Abstract
A new ultra sensitive laser-based analytical technique, intracavity optogalvanic spectroscopy (ICOGS), allowing extremely high sensitivity for detection of 14C-labeled carbon dioxide has recently been demonstrated. Capable of replacing accelerator mass spectrometers (AMS) for many applications, the technique quantifies zeptomoles of 14C in sub micromole CO2 samples. Based on the specificity of narrow laser resonances coupled with the sensitivity provided by standing waves in an optical cavity, and detection via impedance variations, limits of detection near 10−15 14C/12C ratios have been obtained with theoretical limits much lower. Using a 15 W 14CO2 laser, a linear calibration with samples from 5 × 10−15 to >1.5 × 10−12 in 14C/12C ratios, as determined by AMS, was demonstrated. Calibration becomes non linear over larger concentration ranges due to interactions between CO2 and buffer gas, laser saturation effects and changes in equilibration time constants. The instrument is small (table top), low maintenance and can be coupled to GC or LC input. The method can also be applied to detection of other trace entities. Possible applications include microdosing studies in drug development, individualized sub therapeutic tests of drug metabolism, carbon dating and real time monitoring of atmospheric radiocarbon.
Keywords: Laser based isotope analysis, Carbon 14 tracers, accelerator mass spectrometry
Introduction
Carbon 14 (radiocarbon) is an ideal organic tracer having an extremely low natural abundance in living systems, near 1 ppt, and a long half life, 5730 years, ideal for clinical and laboratory tracer experiments. Until recently almost all quantitation of 14C content was by scintillation detection of the low energy beta particle emitted in its decay, a low efficiency method as only 1 beta particle is emitted per minute for every 5 billion 14C atoms present. Only Accelerator Mass Spectrometry (AMS), first developed to extend carbon dating to smaller and older samples, has been able to achieve the specificity and sensitivity required for 14C atom counting. Constraining the wider use of AMS for many studies, however, are the size, cost and complexity of the analysis system as well as the fact that samples must contain of the order of 0.5 mg total carbon.
We have recently reported[1] a new technique- IntraCavity OptoGalvanic Spectroscopy (ICOGS) that also meets the requirements for 14C quantitation at AMS levels. ICOGS combines aspects of the laser assisted ratio analyzer (LARA) technique[2] and intracavity absorption spectroscopy[3] (ICAS). A special 14CO2 laser replaces the tandem accelerator of AMS. Specificity is based on the existence of large isotope shifts in CO2 molecular spectra. Sensitivity is achieved via the laser optogalvanic effect (OGE). As discussed at the previous AMS Conference[4], LARA’s sensitivity and versatility make new classes of isotope ratio measurement systems possible[5].
Optogalvanic Measurement Principle
The basic concept of OGE detection has long been used in atomic and molecular spectroscopy[6]. It is based on the electrical response of a gas discharge to an optical perturbation. There exist several significant advantages of OGE measurements compared to ion beam and other spectrometric stable isotope measurement technologies:
The measured parameter is electrical so that filtering and averaging techniques can be used to achieve extremely low noise.
No optical measurement is required- eliminating all collection and dispersion optics and light transducers.
The measurement is nondestructive, hence the technique makes it possible to recycle samples.
The measurement system can be varied, within limits, in order to enhance signal to background ratios by controlling gas mixtures (typically 5% CO2, 95% N2), pressure (typically 5 to 10 mbar) and electrical discharge power (typically 2 to 5 Watts).
Optogalvanic spectroscopy makes use of the fact that laser resonances in CO2 are isotope dependent [7]. In these studies, a low pressure rf glow is utilized due to its stability and low inherent noise [8]. The laser transition chosen for 14CO2 must be well separated in wavelength from any transition in 12CO2 or 13CO2 and from any transition in a CO2 molecule with 18O (.02% natural abundance) or 17O (.004% natural abundance). The strongest lasing transitions observed in 14CO2 are at 11.8 microns and 11.3 microns, significantly longer in wavelength than lasing transitions of the other “stable” isotope CO2 lasers. Any 14CO2 molecule in the lower or upper laser level is automatically in resonance (absorption or stimulated emission) with the narrow band laser, providing the sharp specificity required for isotope ratio analysis. The nearest 13CO2 and 12CO2 lines are separated by more than 500 linewidths, leading to non resonant cross sections reduced by approximately 10 orders of magnitude. The nonresonant interaction is further reduced for 13CO2 and 12CO2 by rotational state Boltzmann population ratios for the two isotopes. In any case, this small background effect is pressure and laser wavelength dependent, measurable and does not seriously limit the ultimate 14CO2 detection limit.
