Abstract
Previous studies have identified two distinct O-methyltransferases (OMTs) implicated in isoflavonoid biosynthesis in Medicago species, a 7-OMT methylating the A-ring 7-hydroxyl of the isoflavone daidzein and a 4’-OMT methylating the B-ring 4’-hydroxyl of 2,7,4′-trihydroxyisoflavanone. Genes related to these OMTs from the model legume Medicago truncatula cluster as separate branches of the type I plant small molecule OMT family. To better understand the possible functions of these related OMTs in secondary metabolism in M. truncatula, seven of the OMTs were expressed in E. coli, purified, and their in vitro substrate preferences determined. Many of the enzymes display promiscuous activities, and some exhibit dual regio-specificity for the 4′ and 7-hydroxyl moieties of the isoflavonoid nucleus. Protein structure homology modeling was used to help rationalize these catalytic activities. Transcripts encoding the different OMT genes exhibited differential tissue-specific and infection- or elicitor-induced expression, but not always in parallel with changes in expression of confirmed genes of the isoflavonoid pathway. The results are discussed in relation to the potential in vivo functions of these OMTs based on our current understanding of the phytochemistry of M. truncatula, and the difficulties associated with gene annotation in plant secondary metabolism.
Keywords: Isoflavonoid, O-methyltransferase, Secondary metabolism, Molecular modeling, Gene family
Introduction
Isoflavonoids represent a class of bioactive plant natural products with important implications for plant, animal and human health (Dixon and Steele 1999). Over 850 isoflavonoid aglycones have been identified and much of this molecular diversity is generated through the biosynthetic modification of core isoflavonoid chemical scaffolds (Harborne 1994). The majority of naturally occurring isoflavonoids are O-methylated at one or more positions, and over 80% of the reported isoflavonoids in the forage legume alfalfa (Medicago sativa) contain at least one methoxyl group (Bisby et al. 1994). Methylation alters the chemical reactivity and biosynthetic fate of hydroxyl groups, modulates solubility and intracellular localization (Ibrahim et al. 1987), and re-directs biosynthetic intermediates down specific branches of complex metabolic grids (Maxwell et al. 1993).
Enzymatic O-methylation is catalyzed by O-methyltransferases (OMTs), which catalyze transfer of a methyl group from S-adenosyl-l-methionine (SAM) to a hydroxyl moiety of an acceptor molecule. Two distinct groups of small molecule OMTs can be distinguished from plants (Joshi and Chiang 1998). We have defined group I OMTs as 38–43 kDa proteins which do not require a metal ion for activity, and which methylate a variety of acceptors including phenylpropanoids, flavonoids, alkaloids, and coumarins (Noel et al. 2003). Group II OMTs are of lower MW (23–27 kDa), and are Mg2+-dependent enzymes represented by caffeoyl-CoA 3-OMT. Substrate specificities of OMTs can not be accurately predicted on the basis of sequence similarity alone (Schroder et al. 2002), and in some cases a single amino acid change can alter substrate specificity (Frick and Kutchan 1999; Gang et al. 2002). Elucidation of the crystal structures of caffeoyl CoA 3-OMT and three different group I OMTs from alfalfa (Zubieta et al. 2001, 2002; Ferrer et al. 2005) has now made it possible to explore the structural basis of OMT substrate specificity by homology-based modeling (Hoffmann et al. 2001; Gang et al. 2002; Kornblatt et al. 2004; Yang et al. 2004).
Isoflavonoid OMTs catalyze the biosynthesis of natural chemicals that are important for disease resistance in legumes. 6a-Hydroxymaackiain 3-OMT in pea (Pisum sativum) (PsHMM) methylates the relatively nontoxic isoflavonoid-derived compound 6a-hydroxymaackiain to produce the potent antimicrobial chemical pisatin, thereby constituting an essential step in phytoalexin biosynthesis (VanEtten et al. 1982; Preisig et al. 1989).
In addition to PsHMM, two other isoflavonoid O-methyltransferases have been characterized at the molecular level. M. sativa isoflavone 7-OMT (MsI7OMT) catalyzes A-ring 7-O-methylation of isoflavones such as daidzein (8) in vitro (Fig. 1B), and has been studied both biochemically and structurally (He and Dixon 1996; He et al. 1998; Zubieta et al. 2001). Although 7-O-methylated isoflavones such as isoformononetin (9) (7-O-methyl-daidzein) are uncommon in legumes, the induction of I7OMT transcripts and observable enzymatic activity after elicitation or fungal infection in alfalfa suggested a role for this enzyme in the phytoalexin response (Edwards and Dixon 1991; Akashi et al. 2000; He and Dixon 2000). Paradoxically, over-expression of MsI7OMT in alfalfa did not produce isoformononetin, but led to greater accumulation of the 4′-O-methylated isoflavonoids formononetin (4) and medicarpin (5) (Fig. 1A) in elicited leaves, and enhanced resistance to the fungal leaf pathogen Phoma medicaginis (He and Dixon 2000). I7OMT activity is not restricted to alfalfa, and the enzyme has been recently cloned from licorice (Glycyrrhiza echinata) (Akashi et al. 2003).
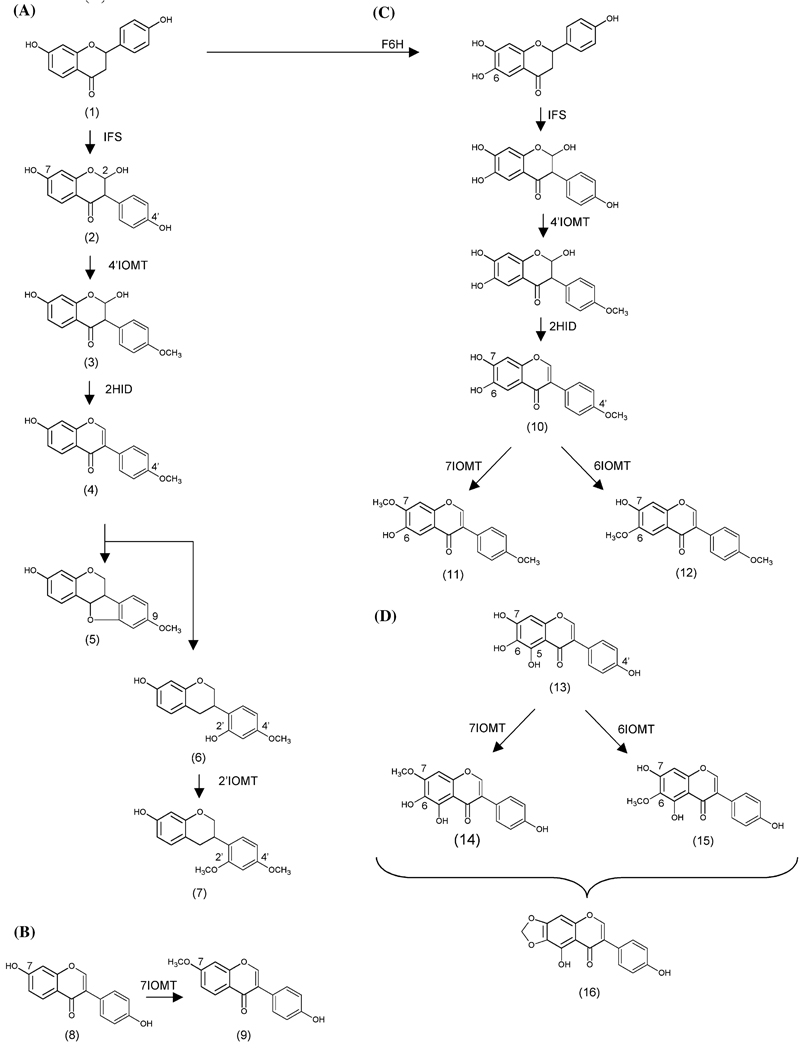
Fig. 1.
Isoflavonoid biosynthetic pathways and IOMT activities. (A) Pathway leading to biosynthesis of formononetin (4), medicarpin (5), and sativan (7). The flavanone liquiritigenin (1) is converted to 2,7,4′-trihydroxyisoflavanone (2) in a reaction catalyzed by isoflavone synthase (IFS). Methylation of (2) by 4′IOMT forms 2,7-dihydroxy, 4′-methoxyisoflavanone (3), which is converted to (4) by 2-hydroxyisoflavanone dehydratase (2HID). Formononetin is a precursor of (5) and (7), and therefore the methyl groups at the 9- (5) and 4′- (7) positions of these compounds originate from 4′IOMT. Sativan (7) is most likely synthesized by 2′-methylation of vestitol (6). (B) Isoflavone 7-OMT activity characterized from legumes. I7OMT methylates daidzein (8) on the 7-position to form isoformononetin (9). (C) Proposed pathway for biosynthesis of alfalone (11) and afromosin (12). Flavanone 6-hydroxylase (F6H) has been shown to introduce a 6-hydroxyl group at the level of flavanone. Subsequent steps may be catalyzed according to the pathway in A and would lead to 6,7-dihydroxy, 4′-methoxyisoflavone (10). Methylation by a 7IOMT would form (11), while methylation by a 6IOMT would form (12). (D) Proposed pathway for biosynthesis of irilone (16). Irilone may be formed by methylation of 4′,5,6,7-tetrahydroxyisoflavone (13) at either the 6- (15) or 7- (14) position followed by ring closure
The most common site of methylation of isoflavo-noids is at the 4′-position of the B-ring (isoflavone numbering), but identification of an IOMT with 4′-specificity eluded efforts until recently (Akashi et al. 2003). Initial work focused on isolation of an isoflavone 4′-OMT (Wengenmayer et al. 1974; Edwards and Dixon 1991; He and Dixon 1996), although it now appears that 4′-O-methylation in isoflavonoid biosynthesis occurs at the level of 2-hydroxyisoflavanone (3, Fig. 1A), the direct product of the “isoflavone syn-thase” (IFS) that constitutes the entry point into the isoflavonoid pathway (Akashi et al. 2000). 2,7,4′-Tri-hydroxyisoflavanone 4′-OMT (HI4′OMT) catalyzes the methylation of the 4′-position of 2-hydroxyisoflavanone to form 2,7-dihydroxy, 4′-methoxyisoflavanone (3), which undergoes dehydration to yield the isoflavone formononetin (4) (Fig. 1A). Formononetin accumulates in several legumes and is also a precursor in the biosynthesis of medicarpin (5) and related ptero-carpanoid phytoalexins. HI4′OMT is closely related to PsHMM at the amino acid sequence level (Akashi et al. 2003), and has been shown to possess 6a-hydroxymaackiain 3-OMT activity (Liu et al. 2005).
On the basis of transgenic studies implicating MsI7OMT in the formation of 4′-O-methylated isoflavonoids in alfalfa (He and Dixon 2000), structural studies suggesting that 2-hydroxyisoflavanone can be accommodated in the active site of MsI7OMT in an orientation favorable for 4′-O-methylation (Zubieta et al. 2001), and cellular imaging studies indicating potential association of the operationally soluble MsI7OMT with IFS on the outer surface of the endoplasmic reticulum (Liu and Dixon 2001), we proposed that the substrate specificity of MsI7OMT may differ in vivo (B- ring 4′-position) from its in vitro specificity (A-ring 7-position). The discovery of a 2-hydroxyisoflavanone-specific 4′-OMT (Akashi et al. 2003) calls into question this earlier interpretation of MsI7OMT’s role in 4′-O-methylation.
