Abstract
In recent decades, temporal patterns in SSB intake have shown a close parallel between the upsurge in obesity and rising levels of SSB consumption. SSBs are beverages that contain added caloric sweeteners such as sucrose, high-fructose corn syrup or fruit-juice concentrates, all of which result in similar metabolic effects. They include the full spectrum of soft drinks, carbonated soft drinks, fruitades, fruit drinks, sports drinks, energy and vitamin water drinks, sweetened iced tea, cordial, squashes, and lemonade, which collectively are the largest contributor to added sugar intake in the US. It has long been suspected that SSBs have an etiologic role in the obesity epidemic, however only recently have large epidemiological studies been able to quantify the relationship between SSB consumption and long-term weight-gain, type 2 diabetes (T2DM) and cardiovascular disease (CVD) risk. Experimental studies have provided important insight into potential underlying biological mechanisms. It is thought that SSBs contribute to weight gain in part by incomplete compensation for energy at subsequent meals following intake of liquid calories. They may also increase risk of T2DM and CVD as a contributor to a high dietary glycemic load leading to inflammation, insulin resistance and impaired β-cell function. Additional metabolic effects from the fructose fraction of these beverages may also promote accumulation of visceral adiposity, and increased hepatic de novo lipogenesis, and hypertension due to hyperuricemia. Consumption of SSBs should therefore be replaced by healthy alternatives such as water, to reduce risk of obesity and chronic diseases.
Introduction
In recent decades the world has seen an unprecedented rise in the prevalence of overweight and obesity as global shifts in diet and lifestyle increasingly promote positive energy balance. The World Health Organization’s latest projections indicate that globally in 2005 approximately 1.6 billion adults were overweight (BMI ≥ 25 kg/m2) and at least 400 million were obese (BMI ≥ 30 kg/m2), numbers which are projected to reach 2.3 billion and 700 million, by 20151. The percentage of overweight and obese adults in the US increased from 47% and 15% respectively in the late 1970’s to over 66% and 33% in 2005–2006, with the greatest proportion of increase observed in Non-Hispanic black and Mexican-American women 2. Of particular concern is the magnitude of increase occurring among children and adolescents. According to national survey data over the past 3 decades, the prevalence of obesity (sex-and age-specific BMI > 95th percentile), has more than doubled across all age groups and is currently 12.4 % in those aged 2–5 years, 17% in those aged 6–11 years and 17.6% in those aged 12–19 years3 (figure 1). These estimates are well above the objectives set for Healthy People 2010, which aim to reduce obesity prevalence to less than 15%4. Similar trajectories of increase are being seen across the globe to varying degrees depending on country, region and stage of epidemiologic transition, as many lower- income countries become increasingly urbanized5, 6. A recent pooling analysis from 106 countries indicates that overweight and obesity are indeed significant and increasing public health challenges in most regions of the world including India, China, South East Asia, the Pacific islands, Latin America, the Middle Eastern crescent, and sub-Saharan Africa7. The implications of obesity are far reaching, from both a health and economic standpoint. Excess bodyweight is the sixth most important risk factor contributing to the overall global burden of disease8. Epidemiologic studies indicate that overweight and obesity are important risk factors for of type 2 diabetes (T2DM), cardiovascular disease (CVD), several cancers and premature death8. In the US, health care costs attributable to obesity were estimated at $147 billion per year by 20089. Such excess costs could have serious repercussions for developing countries which must manage co-existing chronic and infectious disease.
Figure 1. Prevalence of Obesity* Among U.S. Children and Adolescents (Aged 2 – 19 Years). National Health and Nutrition Examination Surveys.

*Sex-and age-specific BMI > 95th percentile based on the CDC growth charts (ref 3)
CDC Data Sources:
Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among U.S. children and adolescents, 1999–2000. JAMA 2002;288:1728–1732.
Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 2004;291:2847–2850.
Ogden CL, Carroll MD, Flegal KM. High Body Mass Index for Age Among US Children and Adolescents, 2003–2006. JAMA 2008;299:2401–2405.
One of the most well established risk factors for T2DM is increased adiposity, particularly around the central depots. Examination of temporal trends in the US show a 10-year lag between the upsurge of obesity and rising rates of T2DM10. Nationally representative data from the US show that T2DM increased from 5.3 % between 1976–1980 to 12.6 % three decades later 9, 11, 12. Paralleling the global trends in obesity prevalence, T2DM has also emerged as a global public health concern. The International Diabetes Federation (IDF) estimated that in 2007, over 240 million people had T2DM worldwide and this number is projected to reach 380 million by 2025, at which time, 80% of the disease burden will be in low and middle income countries13. Asia plays a critical role in this epidemic as it houses some of the worlds’ most populous countries and risk of T2DM occurs at a younger age and lower BMI among individuals of Asian ancestry, compared to other ethnicities5. Certain Pacific Island populations are already facing prevalence rates as high as 40% 14. In the past, T2DM prevalence in sub-Saharan Africa was considered negligible. However, with 70% of the population expected to reside in urban areas by 2025, T2DM and other chronic diseases are rising rapidly 15. In 2007, the IDF estimated that 10.4 million people were living with T2DM in sub-Saharan Africa; a figure expected to reach 18.7 million by 2025, representing an 80% increase 13.
Against a backdrop of a worldwide pandemic of obesity and related chronic diseases, identification of modifiable risk factors for prevention efforts is paramount. For example the American Heart Association recently released a scientific statement recommending reductions in added sugars intake to no more than 100–150 kcal per day for most American women and men respectively, as a means of reducing cardiovascular disease (CVD) risk 16. The statement identified sugar sweetened beverages (SSB) as the primary source of added sugars in the American diet. SSBs which includes the full spectrum of soft drinks, fruit drinks, energy and vitamin water drinks are comprised of naturally derived caloric sweeteners such as sucrose, HFCS or fruit-juice concentrates, all of which have similar metabolic effects. Indeed a number of large scale epidemiological studies have found consistent positive associations between SSB consumption and long-term weight gain and risk of chronic diseases including metabolic syndrome (MetSyn), T2DM and CHD. SSB consumption is thought to lead to weight-gain because of the high added sugars content and low satiety of these beverages and incomplete compensation for total energy at subsequent meals following intake of liquid calories17. In addition, because of their high amounts of rapidly absorbable carbohydrates such as sucrose or high-fructose corn syrup (HFCS), coupled with the large quantities often consumed, SSB’s may increase risk of T2DM independent of obesity as a potential contributor to a high dietary glycemic load (GL) leading to inflammation, insulin resistance, and impaired beta-cell function 18–20. Fructose from any sugar or HFCS may further increase CVD risk by promoting dyslipidemia and deposition of visceral fat, possibly due to increased hepatic de novo lipogenesis and hypertension due to hyperuricemia14, 21 22. Here we discuss temporal trends in SSB intake, the epidemiological evidence linking SSB’s to increased risk for developing obesity, T2DM and MetSyn, other metabolic diseases and overall cardiovascular risk as well as consideration of potential underlying biological mechanisms.
