Abstract
The Brain derived neurotrophic factor (BDNF) is critically involved in neuroplasticity as well as the acquisition, consolidation, and retention of hippocampal- and amygdala-dependent learning. A common functional A→G single nucleotide polymorphism (BDNFval66met) in the pro-domain of the human BDNF gene is associated with abnormal intracellular trafficking and reduced activity-dependent BDNF release. We studied the effect of BDNFval66met in an aversive differential fear conditioning, and a delayed extinction paradigm in fifty-seven healthy participants. Pictures of male faces were used as stimuli and fear learning was quantified by fear potentiated startle (FPS) and skin conductance responses (SCR).
Aware BDNF met-carriers show a behavioral deficit in amygdala-dependent fear conditioning as indicated by an absence of FPS responses in the last acquisition block. This deficit was maintained in the first block of extinction. No genotype differences were found in conditioned SCR discrimination. These data provide evidence for the involvement of BDNF signaling in human amygdala-dependent learning. We suggest that the BDNF met-allele may have a protective effect for the development of affective pathologies that is mediated via reduced synaptic plasticity induced by negative experience.
Keywords: Brain-derived neurotrophic factor, synaptic plasticity, learning, anxiety, fear-potentiated startle
Learning is a major adaptive function that has assisted organisms in tailoring their behavior to environmental contingencies for hundreds of millions of years. This behavioral adaptation reflects evolutionary preserved mechanisms of synaptic plasticity involving cellular/molecular changes in the central nervous system. Pavlovian fear conditioning is a basic learning paradigm for studying how organisms make use of signaling relationships between environmental events where some stimuli (conditioned stimuli, CSs) serve as informative warnings that a potentially damaging stimulus (the unconditioned stimulus, US) is imminent, thus allowing early recruitment of defense responses (conditioned responses, CRs). This emotional memory formation requires synaptic plasticity via activity depended changes in synaptic strength.
The brain-derived neurotrophic factor (BDNF) is the most abundant neutrophin in the mammalian central nervous system (CNS), and it is a major player in mediating synaptic plasticity (e.g. Bramham and Messaoudi, 2005). The involvement of BDNF in hippocampus-dependent cognitive learning and memory has been known from studies in both rodents (for a review see Tyler et al., 2002) and humans (Egan et al., 2003; Hariri et al., 2003). More recently, a similar role of BDNF for Pavlovian fear conditioning, which depends on the amygdala, has been shown (Rattiner et al., 2004a, 2005; Ou and Gean, 2006) as well as an association between a functional polymorphism in the BDNF gene and human fear generalization (Hajcak et al., 2009). Using pharmacological and genetic approaches in rats, it was demonstrated that the binding of mature BDNF to the TrkB receptor within the basolateral amygdala was necessary for acquisition and consolidation of conditioned fear (Rattiner et al., 2004b; Rattiner et al., 2005) as well as for the consolidation of extinguished fear (Chhatwal et al., 2006). The conditioning measure in the animal studies were fear potentiated startle (FPS) responses which reflect direct and indirect effects of the amygdala on the brain-stem startle circuitry (Davis and Whalen, 2001).
As the human BDNF gene harbors a common single nucleotide polymorphism (SNP) that has been shown to affect activity-depended secretion of BDNF and thereby synaptic plasticity, this genetic variation is an excellent candidate for an attempt to bridge the gap between animal and human research. The SNP (BDNFval66met) in the prodomain of the human BDNF gene converts the aminoacid valine (val) to methionine (met) at codon 66 (BDNFval66met). The met-allele leads to a selective impairment in intracellular BDNF trafficking and regulated secretion in neuronal cells without compensatory increase in constitutive secretion (Egan et al., 2003; Chen et al., 2004) and occurs in 20-30% of the Caucasians (Shimizu et al., 2004).
