 nm was determined in a spectrophotometric analyzer (Ou et al. 2006a).
siRNA-Mediated TIEG2 Gene Knockdown
Cells that were stably expressing GAPDH (U-118 MG or SH-SY5Y) were transfected with control-siRNA or TIEG2-siRNA (Santa Cruz) using siPORT Amine Transfection agent (Ambion; Austin, TX, USA) as per manufacturer's instructions. In brief, 15 μl of siPORT Amine Transfection agent in 185 μl of Opti-MEM I medium was mixed with 2 μl siRNA in 198 μl of opti-MEM medium (for a 10-cm dish) for 10 min (final siRNA concentration: 20 nM), and then the mixed complex (the RNA/siPORT Amine transfection agent) was directly applied to cell culture medium (5 ml/dish). During ethanol-induced apoptosis, on day 1, siRNA-transfected cells were treated with 75 mM of ethanol daily for another 2 days. Then cells were subject to MAO B mRNA assay (quantitative RT-PCR), the generation of H2O2 and cell viability assays (MTT).
Statistical Analysis
The statistical significance was evaluated using one-way ANOVA to test for differences between groups and followed by Bonferroni's post hoc t-test. A P-value of 0.05 was considered to be significant.
Results
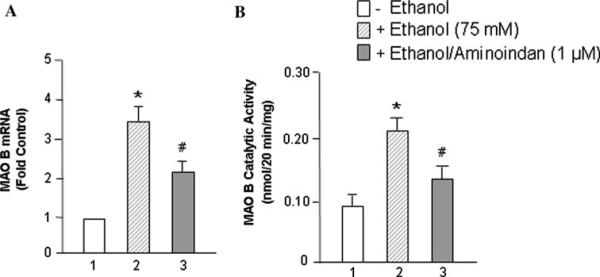
Ethanol Increases MAO B mRNA and Catalytic Activity, but 1-R-aminoindan Decreases Ethanol-Induced MAO B Activity
The expression of MAO B was investigated in two human brain cell lines, glioblastoma U-118 MG and neuroblastoma SH-SY5Y. Cells were seeded in 10-cm dishes. After 24 h, ethanol (0 or 75 mM) for mRNA assay was added into each dish, respectively, for 48 h, or ethanol (0 or 75 mM) for catalytic activity assay was added into each dish for 72 h. MAO B mRNA levels (Fig. 1a) and catalytic activities (Fig. 1b) were determined, respectively. The results indicated that both MAO B mRNA levels (~3.5-fold) and MAO B enzymatic activity (~2.2-fold) were significantly increased with the ethanol treatment in glioblastoma U-118 MG cells (Fig. 1a, b, lane 2 vs. 1). In contrast, the rasagiline metabolite, 1-R-aminoindan (1 μM), significantly decreased the ethanol-induced MAO B mRNA level to ~35% and also inhibited MAO B catalytic activity to ~30% (compare lanes 3 vs. 2 in Fig. 1a, b). A similar result was found in the human neuroblastoma SH-SY5Y cells (data not shown).
Fig. 1.
Effects of ethanol on MAO B mRNA level and catalytic activity. The human brain cell line, glioblastoma U-118 MG, was treated with 75 mM of ethanol for 48 h for mRNA assay or for 72 h for catalytic activity assay. a MAO B mRNA levels, b MAO B catalytic activities were determined, respectively. Controls were untreated cells (ethanol 0 mM), which were taken as 1 in (a). Data represent the mean ± SD of four independent experiments. *P < 0.001 compared with respective controls (ethanol 0 mM) and #P < 0.005 compared with respective ethanol-treated group (without 1-R-aminoindan)
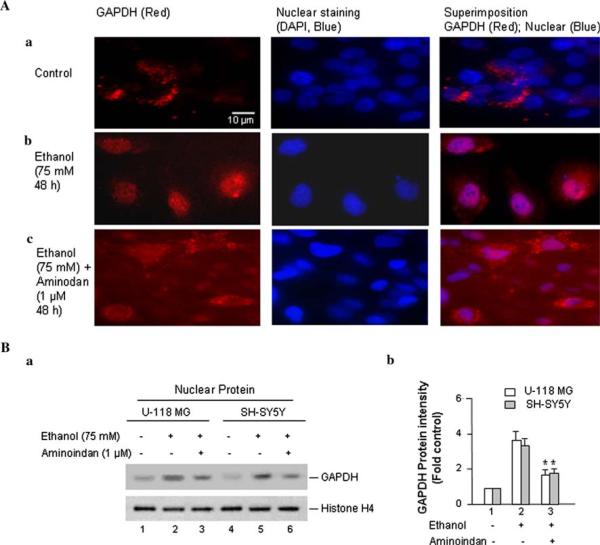
Ethanol also Increases GAPDH Nuclear Translocation, but 1-R-aminoindan Prevents It
GAPDH modulates gene transcription and participation in the initiation of cell death by translocation into nucleus (Hara et al. 2005). Whether ethanol could trigger the translocation of GAPDH from the cytosol into the nucleus was examined by immunocytochemistry in U-118 MG cells (Fig. 2A). In the control group (without ethanol treatment), GAPDH staining (red) was found predominantly in the cytosol (Fig. 2Aa). However, GAPDH signal was found predominantly in the nucleus upon treatment with ethanol (75 mM) for 48 h (Fig. 2Ab). The metabolite of rasagiline, 1-R-aminoindan (1 μM), blocked this nuclear translocation that induced by ethanol (Fig. 2Ac). These results indicate that the GAPDH-mediated ethanol-induced cell death may also require the transcriptional activity of GAPDH in nucleus. Both selegiline and rasagiline also exhibited similar results as 1-R-aminoindan (data not shown), which are consistent with the recent publications (Tatton et al. 2003; Hara et al. 2006). A similar result was found in SH-SY5Y cells as well (data not shown).