Trends of SSB intake
Time-trend data over the past 3 decades has shown a close parallel between the obesity epidemic and rising levels of SSB consumption. Nationally representative estimates from the US show a steady increase in per capita calories from SSBs in both children and adults starting from the mid 1960’s (figure 2)23. At the same time, a decrease in calories consumed from milk, has taken place, particularly among children, while juice consumption has remained relatively stable across all age-groups. The most recent data show that children and adults consume about 172 and 175 kcal per day respectively from SSBs24. It has been estimated that percent of total daily calories from SSBs increased from 3.9% in the late 1970’s to 9.2% in 2001, representing more than a 2-fold increase in intake25. Particularly dramatic increases among individuals under age 40 years are generally driving these trends. Among those aged 2–19 years, SSB intake increased from 4.8% to 10.3% of total energy intake and among those aged 19–39 years intake increased from 5.1% to 12.3% of total energy intake25. Similar trends of increase have been shown in Mexico, where SSBs currently account for 10% of total energy intake26. Large per capita consumption has also been observed across Germany, Australia, Spain and Great Britain27.
Figure 2.

US Trends in Per Capita Calories from Beverages among children and adults.
A lack of large scale dietary intake data from a number of countries makes it difficult to examine global trends in SSB intake. However, food disappearance data show rapid increases in consumption of sugars across the globe, which have been particularly marked in China, India, Vietnam, Thailand, and other Southeast Asian countries27. Sales figures from coca cola’s 2007 annual report show that during 2007, India, and China experienced unit case volume growths of 14% and 18% respectively, which indicates substantial increases in sales at the consumer level28. Findings from small food consumption studies from various countries including South Africa, the Philippines and New Zealand are also indicative of high consumption levels29–31.
Epidemiologic evidence
Cross-sectional studies
A large number of cross-sectional studies have been conducted to evaluate the association between SSB intake and overweight or obesity. Malik and colleagues reviewed the literature until 200632. Of the 13 studies conducted among children and adolescents, the majority found significant positive associations or trends towards a positive association. Larger studies tended to show stronger more consistent results. For example findings from the Growing Up Today study (GUTs) which included > 10,000 children and adolescents from the US showed that in girls consumption of SSBs was associated with a 0.06 unit increase in BMI per serving (p=0.04).33 These findings are supported by those from combined NHANES surveys which showed that consumption of SSBs contributed a higher proportion of total energy in overweight compared to normal weight individuals34. Among studies conducted in adults, one found a greater probability of being overweight in subjects with higher SSB intake levels35 and the other observed that women who consumed SSBs regularly were 0.47 pounds heavier than non-consumers36. Few cross-sectional studies have evaluated metabolic consequences of SSB consumption but in cross-sectional analysis of Framingham Offspring data, the prevalence of MetSyn was significantly higher in soft drink consumers compared to non-consumers37.
Since cross-sectional studies usually evaluate the exposure and outcome at the same point in time they are not able to establish a temporal sequence and infer causality. They are also prone to intractable confounding, reverse causation, and recall bias. For these reasons cross-sectional studies have limited utility in chronic disease epidemiology outside of hypothesis generation. Instead, prospective cohort studies, which study a group of people over time, provide more robust evidence and are the most widely accepted method to capture long term diet and disease relationships. Short term experimental studies are not well-suited to capture long-term patterns since compliance tends to wane with increasing duration but they do provide important insight into potential underlying biological mechanisms.
Prospective cohort studies
Obesity
Findings from prospective cohort studies generally confirm those from cross-sectional data in that greater SSB consumption is positively associated with overweight and obesity. Despite a large degree of diversity between studies in terms of outcome assessment, number of participants and duration of follow-up, which can substantially impact ability to detect an effect, previous reviews and meta-analyses have found consistent results in both children and adults32, 38–40. Associations tend to be stronger in larger studies with longer durations of follow-up, that use longer-term dietary assessment methods such as food frequency questionnaires (FFQs) rather than a single 24-hour diet recall 41, 42. Recently, we conducted a meta-analysis evaluating change in BMI per increase in one 12-oz serving of SSB per day in children and found a clear positive association between SSB intake and weight-gain (0.08; 95% CI: 0.03, 0.13 kg)38 among studies that did not adjust for total energy intake 33, 41, 43–45. Since the association between SSB intake and weight gain is likely mediated in part by an increase in total energy intake, adjusting for total energy is expected to attenuate this effect. This bias was illustrated when by including studies that did adjust for total energy intake, our pooled effect estimate weakened and lost statistical significance (0.03; 95% CI: −0.00, 0.007). These results are also supported by more recent studies. For example, Dubois and colleagues found that in over 2000 Canadian children aged 2.5 y followed for 3 y, those who consumed SSBs between meals had a 2.4-fold greater odds of being overweight compared to non-consumers (p<0.05)46. In contrast, a recent study conducted among 5-year olds in the UK did not find an association between SSB intake and fatness, after 4 years of follow-up, possibly because intake levels were too low47. Studies have also shown that greater SSB consumption in childhood or adolescence predicted a trajectory of weight gain into adulthood48, 49.
Well powered studies in adults that do not adjust for the potential mediating effect of total energy intake also provide clear evidence for an effect of SSBs on weight gain. In a large cohort of over 50,000 nurses followed for 2 four-year time periods (1991–1995 and 1995–1999), those who consumed higher levels of SSBs gained more weight than those with lower intake levels50. After adjustment for potential confounders, women who increased their SSB consumption from 1991 to 1995 and maintained a high level (≥ 1 serv/d) of intake during 1995–1999 (low-high-high) gained on average 8.0 kg over the two time periods while women who decreased SSB intake between 1991 and 1995 and maintained a low level (≤ 1 serv/wk) of intake (high-low-low) gained on average 2.8 kg (Figure 3). Significant between group differences in weight gain were noted between women with low-high-high intake and low-high-low intake and between women with high-low-high intake and high-low-low intake (p=0.02). These findings were later replicated in the Black Women’s health study, another large cohort of over 40,000 women51. After 6 years of follow-up, and adjustment for potential confounding by other diet and lifestyle factors, those who increased their intake of SSBs from ≤ 1 serv/wk to ≥ 1 serv/d gained the most weight while those who decreased their intake gained the least weight (6.8 kg vs. 4.1 kg). Some smaller-scale studies, despite having limited power, have also found a link between habitual SSB consumption and weight gain either in the overall study or in sub-group analyses. For example in a cohort of over 17,000 adults from Germany with about 2 years of follow-up52 SSB intake was significantly associated with weight gain in men but not in women. One reason for the variable findings aside from possibly too short of a duration could be the small (100 g/d) increment of SSB used for estimating risk in these effect estimates. A smaller study from Spain with a similar duration of follow-up found that higher SSB consumption was associated with significant weight- gain among subjects who gained 3 or more kg in the five years before baseline53. Since these participants had a higher absolute intake of SSB at baseline compared to participants with no previous weight-gain, the findings are consistent with an effect of SSBs on weight-gain. In the Framingham Offspring study with over 4000 participants followed for an average of 4 years, those who consumed ≥ 1 soft drink per day had a 37% higher risk of developing obesity compared to non-consumers37. Although this analysis included both diet and regular soft drinks it can be assumed that most of the effect is due to consumption of SSBs. In an observational analysis of the PREMIER trial, 54 reducing SSB intake by 1 serv/d was associated with a weight loss of 0.49 kg (95% CI: 0.11, 0.82; P = 0.006) at 6 month and of 0.65 kg (95% CI: 0.22, 1.09; P = 0.003) at 18 month.