Expressed sequence tag (EST) libraries obtained from the model legume Medicago truncatula contain several related OMT sequences with homology to I7OMT or HI4′-OMT, and therefore annotated, on the basis of sequence identity, as encoding isoflavone 7-OMTs, 2-hydroxyisoflavanone 4′-OMTs, or 6a-hydroxymaackiain OMTs. To better understand the nature of the OMTs catalyzing the 4′ and 7-O-methylation reactions during isoflavonoid biosynthesis in Medicago, and to provide experimental evidence for the tentative annotations based on sequence similarity, we cloned, heterologously expressed and determined relative specificities for (iso)flavonoid substrates of seven putative M. truncatula IOMTs (MtIOMTs).
Materials and methods
Cloning, expression, and purification of M. truncatula IOMTs
Candidate M. truncatula IOMTs were identified by searching MtGI Release 7.0 (May 1, 2003) (http://www.tigr.org/tigr-scripts/tgi/T_index.cgi?species=me-dicago) for ESTs with similarity to M. sativa I7OMT (GB accession number U97125) using BLAST searches. Full-length ESTs for MtIOMT1 (NF070-A11EC1F1082) were obtained from an elicited M. truncatula cell culture library; full length MtIOMT3 ESTs (NF027F03PL1F1029) were obtained from a phosphate-starved leaf library; full length MtIOMT4 ESTs (N201058e) were obtained from a phosphate-starved root library; full length MtIOMT6 (NF031-D06RT1F1046), MtIOMT7 (NF005H09RT1F1079), and MtIOMT8 ESTs (NF031H02RT1F1016) were obtained from a M. truncatula developing root library.
Cloning of MtIOMT5 (corresponding to TC 100926, TIGR MtGI v.8) was as described previously (Liu et al. 2005). To obtain a full-length cDNA for MtIOMT2, specific primers designed based upon the M. truncatula genomic sequence were used to amplify the MtIOMT2 coding sequence from M. truncatula root cDNA. The amplified product was cloned into the pGEM-T easy vector (Promega, Madison WI) and sequenced to confirm its identity. MtIOMTs were cloned into the pET28a vector (EMD Biosciences, Inc., San Diego, CA) using PCR-based amplification with primers designed to introduce EcoRI and XhoI restriction sites; BamHI and XhoI sites were used for cloning MtIOMT6 due to the presence of an internal EcoRI site in this particular cDNA. Because the EST for MtIOMT8 was missing a start codon, the primer designed to clone MtIOMT8 into pET28a contained an additional 2-nucleotide insertion to generate an ATG start site. All MtIOMTs were expressed as translational fusions to an N-terminal His-tag. Expression of MtIOMTs in E. coli BL21(DE3) and purification by Ni2+-NTA chromatography were as described (Zubieta et al. 2001). Proteins were dialyzed against 20 mM Tris pH 8.0, 100 mM NaCl, 10% (v/v) glycerol, and 14 mM β-mercaptoethanol. Protein concentration was determined by Bradford assay using BSA as a standard (Bradford 1976).
OMT assays
Reactions (200 µl) were performed in 0.1 M potassium phosphate, pH 7.4, 10% (w/v) sucrose, 14 mM β-mer-captoethanol with 10 µM protein, 0.5 mM S-adenosyl-l-methionine, and 80 µM phenolic substrates. All substrates were purchased from Indofine (Hillsborough, NJ) except 6,7-dihydroxy-4′-methoxyisoflavone, dihydrodaidzein, and vestitol, which were purchased from Apin (Oxfordshire, UK). 2,7,4′-Trihydroxyisoflavanone was purified from IFS reactions carried out with liquiritigenin as described (Liu et al. 2005). OMT assays were incubated at 30°C for 2 h and extracted with ethyl acetate. Ethyl acetate extracts were dried under N2 and the resultant material was resuspended in 50–100 µl of methanol. Samples were analyzed on an Agilent 1100 HPLC, equipped with a quaternary pump (model # G1311A), a degasser (model # G1322A), an autosampler (model # G1313A), and a diode array detector (model # G1315A). Samples of 20 µl were applied to an ODS2 reverse-phase column (5 µm particle size, 4.6 × 250 mm) and eluted in 1% (v/v) phosphoric acid with an increasing gradient of acetonitrile [0–5 min, 25%; 5–35 min, 25–50%; 35– 39 min, 50–100%] at a flow rate of 1 ml/min. To resolve the 4′-O-methylated and 7-O-methylated products of genistein, samples were applied to a Phenomenex SYNERGI Polar-RP 80Å column (4 µm particle size, 4.6 × 250 mm) and eluted in water with an increasing gradient of acetonitrile [0–5 min, 20%; 5–17 min, 20–38%; 17–33 min, 38%; 33–45 min, 38– 56%; 45–46 min, 56–100%] at a flow rate of 1 ml/min. Chiral chromatography was performed as described (Liu et al. 2005).
For determination of specific activities, reactions (100 µl) were performed in 0.1 M potassium phosphate, pH 7.4, 10% (w/v) sucrose, 14 mM β-mercaptoethanol with 0.2–10 µg protein, 50 µM S-adenosyl-l-methionine (0.025 µCi 14C-SAM per reaction), and 100 µM substrate. Reactions were incubated at 30°C for 15–25 min and stopped by addition of 4 µl of 1N HCl. Products were extracted into 500 µl of ethyl acetate and 400 µl was used for liquid scintillation counting.
Tandem MS analysis
An HP 1100 series II LC system (Hewlett-Packard, Palo Alto, CA) with a photodiode array detector was coupled to a Bruker Esquire ion-trap mass spectrometer (ITMS) equipped with an electrospray ionization source. A reverse phase, C18, 5 µm, 4.6 × 250 mm column (J.T.Baker, Phillipsburg, NJ) was used for separations. The mobile phase consisted of 0.1% (v/v) acetic acid using linear gradients of 5–90% acetonitrile (v/v) in 70 min. The flow rate was 0.8 ml/min, and the temperature of the column was kept at 28 °C. Both positive- and negative-ion mass spectra were acquired. Positive-ion ESI was performed using an ion source voltage of 4.0 KV and a capillary offset voltage of 86.0 V. Nebulization was aided with a coaxial nitrogen sheath gas provided at a pressure of 60 psi. Desolvation was aided using a counter current nitrogen flow set at a pressure of 12 psi and a capillary temperature of 300°C. Mass spectra were recorded over the range 50– 2200 m/z. The ITMS was operated under an ion current control of approximately 10,000 with a maximum acquire time of 100 ms. Tandem mass spectra were obtained in manual mode for targeted masses using an isolation width of 2.0, fragmentation amplitude of 2.2 and threshold set at 6,000.
Measurement of IOMT transcript levels
Infection of M. truncatula with Phoma medicaginis was performed as described (Deavours and Dixon 2005). RNA was extracted from plant tissues using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) according to the manufacturer’s instructions. For RT-PCR, 2 µg of total RNA was transcribed into cDNA using Ready-To-Go RT-PCR beads (Amersham, Piscataway, NJ) and oligo-dT primer. Two µl of cDNA was used in each PCR reaction (50 µl total) with Ex-Taq PCR reagents (Takara Bio Inc., Shiga, Japan) and the primers listed in Table S1. PCR conditions were 94°C, 5 min; 25–32 cycles of 94°C for 1 min, 55°C for 1 min, 72°C for 1 min; followed by 72°C for 10 min. PCR products were resolved on a 1 % (w/v) TAE-agarose gel and visualized with ethidium bromide.
DNA microarray analysis
The Affymetrix DNA chip contained 61,200 probe sets: 32,167 M. truncatula EST/mRNA-based and chloroplast gene-based probe sets (TIGR Gene Index version 8, Jan., 2005, 36,878 unique sequences); 18,733 M. truncatula IMGAG (International Medicago Genome Annotation Group) and phase 2/3 BAC prediction-based probe sets; 1,896 M. sativa EST/mRNA based probe sets; 8,305 Sinorhizobium meliloti gene prediction-based probe sets. For microarray experiments, total RNA was extracted from cell suspension cultures (Suzuki et al. 2005) using TRI Reagent (Molecular Research Center, Inc., Cincinnati, OH) and purified using the RNeasy MinElute Cleanup Kit (Qiagen, Valencia, CA), according to the manufacturer’s instructions. For all samples, 10 µg of total RNA was used for labeling reactions. Labeling, hybridization and data processing were performed according to the manufacturer’s instruction (Affymetrix, Santa Clara, CA). For both YE and MeJA treatments, transcript profiles were examined at early (2 h) and late (24 h) time post-elicitation. For each sample point, two biological replicates were included, and the average signal intensities were used to calculate ratio of treatment to its corresponding control.
Relationship tree
Sequences were aligned using CLUSTAL W (Thompson et al. 1994) and analyzed cladistically using PAUP version 4.0b10 using parsimony optimality criterion and a 1000-replicate bootstrap search using the heurisitic search algorithm. All branches with <70% bootstrap support were judged inconclusive and were collapsed.
Homology modeling and automated substrate docking
The MtIOMT amino acid sequences were aligned to MsI7OMT using CLUSTAL W version 1.82 (Thompson et al. 1994) and homology models were built using the program MODELLER (Marti-Renom et al. 2000) based upon the crystal structure of the MsI7OMT-isoformononetin complex (PDB:1FP2) (Zubieta et al. 2001). For each MtIOMT, five models were generated and ranked by Model Rank in the MODELLER package. The top ranked models were chosen and visualized using O (Jones et al. 1991), and quantitatively evaluated using the program Procheck (Laskowski et al. 1993). For substrate docking analyses, Genetic Optimization for Ligand Docking (GOLD) (Otwinowski and Minor 1997) and DOCK (http://dock.compbio.ucsf.edu/) were used. When GOLD was used for docking runs, the parameters controlling the precise operation of the genetic algorithm were as follows: population size = 100, selection pressure = 1.100000, number of operations = 100,000, number of islands = 5, niche size = 2, crossover weight = 95, mutate weight = 95, and migrate weight = 10. Default parameter values for van der Waals interactions and hydrogen bonding were used throughout the docking process. The volume of the active site was defined using a 15 Å radius centered around the NE2 atom of the catalytic His residue. Ten docking calculations were run for each ligand and the GOLD score was used to identify the lowest energy docking results.
