Abstract
Context
Limited information exists regarding the role of left ventricular function in predicting exercise capacity and impact on age- and sex-related differences.
Objective
To determine the impact of measures of cardiac function assessed by echocardiography on exercise capacity and to determine if these associations are modified by sex or advancing age.
Design
Cross-sectional study of patients undergoing exercise echocardiography with routine measurements of left ventricular systolic and diastolic function by 2D and Doppler techniques. Analyses were conducted to determine the strongest correlates of exercise capacity and the age- and sex-interactions of these variables with exercise capacity.
Setting
Large tertiary referral center in Rochester, MN in 2006.
Participants
Patients undergoing exercise echocardiography using the Bruce protocol (n=2,867). Patients with echocardiographic evidence of exercise-induced ischemia, ejection fraction <50% or significant valvular heart disease were excluded.
Main Outcome Measures
Exercise capacity (metabolic equivalents, METs).
Results
Diastolic dysfunction was strongly and inversely associated with exercise capacity. Compared with normal function those with moderate/severe [-1.3 (-1.52 to – 0.99) METs, p<0.0001], and mild resting diastolic dysfunction [-0.70 (-0.88 to -0.46) METs, p<0.0001] had substantially lower exercise capacity after multivariable adjustment. Variation of left ventricular systolic function within the normal range was not associated with exercise capacity. Left ventricular filling pressures measured by resting E/e’ > 15 [-0.42 (-0.70 to -0.11)METs, p=0.004] or post-exercise E/e'> 15 [-0.41 (-0.71 to -0.11), p<0.0001] were similarly associated with a reduction in exercise capacity, each in separate multivariate analyses. Individuals with impaired relaxation or resting E/e’ ≥15 had a progressive increase in the magnitude of reduction in exercise capacity with advancing age (p<0.001 and p=0.02, respectively). Other independent correlates of exercise capacity were age [unstandardized β coefficient -0.85 (95% CI -0.92 to -0.77) METs per 10 year increment, p<0.0001], female sex [-1.98 ( -2.15 to -1.84) METs, p<0.0001], and body mass index >30 kg/m2 [-1.24 (-1.41 to -1.10) METs, p<0.0001],
Conclusion
In this large cross-sectional study of those referred for exercise echocardiography and not limited by ischemia, abnormalities of left ventricular diastolic function were independently associated with exercise capacity.
Introduction
Many factors including age, female sex, body mass index, and co-morbid medical conditions are known to be associated with a decrement in exercise capacity, as reflected by a decrease in maximal workload achieved or maximal oxygen consumption.1-6 Aerobic exercise capacity decreases progressively with age and is associated with reductions in functional capacity, increases in disability, and decreases in independence and quality of life. Determining the most important parameters affecting exercise performance, especially in relation to age, is complex, given the numerous confounding factors. The most consistently reported mechanism contributing to this decrease in exercise capacity with aging is a reduction in maximal heart rate; this appears to be a non-modifiable and inevitable consequence of aging.7 Similarly, the difference in exercise capacity between men and women has largely been attributed to non-modifiable differences in cardiac output and skeletal muscle mass.8-9 Identifying potentially reversible mechanisms underlying the decline in maximal exercise capacity with aging and between men and women could have important implications.
Elucidating the mechanisms of cardiac-related exercise limitation has been technically difficult to date. Previous studies have suggested that measurements of left ventricular systolic function do not predict maximal exercise time in individuals with normal or impaired left ventricular systolic function.10-11 However, differences in exercise capacity related to small changes in ejection fraction within the normal range would require evaluation in a large population. Doppler echocardiography can now characterize left ventricular diastolic function through a combination of measurements, which show evidence of slowed ventricular relaxation, increased left ventricular stiffness or abnormal left ventricular filling. Doppler echocardiography can also provide an estimate of left ventricular filling pressures, one component of diastolic function that reflects pulmonary capillary wedge pressure. In prior small series, these parameters have been shown to correlate with exercise capacity.12-14 Whether abnormalities of diastolic function explain age- or gender-related changes in exercise capacity are unknown. The aims of this study were 1) to determine the relationship between left ventricular diastolic function parameters as determined by echocardiography and exercise capacity; and 2) to determine if there is a change in the magnitude of association of diastolic function parameters with exercise capacity with advancing age or sex.
Methods
Patient Population
This study was approved by the Mayo Clinic institutional review board, and verbal consent was obtained at the time of the stress echocardiogram. During 2006, 4705 patients had a clinically indicated exercise echocardiogram at Mayo Clinic, Rochester. For this analysis, we excluded patients who underwent exercise testing with a protocol other than the Bruce protocol (n=365), refused to participate in research (n=230), were in atrial fibrillation/flutter at the time of exercise (n=118), had moderate or severe valvular heart disease (n=76), had poor image quality which prohibited a final impression (n=7), had an ejection fraction of < 50% (n=88), or had echocardiographic evidence of exercise-induced myocardial ischemia (n=790). The latter group of patients was excluded because symptoms or signs of ischemia would be expected to contribute to premature termination of exercise. Assessment of diastolic function was not possible in 164 patients because of missing values that were the result of E/A fusion or poor apical windows; baseline characteristics of these patients were similar to those studied. The remaining 2867 patients constituted the study population. Of these, 1402 (49%) were referred for exercise echocardiography for shortness of breath or chest pain, 632 (21%) because of the presence of multiple risk factors for cardiovascular disease, 278 (10%) because of an abnormal resting electrocardiogram, 250 (9%) for evaluation of suspected coronary artery disease, 232 (8%) for preoperative assessment, and 73 (2%) for increased calcium score on CT.
Clinical variables and body mass index were recorded at the time of the exercise echocardiogram. Medication use and a history of diabetes mellitus, hypertension, hyperlipidemia, smoking and coronary artery disease were abstracted from the medical record and entered into a prospectively maintained database by specially trained nurses. Coronary artery disease was defined as previous coronary revascularization and/or history of myocardial infarction.