Figure 3. Mean weight in 1991, 1995, and 1999 according to trends in sugar-sweetened soft drink consumption in 1,969 women who changed consumption between 1991 and 1995 and either changed or maintained level of consumption until 1999*.
* Low and high intakes were defined as ≤1/week and ≥1/day. The number of subjects were: low-high-high=323, low-high-low=461, high-low-high=110, and high-low-low=746. Groups with similar intake in 1991 and 1995 were combined for estimates for these time points. Means were adjusted for age, alcohol intake, physical activity, smoking, postmenopausal hormone use, oral contraceptive use, cereal fiber intake, and total fat intake at each time point. Modified from ref 50.
Type 2 Diabetes and Metabolic Syndrome (MetSyn)
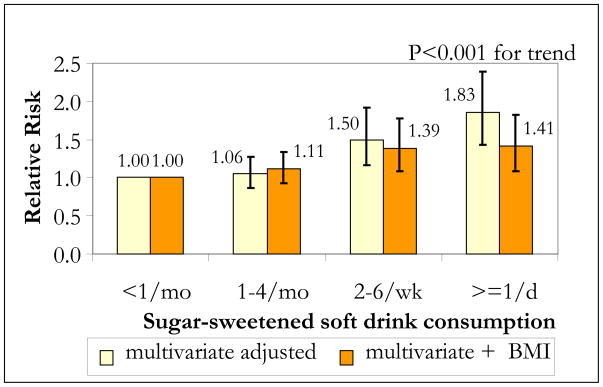
When considering diet in relation to chronic disease etiology, sufficient time is required for causal action and disease initiation and detection to occur. For this reason greater weight should be given to larger studies with longer durations of follow-up. In addition, since adjusting for potential mediators of effect such as BMI or total energy intake will tend to underestimate the association between SSB intake and these outcomes, greater weight should be given to studies that do not adjust for such factors in primary analyses. In the NHS II, a cohort of over 50,000 women, those who consumed ≥ 1 SSB per day had an 83% greater risk of developing T2DM over the course of 8 years compared to those who consumed < 1 per month after adjusting for potential confounders (RR=1.85, 95% CI: 1.42, 2.36; p<0.001 for trend)50 (figure 4). After further adjustment for BMI, the RR comparing extreme categories of intake decreased to 1.41 (95% CI: 1.09, 1.83; p<0.001 for trend), but was still statistically significant, suggesting that BMI accounts for about half of the excess risk50. Similar results were reported in the Black Women’s Health study. Among over 40,000 women followed for 10 years, those who consumed ≥ 2 SSBs per day had a 24% greater risk of developing T2DM compared to those who consumed < 1 SSB per month (RR=1.24, 95% CI: 1.06, 1.45; p=0.002 for trend)51. Additional adjustment for BMI resulted in a loss of statistical significance, suggesting that in this population, the majority of effect was mediated by BMI. In a large cohort of slightly older nurses, daily SSB consumption was associated with a 31% greater risk of developing T2DM compared to infrequent consumption (RR=1.31, 95% CI: 0.99, 1.74; p<0.01 for trend)55. Since this study adjusted for BMI and total energy intake, the effect estimate may have been attenuated. Other smaller studies, which also adjusted for these factors found marginal effects or no association56, 57. The Atherosclerosis Risk in Communities Study did not show a consistent association between SSB intake and incident T2DM after 9 years of follow-up (Men: RR=1.09 (95% CI, 0.89, 1.33), Women RR= 1.17 (95% CI 0.94, 1.46)58. As discussed by the authors, compared to the study by Schulze50, these participants were older (53.6 y vs. 36.1 y) and heavier (27.2 kg/m2 vs. 24.6 kg/m2) at baseline. Since the effect of SSBs on T2DM is partly mediated by BMI, once BMI is increased, it is possible that the additional effect of continued SSB intake is diminished, however, further research is needed to substantiate this hypothesis.
Figure 4. Multivariate relative risks (RRs) of type 2 diabetes according to sugar-sweetened soft drink consumption in the Nurses’ Health Study II 1991–1999*.
*Multivariate RRs were adjusted for age, alcohol (0, 0.1–4.9, 5.0–9.9, 10+ g/d), physical activity (quintiles), family history of diabetes, smoking (never, past, current), postmenopausal hormone use (never, ever), oral contraceptive use (never, past, current), intake (quintiles) of cereal fiber, magnesium, trans fat, polyunsaturated:saturated fat, and consumption of sugar-sweetened soft drinks, diet soft drinks, fruit juice, and fruit punch (other than the main exposure, depending on model). The data were based on ref 50)
Few studies have evaluated the relationship between intake of SSBs and development of MetSyn. Dhingra and colleagues showed that in a cohort of over 6000 adults, those who consumed ≥1 soft drink (diet or regular) per day had a 39% greater risk of developing MetSyn over the course of 4 years37. Other studies have found only marginal effects of SSBs on MetSyn, but since they adjusted for total energy intake, the associations are likely to have been underestimated57, 59.
Hypertension, lipids, inflammation, CHD
Evidence is starting to emerge that also links SSB consumption to the development of hypertension, adverse lipid parameters, inflammation and clinical CHD. The study by Dhingra et al., which also looked at SSB consumption in relation to individual MetSyn components found that individuals who consumed ≥1 soft drink per day had a 22% greater risk of developing hypertension (≥ 135/85 mm hg or on treatment) compared to non-consumers (RR=1.22, 95% CI: 1.05, 1.41)37. Results from the MESA study which had relatively fewer participants (N=3878), found a marginal effect of SSB consumption on incident hypertension (RR=1.14, 95% CI: 0.91, 1.43, comparing daily consumers to non-consumers.57 These findings are supported by similar associations observed in the NHS and NHS II cohorts where women who consumed ≥4 SSBs per day had a 44% (RR=1.44, 95% CI: 0.98, 2.11) and 28% (RR=1.28, 95% CI: 1.01, 1.62) greater risk of developing hypertension respectively, compared to infrequent consumers60. In addition, a recent cross-sectional analysis of NHANES data, found a positive association between SSB consumption and blood pressure in adolescents14. Regarding lipid parameters, Dhingra and colleagues found that daily soft drink consumers had a 22% greater risk of developing hypertriglyceridemia (≥ 1.7 mmol/L or on treatment) (RR=1.22, 95% CI: 1.07, 1.41) and low HDL-cholesterol (<1.03 mmol/L for men and <1.3 mmol/L for women or on treatment) (RR=1.22, 95% CI: 1.04, 1.44), compared to non-consumers37. Similarly among MESA participants, daily SSB consumers had a 28% greater risk of developing hypertriglyceridemia (RR=1.28, 95% CI 1.02, 1.6) and low HDL cholesterol (RR=1.28, 95% CI 0.99, 1.64)57. Indirect evidence for an effect of SSBs on systemic inflammation comes from observational studies that have found positive associations between dietary patterns that are high in SSBs with markers of inflammation including CRP and TNFR261. A higher dietary glycemic load, to which SSB intake is a large contributor, has also been linked to elevated CRP levels62.