The OGE is the electrical response of a gas discharge to an optical perturbation. A laser of intensity I and frequency ν, modulated at a frequency f changes the electrical properties of a glow discharge in phase with the modulation. If the laser is incident on a cylindrical (length L and radius R) weak electrical discharge, the electrical response, S, of the discharge can be expressed by an integral over the laser-discharge interaction volume:
| (1) |
The density of interacting species is n and σ (ν) refers to the laser-species interaction cross section. S can be any discharge parameter related to conductivity and K is a corresponding optogalvanic proportionality constant that depends on the details of the electrical discharge.
To a good approximation, on resonance, for cases where light absorption is small and K is independent of n, the average steady state electrical response simplifies to a product:
| (2) |
Here, n represents the average molecular density of interacting particles, Leff, the effective length of the interaction region, I the average laser intensity and A the average area of the laser beam. For an external cavity Leff is the physical length of the cavity or some multiple of L for a multi-pass cavity. Physically, what happens is that the light changes the equilibrium distribution of species, including excited species in the discharge. This affects collision rates, including those between molecules and electrons, leading to a measurable conductivity change, S, of the ionized gas.
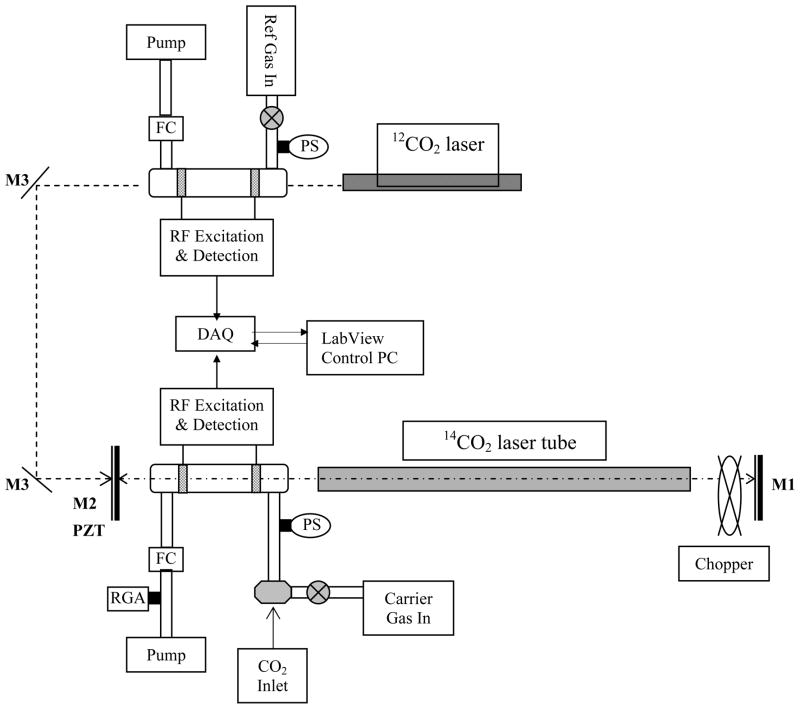
Intracavity Optogalvanic Spectroscopy (ICOGS), is an extension of external cavity studies where the main experimental change is the placement of the analyte cell inside the laser cavity. A schematic drawing of the experimental setup is shown in Figure 1. The laser beam is modulated using an electronically controlled shutter. The laser output beam can be used to monitor power and wavelength as well as for parallel external cell measurements if required. The heart of the instrument is the 14CO2 laser. In principle any 14CO2 laser that can be wavelength stabilized is sufficient. The size of the system (maximum power) depends on the 14CO2 concentration range of interest. For this work a 15 Watt laser was employed. For enriched samples, such as for biomedical tracer studies a lower power laser would suffice. A typical laser tube contains of order 1 to 2 mC of highest enrichment 14CO2. We have used sealed laser tubes so that there is no radioactivity present in the laboratory. In the highly unlikely event of tube breakage, the gaseous species would be rapidly diluted to background levels due to gas diffusion. Safety issues are minimal as the allowable level of intake (ALI) of 14CO2 is 200mC so that in the highly unlikely event of someone inhaling the entire quantity from the laser tube, that person would only receive less than 1% of their annual dose limit.
Figure 1.