Exercise echocardiography
Echocardiography was performed before starting exercise and immediately after symptom-limited treadmill exercise according to the Bruce protocol. The Diastolic Function Initiative of 2006, undertaken at the Mayo Clinic (Rochester, MN), was a routinely performed assessment of left ventricular diastolic function done in addition to assessment of regional wall motion according to the usual exercise echocardiography protocol. The baseline, resting assessment included pulsed wave Doppler measurements of the early (E) and late (A) mitral inflow velocities, deceleration time of early LV filling, the peak early diastolic velocity of the medial mitral annulus (e') with tissue Doppler in the 4-chamber view, and 2D-based measurement of the left atrial size. The ratio E/e', a measurement of left ventricular filling pressures, was feasible in all patients at rest. Shortly after exercise, mitral inflow and annulus Doppler data were obtained again. These were measured in early recovery (within 2-7 minutes of cessation of exercise) after regional wall motion assessment and at the earliest time that the E and A velocities were sufficiently separated to permit measurement. Measurement of post-exercise E/e’ was feasible in 2,366 (82%) patients. E/e’ > 15 was chosen to define increased left ventricular filling pressure at rest and with exercise.15-16 Resting diastolic function was graded as normal, mild (impaired relaxation), moderate (pseudonormal), or severe (restrictive) diastolic dysfunction. The classification of diastolic function was modified from the algorithm outlined by Khouri et al.17 Instead of pulmonary vein flow measurements, left atrial volume was measured as part of our assessment as it has been shown to be a marker of diastolic dysfunction.18-19 Relaxation and restrictive abnormalities were classified based on the mitral inflow patterns, E/A < 0.75 and E/A > 1.5, respectively. To diagnose a restrictive abnormality, both left atrial volume index and E/e’ had to be increased at >28 ml/m2 and >10, respectively, otherwise patients were classified as normal. This is especially important in young people as a normal E/A is often >1.5 due to increased early filling with no other echocardiographic evidence of diastolic dysfunction and should be considered normal.18 To distinguish pseudonormal from normal diastolic function (0.75<E/A<1.5), both left atrial volume index and E/e’ had to be increased as above. Left atrial volume was measured according to the area-length method and indexed to body surface area.20 Left ventricular ejection fraction was assessed by a combination of the modified Quinones method 21 and visual assessment. Wall motion was scored according to a 16-segment model, in which 1 = normal or hyperdynamic, 2=hypokinetic, 3=akinetic, 4=dyskinetic, and 5=aneurysm. The exercise echocardiogram was considered normal if there were no wall motion abnormalities at rest or with exercise. “Fixed” wall motion abnormalities were those present at rest and unchanged with exercise. Global hypokinesis present at rest and improved with exercise was considered to represent cardiomyopathy. Baseline and peak exercise blood pressure, heart rate and pulse pressure (systolic – diastolic blood pressure) were determined. Heart rate increase with exercise was defined as the difference between peak exercise heart rate and baseline heart rate.
Statistics
The primary end point was maximal exercise tolerance defined by the achieved metabolic equivalents (METs). Data are expressed as mean ± SD or n (%). Multiple comparisons of continuous variables were made with analysis of variance. Tukey's HSD approach was used to adjust for multiple comparisons. Categorical data was compared by the chi-square test. Collinearity diagnostics were performed to look for multicollinearity between the independent variables in the linear models. Collinearity between systolic blood pressure and pulse pressure was present and the latter was included in the final multivariate models. Similarly, diastolic function grade and resting E/e’ demonstrated collinearity and two separate multivariate models were constructed to evaluate each of these separately in the 2867 patients with baseline diastolic function assessment. Stepwise multivariate linear regression models were used to estimate the relative contributions of the baseline clinical and echocardiographic variables to exercise performance. Because of the small numbers of patients with moderate and severe diastolic dysfunction, these groups were combined for analysis. In a subgroup of 2366 patients in whom post-exercise E/e’ measurements were also feasible, a separate multivariate linear regression model was used to evaluate the relative contributions of the peak exercise clinical and echocardiographic variables to exercise performance. The distribution of continuous variables was tested to ensure that normality assumptions were fulfilled. Interaction terms of diastolic function parameters with age/gender were also evaluated to determine whether the magnitude of reduction in exercise capacity among those with abnormal diastolic parameters varied with increasing age or between genders. To detect a clinically meaningful difference in exercise capacity of 0.5 METS22 with 90% power, a sample size of n=256 was required in each group (normal, mild and moderate/severe diastolic function groups). This was with a standard deviation of 3.5 METs, assuming a 2-sided analysis and α=0.05. An a priori level of significance was assigned at <0.05. All computations were performed using the JMP statistical software for Windows, version 6.0.
Results
Study Population
The baseline clinical and echocardiographic characteristics are outlined in Tables 1 & 2. Normal diastolic function was present in 1784 (62%), mild diastolic dysfunction in 785 (27%) and moderate/severe diastolic dysfunction in 298 (10%) patients. The exercise echocardiogram was normal in 2655 patients (93%), showed a fixed abnormality in 202 (7%), and was considered to indicate dilated cardiomyopathy in 10 (0.3%) patients. Target heart rate (≥85% age-predicted maximal heart rate) was achieved in 2146 (75%) patients. The primary reason for stopping exercise was fatigue in 1759 (61%), dyspnea in 801 (28%), leg discomfort in 281(10%), arrhythmias in 18 (1%) and atypical chest discomfort in 8 (0.3%) patients.
Table 1.