Genetic association studies optimally study simple behavioral paradigms with a well-defined underlying neural circuitry eliciting robust behavioral responses that are easy to measure and quantify. Fear conditioning is a prototype of such a model as the underlying neural network is well delineated and FPS is used in animal (whole body startle) and human studies (startle blink) to quantify fear learning which facilitates the translation of the findings. In humans, FPS can be measured by the potentiation of the startle reflex to short auditory probes presented during the CS as compared to the interval between CSs (Hamm et al., 1993). The basis for this measurement is that the startle blink response triggered by a sudden burst of noise (startle probe) is larger when the individual is in an aversive or fearful state. The startle blink reflex is particularly appropriate for the study of amygdala-dependent learning as the startle reaction reflects the influence of direct and indirect connections from the amygdala to the startle-reflex pathway in the brainstem (Davis and Whalen, 2001). Animal studies have identified the basolateral amygdala as the primary site of BDNF action during fear conditioning (Rattiner et al., 2004b) and thus FPS can be expected to optimally index BDNF dependent processes related to amygdala-dependent fear conditioning. The (baso-)lateral amygdala receives input from cortical and subcortical sensory sites and houses the molecular machinery for forming associations between the CS and the US (LeDoux, 2000; Fanselow and Poulos, 2005). As a result of this associative process, the CS alone may activate the central nucleus of the amygdala. The central nucleus then recruits pathways to the brainstem, striatum, diencephalon and the midbrain (Davis and Whalen, 2001), which for example promotes the potentiation of the startle response.
The purpose of the present study was to examine whether a functional genetic variation in the pro-domain of the human gene coding for the BDNF (BDNFval66met) affects human amygdala-dependent fear learning and extinction by using fear potentiated startle and skin conductance measurements in an aversive differential conditioning and a 24h-delayed extinction paradigm.
Method
Participants
Sixty-three German university students were recruited by advertisements. They participated in two days of testing, donated 20ml of blood for DNA extraction and genotyping and filled out informed-consent forms (approved by ethics committees at the Karolinska Institute and the University of Greifswald). This is the same sample as reported in a previous publication (Lonsdorf et al., 2009). The experiment and data scoring were performed blind to genotype. Six participants were excluded from all data analysis due to technical problems, leaving 57 participants for analysis. Further nine participants were excluded from major analyses because they were classified as unaware of the CS-US contingencies (see also Procedure and Results for more details). The final sample for the major analyses consisted of 48 participants (25 male).
Genotyping
DNA extraction from whole blood was performed using standard methods (Lindblom and Holmlund, 1988). Genotyping for BDNFval66met was performed using the Taqman allelic discrimination method (5′ nuclease assay, Livak, 1999). PCR reactions, with a total volume of 5μl, were performed in 384-well plates containing 20ng dried-down genomic DNA using the Applied Biosystems (ABI, Foster City, CA) standard protocol and the ABI rs6265 genotyping assay (ABI, Foster City, CA). End-point fluorescence was measured and genotype calling was carried out by the allelic discrimination analysis module an ABI HT7900 (ABI, Foster City, CA) which resulted in clear identification of the three genotypes for the BDNFval66met polymorphism. All genotypes were determined in duplicates.
Stimulus Material
Four different color pictures from the Karolinska Directed Emotional Faces (Lundqvist, Flykt & Öhman, 1998) depicting two different male faces (both with an angry or a neutral expression) were selected to serve as CSs. Each participant saw both male faces and valence of facial expression (angry vs. neutral) was kept constant within participants. A white fixation-cross on black background was presented during the intertrial interval (ITI, 10-18 s). The pictures (visible size: 126 cm × 93 cm) were projected for six seconds onto a screen approximately 2 m in front of the participant using a projector (Sanyo PLC-XU86) located in an adjacent room. Participants were randomly assigned to one of eight stimulus sequences. The US was a 500-Hz monopolar DC-pulse electric stimulation applied above the right ankle in a 10 ms train of 1-ms single pulses. It was generated by a commercial stimulator (Grass Instruments S48K, West Warwick, RI), isolated (SIU5), and transmitted via a constant-current unit (CCU1) to a bipolar electrode (F-E10S2). Startle probes were 50-ms bursts of 95-dB[A] white noise (rise time < 1 ms) presented binaurally over Sony (MDRCD 170) headphones.
Physiological recordings
Startle responses were measured by recording electromyographic (EMG) activity of the left orbicularis oculi muscle using miniature Ag/AgCl surface electrodes. The raw EMG signal was amplified, filtered through a 30-Hz high-pass (Coulbourn S75-01) and a 400-Hz low-pass filter (Kemo KEM-VBF8-03; Beckenham, Kent, United Kingdom), rectified, and integrated with a time constant of 10 ms. Skin conductance was recorded using Hellinge Ag/AgCl standard electrodes placed adjacently on the hypothenar eminence of the right hand (see Weike et al., 2007 for more detailed description of the recording procedure).