Results
Isolation and sequence analysis of MtIOMTs
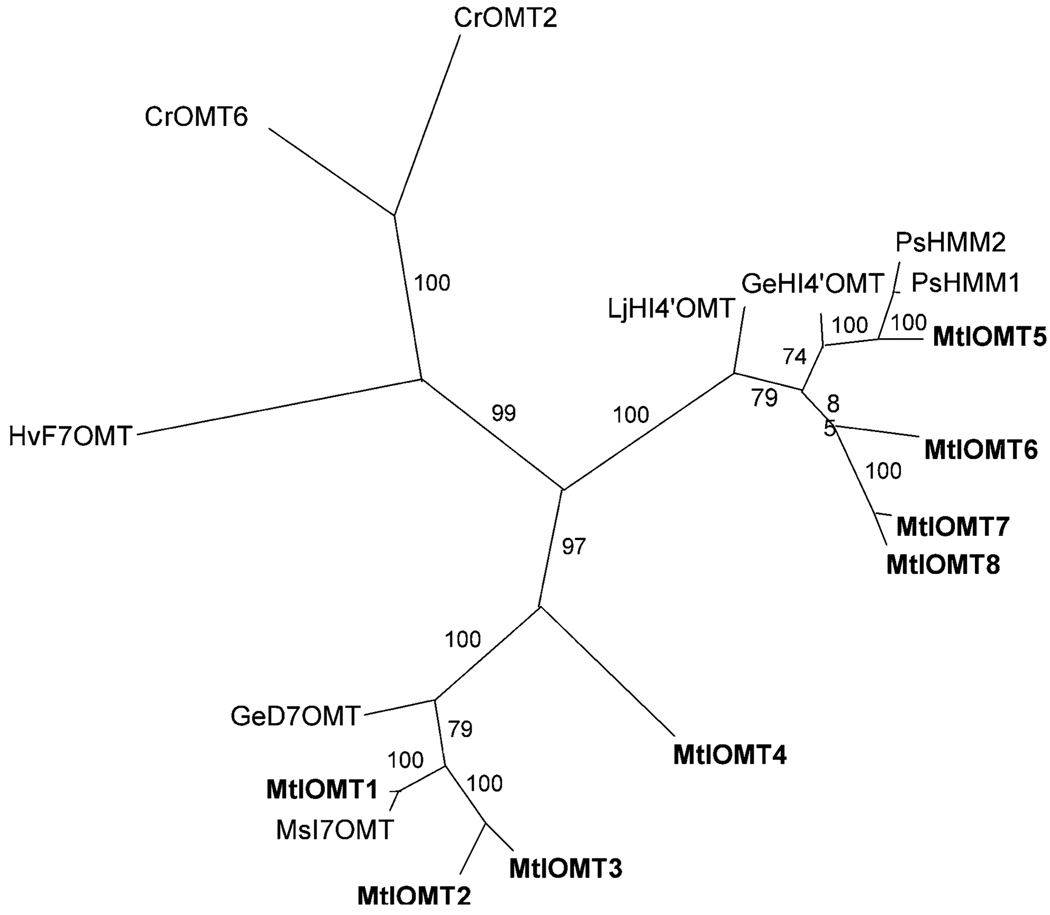
Sequence analysis of M. truncatula EST libraries using M. sativa MsI7OMT (the IOMT8 of He et al. 1998) identified eight putative MtIOMT homologs (here designated as MtIOMT 1–8) with amino acid sequence identities ranging from 51 to 97% relative to MsI7OMT. Phylogenetic analysis indicated that these putative MtIOMTs cluster into two distinct clades separate from known flavonoid OMTs (Fig. 2). Protein sequence identity between OMTs from different clades is less than 50%. One clade, designated as the I7OMT clade, includes I7OMT homologs from alfalfa and Glycyrrhiza echinata and MtIOMTs 1–4. MtIOMTs 1– 3 are 79–89% identical to one another, while MtIOMT4 is 58–59% identical to OMTs within this clade. The second clade includes PsHMM1 and 2, HI4′OMT homologs from G. echinata and Lotus japonicus, and MtIOMTs 5–8. OMTs within this second clade are 73– 76% identical to one another at the amino acid level.
Fig. 2.
Relationship tree of (iso)flavonoid OMTs. CrOMT6, Catharanthus roseus flavonoid 4′-OMT (AY343490); CrOMT2, C. roseus 3′/5′-flavonol OMT (AY127569); HvF7OMT, Hordeum vulgare flavonoid 7-OMT (X77467); GeD7OMT, G. echinata daidzein 7-OMT (AB091685); MsI7OMT, M. sativa isoflavone 7-OMT (U97125); LjHI4′OMT, Lotus japonicus 2-hydroxyisoflavanone 4′-OMT (AB091686); GeHI4′OMT, G. echinata 2-hydroxyisoflavanone 4′-OMT (AB091684); PsHMM1, P. sativum 6a–hydroxymaackiain 3-OMT (U69554). The M. truncatula IOMT sequences are available in the GenBank database under the accession numbers: AY942159, MtIOMT1; DQ419910, MtIOMT2; DQ419911, MtIOMT3; DQ419912, MtIOMT4; AY942158, MtIOMT5; DQ419913, MtIOMT6; DQ419914, MtIOMT7; DQ419915, MtIOMT8
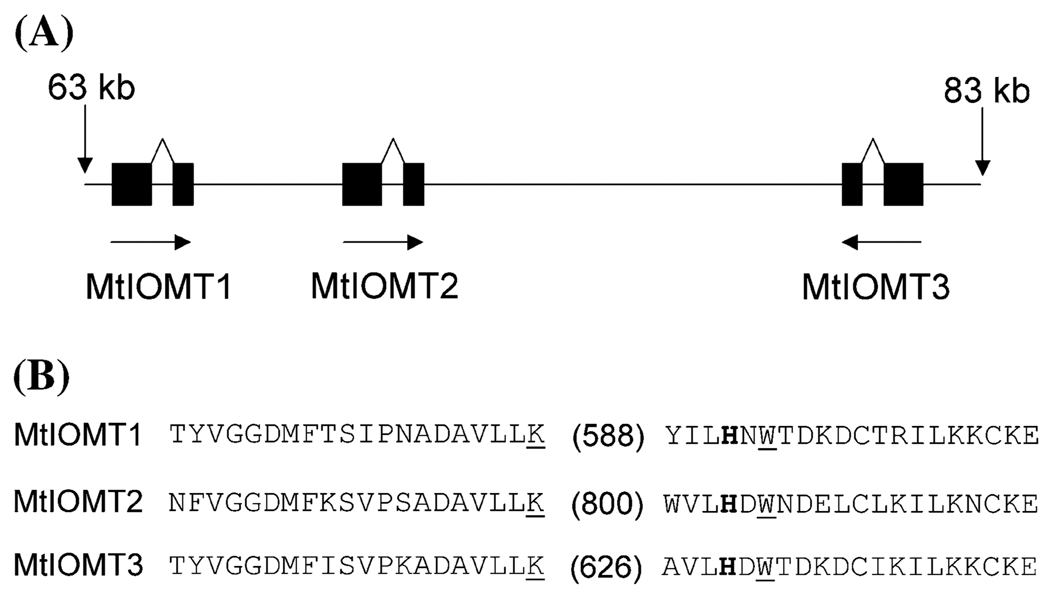
Genomic sequences for MtIOMTs 1–3 were obtained by searching the M. truncatula ongoing genome sequence web site (http://www.genome.ou.edu/medi-cago.html). Genes encoding these three MtIOMTs are located within a 20 kB region on a single BAC clone (AC146549, Fig. 3A). MtIOMT3 is located in the opposite orientation relative to MtIOMT1 and MtIOMT2. All three MtIOMTs have a single intron at the same position in the gene sequence (Fig. 3B), the location of which appears to be highly conserved among plant OMTs (Schroder et al. 2004), while neither intron length nor sequence is conserved. Interestingly, the intron junction neighbors the catalytic His and two residues (Lys and Trp) shown to be important for SAM-binding (Zubieta et al. 2001). These observations suggest that the OMT genes may have arisen from ancient gene duplication events followed by evolution/adaptation.
Fig. 3.
(A) Genomic organization of MtIOMTs 1–3. Black boxes represent exons and arrows indicate relative orientation. Numbering is in reference to BAC clone AC146549. (B) Location of the single intron in the protein sequence of MtIOMT1, MtIOMT2, and MtIOMT3. Numbers in parentheses represents length of intron in bps. Residues involved in SAM-binding (underlined) and catalysis (bold) are indicated (Zubieta et al. 2001). Figure 3B is modeled after Schroder et al. (2004)
Substrate specificity of recombinant IOMTs
Sequence analysis of the MtIOMT EST clones revealed that most were full-length; in the case of MtIOMT2, the full length open reading frame was obtained by PCR from root cDNA using primers based on the previously determined genomic sequence. Hexa-histidine-tagged MtIOMTs were expressed in E. coli, purified by Ni-affinity chromatography, and tested for enzymatic activity using a range of flavonoid and isoflavonoid compounds as potential substrates (Table 1). MtIOMT8 was insoluble upon expression in E. coli and therefore not pursued further. For comparison, we included the previously characterized alfalfa MsI7OMT (alfalfa IOMT8, He and Dixon 1996; He et al. 1998). This enzyme was reported to have highest activity with 6,7,4′-trihydroxyisoflavone (136% of the activity with daidzein) and little activity with the 4′-methoxyisoflavones formononetin and biochanin A (He et al. 1998).
Table 1.
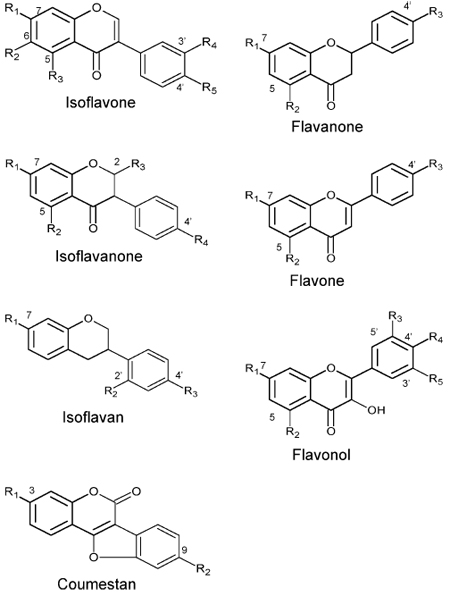
Substrates analyzed with recombinant IOMTs in the present work
| Substrate | R1 | R2 | R3 | R4 | R5 |
|---|---|---|---|---|---|
| Isoflavone | |||||
| 6,7,4′-trihydroxyisoflavone | OH | OH | H | H | OH |
| 6,7-dihydroxy, 4′-methoxyisoflavone | OH | OH | H | H | OCH3 |
| 7,3′,4′-trihydroxyisoflavone | OH | H | H | OH | OH |
| Daidzein | OH | H | H | H | OH |
| Genistein | OH | H | OH | H | OH |
| Glycitein | OH | OCH3 | H | H | OH |
| Isoflavanone | |||||
| 2,7,4′-trihydroxyisoflavanone | OH | H | OH | OH | |
| Dihydrodaidzein | OH | H | H | OH | |
| Isoflavan | |||||
| Vestitol | OH | OH | OCH3 | ||
| Coumestan | |||||
| Coumestrol | OH | OH | |||
| Flavanone | |||||
| Liquiritigenin | OH | H | OH | ||
| Naringenin | OH | OH | OH | ||
| Flavone | |||||
| Apigenin | OH | H | OH | ||
| 5,7,4′-trihydroxyflavone | OH | OH | OH | ||
| Flavonol | |||||
| Myricetin | OH | OH | OH | OH | OH |
 | |||||
MtIOMT1
MtIOMT1 is 97% identical to MsI7OMT and has nearly identical substrate specificity (Table 2). Activity is primarily limited to planar isoflavones, although surprisingly the non-planar isoflavanone dihydrodaidzein (Table 1) also undergoes methylation. MtIOMT1 (and MsI7OMT) have highest activity with the partially methylated 6-methoxyisoflavone glycitein (Table 2) (a natural product of soybean) among the various substrates tested. Methylation of the 4′-hydroxyl group of 6,7,4′-trihydroxyisoflavone drastically reduces activity by more than 80-fold (compare activity with 6,7-dihydroxy-4′-methoxyisoflavone to that with 6,7,4′-trihydroxyisoflavone). MsI7OMT has lower activity with genistein than with daidzein, whereas the activity of MtIOMT1 with genistein is more than 2-fold higher than with daidzein.
Table 2.