Clinical Characteristics
| Variables | Normal n=1784 | Mild DD n=785 | Moderate/Severe DD n=298 |
|---|---|---|---|
| Resting | |||
| Age, years | 53±11 | 67±9* | 66±11* |
| Male | 964 (54%) | 433 (55%) | 172 (53%) |
| Systolic blood pressure, mmHg | 123±18 | 130±19* | 131±19* |
| Pulse pressure, mmHg | 48 ±15 | 54±16* | 56±16* |
| Heart rate, beats/min | 74±13ψξ | 76±14*ξ | 78±13*ψ |
| Body mass index, kg/m2 | 27±5ψξ | 28±4*ξ | 29±6*ψ |
| History CAD | 162 (9%) | 138 (18%)* | 60 (20%)* |
| History of diabetes mellitus | 107 (6%) | 126 (16%)* | 57 (18%)* |
| History of hypertension | 676 (38%) | 496 (63%)* | 232 (71%)*ψ |
| Current/previous smoker | 750 (42%) | 368 (47%)* | 157 (48%)* |
| History of hyperlipidemia | 1015 (57%) | 560 (71%)* | 232 (71%)* |
| Calcium channel blocker | 99 (5%) | 106 (13%)* | 48 (16%)* |
| Beta-blocker | 367 (21%) | 252 (32%)* | 167 (56%)*ψ |
| ACE/ARB | 294 (16%) | 257 (33%)* | 113 (38%)* |
| Peak Exercise | |||
| Duration exercise, min | 9.7±2.6ψξ | 7.6±2.3*ξ | 7.0±2.1*ψ |
| Exercise capacity, METs | 10.7±2.6ψξ | 8.5±2.3*ξ | 8.0±2.1*ψ |
| Peak heart rate, beats/min | 155±21 | 140±21 | 132±22 |
| Heart rate increase, beats/min | 81±19ψξ | 64±18*ξ | 64±19*ψ |
| Systolic blood pressure, mmHg | 165±23ψξ | 166±24 | 165±25 |
| Diastolic blood pressure, mmHg | 79±12 | 81±42 | 78±13 |
Pairwise comparisons of continuous data performed with analysis of variance using Tukey HSD.
p<0.05 compared to patients with normal diastolic function
p<0.05 compared to patients with mild diastolic function.
p<0.05 compared to patients with moderate/severe diastolic function.
Data are mean ± SD or numbers with percentages in parentheses. CAD=coronary artery disease, ACE=angiotensin converting enzyme inhibitor,
ARB=angiotensin receptor blocker.
Heart rate increase = peak exercise heart rate-baseline heart rate.
Table 2.
Echocardiography Characteristics
| Variables | Normal n=1784 | Mild DD n=785 | Moderate/Severe DD n=298 |
|---|---|---|---|
| LV ejection fraction, % | 61±4ψξ | 60±6 | 60±8 |
| LV diastolic dimension, mm | 47±4 | 47±5 | 49±6*ψ |
| Wall motion score index** | 1.0±0.1ψξ | 1.1±0.2 | 1.1±0.3 |
| Deceleration time, ms | 198±34 | 239±51*ξ | 201±39 |
| Left atrial volume index, ml/m2 | 25±7ψξ | 28+9*ξ | 37±10*ψ |
| Resting E/e’ | 8±2ψξ | 10±4*ξ | 14±5*ψ |
| Post-exercise E/e’ | 8±3ψξ | 10±4*ξ | 14±8*ψ |
Pairwise comparisons of continuous data performed with analysis of variance using Tukey HSD.
p<0.05 compared to patients with normal diastolic function
p<0.05 compared to patients with mild diastolic function.
p<0.05 compared to patients with moderate/severe diastolic function. Data are mean ± SD or numbers with percentages in parentheses.
Wall motion score index was the same at rest and exercise as patients with ischemia were excluded.
Clinical and Echocardiographic Correlates of Exercise Capacity
Resting univariate and multivariate correlates of exercise capacity as measured by METs are shown in Tables 3 and 4. Compared to normal diastolic function, the presence of mild and moderate/severe diastolic dysfunction were associated with a reduction in exercise capacity of -2.17 (0.10) and -2.74 (0.15) METs, respectively, before adjusting for other clinical/echocardiographic factors (Table 3). Considering all variables in Table 3 in the multivariate analysis, the strongest independent correlates of reduced exercise tolerance were increasing age, female gender, body mass index >30 kg/m2, moderate/severe diastolic dysfunction vs. normal, and mild diastolic dysfunction vs. normal as shown by the standardized beta coefficients in Table 4. The presence of mild or moderate/severe diastolic dysfunction as compared to normal resulted in a reduction of exercise capacity of 0.70 ± 0.10 (10.6 [95% CI 10.4–10.9] vs. 11.3 [95% CI 11.2-11.6] METs) and 1.30 ± 0.13 METs (10.1[9.8-10.4] vs. 11.4 [95% CI 11.2-11.6] METs), respectively. Every 10 beat increase in resting heart rate was associated with a 0.24±0.03 MET reduction in exercise capacity. In multivariate analysis, increasing age was associated with a reduction of 0.85±0.04 METs per 10 years of age. Overall, females exercised 1.98±0.07 METs less than their male counterparts (9.4 [95% CI 9.2-9.6] vs 11.4 [95% CI 11.2-11.6] METs). Patients with a body mass index >30 kg/m2 exercised 1.24 ± 0.08 METs less than those with a body mass index <30 kg/m2 (10.6 [95%CI 10.3-10.7] vs. 11.8 [95%CI 11.6-11.9] METs). The other correlates in the final multivariate model, although significant, were associated with smaller reductions in exercise capacity: previous/current smoker vs. nonsmoker (10.9 [95% CI 10.8-11.1] vs. 11.3 [95% CI 11.2-11.6] METs); beta-blocker vs. no beta-blocker (10.9[95%CI 10.7-11.2] vs. 11.3[11.2-11.6] METs); hypertension vs. no hypertension (11.1[95%CI 10.9-11.3] vs. 11.4[95%CI 11.2-11.6] METs); diabetes vs. no diabetes (11.1[95%CI 10.8-11.4] vs. 11.5[95%CI 11.2-11.6] METs. The R2 of this model was 0.51; with exclusion of diastolic function grade, R2 decreased to 0.40.
Table 3.