Procedure
On both days, participants were instructed to attend to the pictures but no information about the CS-US contingencies was provided. Before the presentation of the pictures, four acoustic startle probes were presented for habituation on both days. During the experimental sessions, startle probes were presented 4 or 5 s after picture onset for two thirds of the CS presentations and during one third of the inter-trial intervals (ITI).
Day 1 (Fear acquisition)
The experimental procedure was divided into 5 min familiarization with the lab situation followed by habituation (presentation of six different facial stimuli, four of which startled), individual adjustment of the US intensity (“highly annoying but not painful”), and conditioning. During conditioning, participants viewed a mixed series of nine presentations (6 s) of two CS pictures each. One of the pictures (CS+) co-terminated with the US (100% reinforcement), whereas the other picture (CS−) was never coupled with the US. The conditioning phase ended with a standardized post-experimental awareness interview (cf. Bechara et al., 1995), to assess CS-US contingency awareness.
Day 2 (Extinction, approx. 24h later)
During extinction, the CS+ and CS− were presented 18 times each without administration of any further USs. After completing the experiment participants were debriefed and paid 15 Euro.
Data reduction and response definition
The magnitude of the startle eyeblink (in microvolts) was measured from onset to peak, as described previously in detail (Weike et al., 2007). Blink magnitudes were normalized using z-standardization and converted to T scores to ensure that all participants contributed equally to the group means. The T-score calculation, 50+(z ×10), results in a distribution with an overall mean of 50 and a standard deviation of 10 for each participant. SCR magnitude (in microSiemens) was scored as the first response occurring 0.9 to 4.0 s after picture onset. Logarithms were computed for all values to normalize the distribution (Venables and Christie, 1980) and these log values were range-corrected (individual score/individual maximum response) to account for interindividual response level differences (Lykken and Venables, 1971). Startle and SCR measurements showing recording artifacts or excessive baseline activity were discarded.
Data analysis
The acquisition session was split into an early and late block and the extinction session was split into four blocks. Each block consisted of the mean of 3 (startle) or 4 (SCR) consecutive reactions within a stimulus category. Repeated Measures Analyses of Variance with stimulus (CS+, CS−, ITI for startle; CS+, CS− for SCR) and trialblock (acquisition=2 blocks, extinction=4 block) as the repeated factors and BDNFval66met genotype (val/val vs. met-carrier) as the between-subject variable were used to analyze startle responses and SCRs. A significance level <.05 was considered significant and Greenhouse-Geisser adjustments of degrees of freedom were used when appropriate. We report η2 as the estimate of effect size. Hardy Weinberg Equilibrium was calculated via Exact Test using a website (http://ihg2.helmholtz-muenchen.de/cgi-bin/hw/hwa1.pl).
Results
Genotype frequencies
In total we observed 43 individuals homozygous for the BDNF val/val genotype and 14 carriers of the BDNF met-allele (N=3 met/met). Genotype frequencies did not differ significantly from Hardy-Weinberg Equilibrium, p(Exact Test)=0.10.
Contingency Awareness
Whereas only four of 43 participants with the val/val genotype (9.3%) failed to report the CS-US contingency after acquisition, five out of 14 met-carriers (35.7%) failed to do so, resulting in a significant association between BDNFval66met genotype and awareness of the CS-US contingency, Fisher’s Exact Test, p=0.032. Moreover, a significant interaction between the participants’ contingency awareness and conditioning performance was observed for both conditioning measures, i.e., the fear potentiated startle, F(2,106)=5.14, p=0.008, η2=0.09, and the skin conductance responses, F(1,53)=5.00, p=0.03, η2=0.09 (see supplementary figure 1). Importantly, this interaction was further qualified by the BDNFval66met genotype for the fear potentiated startle, F(2,106)=4.49, p=0.015, η2=0.08, but not for SCR conditioning. Because of this strong effect of awareness on both startle and SCR in our dataset (see supplementary figure 1) and because the unaware group (N=9) is too small to allow for reliable testing of BDNFval66met genotype associations, which was the major aim of this study, we limited our major analyses to participants that were aware of the contingencies.
The final sample of 48 participants consisted of 9 carriers of the BDNF66met allele (2 met/met) and 39 homozygous for the BDNF66val allele. Carriers of one or two BDNF66met alleles were combined as a BDNF met-carrier group due to the low frequency of participants homozygous for this allele and according to the literature (e.g. Hariri et al., 2003; Hajcak et al., 2009).
