Abstract
Primary liver cancer is a major health problem worldwide, with more than 500,000 new cases diagnosed yearly. Preliminary results suggest excellent local control rates of intrahepatic malignancies treated with stereotactic body radiation therapy (SBRT), but some patients have experienced life-threatening toxicity because the current approaches cannot accurately estimate residual liver function after treatment. An early-phase trial of SBRT in hepatocellular carcinoma patients, including those with compromised liver function, is described. Patients are treated with three fractions of SBRT, then treatment is paused for 4 weeks and liver function is evaluated by means of an indocyanine green assay. The size of the final two fractions of SBRT is determined based on the patient’s indocyanine green assay after the first three fractions, so that the therapy is personalized to each patient’s sensitivity to radiation. The sensitivity to the liver of the final two fractions of SBRT, compared with the first three fractions, is re-estimated using a Bayesian model throughout the trial, so this is an adaptive trial. The operating characteristics of the trial are described by Monte Carlo simulations.
Keywords: adaptive trial design, hepatocellular carcinoma, indocyanine green, radiation-induced liver disease, radiofrequency ablation, stereotactic body radiation therapy, transarterial chemoembolization
Primary liver cancer is the fifth most common neoplasm, and the third most common cause of cancer-related death [1]. In some areas of Asia, hepatocellular carcinoma (HCC) is the most common cause of death owing to cancer. The American Cancer Society estimates that 30,890 people were diagnosed with HCC, gall-bladder cancer or cholangiocarcinoma in the USA in 2008 [2], and the incidence has been increasing in Europe [3] and in the USA [2,4]. Primary liver cancer is one of four cancer sites for which death rates increased between 1990 and 2004, and is the only cancer that has demonstrated more than a 10% increase [2].
Radiofrequency ablation (RFA) is an established treatment for small, unresectable HCCs, and for cancer metastatic to the liver. However, the effectiveness of RFA is limited by tumor size (typically ≤ 4 cm), number of lesions and the geometry of tumor(s) within the liver. Furthermore, patients with HCC who have undergone previous resections or ablations may have significant underlying liver dysfunction, which makes it difficult to design a safe and effective therapy for all patients without a full understanding of each patient’s tolerance to treatment. Likewise, although transarterial chemoembolization (TACE) has been demonstrated to be superior over the best supportive care for patients with HCC, good performance status and tumors less than 6 cm, essentially all patients will ultimately recur. Thus, improved treatment approaches are needed for patients with intrahepatic cancer who cannot undergo resection or transplant.
Recent advances in the delivery of radiation treatment allow for high-precision, high-dose stereotactic radiation techniques, once reserved for intracranial malignancies, to be applied to extracranial sites. Preliminary results suggest excellent local control rates (above 90%) for liver metastases treated with stereotactic body radiation therapy (SBRT) [5–9] using various dosing algorithms, including 60 Gy in three fractions, 50 Gy in five fractions, or 28–60 Gy in six fractions. However, these studies have been carried out on previously untreated patients with metastatic tumors less than 6 cm in size. We hypothesize that it is possible to extend SBRT to ‘nonstandard’ patients, who have had previous liver treatment (such as resection, TACE, RFA or SBRT) or who have primary HCC. These patients are at risk for toxicity from approaches such as 60 Gy in three fractions, and we still only have a limited ability to predict who will develop toxicity based solely on pretreatment characteristics and volume of liver irradiated, particularly for patients with HCC and cirrhosis.
