Abstract
The interaction between genotype and environment is an important feature of the process of development. We investigate this interaction by examining the influence of postnatal cross-fostering and post-weaning cross-housing on the behavioral development of 129S and B6 mice. Following cross-fostering, we found significant alterations in the frequency of maternal care as a function of maternal strain and pup type as well as interactions between these variables. In adulthood, we find there are sex-specific and strain-specific alterations in anxiety-like behavior as a function of rearing environment, with males exhibiting more pronounced rearing-induced effects. Mixed-strain housing of weanlings was found to lead to alterations in home-cage social and feeding behavior as well as changes in adult anxiety-like responses of 129S mice. Anxiety-like behavior in B6 mice was altered as a function of the interaction between housing condition and weaning weight. These data illustrate the complexpathways through which early and later social experiences may lead to variations in behavior.
Keywords: Cross-fostering, Maternal, Strain differences, Juvenile, Social behavior, Cross-housing
Introduction
Phenotypic differences between strains of mice are commonly ascribed as being related to differences in genetic background, or more specifically to differences between strains in the nucleotide sequence of genes (Beck et al. 2000). Two of the most commonly used families of laboratory inbred mice are the various 129S and C57Bl6/J (B6) sub-strains which are known to differ substantially in many aspects of behavior. In particular, B6 mice typically show increased activity levels, self-grooming, enhanced cognitive performance and decreased anxiety-like behavior in comparison to most sub-strains of 129S mice (Kalueff and Tuohimaa 2004; Murphy et al. 2001; Paulus et al. 1999; Voikar et al. 2001). However, it has long been acknowledged that variations in environmental conditions also lead to alterations in strain specific behavior (Crabbe et al. 1999; Wahlsten et al. 2003). The mechanisms through which these environmental experiences are able to alter gene expression during development and hence adult phenotype are increasingly being explored in rodent models. These studies suggest that early rearing conditions, and in particular, the quality of mother-infant interactions, may lead to long-term developmental effects through epigenetic changes in gene expression that persist into adulthood (Meaney 2001; Weaver et al. 2004).
In rodents, one strategy that is commonly used to investigate the effects of rearing environment on strain differences in behavior is postnatal cross-fostering. However, studies using this methodology have produced mixed results regarding the ability of the foster mother of one strain to shift the behavior of offspring of another strain. For instance, male Balb/cByJ (Balb-C) mice reared by a B6 dam have been shown to exhibit decreased anxiety-like behavior and an attenuated corticosterone response to stress compared to those reared by a dam of their own strain, yet behavioral changes in B6 mice reared by Balb-C dams are not as evident (Caldji et al. 2004; Prakash et al. 2006; Priebe et al. 2005). There are many other instances where cross-fostering between mouse strains does not shift strain-typical phenotypes and these findings are often interpreted as demonstrating that strain differences are ‘genetic’ rather than ‘environmental’ in origin. However, several issues are overlooked when making this assertion. Firstly, it is often assumed when cross-fostering is implemented, that strain differences exist in the maternal care of the two contrasting mouse strains. Though there have certainly been demonstrations of strain differences in maternal care in mice, there are also large individual differences within each strain (Champagne et al. 2007; Shoji and Kato 2006). Therefore, as direct characterization of the maternal behavior of females caring for fostered pups is often not included in these investigations; it may be that the differences in maternal behavior between the strains are insufficient to alter the phenotype. Secondly, the quality of mother-infant interactions may be altered by pup characteristics (Alleva et al. 1989) such that strain differences in maternal care are diminished when caring for pups of a different strain (Ressler 1962; Van Der Veen et al. 2008). In addition, there may be sensitive periods other than during postnatal development which provide alternative experience-dependent opportunities to alter strain differences in behavior (Laviola and Terranova 1998). For instance, B6 mice have been shown to be relatively unaffected by cross-fostering to Balb-C mice but when a B6 embryo is transferred prenatally to a Balb-C dam their behavior is adjusted towards that of the Balb-C strain (Francis et al. 2003). Another sensitive period is during post-weaning juvenile development and several studies have demonstrated that variations in the social environment during this period can permanently alter behavior (Champagne and Curley 2005; Curley et al. 2009; Ruscio et al. 2007). Since mice are known to exhibit strain-specific patterns of play and social behavior (Panksepp and Lahvis 2007; Ricceri et al. 2007), it is possible that strain differences in behavior are reinforced through socialization with peers of the same strain (Hughes 1989). Interestingly, there is some evidence that cross-housing (the housing of individuals of one strain with those of another) post-weaning may be an effective strategy for shaping some behavioral phenotypes in mice (Bhansali et al. 2007; Holmes et al. 2005; Randall and Lester 1975).
Here we investigate the effects of cross-fostering and cross-housing in shaping the behavioral development of 129S and B6 mice. In the first experiment, we explore the interaction between pup type (non-fostered, fostered 129S, fostered B6) and maternal strain (129S, B6 and F1-129SB6 hybrid) on postpartum maternal behavior to determine how the quality of the early environment may differ under various rearing conditions. We have previously observed variations in maternal care amongst these strains, with each engaging in a unique pattern of postpartum behavior that could potentially be associated with offspring outcome measures. These offspring were then tested on a measure of open-field exploration to determine the relationship between sex, strain and rearing conditions on behavioral phenotypes. In the second part of the study, non-fostered 129S and B6 weanlings were either housed with their own strain or cross-housed with same-sex mice of the opposite strain to investigate how this housing dynamic would alter their social behavior. As in the cross-fostering study, these mice were then tested in adulthood on measures of open-field exploration to determine how this differential post-weaning social experience can influence development and how these experiences interact with earlier life-history characteristics.
Methods
Animals and animal husbandry
C57BL/6 J (B6) and 129S1/SvImJ (129S) mice used for the cross-fostering study were wild-type laboratory mice (Mus musculus) bred for over 20 generations in our own facility. F1 hybrid mice were produced by a cross of female 129S mice and male B6 mice. B6 and 129S mice for the cross-housing study were the offspring of mice brought in from Harlan UK. All mice were housed at the Sub-Department of Animal Behaviour at the University of Cambridge in accordance to the UK Home Office regulations. The mice were kept in opaque cages (42 cm × 12.5 cm × 12.5 cm) with steel wire lids on a reverse 12D:12L light cycle under a constant temperature of 21°C and 55% humidity and provided ad libitum water and food (RM1 E rodent chow diet, Lillico, Surrey UK). All behavioral observations and tests took place during the dark period of the light cycle under dim red illumination.
Postnatal cross-fostering of 129S and B6 offspring
129S (n = 30), B6 (n = 30) and hybrid F1-129SB6 (F1; n = 12) females were mated with same-strain males to obtain the litters used in this study. Females (housed 3/cage) were mated for a two-week period and singly housed within 48 h of parturition. On the day of birth (PN0), all pups were removed from their cage and weighed before being returned either to their biological mother or a foster dam. Whole litters were fostered to lactating females who had given birth to a minimum of 4 pups within 12 h of the birth of the litter to be fostered. For each of the maternal strains (129S, B6, F1), 6–7 females per strain were designated to rear either fostered 129S pups, fostered B6 pups, or their own biological offspring (129S and B6 dams only). Home-cage mother-infant interactions were observed from PN1-PN6 and litters were otherwise left undisturbed, with the exception of weekly cage cleaning, until PN28. At weaning (PN28), male and female pups were housed 5/cage with same-sex, same-age, same-rearing condition conspecifics. All mice were ear-punched at the time of weaning to be uniquely identified in adulthood.