Activities of purified MtIOMTs against a range of phenolic substratesa
| Substrate | MtIOMT1 | MtIOMT2 | MtIOMT3 | MtIOMT4 | MtIOMT5 | MtIOMT6 | MtIOMT7 | MsI7OMT |
|---|---|---|---|---|---|---|---|---|
| Isoflavone | ||||||||
| 6,7,4′-trihydroxyisoflavone | 43.9 (7) | 35.3 (7) | 100 (7) | 0 | 0 | 0 | 0 | 51.8 (7) |
| 6,7′-dihydroxy, 4′- methoxyisoflavone |
≤1 | 0 | 0 | 0 | 0 | 0 | 0 | ≤1 |
| 7,3′,4′-trihydroxyisoflavone | 40.8 (7) | 43.1 (7) | 52.9 (4’) | 0 | 0 | 0 | ≤1 | 47.1 (7) |
| Daidzein | 20.6 (7) | 100 (7) | ≤1 | 0 | 0 | 0 | 0 | 21.6 (7) |
| Genistein | 49.2 (7) | 61 (7) | 73.7 (4’) | 0 | 0 | 0 | 0 | 15(7) |
| Glycitein | 100 (7) | 38.9 (7) | 51.6 (7) | 0 | 0 | 0 | ≤1 | 100 (7) |
| Isoflavanone | ||||||||
| 2,7,4′-trihydroxyisoflavanone | NDc | NDc | NDc | NDc | 100 (4′) | NDc | 24.8 (4′) | NDc |
| Dihydrodaidzein | 15.6 (7) | 70.1 (7,4′) | 49.4 (4′) | 0 | 7.9 (4′,7) | 100 (4′,7) | 84.1 (4′,7) | 12.8 (7) |
| Isoflavan | ||||||||
| Vestitol | 0 | ≤1 | 0 | 43.8 | ≤1 | 66.2 | 3.4 | 0 |
| Coumestan | ||||||||
| Coumestrol | 0 | ≤1 | 0 | 100 (3,9) | 0 | 0 | 1.5 | 0 |
| Flavanone | ||||||||
| Liquiritigenin | 0 | ≤1 | 0 | 0 | ≤1 | 0 | 42.2 (4′,7) | 0 |
| Naringenin | 0 | ≤1 | 0 | 0 | ≤1 | 0 | 100 (4′) | 0 |
| Flavone | ||||||||
| Apigenin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7,4′-dihydroxyflavone | 0 | ≤1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Flavonol | ||||||||
| Myricetin | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| pkat/mgb | 3646.7 | 930.2 | 7.4 | 2.6 | 4189.6 | 6.9 | 198.8 | 2742.0 |
Relative activity at 50 µM S-adenosyl methionine and 100 µM acceptor substrate. Reactions were run for several different time periods to ensure reaction rates were in the linear portion with respect to time, and values are normalized with the conversion rate for the most converted substrate as 100%. Numbers in parentheses represent the position of methylation of major products where known.Structures of the substrates, with positions of hydroxyl and methoxyl substitutions marked, are shown in Table 1
Specific activity at 100%
Not determined – initial activity screens revealed very low or no activity
To determine the regio-specificity of methylation, reaction products were subjected to HPLC separation and identified by comparison to available standards, or subjected to tandem MS analysis (Figures S1–S4, Table S2). Only 7-O-methylated products were observed for MtIOMT1 and MsI7OMT. In reactions with 2,7,4′-trihydroxyisoflavanone (2), a minor peak was observed eluting slightly before the peak corresponding to 2,7-dihydroxy, 4′-methoxyisoflavanone (3) (Figure S1j). It is possible that this compound is the 7-O-methylated isoflavanone, since isoformononetin (7-O-methyl daidzein) (9) is also detected in reactions with 2,7,4′-trihydroxyisoflavanone. Isoformononetin may arise either by methylation of daidzein formed from the spontaneous dehydration of the unstable 2,7,4′-trihydroxyisoflavanone (Fig. 1B), or via spontaneous dehydration of 2,4′-dihydroxy-7-methoxyisoflavanone.
MtIOMT2
MtIOMT2 methylates the same isoflavones as MtIOMT1 and MsI7OMT, but exhibits a different overall pattern of substrate specificity (Table 2). The best substrate for MtIOMT2 is daidzein (8), followed by dihydrodaidzein and genistein. Comparable activity is observed for 6,7,4′-trihydroxyisoflavone, 7,3′,4′-trihydroxyisoflavone, and glycitein. MtIOMT2 additionally methylates vestitol (6), coumestrol, liquiritigenin (1), and naringenin, although with low efficiency. As with MsI7OMT and MtIOMT1, methylation occurs on the 7-position of isoflavones, although coumestrol is methylated on the 9-position (equivalent to the 4′-position of isoflavone) (Figure S1). Two peaks were observed in reactions with liquiritigenin and naringenin; reaction products with liquiritigenin were identified as the 7-O-methylated and 7,4′-di-O-methylated products. Similarly, both 7 and 4′-O-methylated products are observed in reactions with dihydrodaidzein (Figure S4, Table S2). We were not able to determine the methylation position on vestitol. Minor unidentified peaks were observed in reactions with 2,7,4′-trihydroxyisoflavanone (2) (Figure S1j).
MtIOMT3
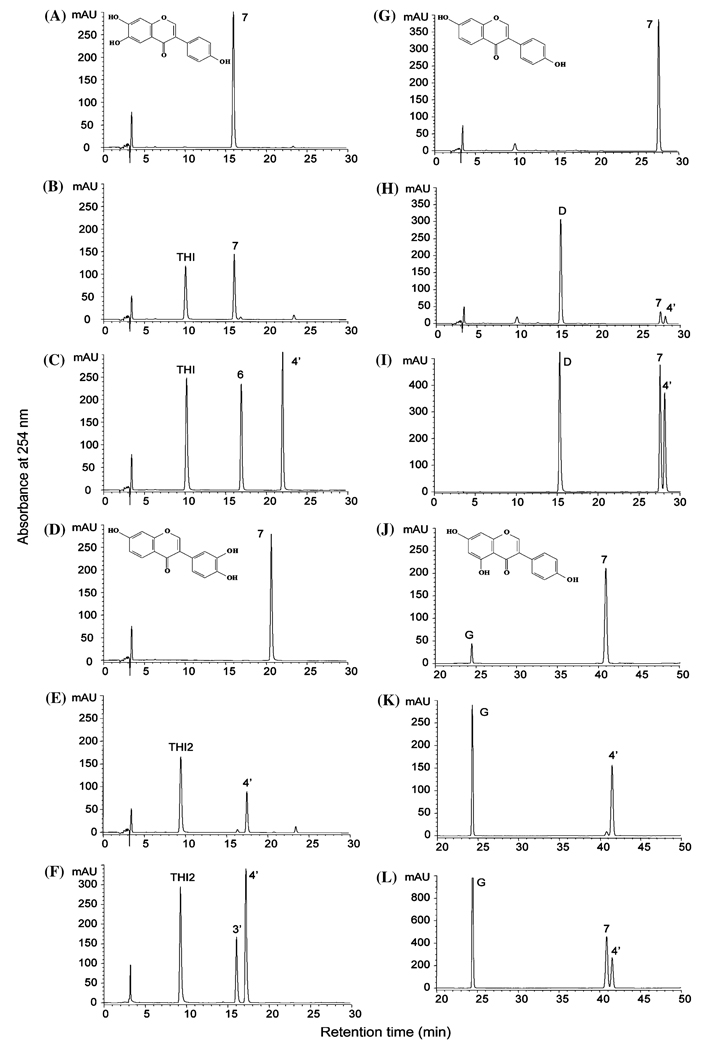
MtIOMT3 methylates the same isoflavone substrates as MsI7OMT, MtIOMT1, and MtIOMT2 (Table 2), and both 7- and 4′-O-methylated products are observed, depending on the substrate used (Fig. 4). Both 6,7,4′-trihydroxyisoflavone and glycitein undergo methylation on the 7-position (Fig. 4B, Figure S1a, e), whereas the major reaction products for 7,3′,4′-trihydroxyisoflavone and genistein are the corresponding 4′-methoxy isoflavones, calycosin and biochanin A (Figs. 4E, K). Minor 7-O-methyl (isoformononetin) (9) and 4′-O-methyl (formononetin) (4) products are observed with daidzein (8) (Fig. 4H). Primarily 4′-O-methylated product is observed in reactions with dihydrodaidzein (Figure S4, Table S2), whereas no products are detected in reactions with 2,7,4′-trihydroxyisoflavanone (2) (Figure S1j).
Fig. 4.
HPLC chromatograms of reaction products of MtIOMT1 (A, D, G, J) and MtIOMT3 (B, E, H, K) with 6,7,4′-trihydroxyisoflavone (A, B), 7,3′,4′-trihydroxyisoflavone (D, E), daidzein (G, H), and genistein (J, K) as substrates. Standards are shown in panels C, F, I, L and methylated standards are labeled according to the position of the methyl group. (C) THI, 6,7,4′-trihydroxyisoflavone; 6, glycitein; 4′, 6,7-dihydroxy, 4′-methoxyisoflavone. (F) THI2, 7,3′,4′-trihydroxyisoflavone; 3′, 3′-methoxydaidzein; 4′, calycosin. (I) D, daidzein; 7, isoformononetin; 4′, formononetin. (L) G, genistein; 7, prunetin; 4′, biochanin A
MtIOMT4
MtIOMT4 possesses low level, trace activity against vestitol (6) and coumestrol (Table 2, Figure S1), the latter being converted to both 3-O-methylcoumestrol and 3,9-O-dimethylcoumestrol, with a trace of 9-O-methylcoumestrol (Figure S1g).
MtIOMT5
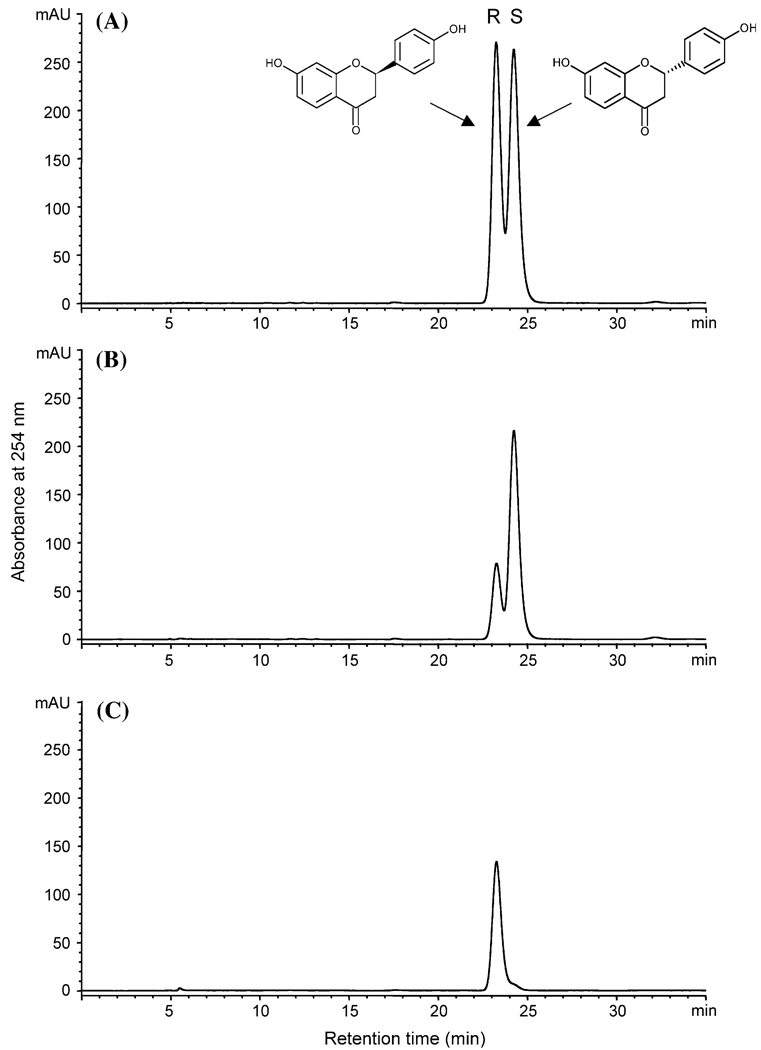
MtIOMTs in the clade containing genes annotated as encoding 2,7,4′-trihydroxyisoflavanone or 6a-hydroxymaackiaian OMTs prefer non-planar substrates with one or more chiral centers. We have recently shown that MtIOMT5 encodes the M. truncatula ortholog of the G. echinata HI4′OMT and methylates 2,7,4′-trihydroxy-isoflavanone (2) (the product of IFS) as well as (+)-6a-hydroxymaackiain (Liu et al. 2005). MtIOMT5 also methylates dihydrodaidzein, and to a lesser extent naringenin, liquiritigenin (1), and vestitol (6) (Table 2). 2,7,4′-Trihydroxyisoflavanone is exclusively methylated on the 4′-position (Figure S1j) whereas 4′-methoxy, 7-methoxy, and 4′,7-dimethoxy products are observed in reactions with dihydrodaidzein (Figure S4i, Table S1). Tandem MS indicated that the flavanones liquiritigenin and naringenin are methylated on the 4′-positions (Figures S2 and S3). Based upon HPLC analysis and quantification of reactions run to completion, the methylated flavanone products never account for more than 50% of the total amount of compound in the reaction mixtures, suggesting that only one stereoisomer of these enantiomeric compounds serves as substrate. Unreacted liquiritigenin remaining in reaction mixtures was primarily (2S)-liquiritigenin, as shown by chiral HPLC chromatography (Fig. 5B), indicating that MtIOMT5 preferentially methylates the (2R)-enantiomer (note that the (2S)-enantiomer of liquiritigenin is the naturally occurring form). We were unable to separate the stereoisomers of vestitol.