Univariate Analysis of Resting Clinical and Echocardiographic Predictors of Exercise Capacity (METs)
| Univariate Analysis | |||
|---|---|---|---|
| Resting Variables | B*Coefficient (SE) | 95% CI | p |
| Clinical | |||
| Age, per 10 years | -1.00 (0.03) | -1.07 to –0.93 | <0.0001 |
| Female gender | -1.81 (0.09) | -1.99 to –1.61 | <0.0001 |
| Systolic blood pressure, per 10 mmHg | -0.31 (0.25) | -0.36 to –0.26 | <0.0001 |
| Pulse pressure, per 10 mmHg | -0.46 (0.03) | -0.52 to –0.40 | <0.0001 |
| Heart rate, per 10 beats/min | -0.19 (0.04) | -0.25 to –0.10 | <0.0001 |
| Body mass index>30kg/m2 | -1.37 (0.10) | -1.56 to –1.15 | <0.0001 |
| History of CAD | -0.65 (0.14) | -0.89 to –0.34 | <0.0001 |
| History of diabetes mellitus | -1.29 (0.15) | -1.59 to –0.93 | <0.0001 |
| History of hypertension | -1.43 (0.09) | -1.63 to –1.25 | <0.0001 |
| History of hyperlipidemia | -0.35 (0.10) | -0.55 to –0.14 | <0.0001 |
| Previous/current smoker | -0.39 (0.09) | -0.58 to –0.19 | <0.0001 |
| Beta-blocker | -1.25 (0.11) | -1.41 to –0.98 | <0.0001 |
| Calcium channel blocker | -1.06 (0.17) | -1.42 to –0.73 | <0.0001 |
| ACE/ARB | -0.85 (0.12) | -1.08 to –0.62 | <0.0001 |
| Echocardiography | |||
| Mild vs. normal diastolic function | -2.17 (0.10) | -2.39 to –1.98 | <0.0001 |
| Moderate/severe vs. normal diastolic function | -2.74 (0.15) | -3.01 to –2.41 | <0.0001 |
| Ejection fraction, per 5% | 0.01 (0.04) | -0.13 to 0.04 | 0.89 |
| Resting E/e’ ≥ 15 | -1.81 (0.19) | -0.23 to –0.18 | <0.0001 |
| LV diastolic dimension, per 1 mm | 0.08 (0.01) | 0.06 to 0.11 | <0.0001 |
| Wall motion score index > 1.18 | -0.41 (0.18) | -1.22 to –0.08 | 0.02 |
| Deceleration time, per 40 ms | -0.34 (0.05) | -0.44 to –0.26 | <0.0001 |
| Left atrial volume index > 30 ml/m2 | -0.45 (0.11) | -0.69 to –0.26 | <0.0001 |
β = Slope of the regression line for each variable and exercise capacity, expressed per unit of each variable or as outlined beside variable (unstandardized coefficient). CAD=coronary artery disease, ACE=angiotensin converting enzyme inhibitor, ARB=angiotensin receptor blocker.
Table 4.
Multivariate Analysis of Resting Clinical and Echocardiographic Correlates of Exercise Capacity (METs)
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Resting Variables | B*Unstandardized (SE) | 95% CI | Bψ Standardized | p |
| Model #1 with Diastolic Function Grade | ||||
| Diastolic function | ||||
| Mild vs. normal | -0.70 (0.10) | -0.88 to –0.46 | -0.12 | <0.0001 |
| Moderate/severe vs. normal | -1.30 (0.13) | -1.52 to –0.99 | -0.16 | <0.0001 |
| Age, years | -0.85 (0.04), per 10 years | -0.92 to –0.77 | -0.40 | <0.0001 |
| Female gender | -1.98 (0.07) | -2.15 to –1.84 | -0.36 | <0.0001 |
| Body mass index >30 kg/m2 | -1.24 (0.08) | -1.41 to –1.10 | -0.21 | <0.0001 |
| Heart rate, beats/min | -0.24 (0.03), per 10 beats/min | -0.30 to –0.18 | -0.11 | <0.0001 |
| Previous/current smoker | -0.43 (0.07) | -0.59 to –0.29 | -0.08 | <0.0001 |
| Beta-blocker | -0.39 (0.09) | -0.61 to –0.21 | -0.07 | <0.001 |
| Pulse pressure, mmHg | -0.10 (0.02), per 10 mmHg | -0.15 to –0.05 | -0.06 | 0.0003 |
| History of hypertension | -0.27 (0.88) | -0.48 to –0.13 | -0.06 | 0.01 |
| History of diabetes mellitus | -0.36 (0.12) | -0.67 to –0.16 | -0.05 | 0.03 |
| Model #2 with Resting E/e’ | ||||
| E/e’ ≥ 15 | -0.41 (0.15) | -0.70 to -0.11 | -0.04 | 0.007 |
| Age, years | -1.00 (0.03), per 10 years | -1.05 to -0.91 | -0.46 | <0.0001 |
| Female gender | -2.00 (0.08) | -2.19 to -1.88 | -0.37 | <0.0001 |
| Body mass index >30 kg/m2 | -1.34 (0.08) | -1.50 to -1.17 | -0.22 | <0.0001 |
| Heart rate, beats/min | -0.28 (0.03), per 10 beats/min | -0.33 to -0.21 | -0.13 | <0.0001 |
| Beta-blocker | -0.53 (0.10) | -0.71 to -0.31 | -0.08 | <0.0001 |
| Previous/current smoker | -0.44 (0.08) | -0.59 to-0.29 | -0.08 | <0.0001 |
| Pulse pressure, mmHg | -0.09 (0.03), per 10 mmHg | -0.15 to -0.04 | -0.05 | 0.0003 |
| History of hypertension | -0.28 (0.10) | -0.44 to -0.06 | -0.04 | 0.004 |
| History of diabetes mellitus | -0.41 (0.13) | -0.64 to -0.12 | -0.04 | 0.002 |
All variables in Table 3 were included in the multivariate analysis.
Diastolic Function Grade Model #1: R2 = 0.51, Intercept 18.3
E/e’ ≥ 15 Model #2: R2 = 0.45, Intercept 19.0
β = Slope of the regression line for each variable and exercise capacity, expressed per unit of each variable or as outlined beside variable (unstandardized coefficient).
β= standardized slope in the same units of measure.
This above multivariate model did not include resting E/e’ because of its collinearity with diastolic function grade, and a separate multivariate model substituting diastolic function grade with resting E/e’ was constructed (Table 4). Resting E/e'≥ 15 was associated with a reduction in exercise capacity of -1.81 (0.19) METs compared to E/e’ <15 in univariate analysis (Table 3). After adjustment for clinical and echocardiographic variables, resting E/e'≥ 15 was associated with a reduction in exercise capacity of -0.41 (0.15) METs compared to E/e'< 15 (11.2 [95%CI 10.8-11.5] vs. 11.6 [95%CI 11.4-11.8] METs) (Table 4).