Recently in the NHS, a higher level of SSB intake was found to increase risk of developing clinical CHD (nonfatal myocardial infarction or fatal CHD)63. In over 88,000 women followed for 24 years, those who consumed ≥ 2 SSBs per day had a 35% greater risk of CHD compared to infrequent consumers, after adjusting for other unhealthy lifestyle factors (RR=1.35, 95% CI: 1.1, 1.7; p<0.01 for trend). Additional adjustment for potential mediating factors including BMI, total energy intake and incident T2DM attenuated the associations but they remained statistically significant, suggesting that the effect of SSBs is not entirely mediated by these factors.
Other Metabolic Diseases: Gout, Hyperuricemia, Gallstone and Renal disease
SBBs may also be associated with development of a number of other metabolic conditions. Findings from the Third NHANES show that serum uric acid levels correlate positively with SSB consumption and those who consumed 1–3 SSB’s per day had a 51% greater odds of developing hyperuricemia (serum uric acid level>7 mg/dl for men and >5.7 mg/dl for women) compared to non-consumers (p=0.003 for trend)64. Modest associations were also noted for orange juice (p= 0.005 for trend) but not diet soda (p=0.46 for trend). SSBs contain large amounts of fructose from added sugars, which is known to increase serum uric acid levels. This is supported by recent findings from NHANES which also showed that SSB consumption increases serum uric acid levels in adolescents14. Hyperuricemia is considered a precursor of gout, which is a common form of inflammatory arthritis arising from deposition of uric acid in articular cartilage21. A recent prospective cohort study in men followed for over 12 years found that consumption of SSBs is strongly associated with development of gout65. Men who consumed ≥ 2 SSBs per day had an 85% greater risk of developing gout compared to infrequent consumers (RR=1.85, 95% CI: 1.08, 3.16; p<0.001 for trend). No association was shown with diet soda. Both gout and hyperuricaemia are associated with hypertension, T2DM, MetSyn, kidney disease and CVD21.
SSB consumption, particularly that of cola, has also been associated with development of albuminuria, a marker for early kidney damage, formation of kidney stones and increased risk of chronic kidney disease66, 67. Findings from a case-control study showed that drinking ≥ 2 colas per day was associated with over a 2-fold increased risk of chronic kidney disease (OR=2.3, 95% CI 1.4, 3.7) compared to infrequent consumption. Similar results were noted for diet cola but not non-cola carbonated beverages67. In the NHS II cohort, sucrose consumption was associated with an increased risk of incident kidney stones68. Women in the highest quintile of sucrose intake had a 31% higher risk of developing kidney stones than those in the first quintile (RR=1.31, 95% CI: 1.07, 1.6). Observational studies have also found that higher intake of refined sugars such as sucrose and fructose and a high dietary glycemic load, of which SSBs is a large contributor, is associated with a higher frequency of gallstones69, 70. A gallstone is a solid mass that forms in the gallbladder from cholesterol, bilirubin and calcium salts, precipitated from bile. In most Western countries, cholesterol is the primary component of gallstones and increasing evidence suggests that the majority of causative factors are related to insulin resistance. While the association between refined sugars and GL and gallstones could be mediated in part by obesity, some data suggests an independent lithogenic effect69. Findings from the NHS and HPFS indicate that a high intake of carbohydrate, dietary glycemic load and glycemic index, significantly increase the risk of developing gallstone disease70, 71. Independent positive associations have also been observed for intakes of sucrose and fructose. It has been estimated that the equivalent of 40 grams of sugar per day doubles the risk of symptomatic gallstones72. Although no studies have directly evaluated the relationship between SSB consumption and gallstone formation, the high amounts of added sugars contained in these beverages and large contribution to a high dietary GL, suggest an etiologic link as one 12-oz serving of SSB contains on average 35–37.5 g of sugar.
Diet Soda
Although not the main focus of our review, some studies also evaluated the effect of diet soda consumption on the risk of developing MetSyn and T2DM. Studies by Schulze et al50., and Palmer et al51., both showed slight non-significant increased risk of T2DM in individuals consuming ≥ 1 diet soda /d compared to those consuming < 1 diet soda/mo after additional adjustment for baseline BMI 50. Findings from MESA participants however, showed significant positive associations between diet soda intake and risk of T2DM and MetSyn, although adjustment for baseline BMI somewhat attenuated the estimates and were no longer significant for MetSyn57. Similar results were found among participants in the ARIC study, which showed that those in the highest tertile of diet soda intake had a 34% greater risk of MetSyn compared to those in the lowest tertile (p<0.001)59. Dhingra et al reported a 39% greater risk of MetSyn among participants in the Framingham Offspring study, however it can be assumed that the majority of this effect was due to regular soft drink consumption since in this study diet and regular soft drinks were evaluated concurrently37. A weak but marginally significant association was observed between diet soda intake and risk of CHD in the NHS, which was no longer significant after adjusting for T2DM, BMI and total energy intake63.
These findings must be interpreted with caution since, longitudinal studies evaluating diet and chronic disease risk are prone to reverse causation i.e. persons change their diet because of subclinical disease or weight-gain, which could result in spurious associations. For example, individuals aware of subclinical disease symptoms or weight gain may increase diet soda intake, which could result in a spurious association between disease and diet soda intake. This may partially explain why some studies evaluating diet soda consumption found strong positive associations37, 57. These studies may also be particularly prone to residual confounding since for example diet soda consumption has been shown to be higher in individuals with diabetes than in those without diabetes73. Studies with longer durations of follow-up and repeated measures of diet and disease status, which are less prone to reverse causation, showed only marginal non-significant associations50, 51, 63. Given the increasingly high prevalence of diet soft drink consumption, for health reasons or body- weight management, further work investigating potential metabolic consequences is clearly warranted.