Fig. 5.
Chiral HPLC chromatography of unreacted liquiritigenin present in reactions with MtIOMT5 (B) and MtIOMT7 (C). Racemic liquiritigenin standard is shown in (A)
MtIOMT6
MtIOMT6 and MtIOMT4 possess the most narrow substrate specificity for (iso)flavonoids of the enzymes analyzed in the present work. Among the compounds tested, dihydrodaidzein and vestitol (2) are the only ones methylated by MtIOMT6, and the specific activity with these compounds is very low. 4′-O-Methylated, 7-O-methylated, and 4′,7-di-O-methylated products are observed in reactions with dihydrodaidzein (Figure S4, Table S2).
MtIOMT7
Naringenin and dihydrodaidzein are the best substrates for MtIOMT7 of the compounds tested (Table 2). Almost all the naringenin is methylated after 2 h, suggesting that MtIOMT7 is able to methylate both (2S) and (2R)-enantiomers (Figure S1l). Surprisingly, the liquiritigenin remaining in reactions after 2 h is the opposite enantiomer (2R) than that observed in reactions with MtIOMT5, indicating that MtIOMT7 preferentially methylates the (2S)-enantiomer (Fig. 5C). Multiple products are observed with both flavanones (Figures S1k, l). The major product with naringenin is 4′-O-methyl-naringenin; an additional peak, which elutes later on reverse phase HPLC, is most likely a dimethylated product (Figure S1l). Three products are observed in reactions with liquiritigenin; these were identified as 7-O-methyl-liquiritigenin, 4′-O-methyl-liquiritigenin, and 7,4′-O-dimethyl-liquiritigenin (Figure S2). Similarly, 4′-O-methylated, 7-O-methylated, and 4′,7-di-O-methylated products are also observed in reactions with dihydrodaidzein (Figure S4, Table S2). Two minor peaks are observed in reactions with 2,7,4′-trihydroxyisoflavanone, one of which corresponds to 2,7-dihydroxy, 4′-methoxyisoflavanone (Figure S1j). MtIOMT7 also methylates vestitol and coumestrol (9-O-methylation). Minor peaks are observed in reactions with 7,3′,4′-trihydroxyisoflavone and glycitein (both 7-O-methylated).
Molecular modeling studies
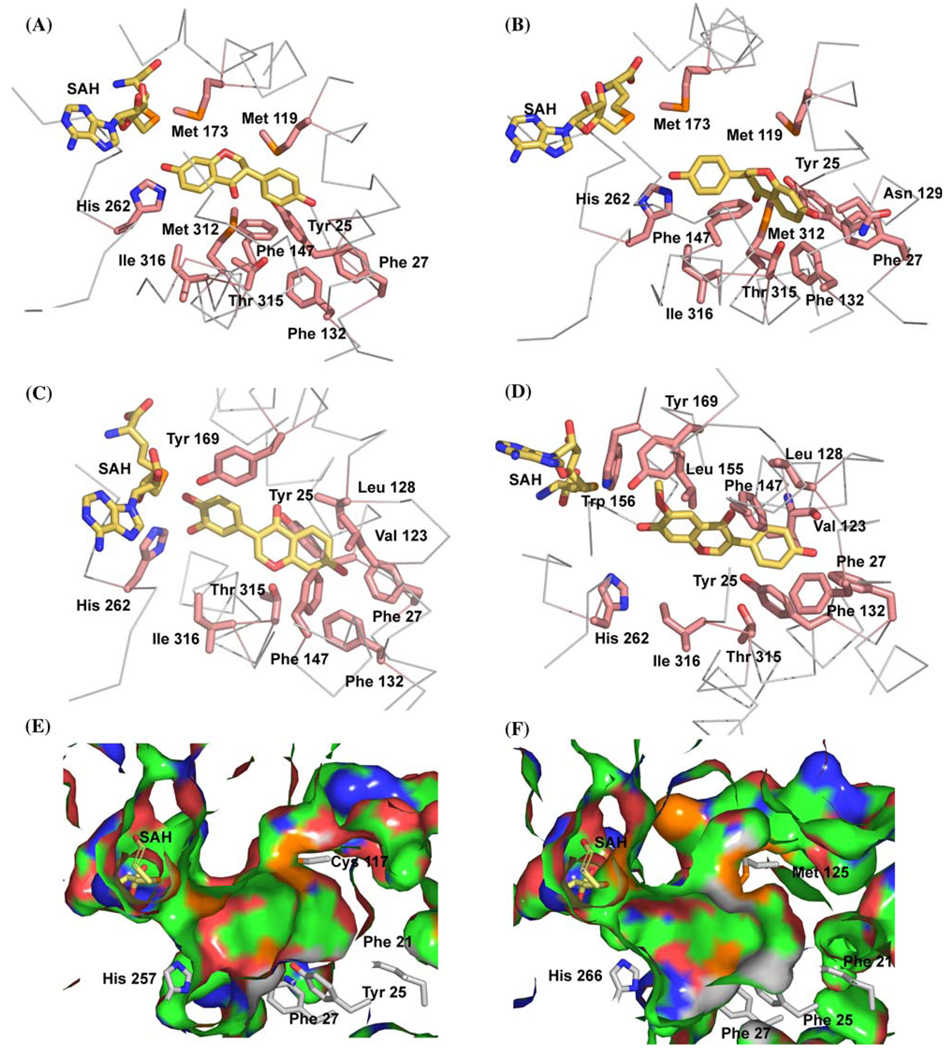
To gain a preliminary insight into the structural basis of IOMT substrate specificity, homology modeling of MtIOMTs from the 7-OMT clade was performed using the previously solved structure of MsI7OMT complexed with isoformononetin (PDB: 1FP2) (Zubieta et al. 2001). Homology modeling was performed using the program MODELLER. The quality of models was evaluated by PROCHECK and visualized using the O program. Subsequently, isoflavonoid substrates were docked using the GOLD program.
Superimposition of the modeled MtIOMT1 structure with that of MsI7OMT revealed that the active site of MtIOMT1 is nearly identical to that of MsI7OMT, consistent with the virtually identical activities of these two enzymes determined using in vitro assays (Table 2). Only one amino acid difference is found in the active site of the MtIOMT1 model; Tyr 25, which forms the back wall of the active site and is contributed from the dyad related monomer, is replaced by a conservative Phe substitution.
In the active site of the MtIOMT2 model, Tyr 127, Asn310, Met311 and Phe150 in MsI7OMT are replaced by Phe132, Thr315, Ile316, and Leu155, respectively (Fig. 6A, B). In total, these changes result in a significant increase in the predicted volume of the substrate binding pocket of MtIOMT2, particularly in the bottom part of the cavity. Furthermore, replacement of Met311 of MsIOMT7 by Ile316 in MtIOMT2 may alter the hydrophobic packing and thioether-mediated interaction of the side chain at this position with the bound substrate/product found experimentally in MsI7OMT complexed with isoformononetin (9) (Zubieta et al. 2001). These structural alterations would be predicted to allow for greater flexibility in substrate positioning in the active site of MtIOMT2 compared with that of MsIOMT7.
Fig. 6.
Homology models of selected members of the MtI7OMT clade. (A, B) Close-up view of the MtIOMT2 active site with dihydrodaidzein docked for 7-O-methylation (A) or 4-O-methylation (B). (C, D) View of 7,3′,4′-trihydroxyisoflavone (C) and glycitein (D) docked into the MtIOMT3 active site. (E, F) Molecular surface views of the experimentally determined MsI7OMT active site (E) and the modeled MtIOMT4 active site (F)
To test this hypothesis, dihydrodaidzein, a substrate that is methylated at either the 7 or 4′-positions in vitro (Table S2), was docked into the active site of the MtIOMT2 homology model by orienting either the 7-or 4′-hydroxy group toward the catalytic general base, His 262, and the reactive methyl group of SAM (Fig. 6A, B). When dihydrodaidzein is oriented for 7-O-methylation, the binding pattern is comparable to that determined experimentally for isoformononetin in the MsI7OMT structure; moreover, the enlarged binding pocket in MtIOMT2 compensates for the more bent isoflavanone conformation (Fig. 6A). This enlarged binding cavity also allows dihydrodaidzein to be docked with its 4′-hydroxyl moiety pointing toward the catalytic His and reactive SAM methyl donor (Fig. 6B). In particular, substitution of Asn 310 and Tyr 127 in MsI7OMT for Thr 315 and Phe 132 respectively in MtIOMT2 create the necessary volume in the binding cavity to accommodate the planes formed by the A- and C-rings of dihydrodaidzein. The edge to face van der Waal’s interactions of the side chain of Phe 147 with the plane formed by the A- and C-rings of the modeled substrate may further accommodate the substrate, while the amide moiety of Asn 129 and the phenolic group of Tyr 25 can potentially form hydrogen bonds with the 7-hydroxyl moiety and the oxygen atom of the ketone group of dihydrodaidzein. Finally, Met 119, Met 173 and Met 312 form a three pronged clamp loosely packing around the modeled substrate molecule (Fig. 6B).
In the active site model of MtIOMT3, Phe 164 in MsI7OMT is changed to Tyr 169 in MtIOMT3 while the diagonally arranged residue, Tyr 127 in MsI7OMT, is replaced by Phe 132 in MtIOMT3 (Fig 6c, d). These substitutions in MtOMT3 diagonally alter the architecture and hydrophobic environment of the active site. As seen in the MtIOMT2 homology model, several active site substitutions increase the volume of the phenolic binding pocket, which may offer an explanation for the ability of MtIOMT3 to methylate at the 7 or 4′-positions. When 7,3′,4′-trihydroxyisoflavone is docked into the active site model of MtIOMT3, the 4′-hydroxyl moiety is placed proximal to the putative catalytic base, His 262, and oriented towards the reactive methyl group of SAM; the 3′-hydroxyl group projects into the extra space created by the substitution of Met 311 in MsIOMT7 by Ile 316 in MtIOMT3. Finally, the A-ring 7-hydroxyl moiety inserts into the small cavity formed by Phe 132, Phe 147, Leu 128, Val 123, Tyr 25 and Phe 27, the latter two residues being contributed from the dyad related monomer (Fig. 6C).