In univariate analysis, post-exercise E/e'≥ 15 was associated with a -1.86 (0.20) MET change in exercise capacity (p<0.0001), every 10 beat increase in the change in heart rate from rest to exercise with a 0.77 (0.02) MET increase in exercise capacity (p<0.0001), and every 10 beat increment in peak exercise heart rate with a 0.59 (0.02) MET increase in exercise capacity (p<0.0001). After adjusting for clinical and echocardiographic parameters in multivariate analysis, the strongest peak exercise correlates of exercise capacity were heart rate increase (0.45 MET increase in exercise capacity per 10 beat increment), and post-exercise E/e'≥15 (0.41 MET reduction in exercise capacity; 10.9 [96%CI 10.6-11.3] vs. 11.3 [95%CI 11.1-11.5] METs) (Table 5).
Table 5.
Multivariate Analysis of Exercise Clinical and Echocardiographic Correlates of Exercise Capacity (METs)
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Exercise Variables | B* Unstandardized (SE) | 95% CI | Bψ Standardized | p |
| Post exercise E/e'≥15 | -0.41 (0.15) | -0.71 to -0.11 | -0.04 | 0.007 |
| Heart rate increase, beats/min | 0.45 (0.02), per 10 beats/min | 0.41 to 0.51 | 0.36 | <0.0001 |
| Age, years | -0.64 (0.04), per 10 years | -0.72 to –0.57 | -0.30 | <0.0001 |
| Female gender | -1.78 (0.08) | -1.92 to –1.59 | -0.32 | <0.0001 |
| Body mass index>30 kg/m2 | -1.18 (0.09) | -1.35 to –1.01 | -0.20 | <0.0001 |
| Peak systolic blood pressure, mmHg | 0.07 (0.02), per 10 mmHg | 0.03 to 0.10 | 0.10 | 0.0001 |
| Previous/current smoker | -0.26 (0.08) | -0.41 to –0.10 | -0.05 | 0.001 |
| History of hypertension | -0.23 (0.10) | -0.43 to –0.04 | -0.03 | 0.02 |
| History of diabetes mellitus | -0.28 (0.14) | -0.54 to –0.01 | -0.03 | 0.04 |
Multivariate analysis (n=2366). Multivariate model also adjusted for ejection fraction, left atrial volume, left ventricular diastolic dimension, CAD, and medications (beta-blockers, calcium channel blockers and ACE/ARB) which were not statistically significant in the final model.
R2 = 0.54 and Intercept 10.49
β = Slope of the regression line for each variable and exercise capacity, expressed per unit of each variable or as outlined beside variable (unstandardized coefficient).
β= standardized slope in the same units of measure.
Heart rate at rest was increased and the increase at peak exercise was blunted with diastolic dysfunction (Table 1). The correlation between resting heart rate and diastolic function was poor (r=0.08, p<0.0001) as was the relationship with resting E/e’ (r=0.13, p<0.0001), and post-exercise E/e’ (r=0.09, p<0.0001). There was only a modest correlation between heart rate increase and worsening diastolic function (r=0.31, p<0.0001), resting E/e’ (r=0.24, p<0.0001) and post-exercise E/e’ (r=0.23, p<0.0001).
Among the 2366 patients with post-exercise E/e', 742 had mild or moderate/severe resting diastolic dysfunction and resting E/e’ < 15. Of these, 57 (8%) developed an E/e’ ≥ 15 post-exercise. This is in contrast to the 1472 individuals with normal diastolic function and resting E/e’ < 15 of whom 38 (2.6%) developed post-exercise E/e’ ≥ 15. Of the 179 patients with post-exercise E/e’ ≥ 15, 95 (53%) had a resting E/e’ < 15. Mild or moderate/severe diastolic dysfunction was present in 138 of the 179 (77%) patients with post-exercise E/e’ ≥ 15.
Age and Sex Interactions with Exercise Capacity
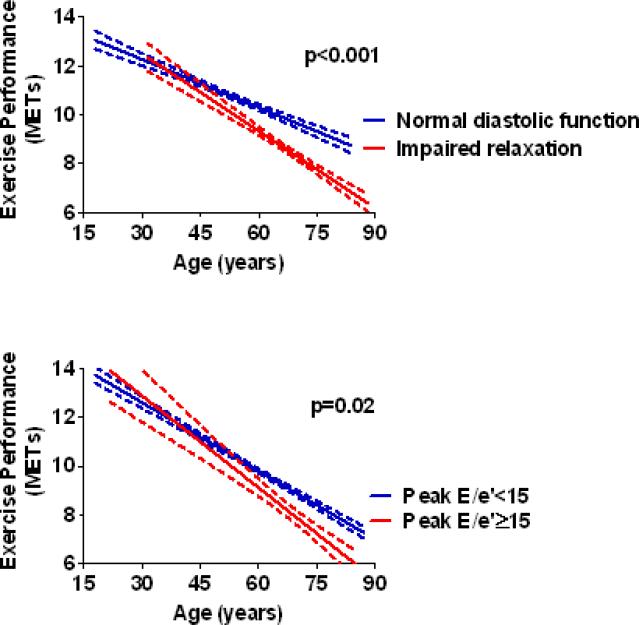
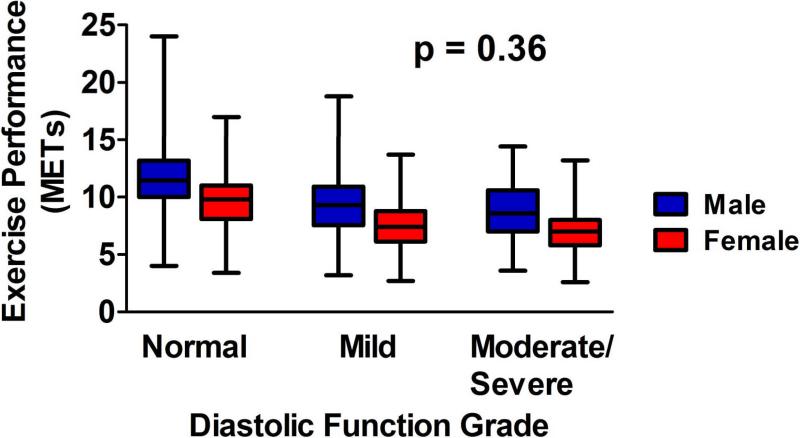
Although males had a greater exercise capacity than females, the magnitude of this difference decreased with age (p<0.0001). Compared to those with normal diastolic function, patients with mild diastolic dysfunction (impaired relaxation) had a progressive increase in the magnitude of reduction in exercise capacity with advancing age (Figure 1a). In contrast, individuals with moderate/severe diastolic dysfunction had a reduced exercise capacity compared to those with normal function, but the magnitude of this reduction was similar across the age spectrum (p=0.46). This was also true for those with resting E/e’ ≥15 (p=0.46). Compared to those with a post-exercise E/e’ < 15, however, individuals with a post-exercise E/e’ ≥15 had a progressive increase in the magnitude of reduction in exercise capacity with advancing age (Figure 1b). Although an increase in resting heart rate had a negative correlation with exercise capacity, the strength of this correlation diminished with age. Specifically, every 10 beat increase in resting heart rate predicted a 0.54 MET decrease in exercise capacity among patients < 50 years and a 0.24 MET decrease in patients >50 years (p<0.0001). Exercise capacity was reduced in females as compared to males, and the magnitude of this reduction in each diastolic dysfunction grade was similar between the sexes (Figure 2). This was also true for resting and post-exercise E/e’ (p=0.14 and p=0.20, respectively).