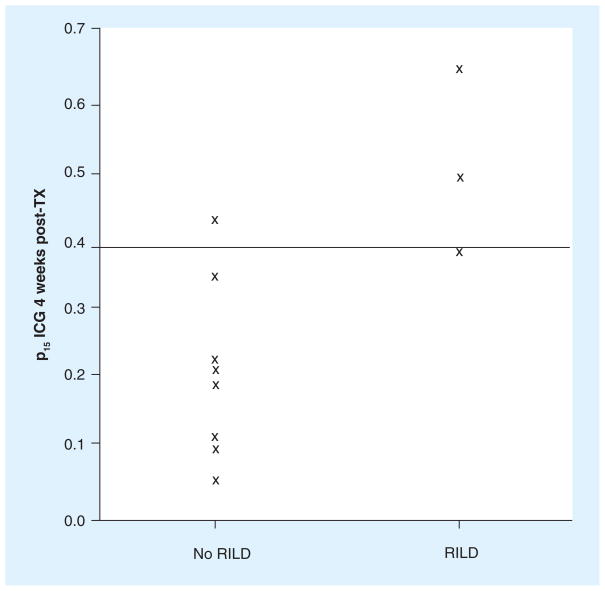
Attempts to deliver high-dose radiotherapy to the liver have been limited by the development of radiation-induced liver disease (RILD) [10,11]. Symptoms generally occur 1–2 months following completion of radiotherapy, and include tender hepatomegaly and weight gain secondary to ascites. The clinical outcomes range from mild, reversible damage to rare fatality [8,12,13]. Investigators at the University of Michigan (MI, USA) have developed and refined a quantitative model to estimate the risk of RILD at the treatment planning stage based on dose to the liver [14], which guides the initial radiation dose selection. In addition, we have adopted the use of an indocyanine green (ICG) assay to monitor patients’ liver function during treatment. Following intravenous injection, ICG is rapidly bound to plasma protein, and is removed from plasma almost exclusively by the hepatic parenchymal cells and secreted entirely into the bile. Therefore, the serum clearance rate, expressed as the proportion of the injected dose retained by the body 15 min after administration (p15), serves as an index of liver function, and has been used in thousands of patients to plan the extent of safe surgical resection [15–19]. Our preliminary study of patients undergoing fractionated radiation (from an open protocol approved by the University of Michigan Institutional Review Board) demonstrates that the pretreatment ICG clearance rate alone is not useful for predicting if a patient will develop RILD after treatment, but that post-treatment ICG clearance is significantly reduced in patients who soon develop RILD (Figure 1). That is, the ICG assay is an assessment of the function of the liver at the current time, but is not an indicator of the sensitivity of the liver to radiation. A large Japanese series of cases has retention of more than 39% determined that p15 of an ICG dose is indicative of an excess risk of clinically significant liver dysfunction [17].
Figure 1. Proportion of indocyanine green retained 15 min after administration in patients studied up to July 2008 in an institutional review board-approved protocol.
The horizontal line at 0.39 indicates the criterion for high likelihood of liver dysfunction used in the protocol [17]. ICG: Indocyanine green; p15: 15 min after administration; RILD: Radiation-induced liver disease; TX: Treatment.
Based on these technologies – SBRT, initial treatment planning that controls the risk of RILD using the normal tissue complication probability (NTCP) model, and ICG assay for ongoing monitoring of liver function – we propose a new approach to SBRT, in which therapy is divided into two stages, with a 4-week pause between stages. This treatment pause permits the assessment of the effect of the first stage of therapy on the patient’s liver function before administering the second, final stage of treatment. Our primary aims are to characterize the safety of this approach and to estimate the proportion of patients experiencing lesion control. Our goal is to achieve the same or superior lesion control rate in patients who have been pretreated or have compromised liver function that has been achieved for previously untreated patients using RFA, TACE or primary SBRT, while controlling the risk of toxicity.
Basic trial design
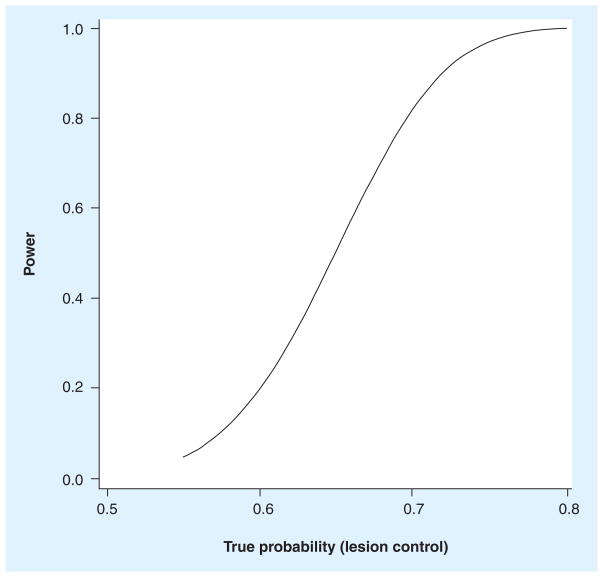
This Phase II, single-arm trial has lesion control, defined as no growth in any treated tumor 6 months after treatment, as the primary end point. Eligible patients include those with hepatic metastases, biopsy-proven HCC, a discrete hepatic tumor (by the Barcelona criteria), or a discrete hypervascular tumor; they must not be eligible for curative resection, and they may have been previously treated. We are not interested in pursuing this treatment modality further if less that 55% of these ‘nonstandard’ patients achieve 6-month lesion control, so the primary null hypothesis is H0: Π < 0.55 (where Π represents the proportion of patients experiencing lesion control); the trial will accrue 70 patients, for 80% power using a one-sided exact test (α = 0.05), as described in Figure 2. There are statistically justified early stopping rules for both inadequate efficacy (lesion control) and excessive toxicity (RILD), with reviews after every ten patients are treated [20]. Accrual to the trial is expected to be complete within 3 years.