When glycitein is docked into the active site of MtIOMT3, the energetically favorable orientation of the compound results in its being flipped horizontally and vertically relative to 7,3′,4′-trihydroxyisoflavone, with the 6-methoxy moiety of the compound pointing into the hole formed by Leu 155, Trp 259, Tyr 169, and Leu 173 (Fig. 6D). Hydrophobic interactions with the 6-methoxyl moiety within this apolar crevice serve to lock down the bound conformation of glycitein modeled into MtIOMT3’s active site pocket.
The active site residues of the modeled MtIOMT4 structure are notably different across the entire active site from those observed in MsI7OMT. These large scale changes likely result in extensive architectural changes of the phenolic substrate binding pocket relative to that of MsIOMT7 (Fig. 6E, F), and most isoflavones examined computationally for docking were unable to be reasonably fitted into the active site model of MtIOMT4. In addition, amino acid residues that potentially contribute to hydrogen bond formation in MsI7OMT (including Asn 310, Cys 117, Cys 313 and the neighboring Tyr 25) are not present in MtIOMT4.
Expression analysis of MtIOMT genes
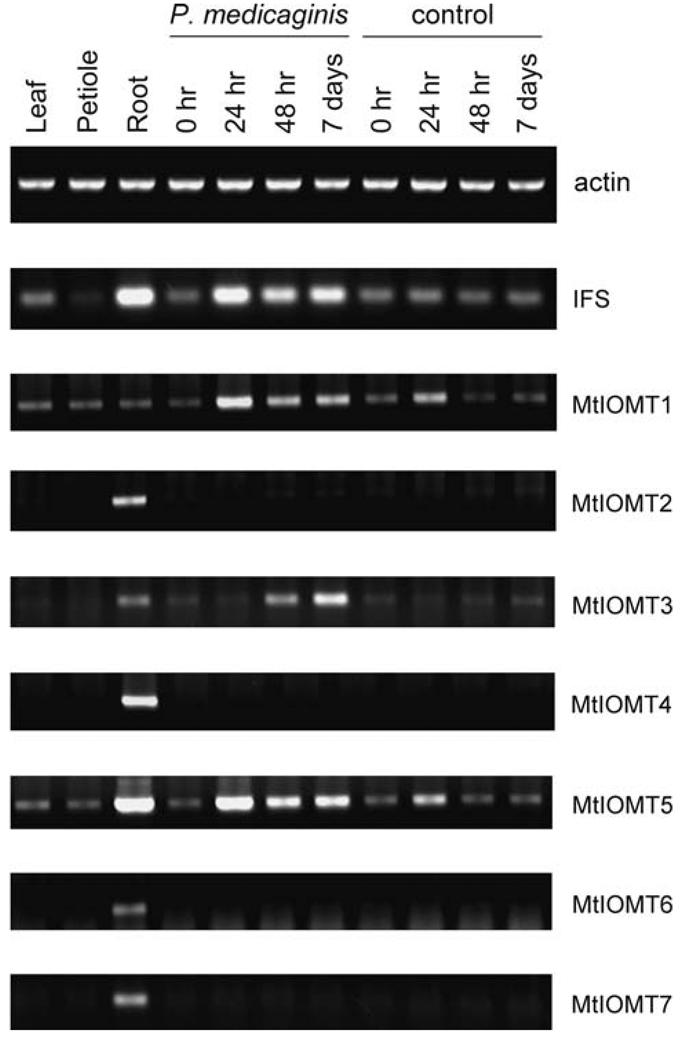
The expression patterns of MtIOMT genes in M. truncatula were examined by RT-PCR using primers specific for each gene (Table S1, Fig. 7). Most MtIOMTs, except MtIOMT1 and MtIOMT5, are primarily expressed in the roots of M. truncatula. MtIOMT1 has comparably low transcript levels in leaf, petiole, and root tissue. In addition to a significant level in root tissue, MtIOMT5 transcripts are also detectable in leaf and petiole.
Fig. 7.
Expression of MtIOMTs in healthy tissues of M. truncatula and in leaves infected with the leaf pathogen P. medicaginis. MtIOMT expression was determined by RT-PCR using primers specific for each MtIOMT. IFS, isoflavone synthase
The fungal leaf spot pathogen Phoma medicaginis is a known inducer of isoflavonoid biosynthesis in alfalfa and causes rapid increases in transcripts encoding isoflavonoid biosynthetic enzymes including MsI7OMT (Paiva et al. 1994; He and Dixon, 2000). Increased expression of three MtIOMTs is observed in compatible interactions following inoculation of M. truncatula with P. medicaginis. MtIOMT1 and MtIOMT5 exhibit a similar induction profile to that of 2-hydroxyisoflavanone synthase (isoflavone synthase; IFS), with maximal transcript levels appearing 14–24 h post-inoculation (Fig. 7). MtIOMT3 is also induced, although transcripts first appear 48 h post-inoculation.
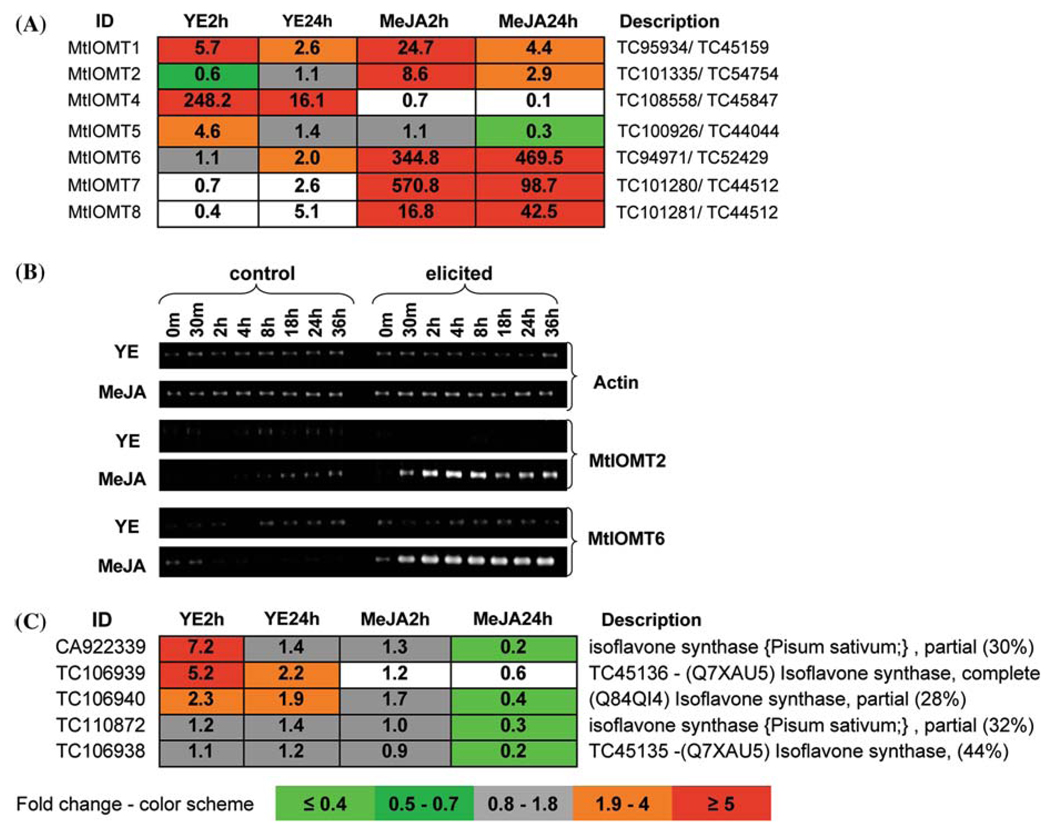
Differential induction of MtIOMTs in response to elicitation was also observed in M. truncatula cell cultures treated with yeast elicitor (YE) or methyl jasmonate (MeJA), which induce different global patterns of genetic re-programming (Suzuki et al. 2005). Changes in IOMT transcripts in elicited cells were inferred from microarray data generated utilizing Affymetrix Medicago genome arrays with 61 K probe sets (http://www.affymetrix.com). Transcript levels for IFS and the MtIOMT 1, 2, 4, 5, 6, 7 and 8 present on the Affymetrix arrays were compared at 2 and 24 h post-elicitation against corresponding control values (Fig. 8). MtIOMT4 was induced approximately 250-fold within 2 h of exposure to YE, but was down-regulated by MeJA. MtIOMT5 was induced 4.6-fold by YE, but not by MeJA. In contrast, MtIOMT6 and MtIOMT7 were very strongly induced by MeJA, but only very weakly by YE (Fig. 8A). RT-PCR analysis confirmed the lack of induction of MtIOMT2 or MtIOMT6 by YE, and their strong induction by MeJA (Fig. 8B).
Fig. 8.
Expression analysis of MtIOMT and IFS genes in elicitortreated cell suspension cultures of M. truncatula. (A) Microarray analysis of seven MtIOMT genes in response to YE and MeJA treatment. TC numbers from TIGR MtGI v. 8/v.5 are given under the description. (B) RT-PCR analysis confirming induction of MtIOMT2 and MtIOMT6 by MeJA. (C) Microarray analysis of IFS expression in response to YE and MeJA treatment. The TC number is from TIGR MtGI v. 8. Note: for (A) and (C), numbers represent ratios of treatment versus control samples. All ratios are coded according to the color scheme at the bottom of the figure. All color coded values had a “presence call:” by the GCOS program in the higher expressed or both sample groups. The non-coded values indicate absence of a “presence call:” in both control and treatment samples
MtIOMTs 6–8 are annotated as encoding 2,7,4′-trihydroxyisoflavanone 4′-OMTs. 2,7,4′-Trihydroxyisoflavanone 4′-OMT is proposed to exist as the central enzyme in a metabolon also comprising IFS and 2,7,4′-trihydroxyisoflavanone dehydratase (2HID) (Akashi et al. 2000, 2003). Array data show that several IFS genes were slightly or moderately induced by YE, but consistently reduced by MeJA 24 h after treatment (Fig. 8C). Based on the array data, only MtIOMT4 and MtIOMT5 have similar expression profiles to that of IFS.
Discussion
M. truncatula contains two distinct but related families of isoflavonoid OMT genes
By searching the available M. truncatula EST and genomic sequences we identified eight MtIOMTs with homology to either MsI7OMT or HI4′OMT enzymes. Members of the two groups of genes cluster together on different branches of the type I plant small molecule OMT family. Overall, the MtIOMTs showed a preference for isoflavonoid rather than flavonoid substrates and the phylogenetic analysis supports their classification as isoflavonoid OMTs. This clade of enzymes forms a separate cluster distinct from known flavonoid OMTs.
The close genomic organization of MtIOMTs 1–3, the fact that they share location of the single intron, and that this conserved intron junction (Schroder et al. 2004) neighbors critical catalytic residues, all suggest that the OMT genes may have arisen from ancient gene duplication events followed by evolution/adaptation. This concept is supported by the preference of MtIOMTs 1–3 for similar substrates.