Post-weaning cross-housing of 129S and B6 mice
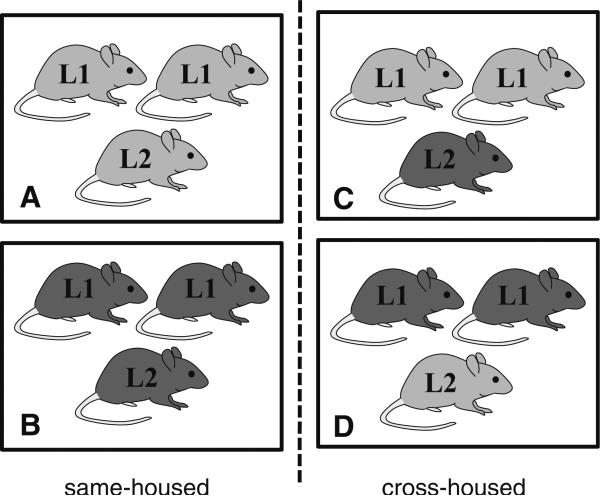
Cross-housed mice were generated from 11 129S and 17 B6 litters born to male and female mice purchased from Harlan UK. After a 2 week habituation period, 129S and B6 females were mated for a 2 week period with same-strain males. Females were singly housed 48 h before parturition and on the day of birth (PN0) litters were weighed and counted. Litters were left undisturbed with the exception of weekly cage cleaning until PN28. At weaning (PN28), male and female pups were weighed and housed 3/cage to create 3 distinct rearing environments for each strain resulting in 4 overall conditions (129S same-housed, 129S cross-housed, B6 same-housed, B6 cross-housed; Fig. 1). Groups consisted of two same-sex mice from the same litter (designated “L1” in Fig. 1) plus a same-sex weanling from a separate litter of either the same or different strain (designated “L2” in Fig. 1). Mice that were housed post-weaning with at least one mouse of a different strain are termed “cross-housed”. Mice that were housed post-weaning with only mice of their own strain are termed as “same-housed”. Mice were assigned so that typically only one but never more than two mice per litter per sex contributed to each group, and such that there were no mean differences between groups in weaning weights. All mice were ear-punched at the time of weaning to be uniquely identified in adulthood. In total we generated the following number of cages: Same-housed: n = 7 female-B6, n = 10 male-B6, n = 7 female-129S, n = 7 male-129S; Cross-housed: n = 17 female-B6, n = 14 male-B6, n = 17 female-129S, n = 14 male-129S.
Fig. 1.
Illustration of the different housing conditions used for 129S (light gray) and B6 (dark gray) mice with L1 indicating same-litter cage-mates and L2 indicating a cage-mate from a different litter. Mice placed in post-weaning conditions A and B are classified as same-housed and those mice placed in conditions C and D are considered cross-housed
Assessment of home-cage maternal behavior
The procedure for assessing maternal behavior in mice has been described previously (Champagne et al. 2007). Maternal behavior was scored from PN1 to PN6. Observers were trained to a high level of inter-rater reliability (i.e., >0.90). Dams were observed in their home cage during the dark-phase of the light cycle under dim red light (<5 lux) and not disturbed for the duration of the 6-day observation period. Each day consisted of 4 observation periods, 2 within the first 5 h following the onset of the dark cycle (0800–1300) and 2 within 7 h of the end of the dark cycle (1300–2000). Each observation was 60 min in duration and no observation session took place within the 1 h period before or after the transition from the light to dark cycle. Within each observation period, the behavior of each mother was scored every 3 min (20 observations/period × 4 periods per day = 80 observations/mother/day = 480 observations per mother over the 6 days). The following behaviors were scored: mother off pups, mother licking and grooming (LG) any pup (both body and anogenital licking were included), mother in nursing posture over pups, nest-building (while in contact with pups, nursing pups, or not in contact with pups), self-grooming, eating and drinking. We have used this method in previous studies of natural variations in maternal care in 129S and B6 mice (Champagne et al. 2007).
Home-cage behavioral observations
Home-cage observations of behavior of same-housed and cross-housed mice were conducted from PN35 to PN45 on alternating days. Observations of 30 min each were carried out four times daily. During observations, the behavior of each animal in the cage was recorded every 3 min (10 observations per period × 4 periods per day = 40 observations per animal per day). The following behaviors were recorded: allo-grooming, nest-building, burying (animal covers itself with wood shavings), cage-climbing, chasing, chewing (animal chews wood shavings), climbing over other mice, darting (fast sprint from one end of the cage to the other), drinking, eating, fighting (attacking and biting another mouse in a playful or antagonistic manner), frisky hops (performance of vertical jumping hops), huddling (sitting or lying next to one or more animals with body contact), jerky movements (a sudden horizontal body twitch), rearing, resting-alone (resting without physical contact to other mice), self-grooming, allo-sniffing (investigate other mice by smelling their nose, mouth, anogenital area or flanks) and general activity (physical activity that does not fit into any of the other categories).
Open-field exploration
The open-field test is a system that provides an unfamiliar environment for measuring exploratory activity in rodents (reviewed in Prut and Belzung 2003). The open-field used was a 90 cm × 90 cm × 60 cm plastic box. Male and female mice were tested in the open-field at approximately PN60 and females were confirmed to be in diestrus on the day of testing following analysis of the cytology of a vaginal smear. On the day of testing, the mouse was removed from its home cage and placed directly into one corner of the open field. After a 10-min session, the mouse was returned to its home cage. Counts of fecal boli were assessed at this time. All testing was conducted under red (dark phase) lighting conditions. During analysis of the video recordings of testing sessions, the field was divided into a grid of 10 × 10 squares. For the purposes of analysis, inner field exploration was defined as the time spent in the inner 9 × 9 squares, activity was defined as the number of grid crossings and pauses in movement within the field were defined as the duration of time spent immobile. In addition, self-grooming behavior, which was observed in some cases amongst cross-fostered offspring, was observed in higher frequencies in the cross-housed mice and was therefore quantified in this cohort.
Results
I: Cross-fostering effects on behavior
Interaction of maternal strain and offspring type/strain on frequency of maternal care
Analyses of the postpartum care of 129S, B6 and F1 females was conducted using a two-way ANOVA (with Tukey's HSD post-hoc analyses) with maternal strain and pup type/strain (biological offspring, fostered 129S or fostered B6) as factors. Frequency of maternal behavior as a function of maternal strain and pup type are provided in Table 1. A significant main effect of maternal strain [F(2,36) = 10.13, p < .001], pup type [F(2,36) = 3.19, p < .05] as well as a maternal strain by pup type interaction [F(2,36) = 4.09, p < .05] was found for frequency of nursing behavior. Consistent with previous studies, Champagne et al. (2007) nursing was found to occur more frequently amongst 129S females (p < .01; Table 1), with F1 females displaying the lowest frequency of this behavior. Exploration of the interaction between maternal strain and pup type indicated that there was a trend for B6 females to engage in nursing more frequently to foster pups compared to their own biological offspring (p = .07). There was also a trend for 129S females to decrease (p = .09) and B6 females to increase (p = .10) the frequency of nursing toward foster B6 pups. Indeed, rearing foster B6 pups effectively abolished strain-differences in frequency of nursing behavior normally observed in 129S and B6 dams. F1 females were found to significantly vary in the level of maternal care dependent on pup type such that when caring for fostered 129S pups, females displayed reduced frequency of nursing compared to those caring for fostered B6 pups (p < .05). A significant main effect of maternal strain [F(2,36) = 38.79, p < .001] and pup type [F(2,36) = 5.83, p < .001] was found in the analysis of frequency of LG behavior, with no significant interaction found between these variables. B6 females engaged in a higher frequency of LG than both 129S (p < .001) and F1 females (p < .001) and B6 foster pups received higher levels of licking/grooming than non-fostered pups (p < .05). Significant main effects of strain were found on the frequency of eating [F(2,36) = 4.08, p < .05] and drinking [F(2,36) = 3.57, p < .05]. 129S females spent significantly less time eating and drinking than both B6 (p < .05) and F1 females (p < .05). No difference in nest-building, self-grooming or frequency of non-nursing contact with pups was found as a function of maternal strain or pup type.