Potential biological mechanisms
SSB consumption is thought to lead to weight gain by virtue of decreased satiety and an incomplete compensatory reduction in energy intake at subsequent meals following intake of liquid calories. On average, one 12-oz serving of SSB contains about 140–150 calories74. If these calories are added to the typical US diet without reducing calories from other sources, one SSB per day could lead to a weight gain of 15 lbs over the course of one year. This is supported by evidence from short term feeding trials that have shown greater energy intake and weight gain following consumption of SSBs compared to non-caloric artificially sweetened beverages. For example, Raben and colleagues showed after 10 weeks of supplementation with either sucrose sweetened or artificially sweetened beverages and foods, those in the sucrose group had increased energy intake, body weight (p<0.001 between group difference), fat mass (p<0.01 between group difference) and blood pressure compare to the sweetener group75. In a cross-over trial, it was also shown that drinking aspartame sweetened beverages for 3 weeks resulted in small weight loss while consumption of high fructose corn syrups (HFCS)- sweetened SSBs resulted in small weight gain 76. In another cross-over study, where participants were offered diet cola, regular cola, orange juice, milk or no beverage at lunch once a week for six weeks77, on the weeks when caloric beverages were consumed, average caloric consumption increased by 104 ± 16 kcal over the level consumed when water, diet cola, and no beverage were offered.77
Another line of evidence for failure of complete compensation stems from studies comparing the effects of consuming isocaloric loads of liquid or solid calories.17 When two groups were assigned to drink 450 kcal/day of a soft drink, or consume the same amount of sucrose energy as jelly beans, the jelly beans reduced their caloric consumption by slightly more than contained in the beans, but subjects drinking the soft drinks failed to reduce their caloric consumption to compensate for the soft drink, and actually increased their consumption of other foods slightly. Subjects consuming soft drinks had a small but significant weight gain over the course of the study, but those eating the jelly beans did not.17 Other studies also demonstrate a lack of compensation for calories consumed as beverages as opposed to solid food.78–83 These studies argue that sugar or HFCS in liquid beverages may not suppress intake of solid foods to the level needed to maintain energy balance, however, the exact mechanism responsible for that weaker compensatory response is unknown.84
Metabolic consequences of SSB consumption may result in part due to their propensity to induce weight gain, but an independent effect may also result from the large quantities of rapidly absorbable carbohydrates such as sucrose or HFCS, used to flavor these beverages. Rapid increases in blood glucose and insulin levels have been observed following consumption of SSBs85, which in conjunction with the large volumes often consumed, contribute to a high dietary glycemic load (GL). High GL diets have been shown to stimulate appetite and induce weight gain and are associated with development of both glucose intolerance and insulin resistance86. In addition, High GL diets have been shown to dysregulate lipid profiles, which taken together may explain how SSB consumption can lead to increased risk of gallstone disease70. Higher dietary GL has also been shown increase levels of inflammatory biomarkers such as C-reactive protein, a known marker of elevated T2DM and CVD risk19, and has been linked to development of CHD in a relatively short time-frame 87 since inflammation impacts development of atherosclerosis as well as plaque stability and thrombosis63. It is also thought that the caramel coloring used in cola type beverages may further increase insulin resistance and inflammation due to the high amounts of advanced glycation end products, produced during the process of caramelization88.
Recently, much attention has been given to the potentially adverse metabolic effects of the fructose fraction of these beverages. Fructose, which is a constituent of both sucrose and HFCS in relatively equal parts is preferentially metabolized to lipid in the liver, leading to increased hepatic de novo lipogenesis, dyslipidemia and insulin resistance 89–92. Fructose consumption has also been shown to promote accumulation of visceral adiposity, which has extremely serious implications for T2DM risk22, 93, 94. In a recent study comparing the effects of consuming glucose or fructose sweetened beverages providing 25% of energy requirements, after 10-weeks, both groups showed similar weight gain, but only the fructose group showed a significant increase in visceral adiposity22. In addition, while fasting plasma triglyceride levels only increased in the glucose group, hepatic de novo lipogenesis, postprandial triglycerides and markers of altered lipid metabolism and lipoprotein remodeling such as fasting apoB and small LDL particles significantly increased in the fructose group22. In addition, fasting plasma glucose and insulin levels increased and insulin sensitivity decreased in the fructose group. Of note, Ghanim and colleagues did not find evidence of oxidative or inflammatory stress following intake of 300 kcal of fructose or orange juice while ROS generation and NF-kB binding was significantly increased after intake of glucose95. However, quantities of fructose contained in SSBs are far greater than those contained in these beverage preloads95.
Fructose is also the only sugar known to increase serum uric levels. Fructose induces uric acid production by increasing ATP degradation to AMP, a uric acid precursor. Fructose phosphorylation in the liver uses ATP and the accompanying phosphate depletion limits regeneration of ATP from ADP, which in turn serves as a substrate for uric acid formation96. The production of uric acid in the liver by xanthine oxidase may reduce endothelial nitric oxide, which could partially mediate the association between SSBs and CHD97. Hyperuricemia often precedes development of obesity, hyperinsulinemia and T2DM, and findings from animal studies suggest that it may have a role in development of MeySyn21. The prevalence of MetSyn has been shown to be as high as 62% in individuals with gout21. Gout has been shown to exacerbate formation of kidney stones and renal disease due to increases in serum urate concentrations21. Phosphoric acid used primarily for acidifying cola beverages may further increase development of kidney stones and chronic kidney disease67.
Growing clinical evidence also suggests that hyperuricemia and fructose consumption may increase blood pressure21. For example, increases in blood pressure have been observed when fructose is administered acutely, an effect not seen with glucose98. In addition, an increase in blood pressure over 10 weeks was found when individuals drank SSB’s, in contrast to aspartame-sweetened beverages.75 Hyperuricemia has also been reported in nearly 90% of children with newly diagnosed hypertension21. Serum uric acid may increase blood pressure by development of renal disease, endothelial dysfunction and activation of the rennin-angiotensin system.21 Evidence from prospective cohort studies also suggest that hyperuricemia is an independent risk factor for CVD and has been associated with increased risk of MI, peripheral artery disease and death99. Fructose intake may also lead to weight gain by decreasing production of insulin and leptin in peripheral tissues, thereby initiating the hunger cascade in the central nervous system, 90. However, further elucidation of this pathway is needed since others have found greater satiety and lower total energy intake following fructose preloads compared to glucose preloads100.
Conclusions and Public Health Implications
The prevalence of obesity and related chronic diseases is rising at unprecedented rates across the globe. Identification of modifiable risk factors is therefore essential to abating this escalating pandemic. Temporal patterns in SSB intake across recent decades have shown a close parallel between the obesity epidemic and rising levels of SSB consumption. Findings from epidemiological studies clearly indicate that regular consumption of SSBs can lead to weight gain and substantially increase risk of developing chronic diseases including MetSyn, T2DM and CHD. In general, longer studies with greater numbers of participants that did not adjust for potential mediators of effect such as total energy intake and adiposity, report stronger and more consistent associations. Evidence for adverse effects on other metabolic conditions including hypertension, inflammation, atherogenic dyslipidemia, hyperurecemia, gout, gallstone, and kidney disease is also starting to emerge. SSBs are the greatest contributor to added sugar intake in the US and are thought to induce weight gain in part by incomplete compensation for liquid calories at subsequent meals. SSBs may also increase T2DM risk independently, as a potential contributor to a high dietary GL leading to inflammation, insulin resistance, and impaired beta-cell function. Additional metabolic effects from the fructose fraction of these beverages may also promote accumulation of visceral adiposity, and atherogenic dyslipidemia due to elevated hepatic de novo, and hypertension due to hyperuricemia. Such excess risk could have serious repercussions for developing countries, which must manage dual burdens of chronic and infectious disease as well as for certain populations such as Hispanics26, 101, 102 or South Asians, which are particularly prone to development of visceral adiposity and T2DM 5. Given the increasingly large quantities of SSBs consumed by children and adolescents, limiting intake is critical to obesity prevention in this population. Childhood obesity is known to increase risk of obesity in adulthood and can lead to serious downstream health effects. For these reasons, a number of public health campaigns to limit intake of SSB’s are underway and strategies such as taxation are currently being considered as a means of reducing intake levels as well as offsetting related health care costs24. Thus amidst a growing pandemic of obesity, ample evidence exists to discourage consumption of these beverages in place of healthy alternatives such as water, to reduce risk of T2DM and CVD, and to improve overall health and quality of life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [Last accessed November 11th, 2009.]; http://www.who.int/mediacentre/factsheets/fs311/en/index.html.