Fear potentiated startle
In aware subjects, repeated measures ANOVAs with stimulus (CS+, CS−, ITI) and trial block (conditioning = 2, extinction = 4) as repeated factors revealed robust startle potentiation as indicated by a significant main effect of stimulus during conditioning, F(2,92)=5.60, p=0.005, η2=0.11, and extinction, F(2,92)=3.49, p=0.038, η2=0.07. The significant main effect of stimulus during conditioning was due to significant CS+ potentiation (CS+ vs. ITI), p=0.003, as well as CS discrimination (CS+ vs. CS−), p=0.034. During extinction the significant main effect for stimulus was due to significant CS discrimination only, F(1,46)=8.74, p=0.005, η2=0.16.
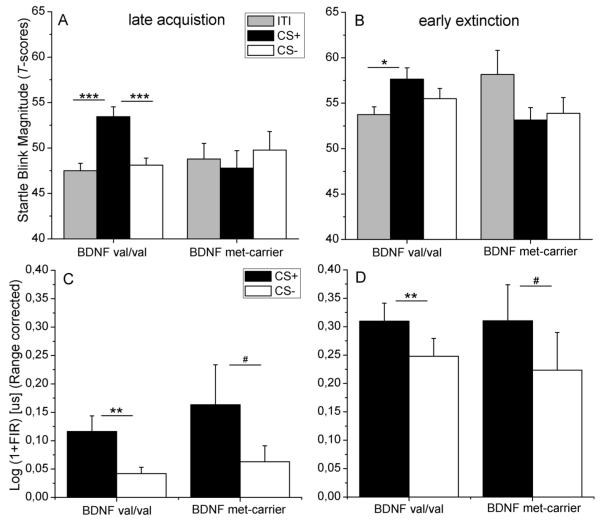
This robust main effect for stimulus was further qualified by a significant trial block x stimulus x BDNFval66met genotype interaction during conditioning, F(2,92)=4.73, p=0.017, η2=0.09, and extinction, F(6,276)=3.22, p=0.013, η2=0.07 (see Figure 1 and 2).
Figure 1.
Fear potentiated startle reactions (T-scores) elicited during the CS+, CS−, and the intertrial interval (ITI) for the BDNFval66met genotype groups (val/val vs. met-carrier) during (A) late acquisition and (B) early extinction as well as skin conductance responses to the CS+ and CS− for the BDNFval66met genotype groups (val/val vs. met-carrier) during (C) late acquisition and (D) early extinction (# p<0.10, * p < .05, ** p < .005, *** p < .001).
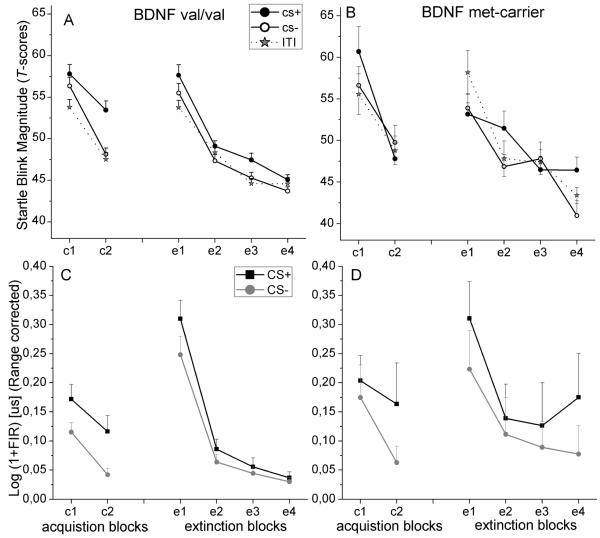
Figure 2.
Fear potentiated startle reactions (T-scores) elicited during the CS+, CS−, and the intertrial interval (ITI) for (A) BDNF val/val and (B) met-carrier during the two acquisition blocks and the four extinction blocks as well as skin conductance responses to the CS+ and CS− for (C) BDNF val/val and (D) met-carrier during the two acquisition blocks and the four extinction blocks.