Substrate specificities of Medicago IOMTs
MtIOMTs in the MsI7OMT clade prefer isoflavone substrates, which adopt a relatively flat conformation, while those in the HI4′OMT clade prefer substrates with chiral centers (isoflavanones or isoflavans) with a bent molecular conformation (Table 2). The ability of the isoflavanone dihydrodaidzein to adopt a relatively flat conformation as modeled in the structure of MtIOMT2 or a sharply bent conformation similar to that observed for 2,7,4′-trihydroxyisoflavanone in the structure of MtIOMT5 (data not shown; Liu et al. 2005) may explain why this compound could be methylated by both groups of MtIOMTs. The similar conformations of flavanones and isoflavanones, both with chiral centers at C2 and/or C3, may explain why MtIOMT5 and MtIOMT7 catalyzed methylation of liquiritigenin and naringenin, in addition to isoflavanone.
Recently, a 4′-OMT from Glycine max (SOMT-2) was reported to methylate daidzein (8), genistein, and naringenin (Kim et al. 2005). SOMT-2 is 67% identical to MsI7OMT and MtIOMT1 and only 48–53% identical to OMTs within the HI4′OMT clade. However, in contrast to the IOMTs characterized in the present report, which did not methylate flavone or flavonol substrates, SOMT-2 methylated apigenin and quercetin.
The presence of an intron at a conserved location within the C-terminal regions of OMTs (Schroder et al. 2004), close to residues involved in SAM binding and catalysis, suggests that variable splicing at intron–exon junctions may contribute to the diversification of OMT substrate-binding and catalysis during the evolution and selection of related plant OMTs.
Structural basis for IOMT substrate specificity
The most frequent substitutions of active site residues in the MtI7OMT clade of plant OMTs were at those amino acid positions forming the bottom and back wall of the substrate binding pocket. These include Tyr 25, Asn 310, Met 311, and Tyr 127 (numbered as in MsI7OMT), which were shown previously to be critical residues for either hydrogen bond formation or van der Waal’s interactions with phenolic substrates in the structure of MsI7OMT complexed with isoformononetin (9). Substitutions of these residues in MtIOMT2 resulted in a change in the shape of the MtIOMT2 binding pocket leading to fewer spatial restraints on substrate recognition and binding, thus explaining the broader substrate- and regio-specificities of MtIOMT2 relative to MsI7OMT and MtIOMT1.
In the homology model of MtIOMT3, hypotheses regarding regio-specificity were governed by structural restraints imposed by the bottom half of the catalytic cavity that served to orient substrates with ortho-substituted groups for SAM-mediated methylation. Thus, when daidzein, which lacks ortho-substituted groups proximal to either the 7- or 4′-hydroxyl groups, was used as an in vitro substrate, both 7- and 4′-O-methylated products were observed.
The gross alteration in the shape of the substrate binding pocket of MtIOMT4 anticipated based upon the computed homology model suggests why this IOMT has only minimal activity with the substrates currently tested in vitro.
We have recently solved the structure of MtIOMT5 in an “open” conformation (Liu et al. 2005) but, due to the large distance between the phenolic substrate binding pocket, the SAM binding cleft, and the catalytic His in the solved structure, we were not able to reliably auto-dock the phenolic substrates to the structural model of other MtIOMTs within this clade. Additional structural data for the other HI4′OMT clade members is necessary for a more concrete understanding of their respective substrate specificities.
In the experimentally determined crystal structure of MtIOMT5 with 2,7,4′-trihydroxyisoflavanone (2), the 2-hydroxyl group forms hydrogen bonds with the side chain of Tyr 25 and a neighboring water molecule that serves to anchor the substrate in the binding cavity (Liu et al. 2005). The importance of this 2-hydroxyl moiety in limiting methylation to the 4′-position was confirmed by the presence of 4′-O-methyl, 7-O-methyl, and 7,4′-di-O-methylated products in reactions with dihydrodaidzein (7,4′-dihydroxyisoflavanone), which lacks a 2-hydroxyl group, while only 4′-O-methylated product is observed in reactions with 2,7,4′-trihydroxyisoflavanone (Figures S1i, j).
The discovery of the 2,7,4′-trihydroxyisoflavanone OMT led to a new hypothesis for the biosynthesis of 4′-O-methylated isoflavonoids (Akashi et al. 2003), and strongly questioned the earlier hypothesis for 4′-O-methylation of 2,7,4′-trihydroxyisoflavanone by an enzyme that also exhibited 7-OMT activity in vitro (Liu and Dixon 2001). Several of the enzymes currently studied exhibited both 7- and 4′-OMT activities, depending on the substrate; however, only MtIOMT1 (7-position specific for isoflavones) and MtIOMT5 (4′-specific for 2-hydroxyisoflavanone) were strongly co-induced with IFS based on RT-PCR results. Microarray data also confirmed the co-expression pattern of MtIOMT5 with IFS genes. The potential physiological function of the dual regiospecificity of MtIOMTs 2–6 with alternative substrates (e.g. dihydrodaidzein) remains unclear.
Annotation of MtIOMTs based on transcript expression profiling
The expression patterns of MtIOMT gene family members, either in healthy or infected plant tissues, or in cell cultures responding to yeast elicitor or methyl jasmonate, provided strong indirect evidence for or against specific gene annotations. For example, MtIOMT5 was co-expressed with IFS in all tissues/ treatments studied, consistent with its functioning as a true 2,7,4′-trihydroxyisoflavanone OMT, a conclusion corroborated by its substrate preference in vitro. However, the closely related MtIOMT6 gene, although expressed in healthy roots, was not co-expressed with IFS; indeed, MeJA massively induced MtIOMT6 and MtIOMT7 but down-regulated IFS genes. Even though MtIOMT6 is 97% identical to true 2,7,4′-trihydroxyisoflavanone OMT, this annotation would appear to be incorrect, as further indicated by in vitro analysis.
MtIOMT1 and MtIOMT3 are induced in Phoma-infected leaves. MtIOMT1 is also induced by yeast elicitor and MeJA in cell cultures. In contrast, MtIOMT4 is massively induced by YE (nearly 250-fold) but down-regulated by MeJA. Clearly, these four genes annotated as isoflavone 7-OMTs most likely encode enzymes with different biochemical functions. Unfortunately, analysis of substrate specificities in vitro fails to clarify these functions in relation to the known natural products of M. truncatula (see below).
In vivo physiological roles of Medicago IOMTs in relation to metabolite composition
Several studies have documented the (iso)flavonoid constituents of Medicago truncatula and related species. These include early studies of intact plant tissues (Bisby et al. 1994), and more recent in-depth metabolomic studies of yeast- and MeJA-elicited cell suspension cultures (Suzuki et al. 2005; M. Farag et al. submitted) or Phoma-infected leaves (Deavours and Dixon 2005) (Fig. 1). Based on such phytochemical analysis, and the present data on in vitro substrate specificities and expression patterns of putative IOMTs, several conclusions can be made.
First, the 4′-O-methyl group of formononetin (4) and compounds derived therefrom (such as medicarpin (5)), originates via IOMT5 (a true 2,7,4′-trihydroxyisoflavanone OMT) in Medicago. 7-Methoxy, 4′,5,6-trihydroxyisoflavone (Fig. 1D, 14), although not yet detected in Medicago, is a potential precursor of irilone (16) and the corresponding tetrahydroxyisoflavone (13) is therefore a candidate substrate for I7OMTs, although the A-ring methylenedioxy group on irilone could also arise via a 6-O-methylated intermediate (Fig. 1D, 15). Assuming that the first methylation reaction of the isoflavone nucleus occurs immediately after aryl ring migration, catalyzed by MtIOMT5, formation of alfalone (Fig. 1C, 11) and afromosin (12) would likely require subsequent OMTs active with 6,7-dihydroxy, 4′-methoxyisoflavone (10). Interestingly, none of the enzymes analyzed in the present work possessed such specificity (Table 2), the presence of the 4′-methoxyl group blocking activity.
Formononetin (4) and medicarpin (5) conjugates occur in M. truncatula roots (Harrison and Dixon 1993). Infection of M. truncatula leaves with P. medicaginis results in accumulation of the isoflavonoids coumestrol (non-methylated), afrormosin (12) and medicarpin (5) in addition to several unidentified compounds (M. Farag et al., submitted). Like MsI7OMT (He and Dixon 2000), MtIOMT1 is induced in response to fungal infection and its expression pattern is nearly identical to that of MtIOMT5. However, the in vitro activity of MtIOMT1 is inconsistent with a role in the biosynthesis of formononetin and/or medicarpin. Microarray data also showed that transcription patterns of MtIOMT1 and MtIOMT5 are different in response to YE and MeJA treatment. MtIOMT1 may be involved in the biosynthesis of other, unidentified compounds that were observed to accumulate in P. medicaginis infected leaves. It is also possible that the product of MtIOMT1 activity does not accumulate to significant levels or that MtIOMT1 may have a signaling, rather than a biosynthetic role.
The phytoalexin sativan (7-hydroxy, 2′,4′-dimethoxyisoflavan, 7) has been reported in M. truncatula (Bisby et al. 1994) and is likely formed by 2′-O-methylation of vestitol (6). MtIOMT2, MtIOMT4, MtIOMT5, MtIOMT6, and MtIOMT7 all methylated vestitol in vitro, although we were not able to confirm the position of methylation.
Based on their reported occurrence in M.sativa and/or M. truncatula, other potential products of MtIOMT activity include the coumestans sativol (7-methoxy, all numbering is relative to isoflavone), wairol (6′-methoxy), 7,12-dihydroxy-11-methoxycoumestan (5′-methoxy), the isoflavans 7-hydroxy-2′,3′,4′-trimethoxyisoflavan (2′,3′,4′-trimethoxy)and 5′-methoxysativan(5′-methoxy),and the pterocarpans 4-methoxymedicarpin (6-methoxy) and methylnissolin (3-methoxy) (Bisby et al. 1994). Due to the unavailability of appropriate substrates we were not able to determine whether any of the characterized MtIOMTs are potentially involved in the synthesis of these compounds.
On the basis of specific activity against the “best” in vitro substrate (Table 2), the seven MtIOMTs and MsI7OMT fell into three groups. MtIOMTs 1 and 5, and MsIOM7, exhibited high activities (2,742– 4,189 pkat/mg protein), MtIOMTs 2 and 9 had intermediate activities (930 and 198 pkat/mg, respectively), whereas MtIOMTs 3, 4 and 6 were poor catalysts (only 2–7 pkat/mg). Although we can not rule out the possibility of poor stability or incorrect folding of MtIOMTs 3, 4 and 6 when expressed in vitro, the results highlight the possibility that alternative substrates may be used by these enzymes in vivo.
All MtIOMTs were expressed in the roots of M. truncatula. Root secreted isoflavonoids are known signaling molecules in the interaction of legumes with the rhizosphere, mediating both positive and negative interactions with symbiotic and pathogenic microorganisms (Phillips and Kapulnik 1995). An extensive metabolite profile of M. truncatula roots or root exudates has not been reported. Further evidence of the role of MtIOMTs in isoflavonoid biosynthesis awaits reverse genetic approaches coupled with extensive metabolic profiling to include secreted compounds.
In summary, the present in-depth analysis of putative isoflavonoid methylating enzymes from M. truncatula highlights the extreme caution that must be applied to interpretation of gene annotations based on sequence alone. A further difficulty for annotation is suggested by the observation that, at least in vitro, some plant small molecule OMTs may form heterodimers with altered substrate preferences and specificities (Frick et al. 2001); it is unclear whether such a phenomenon can occur in vivo. Irrespective of their in vivo function in the plant, the genes described here have potential value as reagents for metabolic engineering, providing a means for regiospecific introduction of methyl groups to (iso)flavonoid skeletons to generate novel bioactive compounds.