Fig. 2.
Effects of ethanol on the accumulation of GAPDH in the nucleus. Cells were treated by 75 mM ethanol for 48 h (for mRNA level and immunofluorescence) or 72 h (for protein level/Western blot). A Immunofluorescence microscopy was performed with anti-GAPDH antibody. U-118 MG cells were plated on a 4-well chamber slide and treated (a) without or (b) with 75 mM of ethanol or (c) with 75 mM of ethanol plus 1 μM of 1-R-aminoindan for 48 h. Then the cells were fixed and immunostained by mouse anti-GAPDH antibody and followed with fluorescein-conjugated anti-mouse secondary antibody (red). Stained slides were mounted in the presence of DAPI for nuclear staining (blue). The GAPDH (red) and nucleus (blue) and the merge of both GAPDH and the nucleus are indicated at the top. B Western blot analysis of nuclear GAPDH protein. (a) A representative of protein expression of GAPDH is shown. The anti-Histone H4 antibody was used as the loading control. (b) Quantitative analysis of Western blot results. A graph of the average optical density of GAPDH (normalized to the density of Histone H4) is shown. The relative intensity (relative optical density × pixel area) of autoradiographic bands from four independent preparations was evaluated using gel analysis software and a computer-assisted image analysis system. Data represent the mean ± SD of four independent experiments. *P < 0.001 compared with ethanol-treated group (without 1-R-aminoindan)
To confirm the result by immunocytochemistry above, the expression of nuclear GAPDH was determined with Western blot analysis in both brain cell lines (Fig. 2B). GAPDH protein expression was significantly increased by ~3.5-fold in the nucleus upon ethanol treatment (75 mM ethanol for 72 h; Fig. 2Bb, lane 2 vs. 1). However, 1-R-aminoindan (1 μM) was able to prevent the ethanol-induced nuclear accumulation of GAPDH to ~50% as compared to those in cells treated with ethanol alone (Fig. 2Bb, lane 3 vs. 2).
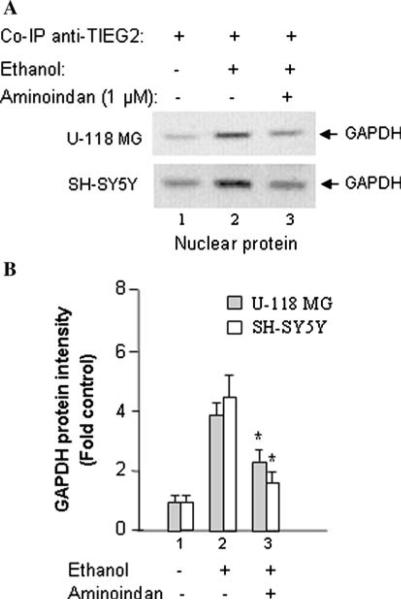
Ethanol Increases GAPDH Interacting with an MAO B Transcriptional Activator, TIEG2, but 1-R-aminoindan Reduces GAPDH/TIEG2 Interaction
The Sp1 family including TIEG2 (transforming growth factor-beta-inducible early gene 2) can bind to Sp1-binding sites in the core promoter of the MAO B gene and activate MAO B promoter activity (Zhang et al. 2001; Ou et al. 2004). Because ethanol can increase the mRNA level of MAO B and also induce GAPDH protein movement into the nucleus, it suggests that GAPDH might regulate the expression of MAO B at the transcriptional level. Therefore, the co-immuno-precipitation (co-IP) assay were performed in both U-118 MG and SH-SY5Y cell lines to test whether GAPDH interacts with an MAO B transcription factor, such as TIEG2.
TIEG2, also called KLF11 (Fernandez-Zapico et al. 2003), has been reported to increase MAO B gene expression (Ou et al. 2004) and inhibit cell proliferation (Zhang et al. 2001). Therefore, the interaction between GAPDH with TIEG2 was analyzed by Co-IP assay.
Nuclear proteins were extracted from cells treated without ethanol (Fig. 3a, lane 1), with 75 mM of ethanol (Fig. 3a, lane 2) or with both ethanol and 1 μM of 1-R-aminoindan (Fig. 3a, lane 3) for 72 h. Then the nuclear proteins were immunoprecipitated by incubation with anti-TIEG2 antibody. The immunoprecipitated proteins were visualized by Western blot using anti-GAPDH antibody (Fig. 3a), and the quantitative analysis of protein band intensity/statistical evaluations were shown in Fig. 3b. The results showed that GAPDH interacted with TIEG2 (Fig. 3b) and the intensity of GAPDH protein band was increased after treatment with ethanol, as compared with the untreated cells (Fig. 3b, lane 2 vs. 1). Further, the intensity of the GAPDH protein band was reduced after treatment with both ethanol and 1-R-aminoindan, as compared with the cells which were treated with ethanol alone (Fig. 3b, lane 3 vs. 2). Nuclear extracts without anti-TIEG2 antibody and nuclear extracts with an irrelevant anti-β-actin antibody (in the immunoprecipitation assay) did not show any bands and were used as negative controls (data not shown).