Figure 2. Estimated statistical power of the trial.
Power to test the null hypothesis that the proportion of patients experiencing lesion control (Π) is less than 0.55, using a one-sided exact binomial test for n = 70.
Treatment personalization of SBRT using ICG
In this protocol, each patient receives three initial SBRT fractions in the first stage of treatment, with the dose chosen to limit the probability of RILD after all five fractions to 0.15, using the established NTCP model. Liver function is assessed by means of an ICG assay at baseline (just prior to the initiation of the first stage of treatment) and approximately 4 weeks after the completion of the first stage of SBRT. An additional two SBRT fractions may be given after this assessment, but patients with unacceptably slow ICG clearance, indicative of compromised liver function, will receive no additional therapy. Patients with normal liver function (as assessed by ICG assay) will receive the full initial dose for the last two fractions. Patients who would be at significant risk of impaired liver function at the end of the treatment (if they received five full-dose fractions) will receive a reduced dose for their final two fractions.
Data from previously treated patients is represented by an estimated parameter in the individualization model, which is updated continually throughout the trial. The primary indicator of liver function at any time is the p15 proportion of ICG. The basic concept is to use the change in liver function over the first treatment stage to predict the change over the second treatment stage, and adjust the second fraction size if it appears that the probability is high that the patient’s liver function will be unacceptably decreased by the fraction size originally planned. In addition, as the trial progresses, the differences in patient ICG responses to the second stage of treatment, compared with their ICG responses to the first stage, will be incorporated into the ICG response model, so that this is an adaptive trial of a personalized therapy.
To make the process explicit, we will record the three observed ICG 15-min retention proportions (p15 before stage 1, before stage 2 and after stage 2) of patient i as k0i, k1i and k2i, and the SBRT stage 1 and stage 2 fraction (radiation dose) sizes as d1i and d2i. The stage 1 fraction size, d1i, will be determined using the NTCP model, and will be typically between 4.5 and 12 Gy per fraction. The sensitivity of liver function to SBRT stage 1 is b1i = (k1i − k0i)/(3 × d1i); the 3 in the formula represents the three fractions in stage 1. Similarly, the sensitivity of the total stage 2 dose equals b2i = (k2i − k1i)/(2 × d2i) (there are only two fractions in stage 2). An estimated parameter γ, representing the ratio b2i:b1i, relates the change in the second stage to the change in the first stage. The algorithm for selecting the stage 2 fraction, the dose d2i, such that P{k2i < 0.39| k1i < 0.39} > 0.9, is:
If i = 1, set γ = 1.5. Otherwise, for each patient who has completed treatment, h = 1, i − 1, calculate the values b1h = (k1h − k0h)/(3 × d1h) and b2h = (k2h − k1h)/(2 × d2h), and the ratio b2h:b1h.
If i > 1, estimate the posterior distribution of γ from the known ratios b2h:b1h on all previously treated patients. Using that distribution, find γ* such that P{γ < γ*} = 0.9.
Set d2i = max(min[(0.39 − k1i)/(2 × γ* × b1i),d1i],0).
The posterior distribution of γ in step 2 above, is estimated by means of Markov chain Monte Carlo [21], assuming that γ~Gaussian(μ, σ2), μ~Gaussian(1.5,0.25) and σ2~Γ(2.5,0.08). γ* is determined from the median of μ + φ−1(0.9)σ2, sampled from the posterior distribution.