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006 Apr 5;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3. [Last accesssed November 11th, 2009.]; http://www.cdc.gov/obesity/childhood/prevalence.html.
- 4.Teff KL, Grudziak J, Townsend RR, et al. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab. 2009 May;94(5):1562–1569. doi: 10.1210/jc.2008-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. Jama. 2009 May 27;301(20):2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 6.Popkin BM. What can public health nutritionists do to curb the epidemic of nutrition-related noncommunicable disease? Nutr Rev. 2009 May;67( Suppl 1):S79–82. doi: 10.1111/j.1753-4887.2009.00165.x. [DOI] [PubMed] [Google Scholar]
- 7.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008 Sep;32(9):1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 8.Haslam DW, James WP. Obesity. Lancet. 2005 Oct 1;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009 Sep–Oct;28(5):w822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 10.Naser KA, Gruber A, Thomson GA. The emerging pandemic of obesity and diabetes: are we doing enough to prevent a disaster? Int J Clin Pract. 2006 Sep;60(9):1093–1097. doi: 10.1111/j.1742-1241.2006.01003.x. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Cadwell BL, Cheng YJ, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004 Dec;27(12):2806–2812. doi: 10.2337/diacare.27.12.2806. [DOI] [PubMed] [Google Scholar]
- 12.Cowie CC, Rust KF, Ford ES, et al. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009 Feb;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block G. Foods contributing to energy intake in the US: data from NHANES III and NHANES 1999–2000. Journal of Food Composition and Analysis. 2004;14:439–447. [Google Scholar]
- 14.Nguyen S, Choi HK, Lustig RH, Hsu CY. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009 Jun;154(6):807–813. doi: 10.1016/j.jpeds.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004 May;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 16.Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009 Sep 15;120(11):1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627. [DOI] [PubMed] [Google Scholar]
- 17.DiMeglio DP, Mattes RD. Liquid versus solid carbohydrate: effects on food intake and body weight. Int J Obes Relat Metab Disord. 2000 Jun;24(6):794–800. doi: 10.1038/sj.ijo.0801229. [DOI] [PubMed] [Google Scholar]
- 18.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004 Aug;80(2):348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Manson JE, Buring JE, Stampfer MJ, Willett WC, Ridker PM. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002 Mar;75(3):492–498. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 20.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001 Jul 18;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Richette P, Bardin T. Gout. Lancet. 2009 Aug 17; [Google Scholar]
- 22.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009 May;119(5):1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007 Nov;15(11):2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- 24.Brownell KD, Farley T, Willett WC, et al. The Public Health and Economic Benefits of Taxing Sugar-Sweetened Beverages. NEJM. 2009 doi: 10.1056/NEJMHPRO905723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen SJ, Popkin BM. Changes in beverage intake between 1977 and 2001. Am J Prev Med. 2004 Oct;27(3):205–210. doi: 10.1016/j.amepre.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Barquera S, Hernandez-Barrera L, Tolentino ML, et al. Energy intake from beverages is increasing among Mexican adolescents and adults. J Nutr. 2008 Dec;138(12):2454–2461. doi: 10.3945/jn.108.092163. [DOI] [PubMed] [Google Scholar]
- 27.Ismail AI, Tanzer JM, Dingle JL. Current trends of sugar consumption in developing societies. Community Dent Oral Epidemiol. 1997 Dec;25(6):438–443. doi: 10.1111/j.1600-0528.1997.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 28.Bray GA. The Battle of the Bulge. Pittsburgh, PA: Dorrance Publishing; 2007. [Google Scholar]
- 29.Steyn NP, Myburgh NG, Nel JH. Evidence to support a food-based dietary guideline on sugar consumption in South Africa. Bull World Health Organ. 2003;81(8):599–608. [PMC free article] [PubMed] [Google Scholar]
- 30.Adair LS, Popkin BM. Are child eating patterns being transformed globally? Obes Res. 2005 Jul;13(7):1281–1299. doi: 10.1038/oby.2005.153. [DOI] [PubMed] [Google Scholar]
- 31.Chacko E, McDuff I, Jackson R. Replacing sugar-based soft drinks with sugar-free alternatives could slow the progress of the obesity epidemic: have your Coke and drink it too. N Z Med J. 2003 Oct 24;116(1184):U649. [PubMed] [Google Scholar]
- 32.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006 Aug;84(2):274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berkey CS, Rockett HR, Field AE, Gillman MW, Colditz GA. Sugar-added beverages and adolescent weight change. Obes Res. 2004 May;12(5):778–788. doi: 10.1038/oby.2004.94. [DOI] [PubMed] [Google Scholar]
- 34.Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and fat intakes of children and adolescents in the united states: data from the national health and nutrition examination surveys. Am J Clin Nutr. 2000 Nov;72(5 Suppl):1343S–1353S. doi: 10.1093/ajcn/72.5.1343s. [DOI] [PubMed] [Google Scholar]
- 35.Liebman M, Pelican S, Moore SA, et al. Dietary intake, eating behavior, and physical activity-related determinants of high body mass index in rural communities in Wyoming, Montana, and Idaho. Int J Obes Relat Metab Disord. 2003 Jun;27(6):684–692. doi: 10.1038/sj.ijo.0802277. [DOI] [PubMed] [Google Scholar]
- 36.French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord. 1994 Mar;18(3):145–154. [PubMed] [Google Scholar]
- 37.Dhingra R, Sullivan L, Jacques PF, et al. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007 Jul 31;116(5):480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 38.Malik VS, Willett WC, Hu FB. Sugar-sweetened beverages and BMI in children and adolescents: reanalyses of a meta-analysis. Am J Clin Nutr. 2009 Jan;89(1):438–439. doi: 10.3945/ajcn.2008.26980. author reply 439–440. [DOI] [PubMed] [Google Scholar]
- 39.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev. 2009 Jan;10(1):68–75. doi: 10.1111/j.1467-789X.2008.00523.x. [DOI] [PubMed] [Google Scholar]
- 40.Vartanian LR, Schwartz MB, Brownell KD. Effects of soft drink consumption on nutrition and health: a systematic review and meta-analysis. Am J Public Health. 2007 Apr;97(4):667–675. doi: 10.2105/AJPH.2005.083782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ludwig DS, Peterson KE, Gortmaker SL. Relation between consumption of sugar-sweetened drinks and childhood obesity: a prospective, observational analysis. Lancet. 2001 Feb 17;357(9255):505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 42.Blum JW, Jacobsen DJ, Donnelly JE. Beverage consumption patterns in elementary school aged children across a two-year period. J Am Coll Nutr. 2005 Apr;24(2):93–98. doi: 10.1080/07315724.2005.10719449. [DOI] [PubMed] [Google Scholar]