Fig. 3.
The effect of 1-R-aminoindan on 75 mM ethanol-induced nuclear accumulation of GAPDH/transcriptional activator complex. The interaction of GAPDH with TIEG2 (an MAO B transcriptional activator) in nucleus was determined by Co-IP assay. a Nuclear proteins isolated from U-118 MG or SH-SY5Y cells which were treated without ethanol (lane 1) or with ethanol (lane 2) or with both ethanol and 1-R-aminoindan (lane 3) for 72 h were immunoprecipitated by incubating with anti-TIEG2 antibody. Protein Co-IP with TIEG2 was analyzed by Western blot with anti-GAPDH antibody (lanes 1–3). b Quantitative analysis. The relative intensity of TIEG2-bound GAPDH band was quantified by Kodak Imaging Analysis System. The GAPDH levels were increased by ethanol but reduced by 1-R-aminoindan. Data represent the mean ±SD of four independent experiments. *P < 0.001 compared with respective ethanol-treated group (without 1-R-aminoindan)
Rasagiline (0.25 nM) and selegiline (0.25 nM) had the same effect on GAPDH binding to TIEG2 (data not shown) as did 1-R-aminoindan. This result suggests that GAPDH may interact with TIEG2 to enhance MAO B expression at the transcriptional level.
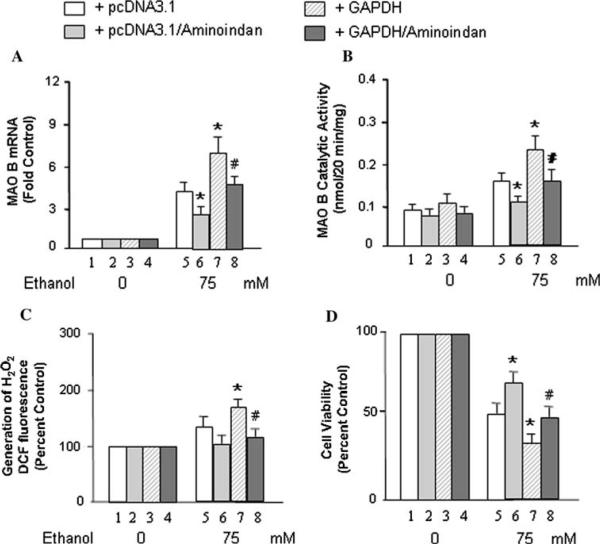
Over-Expressed GAPDH Increases, Whereas 1-R-aminoindan or an MAO B Inhibitor (Rasagiline or Selegiline) Decreases Ethanol-Induced MAO B Expression, the Production of H2O2 and Cell Death
As shown above, the interaction between GAPDH and the transcription factor of MAO B could be disrupted by 1-R-aminoindan (1 μM). Therefore, these drugs may reduce the MAO B mRNA level and catalytic activity; however, GAPDH may increase the MAO B mRNA level and catalytic activity upon ethanol treatment. In order to test this hypothesis, GAPDH or pcDNA3.1 stably transfected cells (U-118 MG) were cultured in medium without or with ethanol (75 mM) or with both ethanol/1-R-aminoindan for 2 days, and the levels of MAO B mRNA were determined by quantitative RT-PCR (Fig. 4a). The results show that the mRNA expression of MAO B was significantly increased in the GAPDH-stably expressed cell line upon ethanol treatment compared to the control group with pcDNA3.1-stably expressed cell line (Fig. 4a, lane 7 vs. 5). However, 1-R-aminoindan (1 μM) significantly decreased the MAO B expression induced by ethanol (Fig. 4a, lane 6 vs. 5) or induced by both ethanol and overexpressed GAPDH (Fig. 4a, lane 8 vs. 7). MAO B inhibitors, rasagiline (0.25 nM) and selegiline (0.25 nM), displayed a similar effect on the inhibition of MAO B; however, a greater inhibitory effect was achieved by rasagiline and selegiline (Table 1).
Fig. 4.
Effects of GAPDH and 1-R-aminoindan on ethanol-induced MAO B mRNA level, catalytic activity, generation of H2O2, and cell proliferation rate (MTT assay). GAPDH- or pcDNA3.1-overexpressed U-118 MG cells were treated by 75 mM of ethanol without or with 1-R-aminoindan (1 μM) as indicated in the figure for 48 h (for mRNA level) or 72 h (for MAO B catalytic activity, generation of H2O2 or MTT assay). a MAO B mRNA levels, b MAO B catalytic activity, c generation of H2O2, and d cell proliferation rate were determined, respectively. Controls were untreated cells (ethanol 0 mM) which were taken as 1 in (a) or taken as 100% in (c) and (d). Data represent the mean ± SD of three independent experiments. *P < 0.01 compared with pcDNA3.1-transfected cells in each ethanol treatment group (lane 5). #P < 0.02 compared with GAPDH-overexpressed cells in each ethanol treatment group (lane 7)
Table 1.