Table 1.
Frequency of postpartum maternal care as a function of maternal and pup strain; pup types: non-fostered 129S (129S), non-fostered B6 (B6), fostered 129S (F-129S), fostered B6 (F-B6)
| Maternal strain | Pups | Nursing | Lick/groom | Nest build | Self-groom | Eating | Drinking | Non-nursing contact |
|---|---|---|---|---|---|---|---|---|
| 129S | 129S | 76.34 ± 2.29 | 2.41 ± .28 | 6.01 ± 1.01 | 3.10 ± .58 | 19.63 ± 2.76 | .92 ± .27 | 1.43 ± .38 |
| F-129S | 73.10 ± 2.97 | 2.66 ± .38 | 6.48 ± 1.68 | 3.11 ± .59 | 15.25 ± 1.68 | .66 ± .16 | 2.09 ± .53 | |
| F-B6 | 67.87 ± 2.79 | 2.83 ± .68 | 9.15 ± 2.59 | 2.87 ± .40 | 17.76 ± 2.64 | .76 ± .21 | 2.63 ± .99 | |
| B6 | B6 | 58.84 ± 2.94 | 7.11 ± 1.38 | 7.52 ± .87 | 3.29 ± .44 | 22.57 ± 1.20 | 1.30 ± .35 | 2.04 ± .47 |
| F-129S | 63.34 ± 2.69 | 13.34 ± 2.07 | 8.01 ± 1.29 | 4.58 ± .67 | 21.70 ± 1.22 | 1.55 ± .32 | 3.96 ± .77 | |
| F-B6 | 68.25 ± 3.76 | 13.54 ± 1.97 | 8.61 ± 1.45 | 3.53 ± .56 | 18.75 ± 2.13 | 1.22 ± .28 | 3.71 ± .78 | |
| F1 | F-129S | 47.01 ± 4.27 | 4.03 ± 1.33 | 9.72 ± 2.82 | 3.05 ± .91 | 27.29 ± 1.75 | 1.46 ± .32 | 4.79 ± 1.65 |
| F-B6 | 61.67 ± 2.52* | 3.82 ± .80 | 4.31 ± 1.66 | 3.26 ± .49 | 20.76 ± 1.98 | 1.32 ± .42 | 2.92 ± 1.18 |
p < .05
F-129S vs. F-B6
Postnatal cross-fostering and offspring open-field behavior
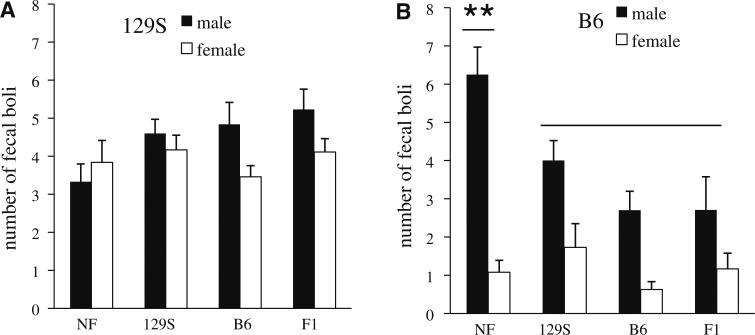
Analysis of open-field behavior during the 10 min testing session was conducted using a three-way ANOVA with sex, strain (129S vs. B6), and rearing condition (reared by own mother, fostered to a 129S dam, fostered to a B6 dam, fostered to an F1 dam) as factors. The 4 measures analyzed were: (1) number of fecal boli, (2) time spent immobile, (3) time spent in the inner area of the field, and (4) total activity (grid crossings). A main effect of sex was found on the number of fecal boli [F(1,201) = 39.04, p < .001] and total activity [F(1,201) = 7.81, p < .01], with males producing more boli (male = 4.33 ± .23 vs. female = 2.59 ± 4.33) and females engaging in a higher number of grid crossings (male = 441.54 ± 29.16 vs. female = 497.60 ± 40.03). A main effect of strain was found on all open-field measures with 129S mice producing more fecal boli [F(1,201) = 38.08, p < .001; 129S = 4.21 ± .18 vs. B6 = 2.60 ± .25], spending more time immobile [F(1,201) = 354.49, p < .001; 129S = 336.23 ± 14.64 vs. B6 = 35.37 ± 1.82], spending less time in the inner area [F(1,201) = 402.73, p < .001; 129S = 18.98 ± 2.76 vs. B6 = 130.35 ± 4.83], and engaging in significantly fewer grid crossings [F(1,201) = 515.46, p < .001; 129S = 179.61 ± 17.65 vs. B6 = 785.97 ± 22.33; see Table 2] compared to B6 mice. Rearing environment was found to have a significant main effect on total activity [F(1,201) = 8.31, p < .001] with post-hoc analysis indicating that mice fostered to F1 dams were more active compared to mice in all other rearing conditions (p < .05; see Table 2). Significant sex by strain interactions were found on the number of fecal boli produced [F(1,201) = 16.02, p < .001] and the total number of grid crossings [F(1,201) = 17.56, p < .001] with sex-differences (males producing more boli and females being more active) only significant amongst B6 mice. A significant sex by rearing environment interaction was found on time spent in the inner area [F(3,201) = 2.80, p < .05] with the elevated levels of inner area exploration in males compared to females found only amongst those offspring fostered at birth to 129S females. Strain by rearing environment interactions were found to be significant on measures of fecal boli [F(3,201) = 5.65, p < .001], time spent immobile [F(3,201) = 2.99, p < .05], and time spent in the inner area of the field [F(3,201) = 5.79, p < .001]. As illustrated in Table 2, rearing environment did not induce variations in fecal boli counts amongst 129S mice, however rearing effects were observed on this measure amongst B6 mice, such that those reared by an F1 or foster B6 dam produced fewer boli. Rearing effects were observed in 129S mice on measures of time spent immobile and open-field exploration (Table 2), with those 129S mice reared by an F1 dam spending significantly less time immobile and more time in the inner area of the field (an effect not observed amongst B6 mice). A significant 3-way sex by strain by rearing environment interaction was found on the measure of fecal boli [F(3,201) = 5.23, p < .01] with a trend towards a significant effect on the measure of inner area exploration [F(3,201) = 2.30, p = .08]. Figure 2 illustrates the sex-differences in boli counts as a function of rearing environment and strain. As can be seen in panel A and B of Fig. 2, variation in fecal boli as a function of rearing environment occurs in male but not female B6 mice, with fostered B6 males producing fewer fecal boli compared to non-fostered B6 males (p < .01), and this interaction is not observed amongst 129S mice.
Table 2.