Experimental configuration: The OGE cell inside the cavity has Brewster windows to reduce losses. The C12 laser incident on the OGE cell provides a “C12 signal” that is used for normalization of the C14 signal. The shutter inside the laser cavity is for modulating the 14CO2 laser. M1: High reflective mirror & grating, M2: 85% reflective output coupler, M3: Gold plate mirror, PS: Pressure Sensor, FC: Flow Controller, RGA: Residual Gas Analyzer, DAQ: Data Acquisition Board
A small laser (2 to 5 Watt) is used for measuring the 12C signal of the sample for normalization. Though only about 10% (The transmission of the 14CO2 laser output coupler) of the 12CO2 laser light traverses the sample cell without back reflection, the 1012 times higher 12CO2 density provides an easily measurable signal. The analyte is placed in a quartz cell, 10 cm in length and 2.5 cm OD, with ZnSe Brewster angle windows. With sample pressure held constant- typically between 1 and 5 mbar, a low power (2 to 5 Watt) rf discharge is ignited and maintained via external copper electrodes. The oscillator power supply circuit also monitors the average rf voltage amplitude across the discharge for the OGE signal. The 12CO2 and 14CO2 laser beams simultaneously passing through the sample cell are chopped at different frequencies, and the resulting voltage variation across the cell is measured. The Fourier Transform values of the voltage signal at the two different frequencies yield the 12CO2 and 14CO2 OGE signals. Measurements could be run in a sealed cell (batch mode) or continuous flow mode with flow rates of 0.1 to 2 sccm.
Results
Time averaged OGE signals were obtained using a National Instruments Data Acquisition System. The virtual instrument designed controlled the shutter, and monitored pressure, 12CO2 concentration and laser wavelength data. An OGE output from the 14CO2 laser tube was used to stabilize laser wavelength and for laser power normalization
This intracavity method is analogous to the well known intra-cavity laser absorption spectroscopy (ICLAS) making the sample subject to full laser saturation power with an enhanced interaction length depending on details of the laser cavity. The major difference from ICLAS is that the detection is via the OGE. The intra-cavity laser absorption, in fact, is far too small to detect.
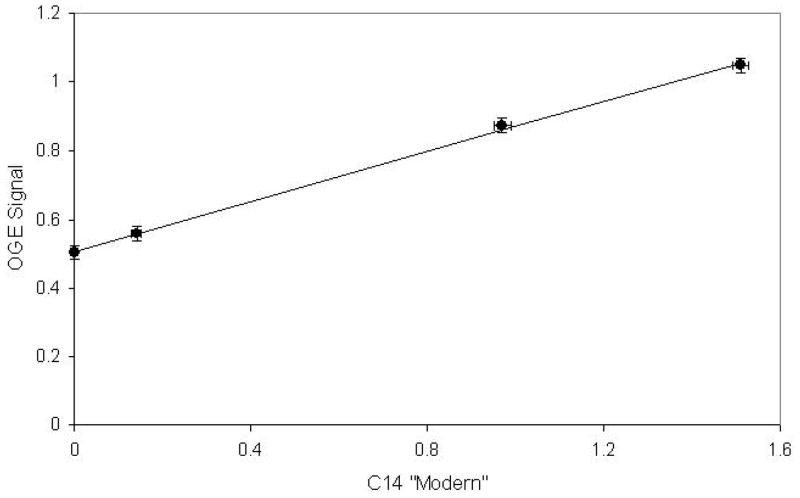
Typical results obtained with the system are shown as a calibration in Figure 2 where the normalized OGE signals are plotted against the actual 14C concentrations measured with AMS. These data demonstrate that the ICOGS system has a linear response in the “dead CO2” to 10 Modern range. Limits of detection are such that 14C in ambient air can be detected. An extrapolation of the present data yields an estimated sensitivity near a 14C/12C ratio of 10−15.
Figure 2.
Calibration Curve: OGE signals obtained with the ICOGS system plotted against the AMS results. The vertical error bars are determined by voltage averaging statistics.