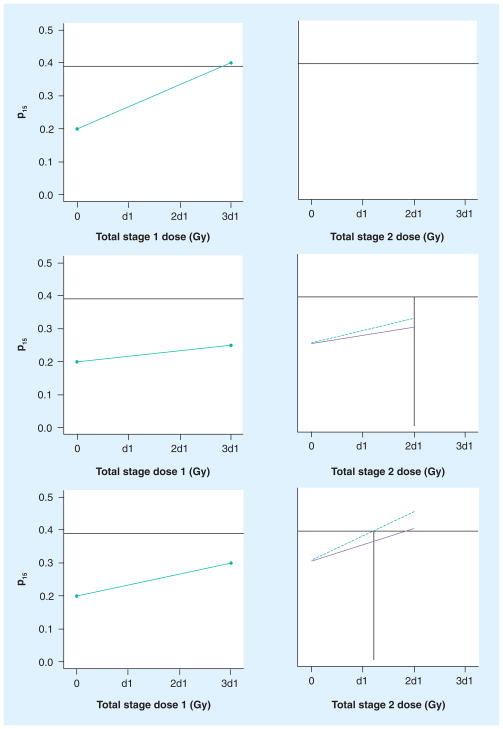
The application of this algorithm is illustrated in Figure 3 by the following cases. In case 1 (top frames of Figure 3), a patient begins the trial with k0i = 0.23 and, after SBRT part 1, has k1i = 0.40. This patient will receive no stage 2 treatment (d2i = 0) because he has already exceeded the upper boundary for liver function. We will be unable to use this patient’s data in the subsequent estimation of γ. In case 2 (middle frames), a patient begins the trial with k0i = 0.18, has k1i = 0.22 after SBRT stage 1, and the current estimate of γ* is 1.3. She will receive full SBRT in stage 2 (d2h = d1h). In case 3 (bottom frames), a patient has k0i = 0.20 and k1i = 0.30, and the current estimate of γ* is 2.3. His stage 2 fraction size will be 0.65 times his stage 1 fraction size (d2h = 0.65 × d1h), since two full fractions would be expected to result in estimated k2h = 0.30 + (0.30–0.20) × 2.3 × 2/3 = 0.45.
Figure 3. Three scenarios for stage 2 dosing.
The left column of frames indicates the observed change in indocyanine green clearance (p15) after treatment in stage 1; the right column of frames indicates the predicted change in p15 clearance in stage 2 under the baseline assumption (solid line) and after consideration of the patient’s observed stage 1 change (dashed line). The vertical lines indicate the calculation of the patients’ total stage 2 dose (horizontal axis). Top: Patient’s p15 exceeds 0.39 after stage 1 and receives no stage 2 treatment. Middle: Patient’s p15 is predicted to be less than 0.39 after full dosing and receives stage 2 fraction size equal to stage 1 size. Bottom: Stage 2 fraction size is reduced compared with stage 1.
d1: Dose per fraction in stage 1; p15: 15 min after administration.
Justification of design
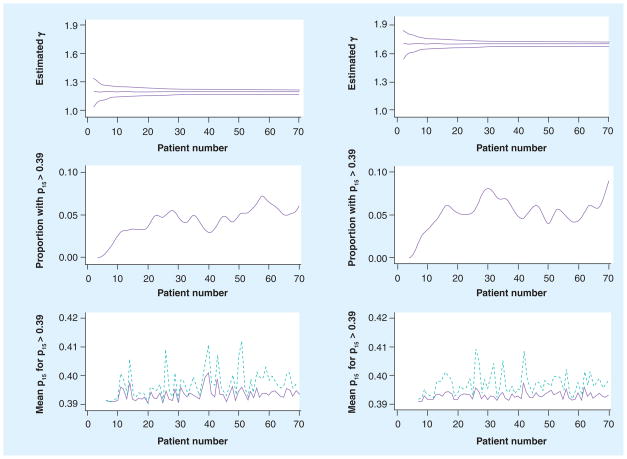
Some of the goals of this trial are typical of early Phase II oncology trials: estimation of the proportion of patients who experience lesion control; characterization of survival and time to progression; description of clinically significant toxicities. Accrual of 70 patients is planned, justified using typical statistical power arguments based on the lesion control end point (Figure 2). However, some features unique to adaptive trials also require justification. Since an adaptive trial has, by definition, one or more control parameters that are re-estimated during the course of the trial, it is of interest to estimate, for instance, how quickly that estimate converges to a value reasonably close to the truth, how the patients are affected by that rate of convergence and how robust the trial design is if the initial assumptions are grossly different from the truth. We described these features using Monte Carlo simulation, as displayed in Figure 4. The trial process, with estimation of γ and stage 2 doses as described above, was simulated. For the simulations, values of k0i and k1iwere drawn from γ distributions, and the true value of γ, the primary parameter of interest, was drawn for each patient from a γ distribution with mean of either 1.2 or 1.7 (representing lower and higher sensitivity to radiation, respectively). A total of 200 trials were simulated. Figure 4 displays, as patients accumulate, the quality of the estimation of γ and its effect on dosing. It is seen that in both cases the estimate of γ is very good, and that the variation in success in dosing at stage 2 is primarily a function of between-patient variation, which is irreducible. The decision rule, as proposed, is very conservative, which is intentional in this early-phase trial where toxicity is more of a concern than potential undertreatments; a positive result in the current trial would motivate a larger trial that would seek to maximize the probability of uncomplicated success, rather than emphasize toxicity control. In addition, since the data are being continually reviewed, a decision could be made during the trial to revise the protocol to reduce the probability of undertreatment.