Open-field behavior as a function of strain and rearing condition averaged across sex
| Strain | Rearing condition | Number of fecal boli | Time spent immobile (s) | Time spent in the inner area (s) | Total activity (# of grid crossings) |
|---|---|---|---|---|---|
| 129S | Non-fostered | 3.65 ± .39 | 365.31 ± 25.72 | 11.71 ± 2.25 | 141.77 ± 25.58 |
| Fostered-129S | 4.32 ± .28 | 374.59 ± 26.05 | 9.64 ± 2.48 | 136.75 ± 31.67 | |
| Fostered-B6 | 4.28 ± .38 | 335.06 ± 27.33 | 16.83 ± 4.65 | 163.28 ± 31.74 | |
| Fostered-F1 | 4.77 ± .36 | 248.15 ± 36.24 | 44.22 ± 10.09 | 311.23 ± 48.60 | |
| B6 | Non-fostered | 3.56 ± .65 | 33.50 ± 3.28 | 117.43 ± 8.05 | 726.60 ± 40.14 |
| Fostered-129S | 3.26 ± .44 | 38.66 ± 3.22 | 137.55 ± 8.01 | 727.53 ± 28.80 | |
| Fostered-B6 | 1.42 ± .30 | 29.46 ± 3.33 | 144.34 ± 13.08 | 800.65 ± 50.14 | |
| Fostered-F1 | 1.74 ± .43 | 40.05 ± 4.92 | 115.33 ± 6.06 | 948.58 ± 56.71 |
Fig. 2.
Open-field behavior (fecal boli counts ± SEM) as a function of strain, sex, and rearing condition in adult 129S and B6 mice. Offspring were either reared by their biological mother (NF), or fostered to a 129S, B6 or F1 dam. Graphs illustrate number of fecal boli produced by a 129S and b B6 mice during open-field testing Comparisons include: non-fostered 129S males (n = 12), 129S females (n = 19), B6 males (n = 12), B6 females (n = 13); 129S fostered 129S males (n = 10), 129S females (n = 18), B6 males (n = 23), B6 females (n = 11); B6 fostered 129S males (n = 19), 129S females (n = 13), B6 males (n = 10), B6 females (n = 16); F1 fostered 129S males (n = 13), 129S females (n = 9), B6 males (n = 7), B6 females (n = 12). ** p < .01
II: Cross-housing effects on behavior
Strain differences in home-cage behavior in same-housed mice
Strain differences in home-cage behavior were investigated in same-housed mice, with the average level of behavior within the cage as an independent data point (i.e. average behavior in cage A vs. B in Fig. 1). Each behavior was analyzed using a two-way ANOVA with sex and strain as main factors (results summarized in Table 3). All mice spent the largest proportion of their time huddling (51% in B6 and up to 88% in 129S), and we found a significant sex by strain interaction [F(1,27) = 6.29, p < .05] on the frequency of huddling. Post-hoc analysis revealed that 129S females and 129S males huddled significantly more frequently than B6 females (p < .001) and B6 males (p < .005) respectively, and that 129S females huddled more than 129S males (p < .005) whereas there was no sex difference in huddling amongst B6 mice. A main effect of strain was found for allo-sniffing [F(1,27) = 4.98, p < .05] and for allo-grooming [F(1,27) = 9.45, p < .005] with 129S mice engaging in allo-sniffing less frequently and allo-grooming more frequently than B6 mice. With regard to play behaviors, a significant main effect of strain indicated that B6 mice were more frequently engaged in frisky hops [F(1,27) = 31.06, p < .001], fighting [F(1,27) = 5.74, p < .05] and chasing [F(1,27) = 10.23, p < .005]. There was no main effect of sex on each of these behaviors. We found a significant interaction between sex and strain in the frequency of darts [F(1,27) = 4.27, p < .05]. B6 females darted more frequently than 129S females (p < .001) and B6 males (p < .05), but there was no difference between 129S males, B6 males and 129S females. Main effects of strain but not of sex were found for frequency of eating [F(1,27) = 41.18, p < .001], drinking [F(1,27) = 21.10, p < .001], rearing [F(1,27) = 8.55, p < .01], cage-climbing [F(1,27) = 9.47, p < .005], jerky movements [F(1,27) = 18.86, p < .001] and nest-building [F(1,27) = 9.58, p < .005], with B6 mice engaging in these behaviors more frequently. A sex by strain interaction was found on the frequency of burying [F(1,27) = 5.50, p < .05], with males burying more frequently than females amongst 129S but not B6 mice. There were no significant effects of sex or strain on general activity, climbing over others, or chewing. Finally, both B6 and 129S males were found to spend more time resting alone [F(1,27) = 28.70, p < .001] and self-grooming [F(1,27) = 7.32, p < .05] compared to same-strain females.
Table 3.
Frequency of home-cage social behavior of same and cross-housed 129S and B6 mice
| B6 |
129S |
|||||||
|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Female |
Male |
|||||
| Same | Cross | Same | Cross | Same | Cross | Same | Cross | |
| Huddling | 51.36 ± 5.16 | 57.60 ± 2.85 | 51.55 ± 3.11 | 52.23 ± 3.75 | 87.62 ± 1.78 | 65.29 ± 3.29 | 68.74 ± 4.45 | 52.55 ± 3.91 |
| Eating | 21.00 ± 1.32 | 17.56 ± 1.18 | 22.23 ± 1.69 | 22.25 ± 1.43 | 10.02 ± 1.06 | 14.06 ± 1.58 | 12.67 ± 1.86 | 18.45 ± 2.18 |
| Drinking | 1.12 ± .19 | 1.03 ± .18 | 1.15 ± .16 | 1.07 ± .14 | .29 ± .08 | .53 ± .14 | .50 ± .18 | 1.04 ± .15 |
| Self-groom | 10.38 ± 1.84 | 13.44 ± 1.20 | 17.08 ± 1.61 | 16.05 ± 1.45 | 13.52 ± 1.26 | 15.07 ± 1.47 | 15.05 ± .85 | 16.32 ± 1.64 |
| General activity | 5.60 ± 1.12 | 5.38 ± .58 | 6.03 ± .64 | 5.18 ± .55 | 3.21 ± .53 | 6.06 ± .70 | 5.93 ± 1.20 | 6.39 ± .90 |
| Resting alone | 4.38 ± .63 | 3.56 ± .60 | 7.73 ± .79 | 7.09 ± 1.08 | 1.95 ± .45 | 8.03 ± .91 | 7.41 ± 1.16 | 13.86 ± 1.98 |
| Nest-building | 2.21 ± .31 | 2.32 ± .35 | 2.58 ± .25 | 1.93 ± .26 | 1.67 ± .41 | 2.09 ± .29 | 1.24 ± .24 | 1.27 ± .25 |
| Cage-climbing | 6.31 ± 2.50 | 5.27 ± 1.62 | 1.67 ± .28 | 1.54 ± .72 | .38 ± .22 | .96 ± .21 | .43 ± .32 | .32 ± .11 |
| Rearing | 1.60 ± .4 | 1.60 ± .32 | 1.17 ± .25 | 1.23 ± .21 | .48 ± .18 | .69 ± .19 | .67 ± .24 | .79 ± .20 |
| Frisky hops | 1.05 ± .22 | 1.07 ± .11 | .78 ± .18 | .57 ± .12 | .05 ± .05 | .19 ± .06 | .05 ± .03 | .07 ± .04 |
| Darts | .64 ± .18 | .68 ± .16 | .30 ± .10 | .30 ± .08 | .05 ± .03 | .41 ± .15 | .14 ± .04 | .32 ± .14 |
| Chasing | .21 ± .09 | .37 ± .14 | .40 ± .12 | .64 ± .14 | .00 ± .00 | .15 ± .07 | .02 ± .02 | .05 ± .04 |
| Fighting | .10 ± .06 | .06 ± .04 | .30 ± .10 | .57 ± .18 | .00 ± .00 | .03 ± .02 | .05 ± .05 | .29 ± .11 |
| Play | 2.69 ± .66 | 2.64 ± .21 | 2.08 ± .34 | 1.96 ± .31 | .09 ± .05 | .77 ± .19 | .26 ± .07 | .71 ± .21 |
| Allo-sniffing | 1.88 ± .29 | 1.69 ± .25 | 1.47 ± .32 | 2.38 ± .37 | .93 ± .13 | 1.03 ± .16 | 1.21 ± .19 | 1.16 ± .25 |