Effects of rasagiline, selegiline, and 1-R-aminoindan on ethanol-induced MAO B activity and neuroprotection (percent control)
| MAO B inhibition |
Inhibition of H2O2 generation (%) | Cell viability (MTT) (%) | ||
|---|---|---|---|---|
| mRNA (%) | Catalytic activity (%) | |||
| Aminoindan (1 μM) | 34 | 33 | 30 | 156 |
| Rasagiline (0.25 nM) | 58* | 61* | 43# | 181# |
| Selegiline (0.25 nM) | 44# | 45# | 30 | 140 |
Neuroprotective effect of MAO B inhibitors, rasagiline, and selegiline, as compared to 1-R-aminoindan (a major metabolite of rasagiline) on the inhibition of MAO B and the survival of a GAPDH-overexpressed cell line (U-118 MG) in ethanol-induced cell death (75 mM; 48 h for mRNA level or 72 h for catalytic activity, H2O2 generation and cell viability analysis). Controls were values obtained from Fig. 4, lane 7 (without drug treatment) which were taken as 100. Data represent the average of three independent experiments.
P < 0.02
P < 0.05 compared with 1-R-aminoindan (Aminoindan)
A similar result was found when MAO B catalytic activity was determined (Fig. 4b). The GAPDH-stably expressed cell line exhibited a significant increase in MAO B catalytic activity upon ethanol treatment (for 3 days) compared to the control group (pcDNA3.1-stably expressed cell line; Fig. 4b, lane 7 vs. 5). However, 1-R-aminoindan (1 μM), significantly decreased the MAO B catalytic activity-induced by ethanol (Fig. 4b, lane 6 vs. 5) or induced by both ethanol and over-expressed GAPDH (Fig. 4b, lane 8 vs. 7).
To test whether the increase in toxic reactive oxygen (H2O2) produced by MAO B enzymatic activity or ethanol neurotoxicity could be enhanced by over-expressed GAPDH, but reduced by 1-R-aminoindan, GAPDH or pcDNA3.1-stably transfected cells were treated without or with ethanol (75 mM) or with both ethanol/1-R-aminoindan (1 μM) for 3 days. Then the intracellular levels of H2O2 were determined. As shown in Fig. 4c, GAPDH-stably expressed cell line resulted in a significant increase in the H2O2 generation (Fig. 4c, lane 7 vs. 5), but 1-R-aminoindan was able to reduce the production of H2O2 significantly (Fig. 4c, lane 8 vs. 7). Rasagiline and selegiline (0.25 nM) showed a similar effect on the reduction of H2O2 generation, but rasagiline exhibited a protective effect significantly greater than either 1-R-aminoindan or selegiline (Table 1).
Furthermore, the evaluation of cell death was performed in both glioblastoma and neuroblastoma cell lines. Cell proliferation rate among those groups was determined by MTT assay (Fig. 4d). The results show that the proliferation of the GAPDH-stably cell line was decreased significantly upon ethanol treatment (Fig. 4d, lane 7 vs. 5). Whereas, the addition of 1-R-aminoindan (1 lM) increased the proliferation rate of pcDNA3.1- or GAPDH-stably overexpressed cell line significantly upon ethanol treatment (Fig. 4d, lanes 6 vs. 5 and 8 vs. 7).
MAO B inhibitors, rasagiline (0.25 nM) and selegiline (0.25 nM), were also used in the above experiments as well as 1-R-aminoindan, and their effects were summarized in Table 1. The results indicated that rasagiline (~2.5-fold) and selegiline (~1.5-fold) had significantly higher inhibitory effects on MAO B catalytic activity than that of 1-R-aminoindan, but rasagiline exhibited significantly more protective effect than either 1-R-aminoindan or selegiline (Table 1). The reason for more protective effect of rasagiline than that of 1-R-aminoindan or selegiline may be because rasagiline inhibits MAO B activity more than 1-R-aminoindan or selegiline, and hence reduces the production of the toxic hydrogen peroxide (H2O2) more than 1-R-aminoindan or selegiline does. Similar results were found in the human neuroblastoma SH-SY5Y cells (data not shown).
siRNA-Mediated TIEG2 Knockdown Decreased Ethanol-Induced MAO B mRNA Level, the Production of H2O2, and Cell Death
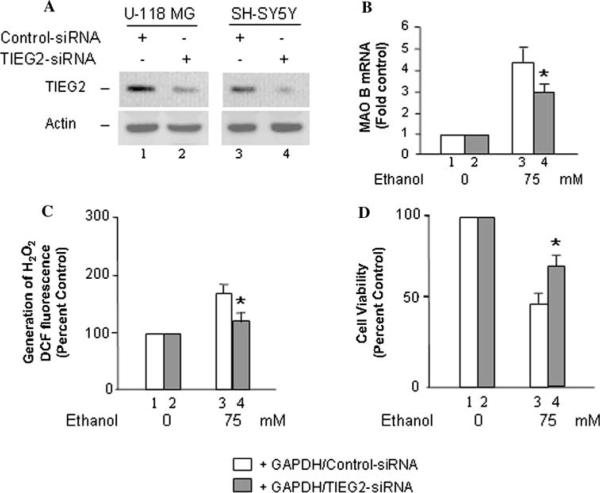
In contrast to over-expression of GAPDH, a TIEG2-knockdown cell line (generated in a GAPDH-stably expressed U-118 MG, or SH-SY5Y cell line; mediated by TIEG2-siRNA technique; Fig. 5a) was used to provide the direct evidence that TIEG2 plays the important role in ethanol-induced apoptosis. The cells were harvested and lysed after siRNA treatment for 3 days. Equal amounts of total protein from each supernatant solution were resolved by SDS/PAGE; resolved TIEG2 was then quantified by Western blot analysis. The TIEG2 protein expression in TIEG2-siRNA-transfected cells was decreased to ~15% compared to those in cells transfected with control-siRNA (normal control cells; Fig. 5a, lanes 2 vs. 1 and 4 vs. 3). After treatment with 75 mM ethanol for 2 days, the TIEG2-knockdown cells displayed a significant decrease in levels of MAO B mRNA, as compared to control siRNA-transfected cells (P < 0.02; Fig. 5b, lane 4 vs. 3). In addition, the production of H2O2 (P < 0.02; Fig. 5c) and cell death (P < 0.02; Fig. 5d) was significantly reduced upon treatment with ethanol in TIEG2-knockdown cells compared to control cells. Similar results were found in SH-SY5Y cells (data not shown). These results suggested that TIEG2 plays the important role in GAPDH-mediated ethanol neurotoxicity.