- 43.James J, Thomas P, Cavan D, Kerr D. Preventing childhood obesity by reducing consumption of carbonated drinks: cluster randomised controlled trial. Bmj. 2004 May 22;328(7450):1237. doi: 10.1136/bmj.38077.458438.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillips SM, Bandini LG, Naumova EN, et al. Energy-dense snack food intake in adolescence: longitudinal relationship to weight and fatness. Obes Res. 2004 Mar;12(3):461–472. doi: 10.1038/oby.2004.52. [DOI] [PubMed] [Google Scholar]
- 45.Ebbeling CB, Feldman HA, Osganian SK, Chomitz VR, Ellenbogen SJ, Ludwig DS. Effects of decreasing sugar-sweetened beverage consumption on body weight in adolescents: a randomized, controlled pilot study. Pediatrics. 2006 Mar;117(3):673–680. doi: 10.1542/peds.2005-0983. [DOI] [PubMed] [Google Scholar]
- 46.Dubois L, Farmer A, Girard M, Peterson K. Regular sugar-sweetened beverage consumption between meals increases risk of overweight among preschool-aged children. J Am Diet Assoc. 2007 Jun;107(6):924–934. doi: 10.1016/j.jada.2007.03.004. discussion 934–925. [DOI] [PubMed] [Google Scholar]
- 47.Johnson L, Mander AP, Jones LR, Emmett PM, Jebb SA. Is sugar-sweetened beverage consumption associated with increased fatness in children? Nutrition. 2007 Jul–Aug;23(7–8):557–563. doi: 10.1016/j.nut.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Nissinen K, Mikkila V, Mannisto S, et al. Sweets and sugar-sweetened soft drink intake in childhood in relation to adult BMI and overweight. The Cardiovascular Risk in Young Finns Study. Public Health Nutr. 2009 May 28;:1–9. doi: 10.1017/S1368980009005849. [DOI] [PubMed] [Google Scholar]
- 49.Viner RM, Cole TJ. Who changes body mass between adolescence and adulthood? Factors predicting change in BMI between 16 year and 30 years in the 1970 British Birth Cohort. Int J Obes (Lond) 2006 Sep;30(9):1368–1374. doi: 10.1038/sj.ijo.0803183. [DOI] [PubMed] [Google Scholar]
- 50.Schulze MB, Manson JE, Ludwig DS, et al. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. Jama. 2004 Aug 25;292(8):927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- 51.Palmer JR, Boggs DA, Krishnan S, Hu FB, Singer M, Rosenberg L. Sugar-sweetened beverages and incidence of type 2 diabetes mellitus in African American women. Arch Intern Med. 2008 Jul 28;168(14):1487–1492. doi: 10.1001/archinte.168.14.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz M, Kroke A, Liese AD, Hoffmann K, Bergmann MM, Boeing H. Food groups as predictors for short-term weight changes in men and women of the EPIC-Potsdam cohort. J Nutr. 2002 Jun;132(6):1335–1340. doi: 10.1093/jn/132.6.1335. [DOI] [PubMed] [Google Scholar]
- 53.Bes-Rastrollo M, Sanchez-Villegas A, Gomez-Gracia E, Martinez JA, Pajares RM, Martinez-Gonzalez MA. Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study 1. Am J Clin Nutr. 2006 Feb;83(2):362–370. doi: 10.1093/ajcn/83.2.362. quiz 394–365. [DOI] [PubMed] [Google Scholar]
- 54.Chen L, Appel LJ, Loria C, et al. Reduction in consumption of sugar-sweetened beverages is associated with weight loss: the PREMIER trial. Am J Clin Nutr. 2009 May;89(5):1299–1306. doi: 10.3945/ajcn.2008.27240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bazzano LA, Li TY, Joshipura KJ, Hu FB. Intake of fruit, vegetables, and fruit juices and risk of diabetes in women. Diabetes Care. 2008 Jul;31(7):1311–1317. doi: 10.2337/dc08-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montonen J, Jarvinen R, Knekt P, Heliovaara M, Reunanen A. Consumption of sweetened beverages and intakes of fructose and glucose predict type 2 diabetes occurrence. J Nutr. 2007 Jun;137(6):1447–1454. doi: 10.1093/jn/137.6.1447. [DOI] [PubMed] [Google Scholar]
- 57.Nettleton JA, Lutsey PL, Wang Y, Lima JA, Michos ED, Jacobs DR., Jr Diet soda intake and risk of incident metabolic syndrome and type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2009 Apr;32(4):688–694. doi: 10.2337/dc08-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paynter NP, Yeh HC, Voutilainen S, et al. Coffee and sweetened beverage consumption and the risk of type 2 diabetes mellitus: the atherosclerosis risk in communities study. Am J Epidemiol. 2006 Dec 1;164(11):1075–1084. doi: 10.1093/aje/kwj323. [DOI] [PubMed] [Google Scholar]
- 59.Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008 Feb 12;117(6):754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- 60.Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. Jama. 2005 Nov 9;294(18):2330–2335. doi: 10.1001/jama.294.18.2330. [DOI] [PubMed] [Google Scholar]
- 61.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005 Sep;82(3):675–684. doi: 10.1093/ajcn.82.3.675. quiz 714–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sorensen LB, Raben A, Stender S, Astrup A. Effect of sucrose on inflammatory markers in overweight humans. Am J Clin Nutr. 2005 Aug;82(2):421–427. doi: 10.1093/ajcn.82.2.421. [DOI] [PubMed] [Google Scholar]
- 63.Fung TT, Malik V, Rexrode KM, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr. 2009 Apr;89(4):1037–1042. doi: 10.3945/ajcn.2008.27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JW, Ford ES, Gao X, Choi HK. Sugar-sweetened soft drinks, diet soft drinks, and serum uric acid level: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2008 Jan 15;59(1):109–116. doi: 10.1002/art.23245. [DOI] [PubMed] [Google Scholar]
- 65.Choi HK, Curhan G. Soft drinks, fructose consumption, and the risk of gout in men: prospective cohort study. Bmj. 2008 Feb 9;336(7639):309–312. doi: 10.1136/bmj.39449.819271.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shoham DA, Durazo-Arvizu R, Kramer H, et al. Sugary soda consumption and albuminuria: results from the National Health and Nutrition Examination Survey, 1999–2004. PLoS One. 2008;3(10):e3431. doi: 10.1371/journal.pone.0003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saldana TM, Basso O, Darden R, Sandler DP. Carbonated beverages and chronic kidney disease. Epidemiology. 2007 Jul;18(4):501–506. doi: 10.1097/EDE.0b013e3180646338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curhan GC, Willett WC, Knight EL, Stampfer MJ. Dietary factors and the risk of incident kidney stones in younger women: Nurses’ Health Study II. Arch Intern Med. 2004 Apr 26;164(8):885–891. doi: 10.1001/archinte.164.8.885. [DOI] [PubMed] [Google Scholar]
- 69.Cuevas A, Miquel JF, Reyes MS, Zanlungo S, Nervi F. Diet as a risk factor for cholesterol gallstone disease. J Am Coll Nutr. 2004 Jun;23(3):187–196. doi: 10.1080/07315724.2004.10719360. [DOI] [PubMed] [Google Scholar]