| Allo-grooming | 1.00 ± .28 | 1.12 ± .25 | 1.35 ± .19 | 1.20 ± .21 | 1.90 ± .42 | 1.22 ± .18 | 2.17 ± .22 | .68 ± .16 |
| Climbing over | .07 ± .03 | .06 ± .03 | .12 ± .04 | .23 ± .07 | .02 ± .02 | .03 ± .03 | .14 ± .07 | .05 ± .04 |
| Jerky movements | .88 ± .32 | .57 ± .13 | .60 ± .14 | .36 ± .12 | .00 ± .00 | .04 ± .03 | .00 ± .00 | .04 ± .04 |
| Chewing | .57 ± .20 | .87 ± .15 | .30 ± .11 | .54 ± .12 | .48 ± .12 | 1.01 ± .30 | .38 ± .09 | .57 ± .20 |
| Burying | .62 ± .19 | .66 ± .23 | .52 ± .12 | .50 ± .10 | .14 ± .06 | .16 ± .07 | .67 ± .13 | .13 ± .06 |
Home-cage behavior in same- and cross-housed mice: sex by housing analysis
Within-strain analysis of social behavior of same-housed and cross-housed mice was conducted by comparison of the average frequency of behavior in same-housing (i.e. average behavior in cage A or B in Fig. 1) vs. average frequency of behavior in cross-housing (i.e. average behavior among same-strain individuals in cage C and D in Fig. 1). Two-way ANOVA was conducted with sex and housing condition as factors. Amongst B6 mice, no significant main effects of housing were found for the frequency of any behavior (see Table 3), suggesting that post-weaning cross-housing did not alter home-cage behavior within this strain. Amongst 129S mice, there were significant effects of housing type on behavior. Cross-housed 129S mice spent less time huddling [F(1,41) = 21.85, p < .001] and allo-grooming [F(1,41) = 20.88, p < .001], and increased their frequency of eating [F(1,41) = 5.49, p < .05], drinking [F(1,41) = 5.51, p < .05] and resting alone [F(1,41) = 15.45, p < .001]. No main effect of housing was found for the frequency of darts, frisky hops, fighting or chasing. However, analysis of a composite measure of these play behaviors indicated that cross-housed 129S mice spent significantly more time playing than same-housed 129S mice [F(1,41) = 6.86, p < .05]. No other main effects of housing were found for other social behaviors in 129S mice, though a significant interaction between sex and housing type was found for burying [F(1,41) = 11.21, p < .005], with post-hoc analysis indicating that same-housed males buried themselves more than same-housed females and cross-housed males (p < .001).
Effects of cage-mate composition on home-cage behavior
We next evaluated whether the frequency of behaviors amongst cross-housed mice varied between those mice housed with only one or two opposite strain cage-mates. Those B6 females housed with two 129S females were found to spend significantly less time resting alone than those housed with only one 129S female [1.9% vs. 5.4%; t(15) = 3.91, p < .001]. Those 129S mice housed with one B6 mouse spent less time huddling than those housed with two B6 mice; an effect found in both females [57.6% vs. 72.2%; t(15) = 2.58, p < .05] and males [44.8% vs. 62.8%; t(12) = 2.80, p < .05]. Both male and female 129S mice housed with one B6 mice ate less frequently than those housed with two B6 mice [females: 10.0% vs. 18.6%, t(15) = 3.62, p < .005; males: 13.6% vs. 22.1%, t(12) = 2.17, p < .0]. We saw no other significant differences in B6 and 129S mice with respect to the number of opposite strain cage-mates.
Effects of post-weaning cross-housing on open-field behavior
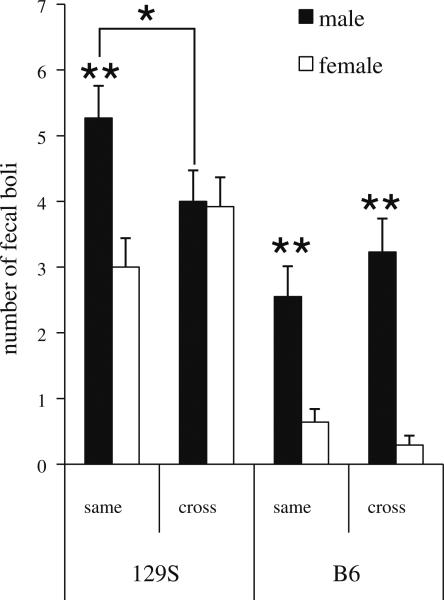
Open-field data were analyzed using a three-way ANOVA with sex, strain and housing condition as factors. Consistent with the open-field data derived from the cross-fostering study, a main effect of sex was found on number of boli produced [F(1,114) = 40.40, p < .001] with males producing more boli than females (male = 3.68 ± .26 vs. female = 2.06 ± .26). The main effects of strain were likewise replicated in this cohort, with 129S mice producing more boli [F(1,114) = 81.58, p < .001; 129S = 4.17 ± .22 vs. B6 = 1.67 ± .24], spending more time immobile [F(1,114) = 65.11, p < .001; 129S = 210.81 ± 25.18 vs. B6 = 11.03 ± 1.32], spending less time exploring the inner area of the field [F(1,114) = 228.34, p < .001; 129S = 43.49 ± 7.57 vs. B6 = 184.76 ± 5.53], and engaging in fewer grid crossings during testing [F(1,114) = 89.04, p < .001; 129S = 352.72 ± 34.20 vs. B6 = 716.06 ± 17.37] compared to B6 mice. In addition to these measures, a main effect of strain on grooming frequency was observed, with 129S mice grooming less frequently that B6 mice [F(1,114) = 66.81, p < .001; 129S = 2.07 ± .17 vs. B6 = 6.97 ± .59] (Table 4). No significant main effects of housing condition were observed, though there was a trend for cross-housed mice to engage in higher levels of grooming that same-housed mice [F(1,114) = 3.64, p = .06]. Significant sex by strain interactions were found on the number of fecal boli produced [F(1,114) = 5.78, p < .05; see Fig. 3] and the frequency of grooming [F(1,114) = 12.68, p < .001] with sex-differences (males producing more boli and engaging in grooming behavior more frequently compared to females) only significant amongst B6 mice. Though there were no significant strain by housing condition interactions on the open-field measures in this cohort, there was a trend for an interaction on the time spent in the inner area of the field [F(1,114) = 2.73, p = .10]. As can be seen from the mean ± SD for open-field measures as a function of strain and housing condition presented in Table 4, there is a trend for an effect of housing amongst B6 but not 129S mice on time spent in the inner area, with cross-housed B6 mice spending less time exploring the inner area compared to same-housed B6 mice. Similar to the open-field analysis in the fostering study, a significant 3-way ANOVA was found between sex, strain, and housing condition on the number of fecal boli produced during testing [F(1,114) = 7.30, p < .01]. As can be seen in Fig. 3, there is a sex by housing condition interaction on boli counts amongst 129S but not B6 mice, such that there are housing effects in male but not female 129S mice, with cross-housing inducing a decrease in fecal boli. In addition, sex-differences (males producing more boli than females) are present in same-housed but not cross-housed 129S mice, whereas amongst B6 mice, sex differences are apparent in both housing conditions.
Table 4.