During acquisition, the trial block x stimulus x BDNFval66met genotype interaction reflected more pronounced CS+ potentiation, F(1,46) =4.40, p=0.042, η2=0.09, and CS discrimination, F(1,46) =6.98, p=0.011, η2=0.13, in the val/val group as compared to met-carriers in the second trial block. This was confirmed by a statistically significant stimulus x BDNFval66met genotype interaction only during the last block of acquisition, F(2,92)=6.20, p=0.004, η2=0.12. During extinction, the trial block x stimulus x BDNFval66met genotype interaction reflected significantly larger CS+ potentiation in the val/val group as compared to the met-carriers, F(1,46)=5.25, p=0.027, η2=0.10, only during the first trial block. Follow-up analyses showed a statistically significant stimulus x BDNFval66met genotype interaction in the first block of extinction, F(2,92)=3.50, p=0.04, η2=0.07 (see Figure 1B).
Within group analyses for the last conditioning block for the BDNF genotype groups showed a significant main effect for stimulus in the BDNF val/val group, F(2,76)=20.04, p<0.000, η2=0.35, indicating robust CS+ potentiation, F(1,38)=24.82, p<0.000, η2=0.40, and robust CS discrimination, F(1,38)=34.26, p<0.000, η2=0.47. In the BDNF met-carrier group no main effect for stimulus was found, F(2,16)<1 (see Figure 1A).
Neither a main effect for BDNFval66met genotype on either day F(1,46)<1, nor a significant main effect of BDNFval66met genotype on mean ITI raw-score magnitudes (in microvolts) was found, even though met-carriers showed higher magnitudes on a descriptive level during acquisition and extinction. Entering valence of the faces presented as a factor in the analyses had no effect on the results.
We have recently reported an association of the 5-HTTLPR and the COMTval158met polymorphisms on FPS during fear acquisition and extinction respectively on the same group of subjects (Lonsdorf et al., 2009). Additional analyses (see supplementary material) provide support that the present results are not due to stratification of the BDNFval66met genotype groups by these other polymorphisms.
Skin conductance responses
In aware participants, robust conditioned SCR discrimination was demonstrated by a significant main effect for stimulus, indicating differential conditioning, during both conditioning, F(1,46)=10.57, p=0.02, η2=0.19, and extinction F(1,45)=13.31, p=0.01, η2=0.23. This effect was however not further qualified by the BDNFval66met genotype nor was any main effect for BDNF genotype found, F(1,46)<1 (see Figure 1C and D). Furthermore BDNF genotype did not differ in their unconditioned SCR to the US, F(1,46)<1.
Discussion
Our data are consistent with a role of BDNF signaling in the acquisition and retention of conditioned fear in humans. Carriers of the BDNF66 met-allele were behaviorally impaired in amygdala-dependent learning as measured by FPS in a classical differential fear conditioning paradigm and showed no retention of fear approximately 24 hours later. During fear conditioning we show during late acquisition, when learning-dependent changes typically are observed, deficient CS+ Potentiation and Discrimination of FPS in aware carriers of the BDNF met-allele, but also more frequent failures to cognitively learn and report the US-CS contingencies when considering the whole sample. Furthermore, during early extinction, which most likely reflects retention of acquisition, BDNF met-carriers show attenuated CS+ and CS− Potentiation as compared to val/val individuals.
In contrast to FPS reactions, we did not find an effect of the BDNFval66met polymorphism on SCRs. While FPS has been suggested to reflect an affective level of learning (Öhman and Mineka, 2001; Hamm and Weike, 2005), SCRs predominantly reflect cognitive contingency awareness and general arousal (Lovibond and Shanks, 2002). In line with this proposition, SCR can be dissociated from amygdala activation during human fear conditioning (Tabbert et al., 2006).
These results replicate and extend a recent report of impaired FPS in carriers of the BDNF66met allele during a generalization fear conditioning paradigm in which participants were explicitly instructed about the CS-US contingency (Hajcak et al., 2009). Even though the paradigm chosen by Hajcak et al. differs in several respects from the paradigm chosen by us, we report similar findings: an attenuated potentiation of the FPS (specifically to the CS+) but also attenuated CS discrimination in the BDNF met-carrier group.
In contrast to the generalization conditioning task used by Hajcak’s group, our task represents a fear conditioning paradigm much more similar to the paradigms used in animal studies. Keeping as close as possible to the behavioral methods used in animal studies is critical for translational research opting to generalize conclusions about physiological mechanisms from animals to humans. However, most animal studies demonstrating a critical involvement of BDNF signaling for successful fear conditioning use methods that are not available for human studies due to ethical constraints. Thus, when studying the specific biological underpinnings of fear learning and extinction we are dependent on the study of genetic polymorphisms, pharmacological challenge tests and molecular imaging methods.