Figure 1. Effects of Diastolic Parameters on Exercise Capacity with Aging.
Figure 1A. Plot of exercise capacity by age for all patients with normal and mild diastolic dysfunction. The curves were fit to the data by group (normal and mild diastolic dysfunction/impaired relaxation) using linear regression analysis with 95% confidence intervals shown. Exercise capacity was reduced in the group with mild diastolic dysfunction vs. normal. However, the magnitude of this reduction progressively increased with advancing age as shown by the steeper slope in the mild diastolic dysfunction group. The p value, derived from age interaction analysis, shows that the slopes of the two lines are statistically different.
Figure 1B. Plot of exercise capacity by increasing age for all patients with post-exercise E/e'≥15 and < 15. The curves were fit to the data by group using linear regression analysis with 95% confidence intervals shown. Exercise capacity was reduced in the group with a post-exercise E/e’ ≥15 vs < 15. However, the magnitude of this reduction increased with advancing age as shown by the steeper slope in the E/e’ ≥15 group. The p value, derived from age/diastolic function interaction analysis, shows that the slopes of the two lines are statistically different.
Figure 2. Effects of Diastolic Function Grade on Exercise Capacity by Sex.
Box and whisker plot of exercise capacity by diastolic function grade grouped by sex. Compared to men, women had a lower exercise capacity within each diastolic function grade. However, the p value derived from gender/diastolic function interaction analysis (p=0.36) was nonsignificant, suggesting that the magnitude of reduction in exercise capacity within each diastolic function grade is similar between the sexes.
Discussion
In a large, consecutive population free of valvular heart disease or exercise-induced ischemia referred for exercise echocardiography, we found resting diastolic function to be the strongest echocardiographic correlate of exercise tolerance. This was superseded only by the clinical factors of advancing age, female gender and increasing body mass index. This relationship remained significant after taking into account resting heart rate, blood pressure, medication use, co-morbid medical conditions and other echocardiographic parameters. Unlike previous studies that have shown modest correlations of E/A ratio and deceleration time with exercise capacity, we found that diastolic function grade was strongly associated with a decrement in exercise capacity. Similarly, resting left ventricular filling pressure (E/e') was also found to correlate with exercise capacity although this association was less robust. E/e’ could be used as a surrogate if assessment of diastolic function grade is not feasible. In a previous smaller study, exercise E/e’ ≥ 15 was shown to strongly correlate with invasively determined left ventricular end-diastolic pressure at peak exercise;16 we found that increased filling pressures with exercise were also associated with a decrement in exercise capacity. We found that exercise capacity is not importantly influenced by variations of ejection fraction within the broad range of normal values; for example, an ejection fraction of 70% would not portend a better exercise capacity than an ejection fraction of 55%. We also documented that a history of previous or current smoking is associated with exercise limitation. An increase in resting heart rate was also a marker of poor exercise capacity; resting heart rate was higher in those with worse diastolic function. However, this may be a marker of overall deconditioning and had a minimal relationship with the extent of diastolic dysfunction or filling pressures. Similarly, a large chronotropic response was associated with improved exercise capacity, but was only modestly associated with diastolic function grade and filling pressures. Clearly, the mechanisms underlying relationships of heart rate with exercise capacity are complex and cannot be entirely explained by diastolic dysfunction parameters.
One mechanism by which diastolic parameters may affect exercise capacity relates to their role in generating a maximal cardiac output. During exercise, the maintenance of adequate left ventricular filling to ensure a normal cardiac output includes the ability to achieve diastolic filling rates greater than the ejection rates during systole. In the setting of exercise-induced tachycardia, abnormalities in diastolic relaxation and filling of the left ventricle can result in filling rates that might be too low to achieve adequate cardiac output during exercise even if ventricular systolic properties are normal.23 Also, left atrial pressure must increase to a level that creates a pressure gradient large enough to provide adequate ventricular filling during exercise in the setting of impaired left ventricular relaxation.12 It has been suggested that stimulation of J receptors in the lungs by congestion or increases in transmitted left atrial pressures to the pulmonary vascular system would tend to result in more tachypneic breathing, thus, altering the normal breathing patterns and resulting in exercise intolerance.24,25 A normal diastolic function response to exercise is characterized by normal and similar resting and exercise E/e’ measurements.26 In our study, just over half of the patients that developed increased filling pressures with exercise had a resting E/e’ < 15 suggesting that they adapted poorly to the cardiac physiology of exercise contributing to exercise intolerance. In part, this may be related to resting diastolic abnormalities, as 77% of patients with post-exercise E/e’ ≥ 15 had evidence of resting diastolic dysfunction. Additionally, only 8% of patients with resting diastolic dysfunction and E/e < 15 developed increased filling pressures post exercise. This suggests that the mechanism by which diastolic dysfunction contributes to exercise intolerance is not limited to the development of increased filling pressures.