Figure 4. Operating characteristics of the adaptive aspects of the trial.
The left column is for simulated trials where the true value of γ is 1.2, and the right column where the true value is 1.7. The top row displays the median and 5 and 95% percentiles of posterior distribution of γ as the patient number increases. The middle row shows the proportions of patients with k2i > 0.39. The bottom row displays the mean (solid line) and maximum (dashed line) of k2i for those patients with k2i > 0.39.
p15: 15 min after administration.
Because we do not expect a significant number of clinically significant RILD cases, re-evaluation during the trial of the relationship between the ICG criterion for unacceptable liver function (p15 > 0.39) and RILD is not a primary objective, although that may occur at the end of the trial.
Discussion
Radiation therapy for cancer is already personalized, in that the dose is sculpted to match the size and position of the patient lesions, and delivery is planned to minimize the exposure of normal functioning organs to potential damage. The trial presented here is novel, because each patient’s therapy is re-planned based upon the actual effect the first part of therapy has on his or her normal organ function. In the future, as metabolic and functional scans are validated to clinical outcomes, we expect function to be monitored and therapy to be adjusted throughout each patient’s treatment, to maximize the expected normal function at the end of treatment. This trial represents an early step in that direction.
This is the first use of a biomarker-adaptive design in a therapeutic trial and of SBRT in liver cancer at the University of Michigan, and accordingly, the trial has some features that are conservative. The second stage radiation dose fraction size (d2i) is constrained to be no more than the first stage fraction size, the calculation of which will be based on parameters estimated from an extensive series of patients treated there; those parameters will not be adaptively re-estimated during the trial. While it is well established that increasing the radiation dose results in improved probability of tumor control, at the cost of increased probability of RILD, it was felt that any excess toxicity in patients with compromised liver function (who currently have limited treatment options) would preclude further development of this potentially beneficial treatment, and that increasing the radiation dose at stage 1 or within patients at stage 2, would have to await the demonstration that RILD could be successfully controlled in the population of patients with potentially compromised hepatic function when treated with SBRT. Further advancement of the dose, by allowing increases in the second stage dose, is a goal of the research program, but was not thought to be feasible in the current trial.
This trial has several features common to early-phase personalized trials. The first feature is that of the adaptive design, where the personalization parameter (here, the angle of the elbow of the piecewise model, γ) is continually re-estimated throughout the trial. It should be expected that early trials of a given therapeutic strategy will be based on some sort of parameterized model, explicit or possibly inherent in a treatment algorithm, such as a dose–response model or assay cut point, which is derived from animal data or a retrospective review that needs to be validated as soon as possible for the personalized therapy to be successful. Given contemporary statistical tools, there is no reason to wait until the end of the trial to use the valuable data that are accumulated by the trial for that validation.
The second feature is the reliance upon a simple model relating liver function to accumulated radiation that we acknowledge is, at best, a rough approximation to reality. There are a number of models already described in the literature relating radiation dose to tissue damage that would be expected to also relate radiation dose to liver function (since we would expect a consequence of tissue damage to be a reduction in function), including the NTCP model employed for initial dose planning. However, these models have multiple parameters, which we would be unable to effectively re-estimate until the trial was completed, while a useful estimate of the single parameter describing the elbow of our piecewise linear model can be obtained relatively early in the present trial, as shown by Figure 4. At the end of the trial, data analysis will not be constrained to the piecewise linear model; given the three ICG assessments over each of 70 patients, we will have a rich set of data from which to construct models that better represent the change in liver function in response to accumulated radiation doses. Designers of trials with adaptive re-estimation of personalization parameters should not be embarrassed to employ simple models that are explicitly provisional, and view those models as management tools for the trial as it is conducted, and then should not be restricted to using those provisional models when performing the final analysis. We have used this strategy successfully, for instance, in our use of the time-to-event continual reassessment method paradigm for Phase I dose-escalation trials with late-onset toxicities [22,23].