Supplementary Material
Acknowledgements
We thank Drs Luis Marquez and Marilyn Roossinck for help with PAUP analysis, and Drs Xiaoqiang Wang and Luzia Modolo for critical reading of the manuscript. This work was supported by grants from the Oklahoma Center for the Advancement of Science and Technology Health Sciences Program to RAD, the National Science Foundation under Grant No. 0236027 to JPN and under Grant No. DBI 0109732 to RAD, and a Noble Foundation Postdoctoral Fellowship at the Salk Institute and a Laboratory Directed Research and Development Award (LDRD) at Brookhaven National Laboratory under contract with U.S. Department of Energy to C-JL. JPN is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- EST
expressed sequence tag
- IFS
isoflavone synthase
- MeJA
methyl jasmonate
- OMT
O-methyltransferase
- TC
tentative consensus
- YE
yeast elicitor
Footnotes
Electronic Supplementary Material Supplementary material is available to authorised users in the online version of this article at http://dx.doi.org/10.1007/s11103-006-9050-x.
Contributor Information
Bettina E. Deavours, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA Department of Biology, Colorado State University, Fort Collins, CO 80523, USA.
Chang-Jun Liu, Howard Hughes Medical Institute, Jack Skirball Chemical Biology and Proteomics Laboratory, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92036, USA; Biology Department, Brookhaven National Laboratory, Upton, NY 11973, USA.
Marina A. Naoumkina, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA
Yuhong Tang, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA.
Mohamed A. Farag, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA
Lloyd W. Sumner, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA
Joseph P. Noel, Howard Hughes Medical Institute, Jack Skirball Chemical Biology and Proteomics Laboratory, The Salk Institute for Biological Studies, 10010 N. Torrey Pines Road, La Jolla, CA 92036, USA
Richard A. Dixon, Email: radixon@noble.org, Plant Biology Division, Samuel Roberts Noble Foundation, 2510 Sam Noble Parkway, Ardmore, OK 73401, USA.
References
- Akashi T, Sawada Y, Aoki T, Ayabe S-I. New scheme of the biosynthesis of formononetin involving 2,7,4′-trihydroxyisoflavanone but not daidzein as the methyl acceptor. Biosci Biotechnol Biochem. 2000;64:2276–2279. doi: 10.1271/bbb.64.2276. [DOI] [PubMed] [Google Scholar]
- Akashi T, Sawada Y, Shimada H, Sakurai N, Aoki T, Ayabe S. cDNA cloning and biochemical characterization of S-adenosyl-L-methionine: 2,7,4′-trihydroxyisoflavanone 4′-O-methyltransferase, a critical enzyme of the legume isoflavonoid phytoalexin pathway. Plant Cell Physiol. 2003;44:103–112. doi: 10.1093/pcp/pcg034. [DOI] [PubMed] [Google Scholar]
- Bisby FA, Buckingham J, Harborne JB, editors. Plants and their constituents. New York: Chapman and Hall; 1994. Phytochemical dictionary of the Leguminosae, Vol I. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deavours BE, Dixon RA. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol. 2005;138:2245–2259. doi: 10.1104/pp.105.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Steele CL. Flavonoids and isoflavonoids- a gold mine for metabolic engineering. Trends Plant Sci. 1999;4:394–400. doi: 10.1016/s1360-1385(99)01471-5. [DOI] [PubMed] [Google Scholar]
- Edwards R, Dixon RA. Isoflavone O-methyltransferase activities in elicitor-treated cell suspension cultures of Medicago sativa. Phytochemistry. 1991;30:2597–2606. [Google Scholar]
- Ferrer J-L, Zubieta C, Dixon RA, Noel JP. Crystal structure of alfalfa caffeoyl coenzyme A 3-O-methyltransferase. Plant Physiol. 2005;137:1009–1017. doi: 10.1104/pp.104.048751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick S, Kutchan TM. Molecular cloning and functional expression of O-methyltransferases common to isoquinoline alkaloid and phenylpropanoid biosynthesis. Plant J. 1999;17:329–339. doi: 10.1046/j.1365-313x.1999.00379.x. [DOI] [PubMed] [Google Scholar]
- Frick S, Ounaroon A, Kutchan TM. Combinatorial biochemistry in plants: the case of O-methyltransferases. Phytochemistry. 2001;56:1–4. doi: 10.1016/s0031-9422(00)00378-2. [DOI] [PubMed] [Google Scholar]
- Gang DR, Lavid N, Zubieta C, Chen F, Beuerle T, Lewinsohn E, Noel JP, Pichersky E. Characterization of phenylpropene O-methyltransferases from sweet basil: facile change of substrate specificity and convergent evolution within a plant O-methyltransferase family. Plant Cell. 2002;14:505–519. doi: 10.1105/tpc.010327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harborne JB, editor. The Flavonoids, advances in research since 1986. London: Chapman & Hall; 1994. [Google Scholar]
- Harrison MJ, Dixon RA. Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol Plant-Microbe Interact. 1993;6:643–654. [Google Scholar]
- He X-Z, Dixon RA. Affinity chromatography, substrate/ product specificity and amino acid sequence analysis of an isoflavone O-methyltransferase from alfalfa (Medicago sativa L.) Arch Biochem Biophys. 1996;336:121–129. doi: 10.1006/abbi.1996.0539. [DOI] [PubMed] [Google Scholar]
- He X-Z, Dixon RA. Genetic manipulation of isoflavone 7-O-methyltransferase enhances the biosynthesis of 4′-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa. Plant Cell. 2000;12:1689–1702. doi: 10.1105/tpc.12.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X-Z, Reddy JT, Dixon RA. Stress responses in alfalfa (Medicago sativa L.) XXII. cDNA cloning and characterization of an elicitor-inducible isoflavone 7-O-methyltransferase. Plant Mol Biol. 1998;36:43–54. doi: 10.1023/a:1005938121453. [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Maury S, Bergdoll M, Thion L, Erard M, Legrand M. Identification of the enzymatic active site of tobacco caffeoyl-coenzyme A O-methyltransferase by site-directed mutagenesis. J Biol Chem. 2001;276:36831–36838. doi: 10.1074/jbc.M104977200. [DOI] [PubMed] [Google Scholar]
- Ibrahim RK, De Luca V, Khouri H, Latchinian L, Brisson L, Charest PM. Enzymology and compartmentation of polymethylated flavonol glucosides in Chrysosplenium americanum. Phytochemistry. 1987;26:1237–1245. [Google Scholar]
- Jones TA, Zou J-Y, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta. Crystallogr. 1991;A49:148–157. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Joshi CP, Chiang VL. Conserved sequence motifs in plant S-adenosyl-L-methionine-dependent methyltransferases. Plant Mol.Biol. 1998;37:663–674. doi: 10.1023/a:1006035210889. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim B-G, Lee Y, Ryu JY, Lim Y, Hur H-G, Ahn JH. Regiospecific methylation of naringenin to ponciretin by soybean O-methyltransferase expressed in Escherichia coli. J Biotech. 2005;119:155–162. doi: 10.1016/j.jbiotec.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kornblatt J, Muzac I, Lim Y, Ahn JH, Ibrahim RK. Role of serine 286 in cosubstrate binding and catalysis of a flavonol O-methyltransferase. Biochem. Cell Biol. 2004;82:531–537. doi: 10.1139/o04-054. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, MacArthur MW, Moss DS, Throrton JM. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993;26:283–291. [Google Scholar]
- Liu C-J, Deavours BE, Richard SB, Ferrer J-L, Dixon RA, Noel JP. Dual functionality of isoflavonoid O-methyltransferases in the evolution of plant defense responses. Plant Cell (in review) 2005 doi: 10.1105/tpc.106.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-J, Dixon RA. Elicitor-induced association of isoflavone O-methyltransferase with endomembranes prevents formation and 7-O-methylation of daidzein during isoflavonoid phytoalexin biosynthesis. Plant Cell. 2001;13:2643–2658. doi: 10.1105/tpc.010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Renom MA, Stuart A, Fiser A, Sanchez R, Melo F, Sali A. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- Maxwell CA, Harrison MJ, Dixon RA. Molecular characterization and expression of alfalfa isoliquiritigenin 2′-O-methyltransferase, an enzyme specifically involved in the biosynthesis of an inducer of Rhizobium meliloti nodulation genes. Plant J. 1993;4:971–981. doi: 10.1046/j.1365-313x.1993.04060971.x. [DOI] [PubMed] [Google Scholar]
- Noel JP, Dixon RA, Pichersky E, Zubieta C, Ferrer J-L. Structural, functional, and evolutionary basis for methylation of plant small molecules. Rec Adv Phytochemistry. 2003;37:37–58. [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. In: Carter CWJ, Sweet RM, editors. Methods in enzymology: macromolecular crystallography, part A. Vol 276. New York: Academic Press; 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- Paiva NL, Oommen A, Harrison MJ, Dixon RA. Regulation of isoflavonoid metabolism in alfalfa. Plant Cell Tissue Organ Cult. 1994;38:213–220. [Google Scholar]
- Phillips DA, Kapulnik Y. Plant isoflavonoids, pathogens and symbionts. Trends Microbiol. 1995;3:58–64. doi: 10.1016/s0966-842x(00)88876-9. [DOI] [PubMed] [Google Scholar]
- Preisig CL, Matthews DE, VanEtten HD. Purification and characterization of S-adenosyl-L-methionine:6a–hydroxymaackiain 3-O-methyltransferase from Pisum sativum. Plant Physiol. 1989;91:559–566. doi: 10.1104/pp.91.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder G, Wehinger E, Lukacin R, Wellmann F, Seefelder W, Schwab W, Schroder J. Flavonoid methylation: a novel 4′-O-methyltransferase from Catharanthus roseus, and evidence that partially methylated flavanones are substrates of four different flavonoid dioxygenases. Phytochemistry. 2004;65:1085–1094. doi: 10.1016/j.phytochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Schroder G, Wehinger E, Schroder J. Predicting the substrates of cloned plant O-methyltransferases. Phytochemistry. 2002;59:1–8. doi: 10.1016/s0031-9422(01)00421-6. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Reddy MSS, Naoumkina M, Aziz N, May GD, Huhman DV, Sumner LW, Blount JW, Mendes P, Dixon RA. Methyl jasmonate and yeast elicitor induce differential genetic and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta. 2005;220:698–707. doi: 10.1007/s00425-004-1387-2. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEtten HD, Matthews DE, Smith DA. Metabolism of phytoalexins. In: Bailey JA, Mansfield JW, editors. Phytoalexins. Glasgow: Blackie; 1982. pp. 180–217. [Google Scholar]
- Wengenmayer H, Ebel J, Grisebach H. Purification and properties of a S-adenosylmethionine: isoflavone 4′-O-methyltransferase from cell suspension cultures of Cicer arietinum L. Eur J Biochem. 1974;50:135–143. doi: 10.1111/j.1432-1033.1974.tb03881.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Ahn JH, Ibrahim RK, Lee S, Lim Y. The three-dimensional structure of Arabidopsis thaliana O-methyltransferase predicted by homology-based modeling. J. Mol Graph Model. 2004;23:77–87. doi: 10.1016/j.jmgm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Zubieta C, He X-Z, Dixon RA, Noel JP. Structures of two natural product methyltransferases reveal the basis for substrate specificity in plant O-methyltransferases. Nature Struct Biol. 2001;8:271–279. doi: 10.1038/85029. [DOI] [PubMed] [Google Scholar]
- Zubieta C, Kota P, Ferrer J-L, Dixon RA, Noel J. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell. 2002;14:1265–1277. doi: 10.1105/tpc.001412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.