Fig. 5.
Effects of TIEG2-knockdown on ethanol-induced MAO B mRNA level, generation of H2O2, and cell proliferation rate (MTT assay). GAPDH-overexpressed U-118 MG cells were transfected with TIEG2-siRNA or control-siRNA. a After transfected with siRNA for 3 days, TIEG2 protein from each cell line was evaluated by Western blot analysis. b After transfected with siRNA for 24 h, cells were treated with 75 mM ethanol, and then harvested after 2 days for determining MAO B mRNA level, c the production of H2O2 and d cell proliferation rate/viability. Controls were untreated cells (ethanol 0 mM) which were taken as 1 in (b) or taken as 100% in (c) and (d). Data represent the mean ± SD of three independent experiments.
*P < 0.02 compared to control-siRNA-transfected cells in each ethanol treatment group (lane 3)
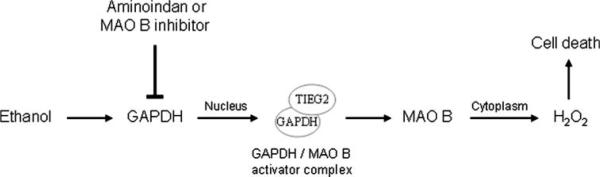
Based on our data, we summarize that upon ethanol treatment, the levels of nuclear GAPDH and MAO B activity are increased. GAPDH forms a complex with transcription factor TIEG2 (the transcriptional activator for MAO B), and increases the expression of MAO B indirectly. The MAO B inhibitors and 1-R-aminoindan block the movement of the GAPDH and TIEG2 complex into the nucleus, and therefore decrease the MAO B gene expression. Thus the MAO B inhibitors and 1-R-aminoindan could disrupt GAPDH/MAO B-mediated cell stress/apoptotic signaling pathway induced by ethanol. The schematic representation of the proposed model for ethanol-induced brain cell death mediated by GAPDH/MAO B and its inhibition by rasagiline and 1-R-aminoindan is shown in Fig. 6.
Fig. 6.
Proposed mechanism for ethanol-induced brain cell death-mediated by GAPDH and MAO B, and the inhibition by 1-R-aminoindan or rasagiline. Arrows and dashed lines indicate activation and repression of the following targets, respectively
Discussion
GAPDH exhibits multiple roles in numerous intracellular activities and is associated with several neurodegenerative diseases, such as Alzheimer's (Li et al. 2004) and Huntington's diseases (Senatorov et al. 2003; Chuang et al. 2005; Bae et al. 2006). Recently, GAPDH protein level has reportedly increased significantly in the brains of human subjects with alcoholism (Alexander-Kaufman et al. 2006). Our current study has first shown that nuclear GAPDH was increased upon ethanol treatment in brain-derived cell lines. Additionally, our study firstly demonstrates the link between GAPDH and MAO B, which suggests that the GAPDH-MAO B-mediated cell stress/cell death pathway may underlie the molecular pathogenesis of ethanol neurotoxicity.
The aberrant increase in MAO B activity has been implicated in alcoholism (Carlsson et al. 1980). Similar to GAPDH, the MAO B expression is increased by ethanol in brain cell lines. Previously, ethanol has been found to increase MAO B promoter activity and altered the transcription factors binding to the MAO B promoter in SH-SY5Y cells (Ekblom et al. 1996), suggesting that ethanol may directly enhance the MAO B expression by induction of transcription factor(s). Recently, we found that ethanol significantly increased TIEG2 expression in SH-SY5Y cells (Lu et al. 2008). Here, we further report that GAPDH interacted with TIEG2, and this interaction was increased in the nucleus upon ethanol treatment, suggesting that TIEG2 may be a direct activator for MAO B induced by ethanol. In turn, the abnormal increase in MAO B could produce more reactive oxygen (H2O2), which may activate apoptosis and cause cell death.
Another transforming growth factor inducible gene, TIEG1, was also involved in apoptosis (Wang et al. 2006) and cell proliferation inhibition (Cook and Urrutia 2000). Although ethanol also induces TIEG1 mRNA expression (Fang et al. 1998), TIEG1 does not increase the MAO B promoter activity as determined by transient transfection and luciferase assay (data not shown). Therefore, TIEG1 is probably through another signaling pathway to mediate ethanol-induced cell death, which needs to be investigated in the future.