As shown in our study and others, older age is a strong predictor of decreasing exercise capacity. Mechanisms proposed to explain this association include reduced peak heart rates or a decrease in arteriovenous oxygen content difference affecting maximal cardiac output generation.27,28 Aging is also associated with a reduction in skeletal muscle mass and decreases in muscle capillarization and mitochondrial enzyme activity, all of which can also contribute to reduced exercise capacity.8 While aging is known to be associated with an increasing prevalence of impaired relaxation, our study shows that even after adjusting for age, diastolic function was a strong predictor of exercise capacity. Moreover, our findings reveal that an important interaction between age and diastolic dysfunction exists, such that absolute reduction in exercise capacity among those with impaired relaxation vs. those with normal diastolic function progressively increases with advancing age. This remains the case for those with elevated exercise filling pressures. Although women have a lower exercise capacity compared to men, our findings confirm that there is a greater age-associated decline in exercise capacity in men.3, 29 Further, our data suggest that the absolute reduction in exercise capacity in women vs. men is similar across the spectrum of diastolic dysfunction, so that diastolic parameters do not account for the sex differences in exercise tolerance.
In identifying diastolic function parameters as strong correlates of exercise capacity, we have identified potentially modifiable and preventable factors in the development of exercise intolerance. It is well known that exercise training improves diastolic function in healthy subjects, demonstrating an increase in peak diastolic filling rates.30,31 However, although patients with diastolic dysfunction show an improvement in exercise tolerance with training, the effects of training on diastolic function are less clear. 32,33 Similarly, pharmacologic treatment of patients with diastolic dysfunction enhances exercise capacity but improvement in diastolic function is limited and occurs in only few patients.34,35 Treatment with angiotensin receptor blockers has appeared to be most promising as it blocks AT II action that is thought to be responsible for slowed left ventricular relaxation during exercise. Although data with respect to modifying diastolic function is unclear and merits further study, current approaches should include aggressive treatment of risk factors such as hypertension and coronary artery disease to prevent development of diastolic abnormalities and related exercise limitations.
Limitations
Although this is a very large study characterizing the impact of age and sex on correlates of exercise capacity, results are limited to subjects in whom a complete echocardiographic assessment as outlined was possible. This should not affect overall results, as the demographics of patients who did not have baseline diastolic function assessment were similar to those who did. The mechanism by which diastolic dysfunction affects exercise capacity may relate to cardiac output. This was not measured in these patients and was not our focus, but merits further study. As patients recruited into our study were middle-aged and referred for a clinically indicated stress echocardiogram, there is the potential for a referral bias related to a higher prevalence of co-morbidities. However, compared to a contemporary community-based cohort of Olmsted County, the prevalence of systolic and diastolic dysfunction was only slightly higher. Our results should be validated in other populations.36 The presence of anemia and obstructive lung disease were not ascertained, although we did account for a smoking history. Lastly, we used calculated METs rather than oxygen consumption as a measure of exercise tolerance. Although the latter is preferable, calculation of achieved METs is a widely accepted clinical tool for determining functional capacity that is relevant to the daily activities of patients. Absolute exercise capacity measured in METs has been shown to be the most powerful predictor of long-term mortality.22
Conclusion
In this large population referred for exercise echocardiography and not limited by ischemia, we demonstrated that diastolic dysfunction was strongly related to decreased exercise capacity. Increased resting and post-exercise left ventricular filling pressures are also associated with a reduction in exercise capacity. Other correlates of exercise intolerance include age, gender and body mass index. While these data require confirmation in prospective studies, they point to a potential modifiable factor that might be a target for interventions that could maintain exercise capacity with aging. Unlike many other factors that are an inevitable consequence of aging, diastolic dysfunction may be a preventable factor in the development of exercise intolerance.
Disclosure
Support for biostatistical analysis was provided by Grant Number 1 UL1 RR024150 from the National Center for Research Resources, a component of the National Institutes of Health, and the NIH Roadmap for Medical Research. Support for data management was provided by the Mayo Foundation. There was no support for the design and conduct of the study; collection, and interpretation of the data; and preparation, review, or approval of the manuscript. The authors have no other financial disclosures. Only the authors had access to the data and none received compensation.