In emotional memory formation, BDNF modulates a neural circuit centered on the amygdala. Elevations of BDNF levels during/after fear conditioning may mediate emotion-induced synaptic plasticity and thereby restructuring of synapses at critical sites of the fear network. BDNF translates environmental stimulation via neuronal activity into structural changes in the brain. However, there may be a dark side of synaptic plasticity as anxiety and affective disorders are known to involve an (over-) activation of the brain’s fear circuit and may very well be associated with exaggerated synaptic plasticity in the amygdala (cf. Rattiner et al., 2005). In line with this hypothesis, a recent study (Gatt et al., in press) demonstrated that individuals homozygous for the BDNF66val allele that had been exposed to stressful events early in life had increased amygdala and medial prefrontal gray matter volumes and displayed elevated anxiety. Chronic stress enhances dendritic arborisation in the rat amygdala (Vyas et al., 2002), a process clearly reflecting synaptic plasticity and enlarged amygdala volumes have been reported in anxiety disorder patients (e.g. Juranek et al., 2006).
Hippocampal activations appear related to awareness of the CS-US contingency in human fear conditioning (Bechara et al., 1995; Knight et al., 2009), whereas amygdala activation is not (Tabbert et al., 2006; Knight et al., 2009). Therefore, it is interesting to note that our data suggest, that carriers of the BDNF66 met-allele also may show impairments in correctly reporting the CS-US contingencies. Thus, our data may provide evidence for a relationship of the BDNFval66met genotype with both amygdala (FPS) and hippocampus (contingency awareness) dependent learning in our fear conditioning paradigm. However, even though significantly more BDNF met-carriers failed to report the correct CS-US contingency after acquisition, the number of subjects in this comparison was very small and therefore, did not allow for analyses that subdivide this group further by the BDNFval66met genotype. Therefore, future studies with larger sample sizes and/or studies experimentally manipulating contingency awareness are warranted to follow up on this interesting suggestive evidence as it may have important implications for individuals placed in situations where it is critical to be able to learn to discriminate between danger and safety (e.g. military personnel or law enforcement officers).
Because our final sample included only two individuals homozygous for the BDNF met/met allele we were unable to discriminate between a possible linear effect or a heterosis (Chen et al., 2004) of the BDNFval66met genotype on FPS. It is important to note that excluding the two homozygous met-carriers from the analyses yielded the same statistical results. Given the infrequency of this genotype most human studies to date have merged carriers of one or two met-alleles to one met-carrier group. Despite the fact that in many studies no or very few met/met individuals are observed, authors usually talk about met-carriers when in fact only inferences about heterozygotes can be drawn. It is important for future studies to elucidate if heterozygotes and individuals homozygous for the BDNF66met allele in fact share the same phenotype.
Our results may have important implications for the genetic risk of developing affective psychophathologies. Hajcak et al. (2009) argue that carriers of the met-allele may be at risk for affective pathologies. However, following our arguments above, we take a different perspective. We suggest that the BDNF met-allele may rather be a protective factor for anxiety disorders (e.g. PTSD) due to the putative impairment of aversive memory acquisition/consolidation via reduced experience-induced synaptic plasticity within the amygdala. Indeed the BDNF met-allele has been associated with reduced neuroticism scores (for a meta-analysis see Frustaci et al., 2008) and has been shown to protect for structural abnormalities in the amygdala-anterior cingulated loop which are seen in carriers of the short-allele of the 5-HTTLPR polymorphism, which is a genetic variant associated with negative affect (Pezawas et al., 2008). However, studies on BDNF val66met knock-in mice suggest that the met-allele may be associated with anxiety (Chen et al., 2006; Yu et al., 2009). Associative (fear) learning is an adaptive process and impairments certainly threaten behavioral adaptation and ultimately successful living. Thus the role of the BDNFval66met polymorphism in clinical anxiety as well as the translation from knock-in mice to human studies needs to be elucidated by future studies using larger sample sizes.