Open-field behavior in same- and cross-housed 129S and B6 mice averaged across sex
| Strain | Housing condition | # of fecal boli | Time spent immobile (s) | Time spent in the inner area (s) | Total activity (# of grid crossings) | Frequency of grooming |
|---|---|---|---|---|---|---|
| 129S | Same | 4.18 ± .35 | 228.48 ± 40.51 | 40.75 ± 9.11 | 357.07 ± 55.10 | 1.75 ± .20 |
| Cross | 4.17 ± .27 | 194.31 ± 31.03 | 46.05 ± 12.04 | 348.67 ± 42.48 | 2.37 ± .26 | |
| B6 | Same | 1.76 ± .32 | 10.02 ± 1.50 | 195.60 ± 7.82 | 719.91 ± 24.22 | 6.47 ± .77 |
| Cross | 1.57 ± .36 | 12.17 ± 2.26 | 172.48 ± 7.29 | 711.70 ± 25.31 | 7.53 ± .92 |
Fig. 3.
Open-field behavior (number of fecal boli produced ± SEM) as a function of strain, sex, and post-weaning housing condition in adult 129S and B6 mice. Weanlings were either housed with same-strain (same) cage-mates or housed in mixed-strain groups (cross). Comparisons include: same-housed 129S males (n = 14), 129S females (n = 14), B6 males (n = 20), B6 females (n = 14) and cross-housed 129S males (n = 13), 129S females (n = 17), B6 males (n = 13), B6 females (n = 17). * p < .05, ** p < .01
Interaction between early environment and cross-housing on open-field behavior
To address the issue of whether individual mice within each strain are more susceptible or resilient to post-weaning environmental manipulation of housing, we conducted a three-way ANOVA for each open-field measure with sex, housing and either high/low weaning weight as fixed factors. Mice were assigned as being high or low for weaning weight (at PN28) based upon strain and sex specific median splits. We used this measure as a proxy measure for variations in early life experiences. A main effect of weaning weight was not found for any of the open-field measures, however, there was a significant interaction between weaning weight and housing for time spent in the inner area by B6 mice [F(1,56) = 8.10, p < .01] and a trend towards an interaction between housing condition and weaning weight for time spent immobile [F(1,56) = 3.60, p = .06]. B6 mice that were small at weaning (low weaning weight) and then cross-housed, spent less time in the inner area and more time immobile than any of the other groups (see Table 5). There were no main effects of weaning weight or any significant interactions with this variable for other measures of open-field behavior or for any measure in 129S mice. Although this analysis is limited, it is strongly suggestive that variations in early life history variables can interact with the quality of the post-weaning environment to determine behavioral outcomes.
Table 5.
Interaction between weaning weight and post-weaning housing conditions in B6 mice
| Housing-weaning weight | Time spent in inner area (s) |
Time spent immobile (s) |
||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Same-high | 181.6 ± 10.8 | 182.8 ± 24.4 | 13.0 ± 3.2 | 7.1 ± 2.6 |
| Same-low | 194.7 ± 13.3 | 225.4 ± 16.4 | 11.8 ± 2.9 | 6.1 ± 2.3 |
| Cross-high | 184.5 ± 10.8 | 189.5 ± 14.4 | 13.4 ± 4.3 | 4.1 ± 0.7 |
| Cross-low | 168.9 ± 11.4* | 141.5 ± 13.8* | 15.8 ± 4.7† | 19.0 ± 6.6† |
Cross-high vs. cross-low post-hoc comparisons
p < .05
p = .07
Discussion
Overall, these studies were designed to address three key questions: (1) Does maternal care vary as a function of strain and pup type?, (2) Does neonatal cross-fostering induce shifts in adult behavior as a function of strain and rearing environment?, and (3) Do the characteristics of the social environment experienced during juvenile development induce both short- and long-term effects on behavior as a function of strain? Importantly, these studies were conducted examining both male and female offspring to determine sex-specific effects. Our results provide insight into the complex nature of the effects of social environment at early and later stages of development on adult behavior in mice. Though strain differences in phenotype of 129S and B6 mice are certainly not reversed by altering the quality of the postnatal or post-weaning environment, here we demonstrate that significant within-strain shifts in development can be achieved through cross-fostering and cross-housing. These findings illustrate the importance of considering sex, strain and the quality of social interactions during these periods as moderating variables in the pathways to adult behavior (Laviola and Terranova 1998).
Interaction between maternal strain and pup characteristics in the cross-fostering paradigm
There is increasing evidence in rodent models for the role of maternal care as a mediating variable in offspring development and there is evidence for within-strain stability of individual differences in maternal behavior regardless of the characteristics of same-strain pups. In these paradigms, cross-fostering is very effective in shifting offspring behavioral patterns in a predicted direction (Champagne et al. 2003). In the current study we replicate the finding that B6 and 129S dams provide different styles of maternal care, with B6 exhibiting high levels of LG and low levels of nursing in contrast to 129S mice, who display the opposite behavioral pattern when rearing biological offspring (Champagne et al. 2007). However, we also find that levels of particular aspects of maternal care can be altered when females are caring for fostered offspring. In particular, B6 dams were found to exhibit remarkably elevated levels of LG towards fostered B6 offspring compared to biological B6 offspring whereas 129S females did not alter this aspect of their maternal behavior (Table 1). These findings are consistent with previous reports of the sensitivity of B6 dams to fostering effects on mother-infant interactions (Van Der Veen et al. 2008) and the insensitivity of other strains to these rearing conditions (Bartolomucci et al. 2004; Meek et al. 2001; Van Der Veen et al. 2008). Though the factors contributing to this strain-pup type interaction on LG behavior have yet to be elucidated, it is certainly possible that B6 dams are more sensitive to olfactory, auditory or sensory cues that differentiate biological vs. foster offspring.
Perhaps the most distinct rearing condition created in this study, associated with the most profound impact on offspring development, was fostering to an F1 hybrid dam. In contrast to 129S and B6 maternal styles, these F1 females engage in low levels of both nursing and LG and significantly alter their nursing behavior when fostering pups of a different strain (Table 1). The sensitivity of nursing behavior but not of LG to pup strain has previously been demonstrated in AKR and C3H dams fostering B6 compared to DBA pups (Van Der Veen et al. 2008). Similar effects have been observed when cross-fostering between two inbred lines of wild-derived mice selected for aggressiveness, with the less aggressive line (LAL) increasing nursing but not LG when fostering pups from the aggressive SAL (short attack latency) line (Benus and Rondigs 1996). These findings are also consistent with our previous reports that nursing but not LG is significantly altered by characteristics of the litter, such as litter size (Champagne et al. 2007). It is also interesting to note that the strain differences in nursing but not LG between 129S and B6 dams were abolished only when rearing foster B6 pups. The challenge raised by these studies is in the prediction of how pup characteristics will augment the behavior of a female depending on maternal strain/phenotype. In addressing this issue it may be important to consider the “match” between maternal and offspring behaviors as is common in the study of human mother-infant attachment and sensitivity (Smith and Pederson 1988). The effects of match and mismatch of maternal and offspring characteristics is demonstrated in a previous study of Swiss-Webster mice exposed to prenatal stress which then foster either non-stressed or prenatally-stressed offspring. Though non-stressed dams decrease maternal behavior towards stressed foster pups, the converse is true of stressed dams caring for stressed offspring (Meek et al. 2001). It is likely that post-parturient females are either genetically or prenatally primed to detect and respond to specific pup characteristics in order to activate their strain-specific levels of care.