The MAO B inhibitors, deprenyl (selegiline) and Azilect (rasagiline), have been used in the therapy of neurodegenerative diseases (such as Parkinson's Disease) (Paterson et al. 1997; Tatton et al. 2000; Maruyama et al. 2001; Fernandez and Chen 2007), mental disorders, such as depression (Goodnick 2007; Robinson and Amsterdam 2008), and senile dementia (Tariot et al. 1987; Youdim et al. 2005; Youdim 2006). Neuroprotection by MAO B inhibitors (at a low concentration) has been suggested to be independent of MAO B (Ansari et al. 1993; Carlile et al. 2000), and through the blockage of a GAPDH-mediated cell death signaling pathway. In our studies, we also used a low concentration of rasagiline and selegiline (0.25 nM). Upon 3 days' treatment, this subnanomolar concentration can inhibit the ethanol-induced MAO B catalytic activity significantly (Table 1). Therefore, our findings provide a new insight of the anti-apoptotic effect of MAO B inhibitors. In addition to the MAO B-independent mechanism such as GAPDH-Siah1-mediated cell death cascade (Hara et al. 2005), the neuroprotective effect of rasagiline and selegiline on ethanol induced, MAO B-involved apoptosis may also depend on the inhibition of MAO B.
In regards to the metabolites of MAO B inhibitors, selegiline is metabolized to potentially toxic amphetaminic metabolites, whereas, rasagiline is mainly metabolized to 1-R-aminoindan (Blandini 2005), which exhibited neuroprotective properties in vitro (Bar-Am et al. 2007), although 1-R-aminoindan is not an MAO B inhibitor. Whether 1-R-aminoindan also inhibits nuclear translocation of GAPDH was yet unclear. In this study, using 1-R-aminoindan effectively prevented the nuclear translocation of GAPDH and decreased ethanol-induced cell death. It suggests that the neuroprotective effect of 1-R-aminoindan, produced by the oral administration of rasagiline, was through the blockage of a GAPDH-mediated cell death signaling pathway as was rasagiline (Maruyama et al. 2001) and secondly via the inhibition of MAO B activity (Fig. 4a, b). Indeed, recent in vivo studies further support that 1-R-aminoindan (Youdim et al. unpublished data) as well as rasagiline (Blandini et al. 2004) displayed neuroprotective activity in a 6-hydrodopamine model of Parkinson's disease, suggesting that 1-R-aminoindan may contribute to the overall neuroprotective and anti-apoptotic activity of rasagiline.
In summary, ethanol-induced cell death, attenuated by MAO B inhibitors and 1-R-aminoindan, may result from disrupting the movement of GAPDH with the transcriptional activator into the nucleus and secondly inhibit MAO B gene expression. Therefore, GAPDH may be a novel player between MAO B and its inhibitors. Hence, an inhibitor directly targeting both GAPDH and MAO B may be therapeutically useful in combating the harmful effects of neurobiological diseases including alcohol-use disorders.
Acknowledgments
This study was supported by Public Health Service Grants P20 RR17701, a NARSAD Young Investigator Award, NIMH Grant R37 MH39085 (Merit Award), RO1 MH67968, Boyd and Elsie Welin Professor, and by an Intramural Research Support grant from The University of Mississippi Medical Center (Jackson, MS). We thank Dr. Craig. A. Stockmeier from The University of Mississippi Medical Center for his helpful advice, Dr. Warren May from The University of Mississippi Medical Center for statistical analysis, and Hailin Zheng from Rappaport Family Research Institute (Haifa, Israel), for synthesizing rasagiline.
Abbreviations
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- MAO
Monoamine oxidase
- Co-IP
Co-immunoprecipitation assay
- TIEG2
Transforming growth factor-beta-inducible early gene 2
- MTT
3-(4,5-Dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
- siRNA
Small interfering RNA
- PBS
Phosphate buffered saline
- DTT
Dithiothreitol
- EDTA
Ethylenediaminetetraacetic acid disodium salt dehydrate
References
- Adickes ED, Mollner TJ, Lockwood SK. Closed chamber system for delivery of ethanol to cell cultures. Alcohol Alcohol. 1988;23:377–381. doi: 10.1093/oxfordjournals.alcalc.a044832. [DOI] [PubMed] [Google Scholar]
- Alexander-Kaufman K, James G, Sheedy D, Harper C, Matsumoto I. Differential protein expression in the prefrontal white matter of human alcoholics: a proteomics study. Mol Psychiatry. 2006;11:56–65. doi: 10.1038/sj.mp.4001741. [DOI] [PubMed] [Google Scholar]
- Ansari KS, Yu PH, Kruck TP, Tatton WG. Rescue of axotomized immature rat facial motoneurons by R(-)-deprenyl: stereospecificity and independence from monoamine oxidase inhibition. J Neurosci. 1993;13:4042–4053. doi: 10.1523/JNEUROSCI.13-09-04042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae BI, Hara MR, Cascio MB, Wellington CL, Hayden MR, Ross CA, Ha HC, Li XJ, Snyder SH, Sawa A. Mutant huntingtin: nuclear translocation and cytotoxicity mediated by GAPDH. Proc Natl Acad Sci U S A. 2006;103:3405–3409. doi: 10.1073/pnas.0511316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Am O, Amit T, Youdim MB. Aminoindan and hydroxyaminoindan, metabolites of rasagiline and ladostigil, respectively, exert neuroprotective properties in vitro. J Neurochem. 2007;103:500–508. doi: 10.1111/j.1471-4159.2007.04777.x. [DOI] [PubMed] [Google Scholar]