Supplementary Material
Acknowledgments
This work was supported by grants from the Swedish Science Research Council (A.Ö), the Nordic Research Council for the Humanities and Social Sciences (NOS-HS, A.Ö.), and the National Institute of Mental Health, Center for the Study of Emotion and Attention (A.Ö.) and a grant from the Swedish Research Council (M.S.). T.B.L. was supported by the German Academic Exchange Service (Deutscher Akademischer Austauschdienst, DAAD). Thanks to Dr. Carmen Hamm for blood sampling and Dr. Heino Mohrmann for technical assistance.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/bne
Contributor Information
Tina B. Lonsdorf, Karolinska Institutet, Stockholm, Sweden; Stockholm Brain Institute, Stockholm, Sweden; Nordic Center of Excellence in Cognitive Control, NIH Center for the Study of Emotion and Attention.
Almut I. Weike, Ernst-Moritz-Arndt University of Greifswald, Greifswald, Germany
Armita Golkar, Karolinska Institutet, Stockholm, Sweden; Stockholm Brain Institute, Stockholm, Sweden; Nordic Center of Excellence in Cognitive Control, NIH Center for the Study of Emotion and Attention.
Martin Schalling, Karolinska Institutet, Stockholm Sweden.
Alfons O. Hamm, Ernst-Moritz-Arndt University of Greifswald, Greifswald, Germany
Arne Öhman, Karolinska Institutet, Stockholm, Sweden; Stockholm Brain Institute, Stockholm, Sweden; Nordic Center of Excellence in Cognitive Control, NIH Center for the Study of Emotion and Attention.
References
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. Journal of Neuroscience. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing DQ, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nature Neuroscience. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annual Review of Psychology. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Pozzi G, Gianfagna F, Manzoli L, Boccia S. Meta-Analysis of the Brain-Derived Neurotrophic Factor Gene (BDNF) Val66Met Polymorphism in Anxiety Disorders and Anxiety-Related Personality Traits. Neuropsychobiology. 2008;58:163–170. doi: 10.1159/000182892. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams HJ. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Molecular Psychiatry. doi: 10.1038/mp.2008.143. in press. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Castille C, Olvet DM, Dunning JP, Roohi J, Hatchwell E. Genetic variation in brain-derived neurotrophic factor and human fear conditioning. Genes Brain and Behavior. 2009;8:80–85. doi: 10.1111/j.1601-183X.2008.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm AO, Greenwald MK, Bradley MM, Lang PJ. Emotional learning, hedonic change, and the startle probe. Journal of Abnormal Psychology. 1993;102:453–465. doi: 10.1037//0021-843x.102.3.453. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val(66)met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juranek J, Filipek PA, Berenji GR, Modahl C, Osann K, Spence MA. Association between amygdala volume and anxiety level: Magnetic resonance imaging (MRI) study in autistic children. Journal of Child Neurology. 2006;21:1051–1058. doi: 10.1177/7010.2006.00237. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA. Neural substrates of explicit and implicit fear memory. Neuroimage. 2009;45:208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lindblom B, Holmlund G. Rapid DNA purification for restriction fragment length polymorphism analysis. Gene Analysis Techniques. 1988;5:97–101. doi: 10.1016/0735-0651(88)90003-9. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5 ′ nuclease assay. Genetic Analysis-Biomolecular Engineering. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Ohman A. Genetic Gating of Human Fear Learning and Extinction: Possible Implications for Gene-Environment Interaction in Anxiety Disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology-Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska Directed Emotional Faces (KDEF) [CD-ROM] Karolinska Institutet, Department of Clinical Neuroscience, Psychology Section; Stockholm: 1998. [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance – proposal for standardization. Psychophysiology. 1971;8:656. doi: 10.1111/j.1469-8986.1971.tb00501.x. &. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchninski BA, Chen G, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Molecular Psychiatry. 2008;13:709–716. doi: 10.1038/mp.2008.32. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learning & Memory. 2004a;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. Journal of Neuroscience. 2004b;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2004;126B:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage. 2006;32:761–770. doi: 10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learning & Memory. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Venables PH, Christie MJ. Electrodermal activity. In: Martin I, Venables PH, editors. Techniques in psychophysiology. Wiley; Chichester: 1980. pp. 3–67. [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wang Y, Pattwell S, Jing D, Liu T, Zhang Y, Bath KG, Lee FS, Chen Z-Y. Variant BDNF Val66Met Polymorphism Affects Extinction of Conditioned Aversive Memory. J. Neurosci. 2009;29:4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.