Influence of rearing environment on adult behavior
The goal of the cross-fostering paradigm is typically to induce a shift in the neurobiological and behavioral development of offspring and we certainly see these effects in B6 mice during a test of open-field exploration. B6 mice fostered to B6 dams were found to emit fewer fecal boli during testing in comparison to non-fostered B6 mice, though these effects are sex-specific and observed in males and not females. These findings are consistent with the evidence that LG, which is significantly increased in dams caring for fostered B6 pups, may be a mediating variable in this aspect of anxiety-like behavior (although these effects are strain-dependent, as similar effects on fostered 129S mice are not observed). Previous studies in the rat have demonstrated that increased tactile stimulation received early in life in the form of LG is related to a decreased hypothalamic–pituitary–adrenal (HPA) response to stress, increased exploration of novelty, and several neuroendocrine changes including increased hippocampal glucocorticoid receptor (GR) mRNA (Liu et al. 1997; Meaney 2001). The pathway through which maternal LG leads to changes in GR expression may involve epigenetic regulation of GR promotor activity which leads to long-term changes in the transcriptional activity of this gene with consequences for anxiety-like behavior (Weaver et al. 2004). However, cross-fostering effects may not be limited to mediation via maternal care as previous studies have illustrated that amongst Swiss CD1 mice, fostering increases exploration of a novel environment without associated alterations in the maternal behavior of foster dams (Bartolomucci et al. 2004). However, consistent with the notion that the patterns of maternal care of foster dams may account for behavioral outcomes in offspring we find that mice reared by foster F1 dams, which are characterized by short duration nursing bouts, develop a more active phenotype. Overall, these data illustrate the importance of considering strain and fostering effects on maternal care when predicting the consequence of postnatal cross-fostering.
Social interactions beyond the postnatal period as a critical influence on behavior
There is increasing appreciation that social experiences occurring post-weaning during the early juvenile period can have a significant effect on behavioral development in rodents (Champagne and Curley 2005; Curley et al. 2009; Ruscio et al. 2007). The most commonly used paradigms for inducing variations in post-weaning social experience involve either depriving animals of social housing by rearing them in isolation, or exposing animals to socially complex environments (Champagne and Meaney 2007; Laviola and Terranova 1998). Cross-housing represents a different type of variation in post-weaning social environment, by altering not just the quantity of social interactions but the quality. Strain differences in juvenile social behaviors including time spent in olfactory investigation, grooming, chasing, fighting and social approach have been established for a number of mouse strains (Brodkin 2007; Panksepp and Lahvis 2007; Ricceri et al. 2007; Yang et al. 2007). However, these differences have typically been measured in discrete tests of social behavior normally involving dyads in a novel arena rather than detailed observations of home-cage social behavior. The challenge of more naturalistic approaches to the assessment of social behavior, such as the home-cage observational data presented in Table 3, is the low frequency of specific play behaviors in mice which requires lengthy and detailed observations and coding. However, when such an approach is implemented successfully, it can yield important insights into strain differences in social dynamics. We find that B6 and 129S mice differ greatly in their home-cage juvenile social behavior with 129S mice spending significantly more time huddling and allo-grooming than B6 mice, whereas B6 mice engaged more frequently in play-type behaviors such as frisky hops, fighting and chasing, as well as exploratory behaviors such as rearing, cage-climbing, and allo-sniffing. When cross-housed with 129S mice, B6 mice were found to maintain these strain-specific patterns of behavior whereas when crossed-housed with B6 mice, 129S mice significantly reduced huddling and allo-grooming and increased play, eating, drinking and resting alone. Thus, 129S mice appear to become more B6-like in their social behavior. Interestingly, eating and huddling were both subject to a cage-mate effect with eating being most increased and huddling most decreased when 129S mice are housed with two B6 mice. These “dose” effects of the number of same or opposite-strain cage-mates provides further support for the notion that the social dynamic of the rearing environment can have a significant developmental impact.
There are three possible explanations for the increased feeding by cross-housed 129S mice that are interesting to consider. Firstly, this behavioral change may be the result of a social facilitation whereby 129S mice learn to eat more frequently by observing B6 mice that are eating more frequently (Clayton 1978). Secondly, the increased feeding of cross-housed 129S mice may be due to the experience of chronic social defeat by aggressive B6 cage-mates and there is evidence from studies of Syrian hamsters and subordinate mice that long-term increases in food intake are associated with these experiences (Moles et al. 2006; Solomon et al. 2007). The increased levels of agonistic behaviors exhibited by B6 mice towards 129S mice that we observed have also been found amongst mixed strain groups when observed in a novel testing arena (Hughes 1989). Other cues derived from social housing that may influence food intake include hormonal changes associated with inhibition of the reproductive system or changes in metabolic rate due to the reduced huddling and hence change in thermoregulation. Differences in metabolic/activity rate likely account for the strain differences in eating frequency observed between 129S and B6 mice and it is interesting to note the social transmission of this phenotype to cross-housed mice. Similar social effects on ingestive behaviors, and in particular ethanol preference, have been found when DBA mice are cross-housed with B6 mice (Randall and Lester 1975). Cross-housing may therefore be an excellent model to investigate how variations in social experience during the juvenile period influence neural mechanisms of feeding, drinking and reward.
The long-term effect of cross-housing during juvenile development on anxiety-like behavior
Though the effects of cross-fostering and cross-housing on adult behavior are certainly complex, it is interesting to note that in the analysis of open-field behavior (number of fecal boli) the significant three-way interaction between sex, strain, and environment is due to strain-specific sex by environment interactions in B6 mice when considering the early rearing environment (cross-fostering) and 129S mice when considering the later juvenile environment (cross-housing). This sensitivity of 129S mice to variations in the juvenile environment is consistent with the housing-induced changes in social behavior which are observed in 129S rather than B6 mice. These interactions highlight the notion that there is no consistent direction of effect of housing environment on anxiety-like behavior. Rather, the response to this aspect of the social environment is strain and sex-dependent. Though 129S males appear to experience a decrease in anxiety-like behavior, as indicated by the decrease in fecal boli during testing, there is a tendency for B6 mice to exhibit increased anxiety-like behavior (decreased inner area exploration) when cross-housed. To our knowledge only one other study has employed the post-weaning cross-housing approach to investigate anxiety-like behavior (Holmes et al. 2005). Male AJ mice cross-housed post-weaning with B6 males in ratios of either 2:3 or 3:2 were more exploratory of a novel environment whereas B6 mice were unaltered by this experience. This study did not investigate what differential social experiences B6 and AJ mice encounter when cross-housed so it is not clear as to why B6 were unaffected in this context. Certainly it is known that juvenile mice of both the B6 and 129S strains alter their social and anxiety-like behavior following post-weaning social enrichment, so it is unlikely to be that either strain are resilient to juvenile social experiences (Abramov et al. 2008). Future studies are needed to explore the specific quality of social interactions during the post-weaning period that uniquely alter strain-specific development.
The interaction between early and later life experiences is an important consideration in predicting long-term neuroendocrine and behavioral outcomes. Though, as we illustrate in the results of the current study, there are certainly significant effects of the postnatal and post-weaning environment on development, under more naturalistic conditions there is likely to be varied experiences in each of these periods within the life-history of an organism. Moreover, an important issue highlighted by these results is the notion of risk vs. resilience with strain and sex showing significant interactions with the quality of the environment in predicting long-term outcomes. Overall, it would appear that males are particularly sensitive to environmentally-induced behavioral changes occurring during both early and later periods of development. In addition, we demonstrate that an early-life variable, weaning weight, predicts differential responses of individuals to later cross-housing. As weaning weight is likely to be determined by the quality of the pre-weaning period, these data suggest an early vs. late environment interaction, an effect that has previously been demonstrated in studies of differential maternal care, post-weaning environment and long-term changes in response to novelty and reproductive behavior associated with changes in neuropeptide levels (Champagne and Meaney 2007). The incorporation of a life-history approach to the study of the moderating and mediating variables in behavioral development may be critical in generating novel hypotheses regarding the neurobiological and molecular substrates through which similar genotypes lead to multiple phenotypes.