Dr. Patricia Pellikka had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Woo JS, Derleth C, Stratton JR, Levy WC. The Influence of Age, Gender, and Training on Exercise Efficiency. J Am Coll Cardiol. 2006;47(5):1049–1057. doi: 10.1016/j.jacc.2005.09.066. [DOI] [PubMed] [Google Scholar]
- 2.Hossack KF, Bruce RA. Maximal cardiac function in sedentary normal men and women: comparison of age-related changes. J Appl Physiol. 1982;53(4):799–804. doi: 10.1152/jappl.1982.53.4.799. [DOI] [PubMed] [Google Scholar]
- 3.Weiss EP, Spina RJ, Holloszy JO, Ehsani AA. Gender differences in the decline in aerobic capacity and its physiological determinants during the later decades of life. J Appl Physiol. 2006;101(3):938–944. doi: 10.1152/japplphysiol.01398.2005. [DOI] [PubMed] [Google Scholar]
- 4.Shubair MM, Kodis J, McKelvie RS. Metabolic profile and exercise capacity outcomes: their relationship to overweight and obesity in a Canadian rehabilitation setting. J Cardiopulm Rehabil. 2004;24(6):405–413. doi: 10.1097/00008483-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Enright PL, McBurnie MA, Bittner V, et al. The 6-min walk test: a quick measure of functional status in elderly adults. Chest. 2003;123(2):387–398. doi: 10.1378/chest.123.2.387. [DOI] [PubMed] [Google Scholar]
- 6.Ellstad MH. Stress testing: principles and practice. FA Davis; Philadelphia, PA: 1996. pp. 75–110. [Google Scholar]
- 7.Ogawa T, Spina RJ, Martin WH, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86(2):494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 8.Coggan AR, Spina RJ, King DS, et al. Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol. 1992;47(3):B71–6. doi: 10.1093/geronj/47.3.b71. [DOI] [PubMed] [Google Scholar]
- 9.Proctor DN, Beck KC, Shen PH, Eickhoff TJ, Halliwill JR, Joyner MJ. Influence of age and gender on cardiac output-VO2 relationships during submaximal cycle ergometry. J Appl Physiol. 1998;84(2):599–605. doi: 10.1152/jappl.1998.84.2.599. [DOI] [PubMed] [Google Scholar]
- 10.Franciosa JA, Pork M, Levine TB. Lack of correlation between exercise capacity and indexes of resting left ventricular performance in heart failure. Am J Cardiol. 1981;47(1):33–39. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 11.Higginbotham MB, Morris KG, Cohn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51(1):52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 12.Hiroyuki O, Hiiroo I, Miyo T, et al. Impact of Doppler derived left ventricular diastolic performance on exercise capacity in normal individuals. Am Heart J. 2000;139(4):716–722. doi: 10.1016/s0002-8703(00)90054-1. [DOI] [PubMed] [Google Scholar]
- 13.Vanoverschelde JJ, Essomri B, Vanbutsele R, d'Hondt A, Cosyns JR, Detry JR. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol. 1993;74(5):2225–2233. doi: 10.1152/jappl.1993.74.5.2225. [DOI] [PubMed] [Google Scholar]
- 14.Skaluba SJ, Litwin SE. Mechanisms of Exercise Intolerance: Insights from Tissue Doppler Imaging. Circulation. 2004;109(8):972–977. doi: 10.1161/01.CIR.0000117405.74491.D2. [DOI] [PubMed] [Google Scholar]
- 15.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102(15):1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 16.Burgess MI, Jenkins C, Sharman JE, Marwick TH. Diastolic Stress Echocardiography: Hemodynamic Validation and Clinical Significance of Estimation of Ventricular Filling Pressure with Exercise. J Am Coll Cardiol. 2006;47(9):1891–1900. doi: 10.1016/j.jacc.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Khouri S, Maly G, Suh D, Walsh T. A Practical Approach to the Echocardiographic Evaluation of Diastolic Function. J Am Soc Echocardiogr. 2004;17(3):290–297. doi: 10.1016/j.echo.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Gilman G, Nelson T, Hansen W, Khanderia B, Ommen S. Diastolic function: A Sonographers Approach to the Essential Echocardiographic Measurements of Left Ventricular Diastolic Dysfunction. J Am Soc Echocardiogr. 2007;20(2):199–209. doi: 10.1016/j.echo.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90(12):1284–1289. doi: 10.1016/s0002-9149(02)02864-3. [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Bierig M, Devereaux RB, et al. Members of the Chamber Quantification Writing Group Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Society of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Quinones MA, Waggoner AD, Reduto LA, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64(4):744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 22.Myers J, Prakash M, Froelicher, et al. Exercise capacity and mortality among men referred for exercise testing. NEJM. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 23.Oldershaw PJ, Dawkins KD, Ward DE, Gibson DG. Diastolic mechanisms of impaired exercise tolerance in aortic valve disease. Br. Heart J. 1983;49(6):568–573. doi: 10.1136/hrt.49.6.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol. 1969;203(3):511–532. doi: 10.1113/jphysiol.1969.sp008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AM, Bhattacharya J, Schultz HD, et al. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58(4):512–522. doi: 10.1161/01.res.58.4.512. [DOI] [PubMed] [Google Scholar]
- 26.Ha JW, Lulic F, Bailey KR, et al. Effects of Treadmill Exercise on Mitral Inflow and Annular Velocities in Healthy Adults. Am J Cardiol. 2003;91(1):114–115. doi: 10.1016/s0002-9149(02)03016-3. [DOI] [PubMed] [Google Scholar]
- 27.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57(15):1374–1379. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 28.Julius S, Amery A, Whitlock LS, Conway J. Influence of age on the hemodynamic response to exercise. Circulation. 1967;36(2):222–230. doi: 10.1161/01.cir.36.2.222. [DOI] [PubMed] [Google Scholar]
- 29.Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc. 1987;46(5):1824–1829. [PubMed] [Google Scholar]
- 30.Brandao MU, Wajngarten M, Rondon E, et al. Left ventricular function during dynamic exercise in untrained and moderately trained subjects. J Appl Physiol. 1993;75(5):1989–1995. doi: 10.1152/jappl.1993.75.5.1989. [DOI] [PubMed] [Google Scholar]
- 31.Levy WC, Cerqueira MD, Abrass IB, et al. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88(1):116–126. doi: 10.1161/01.cir.88.1.116. [DOI] [PubMed] [Google Scholar]
- 32.Smart N, Haluska B, Jeffriess L, et al. Exercise training in systolic and diastolic dysfunction: effects on cardiac function,functional capacity, and quality of life. Am Heart J. 2007;153(4):530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Brassard P, Legault S, Garneau C, et al. Normalization of diastolic dysfunction in type 2 diabetics after exercise training. Med Sci Sports Exerc. 2007;39(11):1896–1901. doi: 10.1249/mss.0b013e318145b642. [DOI] [PubMed] [Google Scholar]
- 34.Warner JG, Jr, Metzger DC, Kitzman DW, et al. Losartan improves exercise tolerance in patients with diastolic dysfunction and a hypertensive response to exercise. J Am Coll Cardiol. 1999;33(6):1567–1572. doi: 10.1016/s0735-1097(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 35.Little WC, Zile MR, Klein A, et al. Effect of losartan and hydrochlorothiazide on exercise tolerance in exertional hypertension and left ventricular diastolic dysfunction. Am J Cardiol. 2006;98(3):383–385. doi: 10.1016/j.amjcard.2006.01.106. [DOI] [PubMed] [Google Scholar]
- 36.Redfield MM, Jacobsen SJ, Burnett JC. Burden of Systolic and Diastolic Dysfunction in the Community: Appreciating the Scope of the Heart Failure Epidemic. J Am Med Assoc. 2003;289(2):194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]