- Blandini F. Neuroprotection by rasagiline: a new therapeutic approach to Parkinson's disease? CNS Drug Rev. 2005;11:183–194. doi: 10.1111/j.1527-3458.2005.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, Fancellu R, Blaugrund E, Nappi G. Neuroprotective effect of rasagiline in a rodent model of Parkinson's disease. Exp Neurol. 2004;187:455–459. doi: 10.1016/j.expneurol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Carlile GW, Chalmers-Redman RM, Tatton NA, Pong A, Borden KE, Tatton WG. Reduced apoptosis after nerve growth factor and serum withdrawal: conversion of tetrameric glyceraldehyde-3-phosphate dehydrogenase to a dimer. Mol Pharmacol. 2000;57:2–12. [PubMed] [Google Scholar]
- Carlsson A, Adolfsson R, Aquilonius SM, Gottfries CG, Oreland L, Svennerholm L, Winblad B. Biogenic amines in human brain in normal aging, senile dementia, and chronic alcoholism. Adv Biochem Psychopharmacol. 1980;23:295–304. [PubMed] [Google Scholar]
- Checkoway H, Powers K, Smith-Weller T, Franklin GM, Longstreth WT, Jr, Swanson PD. Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am J Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- Chuang DM, Hough C, Senatorov VV. Glyceraldehyde-3-phosphate dehydrogenase, apoptosis, and neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 2005;45:269–290. doi: 10.1146/annurev.pharmtox.45.120403.095902. [DOI] [PubMed] [Google Scholar]
- Cook T, Urrutia R. TIEG proteins join the Smads as TGF-beta-regulated transcription factors that control pancreatic cell growth. Am J Physiol Gastrointest Liver Physiol. 2000;278:G513–G521. doi: 10.1152/ajpgi.2000.278.4.G513. [DOI] [PubMed] [Google Scholar]
- Ekblom J, Zhu QS, Chen K, Shih JC. Monoamine oxidase gene transcription in human cell lines: treatment with psychoactive drugs and ethanol. J Neural Transm. 1996;103:681–692. doi: 10.1007/BF01271228. [DOI] [PubMed] [Google Scholar]
- Fang C, Lindros KO, Badger TM, Ronis MJ, Ingelman-Sundberg M. Zonated expression of cytokines in rat liver: effect of chronic ethanol and the cytochrome P450 2E1 inhibitor, chlormethiazole. Hepatology. 1998;27:1304–1310. doi: 10.1002/hep.510270516. [DOI] [PubMed] [Google Scholar]
- Fernandez HH, Chen JJ. Monamine oxidase inhibitors: current and emerging agents for Parkinson disease. Clin Neuropharmacol. 2007;30:150–168. doi: 10.1097/01.wnf.0000240956.49315.be. [DOI] [PubMed] [Google Scholar]
- Fernandez-Zapico ME, Mladek A, Ellenrieder V, Folch-Puy E, Miller L, Urrutia R. An mSin3A interaction domain links the transcriptional activity of KLF11 with its role in growth regulation. EMBO J. 2003;22:4748–4758. doi: 10.1093/emboj/cdg470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276:9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double KL, Youdim MB, Riederer P. Potential sources of increased iron in the substantia nigra of parkinsonian patients. J Neural Transm Suppl. 2006;(70):133–142. doi: 10.1007/978-3-211-45295-0_21. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ. Seligiline transdermal system in depression. Expert Opin Pharmacother. 2007;8:59–64. doi: 10.1517/14656566.8.1.59. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah 1 binding. Nat Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Hara MR, Thomas B, Cascio MB, Bae BI, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder SH. Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc Natl Acad Sci U S A. 2006;103:3887–3889. doi: 10.1073/pnas.0511321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen JH, Gronbaek M, Moller S, Bendtsen F, Becker U. Carbohydrate deficient transferrin (CDT) in alcoholic cirrhosis: a kinetic study. J Hepatol. 1997;26:287–292. doi: 10.1016/s0168-8278(97)80043-8. [DOI] [PubMed] [Google Scholar]
- Li Y, Nowotny P, Holmans P, Smemo S, Kauwe JS, Hinrichs AL, Tacey K, Doil L, van Luchene R, Garcia V, Rowland C, Schrodi S, Leong D, Gogic G, Chan J, Cravchik A, Ross D, Lau K, Kwok S, Chang SY, Catanese J, Sninsky J, White TJ, Hardy J, Powell J, Lovestone S, Morris JC, Thal L, Owen M, Williams J, Goate A, Grupe A. Association of late-onset Alzheimer's disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci U S A. 2004;101:15688–15693. doi: 10.1073/pnas.0403535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Baliga M, Bigler SA, Baliga R. Role of cytochrome P450 2B1 in puromycin aminonucleoside-induced cytotoxicity to glomerular epithelial cells. Nephron Exp Nephrol. 2003;94:e17–e24. doi: 10.1159/000070815. [DOI] [PubMed] [Google Scholar]
- Lu D, Johnson C, Johnson S, Tazik S, Ou XM. The neuroprotective effect of antidepressant drug via inhibition of TIEG2-MAO B mediated cell death. Drug Discov Ther. 2008;2:289–295. [PMC free article] [PubMed] [Google Scholar]
- Luo J, Miller MW. Ethanol inhibits basic fibroblast growth factor-mediated proliferation of C6 astrocytoma cells. J Neurochem. 1996;67:1448–1456. doi: 10.1046/j.1471-4159.1996.67041448.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21:1186–1194. [PubMed] [Google Scholar]