The observed enhancement, compared to a single-pass configuration, is due to the nature of the optogalvanic effect in the optical cavity. In equation 2 above I is the circulating internal laser power, around 50 W in our case, and Leff the effective interaction length is now greatly enhanced due to the standing wave. A simple estimate would have L enhanced only by the average number of reflections of a single photon, where the major loss is due to the output coupler. However, for the cw laser system, loss in the OGE cell is balanced by the gain of the lasing medium keeping the internal circulating power constant. Now, the effective interaction length is given by Leff=cΔt, where Δt is given by half the chopping period, or the time that the laser is on. For a modulation frequency of 63 Hz, Δt is 7.9 msec yielding an effective interaction length of almost 2500 km. This value is close to our observed maximum enhancement of ~107 for the intracavity configuration compared to single pass. However, there are complex saturation effects that can limit Leff in many instances [9]. In a multimode ICAS experiment, Δt is given by the spectral saturation time and can be as long as several seconds[3]. In an ICAS experiment what is observed is a change in laser output. The effect on laser output of the few thousand 14CO2 molecules in the OGE analysis cell is negligible compared to the 1019 active 14CO2 molecules in the laser cavity. However, all photon interactions in the intracavity cell contribute to the OGE.
It should be noted that the optogalvanic proportionality factor for 14CO2 in equation 1 is a complicated function of partial pressure for all gas constituents as well as discharge conditions [8], even changing sign as the system goes from absorption to gain conditions. Also, equation 1 assumes thermal equilibrium, a situation only easily achieved at low concentration and long time. These effects make a linear calibration of the system over a dynamic range in 14CO2 concentration difficult. However, we believe that an automated continuous flow system can be engineered to vary pressure as well as laser power in order to extend the dynamic range up to the scintillation counting regime.
Comparisons with AMS
Though both 14C LARA and AMS aim to quantify 14C in carbon containing samples, there are several significant differences in the technologies. These differences are briefly indicated in the table below (Table 1). Unlike the AMS technique, it is not necessary to separate the analyte chemically for the LARA method, since it is chemical and isotopic species selective via a narrow laser resonance. In the LARA method, the sample is analyzed via the measurement of electrical conductivity whereas the detection is sample destructive via ion current collection for AMS analysis. The LARA device can handle samples of smaller sizes, in the range of micrograms, as opposed to mg sample sizes needed for AMS, hence there is no need to dilute samples with carrier. Furthermore, the analyte in the LARA technique must be in the form of CO2 while AMS machines, at present, require the sample to be in the form of graphite. LARA sensitivity can be amplified by increasing laser power providing a “gain” over single atom counting. The 12C and/or 13C normalization is straightforward with our system for samples of any enrichment, using 12CO2 and 13CO2 lasers as is now done with existing breath and environmental monitoring instruments.
Table 1.
Comparison of AMS to ICOGS
| AMS | ICOGS | |
|---|---|---|
| SPECIFICITY | Mass via trajectory and specific energy loss of ion | Species via laser resonance |
| DETECTION | Particle counts (≈1%) (destructive) | Electrical conductivity (non destructive) |
| GAIN | NO | YES |
| SAMPLE PREPARATION | Reduction to Elemental carbon | Oxidation to Carbon dioxide |
| SAMPLE SIZE | Of order 1 mg | ≥10 μg |
| SENSITIVITY | ≈10−15 | ≈10−15 or better |
| NORMALIZATION | 12C−, 13C n+ | 12CO2, 13CO2 |
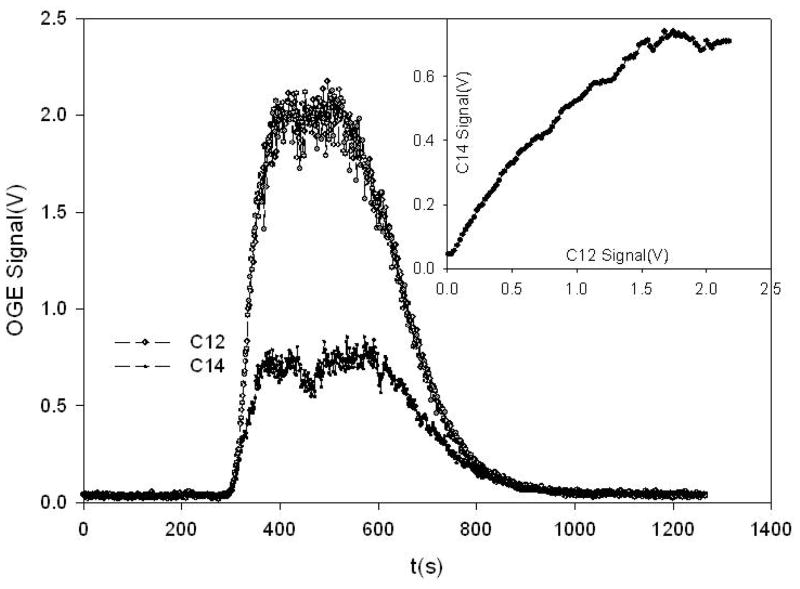
In addition, the small sample required and the possibility of continuous flow or batch processing should permit development of combined instrumentation with gas chromatographs or liquid chromatographs coupled to an oxidizer as a front end[10,11]. The ICOGS system can run with continuous flow as shown in figure 3 for 1ml CO2 injected into a gas stream of CO2 free air as carrier. The buildup and loss of 12C and 14C is obvious. The saturation effect present when the concentration reaches around 1% is also obvious. Modeling of this effect using ICAS theory, [3,9] and OGE theory [12] is in progress.