Acknowledgments
This research was supported by Grant Number DP2OD001674 from the Office of the Director, National Institutes of Health, a Nuffield Foundation Studentship to AM, and the Leverhulme Trust.
Contributor Information
J. P. Curley, Department of Psychology, Columbia University, Room 406 Schermerhorn Hall, 1190 Amsterdam Avenue, New York, NY 10027, USA
V. Rock, Sub-Department of Animal Behaviour, University of Cambridge, Cambridge, UK Department of Biological Sciences, University of Lincoln, Riseholme Park, Lincoln, UK.
A. M. Moynihan, Institute of Evolutionary Biology, University of Edinburgh, Edinburgh, UK
P. Bateson, Sub-Department of Animal Behaviour, University of Cambridge, Cambridge, UK
E. B. Keverne, Sub-Department of Animal Behaviour, University of Cambridge, Cambridge, UK
F. A. Champagne, Department of Psychology, Columbia University, Room 406 Schermerhorn Hall, 1190 Amsterdam Avenue, New York, NY 10027, USA
References
- Abramov U, Puussaar T, Raud S, Kurrikoff K, Vasar E. Behavioural differences between C57BL/6 and 129S6/SvEv strains are reinforced by environmental enrichment. Neurosci Lett. 2008;443:223–227. doi: 10.1016/j.neulet.2008.07.075. [DOI] [PubMed] [Google Scholar]
- Alleva E, Caprioli A, Laviola G. Litter gender composition affects maternal behavior of the primiparous mouse dam (Mus musculus). J Comp Psychol. 1989;103:83–87. doi: 10.1037/0735-7036.103.1.83. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Gioiosa L, Chirieleison A, Ceresini G, Parmigiani S, Palanza P. Cross fostering in mice: behavioral and physiological carry-over effects in adulthood. Genes, Brain, Behav. 2004;3:115–122. doi: 10.1111/j.1601-183x.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Benus RF, Rondigs M. Patterns of maternal effort in mouse lines bidirectionally selected for aggression. Anim Behav. 1996;51:67–75. [Google Scholar]
- Bhansali P, Dunning J, Singer SE, David L, Schmauss C. Early life stress alters adult serotonin 2C receptor pre-mRNA editing and expression of the alpha subunit of the heterotrimeric G-protein G q. J Neurosci. 2007;27:1467–1473. doi: 10.1523/JNEUROSCI.4632-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodkin ES. BALB/c mice: low sociability and other phenotypes that may be relevant to autism. Behav Brain Res. 2007;176:53–65. doi: 10.1016/j.bbr.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Anisman H, Meaney MJ. Maternal behavior regulates benzodiazepine/GABAA receptor subunit expression in brain regions associated with fear in BALB/c and C57BL/6 mice. Neuropsychopharmacology. 2004;29:1344–1352. doi: 10.1038/sj.npp.1300436. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP. How social experiences influence the brain. Curr Opin Neurobiol. 2005;15:704–709. doi: 10.1016/j.conb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Curley JP, Keverne EB, Bateson PP. Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav. 2007;91:325–334. doi: 10.1016/j.physbeh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Clayton DA. Social facilitation of behavior. Q Rev Biol. 1978;53:373. [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Curley JP, Jordan ER, Swaney WT, Izraelit A, Kammel S, Champagne FA. The meaning of weaning: influence of the weaning period on behavioral development in mice. Dev Neurosci. 2009;31:318–331. doi: 10.1159/000216543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nat Neurosci. 2003;6:445–446. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–1346. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Hughes AL. Interaction between strains in the social relations of inbred mice. Behav Genet. 1989;19:685–700. doi: 10.1007/BF01066031. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Tuohimaa P. Contrasting grooming phenotypes in C57Bl/6 and 129S1/SvImJ mice. Brain Res. 2004;1028:75–82. doi: 10.1016/j.brainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Laviola G, Terranova ML. The developmental psychobiology of behavioural plasticity in mice: the role of social experiences in the family unit. Neurosci Biobehav Rev. 1998;23:197–213. doi: 10.1016/s0149-7634(98)00021-9. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Meek LR, Dittel PL, Sheehan MC, Chan JY, Kjolhaug SR. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav. 2001;72:473–479. doi: 10.1016/s0031-9384(00)00431-5. [DOI] [PubMed] [Google Scholar]
- Moles A, Bartolomucci A, Garbugino L, Conti R, Caprioli A, Coccurello R, Rizzi R, Ciani B, D'Amato FR. Psychosocial stress affects energy balance in mice: modulation by social status. Psychoneuroendocrinology. 2006;31:623–633. doi: 10.1016/j.psyneuen.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lam HA, Maidment NT. A comparison of morphine-induced locomotor activity and mesolimbic dopamine release in C57BL6, 129 Sv and DBA2 mice. J Neurochem. 2001;79:626–635. doi: 10.1046/j.1471-4159.2001.00599.x. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes, Brain, Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- Prakash P, Merali Z, Kolajova M, Tannenbaum BM, Anisman H. Maternal factors and monoamine changes in stress-resilient and susceptible mice: cross-fostering effects. Brain Res. 2006;1111:122–133. doi: 10.1016/j.brainres.2006.06.089. [DOI] [PubMed] [Google Scholar]
- Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, Brake WG. Maternal influences on adult stress and anxiety-like behavior in C57BL/6 J and BALB/cJ mice: a cross-fostering study. Dev Psychobiol. 2005;47:398–407. doi: 10.1002/dev.20098. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Randall CL, Lester D. Social modification of alcohol consumption in inbred mice. Science. 1975;189:149–151. doi: 10.1126/science.1138373. [DOI] [PubMed] [Google Scholar]
- Ressler RH. Parental handling in two strains of mice reared by foster parents. Science. 1962;137:129–130. doi: 10.1126/science.137.3524.129. [DOI] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res. 2007;176:40–52. doi: 10.1016/j.bbr.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ruscio MG, Sweeny T, Hazelton J, Suppatkul P, Carter CS. Social environment regulates corticotropin releasing factor, corticosterone and vasopressin in juvenile prairie voles. Horm Behav. 2007;51:54–61. doi: 10.1016/j.yhbeh.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol Behav. 2006;89:320–328. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Smith PB, Pederson DR. Maternal sensitivity and patterns of infant-mother attachment. Child Dev. 1988;59:1097–1101. doi: 10.1111/j.1467-8624.1988.tb03262.x. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–R290. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- van der Veen R, Abrous DN, de Kloet ER, Piazza PV, Koehl M. Impact of intra- and interstrain cross-fostering on mouse maternal care. Genes, Brain, Behav. 2008;7:184–192. doi: 10.1111/j.1601-183X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Wahlsten D, Metten P, Phillips TJ, Boehm SL, 2nd, Burkhart-Kasch S, Dorow J, Doerksen S, Downing C, Fogarty J, Rodd-Henricks K, Hen R, McKinnon CS, Merrill CM, Nolte C, Schalomon M, Schlumbohm JP, Sibert JR, Wenger CD, Dudek BC, Crabbe JC. Different data from different labs: lessons from studies of gene-environment interaction. J Neurobiol. 2003;54:283–311. doi: 10.1002/neu.10173. [DOI] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T? tf/J, C57BL/6 J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]