- 70.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Dietary carbohydrates and glycaemic load and the incidence of symptomatic gall stone disease in men. Gut. 2005 Jun;54(6):823–828. doi: 10.1136/gut.2003.031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Glycemic load, glycemic index, and carbohydrate intake in relation to risk of cholecystectomy in women. Gastroenterology. 2005 Jul;129(1):105–112. doi: 10.1053/j.gastro.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Scragg RK, McMichael AJ, Baghurst PA. Diet, alcohol, and relative weight in gall stone disease: a case-control study. Br Med J (Clin Res Ed) 1984 Apr 14;288(6424):1113–1119. doi: 10.1136/bmj.288.6424.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mackenzie T, Brooks B, O’Connor G. Beverage intake, diabetes, and glucose control of adults in America. Ann Epidemiol. 2006 Sep;16(9):688–691. doi: 10.1016/j.annepidem.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Apovian CM. Sugar-sweetened soft drinks, obesity, and type 2 diabetes. Jama. 2004 Aug 25;292(8):978–979. doi: 10.1001/jama.292.8.978. [DOI] [PubMed] [Google Scholar]
- 75.Raben A, Vasilaras TH, Moller AC, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002 Oct;76(4):721–729. doi: 10.1093/ajcn/76.4.721. [DOI] [PubMed] [Google Scholar]
- 76.Tordoff MG, Alleva AM. Effect of drinking soda sweetened with aspartame or high-fructose corn syrup on food intake and body weight. Am J Clin Nutr. 1990 Jun;51(6):963–969. doi: 10.1093/ajcn/51.6.963. [DOI] [PubMed] [Google Scholar]
- 77.DellaValle DM, Roe LS, Rolls BJ. Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite. 2005 Apr;44(2):187–193. doi: 10.1016/j.appet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Poppitt SD, Eckhardt JW, McGonagle J, Murgatroyd PR, Prentice AM. Short-term effects of alcohol consumption on appetite and energy intake. Physiol Behav. 1996 Oct;60(4):1063–1070. doi: 10.1016/0031-9384(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 79.Beridot-Therond ME, Arts I, Fantino M, De La Gueronniere V. Short-term effects of the flavour of drinks on ingestive behaviours in man. Appetite. 1998 Aug;31(1):67–81. doi: 10.1006/appe.1997.0153. [DOI] [PubMed] [Google Scholar]
- 80.Hagg A, Jacobson T, Nordlund G, Rossner S. Effects of milk or water on lunch intake in preschool children. Appetite. 1998 Aug;31(1):83–92. doi: 10.1006/appe.1997.0152. [DOI] [PubMed] [Google Scholar]
- 81.Blackburn GL, Kanders BS, Lavin PT, Keller SD, Whatley J. The effect of aspartame as part of a multidisciplinary weight-control program on short- and long-term control of body weight. Am J Clin Nutr. 1997 Feb;65(2):409–418. doi: 10.1093/ajcn/65.2.409. [DOI] [PubMed] [Google Scholar]
- 82.Van Wymelbeke V, Beridot-Therond ME, de La Gueronniere V, Fantino M. Influence of repeated consumption of beverages containing sucrose or intense sweeteners on food intake. Eur J Clin Nutr. 2004 Jan;58(1):154–161. doi: 10.1038/sj.ejcn.1601762. [DOI] [PubMed] [Google Scholar]
- 83.De Castro J. The effects of the spontaneous ingestion of particular foods or beverages on the meal pattern and overall nutrient intake of humans. Physiol Behav. 1993;53(6):1133–1144. doi: 10.1016/0031-9384(93)90370-u. [DOI] [PubMed] [Google Scholar]
- 84.Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006 Mar;83(3):529–542. doi: 10.1093/ajcn.83.3.529. [DOI] [PubMed] [Google Scholar]
- 85.Janssens JP, Shapira N, Debeuf P, et al. Effects of soft drink and table beer consumption on insulin response in normal teenagers and carbohydrate drink in youngsters. Eur J Cancer Prev. 1999 Aug;8(4):289–295. doi: 10.1097/00008469-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 86.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Jama. 2002 May 8;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, Willett WC, Stampfer MJ, et al. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am J Clin Nutr. 2000 Jun;71(6):1455–1461. doi: 10.1093/ajcn/71.6.1455. [DOI] [PubMed] [Google Scholar]
- 88.Vlassara H, Cai W, Crandall J, et al. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15596–15601. doi: 10.1073/pnas.242407999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elliott SS, Keim NL, Stern JS, Teff K, Havel PJ. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002 Nov;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 90.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev. 2005 May;63(5):133–157. doi: 10.1301/nr.2005.may.133-157. [DOI] [PubMed] [Google Scholar]
- 91.Bray GA. How bad is fructose? Am J Clin Nutr. 2007 Oct;86(4):895–896. doi: 10.1093/ajcn/86.4.895. [DOI] [PubMed] [Google Scholar]
- 92.Basciano H, Federico L, Adeli K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr Metab (Lond) 2005 Feb 21;2(1):5. doi: 10.1186/1743-7075-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stanhope KL, Griffen SC, Bair BR, Swarbrick MM, Keim NL, Havel PJ. Twenty-four-hour endocrine and metabolic profiles following consumption of high-fructose corn syrup-, sucrose-, fructose-, and glucose-sweetened beverages with meals. Am J Clin Nutr. 2008 May;87(5):1194–1203. doi: 10.1093/ajcn/87.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stanhope KL, Havel PJ. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am J Clin Nutr. 2008 Dec;88(6):1733S–1737S. doi: 10.3945/ajcn.2008.25825D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghanim H, Mohanty P, Pathak R, Chaudhuri A, Sia CL, Dandona P. Orange juice or fructose intake does not induce oxidative and inflammatory response. Diabetes Care. 2007 Jun;30(6):1406–1411. doi: 10.2337/dc06-1458. [DOI] [PubMed] [Google Scholar]
- 96.Johnson RJ, Segal MS, Sautin Y, et al. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr. 2007 Oct;86(4):899–906. doi: 10.1093/ajcn/86.4.899. [DOI] [PubMed] [Google Scholar]
- 97.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis: fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clin Pract Nephrol. 2005 Dec;1(2):80–86. doi: 10.1038/ncpneph0019. [DOI] [PubMed] [Google Scholar]
- 98.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008 Mar;294(3):R730–737. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 99.Gagliardi AC, Miname MH, Santos RD. Uric acid: A marker of increased cardiovascular risk. Atherosclerosis. 2009 Jan;202(1):11–17. doi: 10.1016/j.atherosclerosis.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 100.Rodin J. Comparative effects of fructose, aspartame, glucose, and water preloads on calorie and macronutrient intake. Am J Clin Nutr. 1990 Mar;51(3):428–435. doi: 10.1093/ajcn/51.3.428. [DOI] [PubMed] [Google Scholar]
- 101.Maskarinec G, Grandinetti A, Matsuura G, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis. 2009 Winter;19(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- 102.Zimmet PZ, McCarty DJ, de Courten MP. The global epidemiology of non-insulin-dependent diabetes mellitus and the metabolic syndrome. J Diabetes Complications. 1997 Mar–Apr;11(2):60–68. doi: 10.1016/s1056-8727(96)00090-6. [DOI] [PubMed] [Google Scholar]