Figure 3.
Variation of OGE for 12C (top) and 14C (bottom) as 1 ml is injected into the CO2 free buffer gas flowing at 10 SCCM. As shown in the inset, the signals scale at low concentrations, but the 14C signal saturates at a much lower concentration than the 12C.
The measurement sensitivity is so great that carbon dating using microgram samples can easily be envisioned. Limits of precision and sensitivity are yet to be determined but should equal or exceed those of AMS. Use of an external calibration cell and double ratio measurements as is done to determine 13C variations at the part per 10 thousand[5] level should also be possible with 14C. The engineering challenge will be in the handling of such small samples and eliminating the ubiquitous background 14CO2. We further note that the technique may be extended to other trace atom or compound analysis with tunable or fixed frequency lasers.
In summary, we have shown that the 14C LARA technique has a measurement sensitivity at the order 10−15 Modern levels. Possible applications include microdosing studies in drug development, individualized sub-therapeutic tests of drug metabolism, carbon dating of small samples and real time monitoring of atmospheric radiocarbon. The operational, size and potential cost advantages make the LARA device a promising alternative to AMS.
Acknowledgments
This work has been supported by the US NIH (Grant 5R33RR018280), NSF (Grant DBI0456241) and Merck Research Laboratories as well as by the NIH Resource for Biomedical AMS at Lawrence Livermore National Laboratory who have supplied reference samples. Helpful discussions with John Vogel, Ted Ognibene and Martin Schaden are appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murnick Daniel E, Dogru Ozgur, Ilkmen Erhan. Analytical Chemistry. 2008;80:4820–4824. doi: 10.1021/ac800751y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murnick DE, Peer BJ. Science. 1994;263:945–94. doi: 10.1126/science.8310291. [DOI] [PubMed] [Google Scholar]
- 3.Baev VM, Latz T, Toschek PE. App Phys B. 1999;69(3):171–202. [Google Scholar]
- 4.Murnick DE, Dogru Ozgur, Ilkmen Erhan. Nuclear Instruments and Methods in Physics Research B. 2007;259:786–789. doi: 10.1016/j.nimb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murnick DE, Okil JO. Isotopes in Environmental and Health Studies. 2005;41(4):363–371. doi: 10.1080/10256010500384440. [DOI] [PubMed] [Google Scholar]
- 6.Barbieri B, Beverini N, Sasso A. Optogalvanic spectroscopy. Rev Mod Phys. 1990;62:603–644. [Google Scholar]
- 7.Freed C. Tunable Lasers Handbook. Chap 4. Academic Press; New York: 1995. CO2 Isotope Lasers and Their Applications in Tunable Laser Spectroscopy. [Google Scholar]
- 8.Moffet S, Smith ALS. Phys D: Appl Phys. 1984;17:59–70. [Google Scholar]
- 9.Atmanspacher H, Scheingraber H, Vidal CR. Phys Rev A. 1985;32:254–267. doi: 10.1103/physreva.32.254. [DOI] [PubMed] [Google Scholar]
- 10.Krummen, et al. Rapid Comm in Mass Spectrometry. 2004;18:2260–2266. doi: 10.1002/rcm.1620. [DOI] [PubMed] [Google Scholar]
- 11.Liberman Rosa G, Tannenbaum Steven R, Hughey Barbara J, Shefer Ruth E, Klinkowstein Robert E, Prakash Chandra, Harriman Shawn P, Skipper Paul L. Anal Chem. 2004;76:328–334. doi: 10.1021/ac030181y. [DOI] [PubMed] [Google Scholar]
- 12.Smith ALS, Moffet S. J Phys D: Appl Phys. 1984;17:71–78. [Google Scholar]