- Maruyama W, Akao Y, Youdim MB, Davis BA, Naoi M. Transfection-enforced Bcl-2 overexpression and an anti-Parkinson drug, rasagiline, prevent nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase induced by an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol. J Neurochem. 2001;78:727–735. doi: 10.1046/j.1471-4159.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- Ou XM, Partoens PM, Wang JM, Walker JH, Danks K, Vaughan PF, De Potter WP. The storage of noradrenaline, neuropeptide Y and chromogranins in and stoichiometric release from large dense cored vesicles of the undifferentiated human neuroblastoma cell line SH-SY5Y. Int J Mol Med. 1998;1:105–112. doi: 10.3892/ijmm.1.1.105. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine oxidase (MAO) B gene. J Biol Chem. 2004;279:21021–21028. doi: 10.1074/jbc.M312638200. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006a;103:10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Glucocorticoid and androgen activation of monoamine oxidase A are regulated differently by R1 and SP1. J Biol Chem. 2006b;29:54. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- Pantazis NJ, Dohrman DP, Luo J, Goodlett CR, West JR. Alcohol reduces the number of pheochromocytoma (PC12) cells in culture. Alcohol. 1992;9:171–180. doi: 10.1016/0741-8329(92)90048-f. [DOI] [PubMed] [Google Scholar]
- Paterson IA, Barber AJ, Gelowitz DL, Voll C. (-)Deprenyl reduces delayed neuronal death of hippocampal pyramidal cells. Neurosci Biobehav Rev. 1997;21:181–186. doi: 10.1016/s0149-7634(96)00008-5. [DOI] [PubMed] [Google Scholar]
- Robinson DS, Amsterdam JD. The selegiline transdermal system in major depressive disorder: a systematic review of safety and tolerability. J Affect Disord. 2008;105:15–23. doi: 10.1016/j.jad.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Senatorov VV, Charles V, Reddy PH, Tagle DA, Chuang DM. Overexpression and nuclear accumulation of glyceraldehyde-3-phosphate dehydrogenase in a transgenic mouse model of Huntington's disease. Mol Cell Neurosci. 2003;22:285–297. doi: 10.1016/s1044-7431(02)00013-1. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser Z, Levy R, Cohen S. Effects of N-propargyl-1-(R)aminoindan (rasagiline) in models of motor and cognition disorders. J Neural Transm Suppl. 1998;52:287–300. doi: 10.1007/978-3-7091-6499-0_29. [DOI] [PubMed] [Google Scholar]
- Tariot PN, Cohen RM, Sunderland T, Newhouse PA, Yount D, Mellow AM, Weingartner H, Mueller EA, Murphy DL. L-deprenyl in Alzheimer's disease. Preliminary evidence for behavioral change with monoamine oxidase B inhibition. Arch Gen Psychiatry. 1987;44:427–433. doi: 10.1001/archpsyc.1987.01800170041007. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Ju WY, Holland DP, Tai C, Kwan M. (-)-Deprenyl reduces PC12 cell apoptosis by inducing new protein synthesis. J Neurochem. 1994;63:1572–1575. doi: 10.1046/j.1471-4159.1994.63041572.x. [DOI] [PubMed] [Google Scholar]
- Tatton WG, Chalmers-Redman RM, Elstner M, Leesch W, Jagodzinski FB, Stupak DP, Sugrue MM, Tatton NA. Glyceraldehyde-3-phosphate dehydrogenase in neurodegeneration and apoptosis signaling. J Neural Transm Suppl. 2000;7:7–100. doi: 10.1007/978-3-7091-6301-6_5. [DOI] [PubMed] [Google Scholar]
- Tatton W, Chalmers-Redman R, Tatton N. Neuroprotection by deprenyl and other propargylamines: glyceraldehyde-3-phosphate dehydrogenase rather than monoamine oxidase B. J Neural Transm. 2003;110:509–515. doi: 10.1007/s00702-002-0827-z. [DOI] [PubMed] [Google Scholar]
- Wang CL, Wan YL, Liu YC, Huang ZQ. TGF-beta1/SMAD signaling pathway mediates p53-dependent apoptosis in hepatoma cell lines. Chin Med Sci J. 2006;21:33–35. [PubMed] [Google Scholar]
- Wong WK, Chen K, Shih JC. Regulation of human monoamine oxidase B gene by Sp1 and Sp3. Mol Pharmacol. 2001;59:852–859. doi: 10.1124/mol.59.4.852. [DOI] [PubMed] [Google Scholar]
- Yao Z, Zhang J, Dai J, Keller ET. Ethanol activates NFkappaB DNA binding and p56lck protein tyrosine kinase in human osteoblast-like cells. Bone. 2001;28:167–173. doi: 10.1016/s8756-3282(00)00425-7. [DOI] [PubMed] [Google Scholar]
- Youdim MB. The path from anti Parkinson drug selegiline and rasagiline to multifunctional neuroprotective anti Alzheimer drugs ladostigil and m30. Curr Alzheimer Res. 2006;3:541–550. doi: 10.2174/156720506779025288. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Wadia A, Tatton W, Weinstock M. The anti-Parkinson drug rasagiline and its cholinesterase inhibitor derivatives exert neuroprotection unrelated to MAO inhibition in cell culture and in vivo. Ann N Y Acad Sci. 2001;939:450–458. doi: 10.1111/j.1749-6632.2001.tb03656.x. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Maruyama W, Naoi M. Neuropharmacological, neuroprotective and amyloid precursor processing properties of selective MAO-B inhibitor anti-parkinsonian drug, rasagiline. Drugs Today (Barc) 2005;41:369–391. doi: 10.1358/dot.2005.41.6.893613. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]