The third aspect of this trial that is, or should be, common to adaptive and personalized trials, is the use of the Bayesian modeling paradigm for re-estimation of trial control parameters while the trial is ongoing. We believe that this should be a part of every experiment that translates results from animal models or retrospective studies into a clinical context. The Bayesian context of the prior distribution expresses the state of our knowledge at the beginning of a trial well; investigators have initial beliefs regarding the values of trial control parameters based on previous rigorous scientific work, but owing to differences in context, they express uncertainty regarding those beliefs. Some trialists are uncomfortable with the concept of the prior distribution, because it has sometimes been presented as an arbitrary or manipulative concept, but if viewed simply as a mechanism for expressing uncertainty at the outset of the trial quantitatively, its utility in early-phase assessments of drugs or treatments becomes apparent. The prior distribution is continually updated using the data that accumulate throughout the trial (generating the posterior distributions), so that each patient is treated based on a model using all the data available at the time the patient arrives. This is especially important when treating patients with therapeutic intent where the possibility of serious, irreversible toxicity is present.
In summary, we believe that the use of SBRT with monitoring of liver function during treatment and subsequent personalized recalculation of dose has the potential to increase the therapeutic benefit to patients with extremely serious disease, while controlling the probability of toxicity, and that the trial of that therapy presented here represents a paradigm for the development of early-phase studies of personalized therapy.
Future perspective
The use of ICG as a monitor of whole-liver function is an intermediate technology, which will be superseded by noninvasive scans that measure localized liver function. Once one or more of these scanning technologies have been validated, initial liver radiation treatment plans will be customized to maximize expected liver function post-treatment, given pretreatment variations within a patient’s liver. Subsequently, localized liver function will be measured during the course of treatment to assess unanticipated responses to radiation treatment, thereby replacing the single parameter γ described here with a 3D array of parameters that describe localized sensitivity to radiation. The integration of 3D functional scanning with complex treatment modalities, such as SBRT, presents a significant challenge to radiation treatment planners and statisticians alike.
Executive summary
Radiofrequency ablation and transarterial chemoembolization are established treatments for hepatic cancer patients with good performance status and small tumors, but essentially all patients’ disease ultimately recurs.
Preliminary results suggest excellent local control rates of intrahepatic malignancies treated with stereotactic body radiation therapy.
Patients who have been heavily pretreated and/or have compromised liver function are highly variable in their sensitivity to radiation, so each patient’s sensitivity to radiation informs their treatment plan.
A Phase II clinical trial is described. The primary end point of the trial is lesion control at 6 months, and there are statistically justified stopping rules for excess toxicity and lack of efficacy.
Treatment is administered in five fractions, with a pause after the third fraction to assess the patients’ sensitivity using indocyanine green, an established assay of liver function.
The relationship between patients’ change in liver function owing to the last two fractions, compared with the first three fractions, is a value re-estimated throughout the trial using Bayesian statistical modeling.
The size of the final two fractions is conservatively determined to keep the probability of clinically significant liver damage at the end of treatment below a prespecified level.
Since the quality of estimates of parameters used in trial management changes during the course of the trial, its operating characteristics must be assessed by Monte Carlo simulation.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The following NIH grants supported the development of this manuscript: P01 CA59827, P30 CA46592 and P30 CA4790413. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Parkin D, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Deuffic S, Poynard T, Buffat L, Valleron A. Trends in primary liver cancer. Lancet. 1998;351(9097):214–215. doi: 10.1016/S0140-6736(05)78179-4. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag H, Davila J, Petersen N, Mc Glynn K. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139(10):817–823. doi: 10.7326/0003-4819-139-10-200311180-00009. [DOI] [PubMed] [Google Scholar]
- 5.Tse R, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26(4):657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 6.Herfarth K, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a Phase I/II trial. J Clin Oncol. 2001;19(1):164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 7.Kavanagh B, Schefter T, Cardenes H, et al. Interim analysis of a prospective Phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45(7):848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 8.Schacter L, Crum E, Spitzer T, Maksem J, Diwan V, Kolli S. Fatal radiation hepatitis: a case report and review of the literature. Gynecol Oncol. 1986;24(3):373–380. doi: 10.1016/0090-8258(86)90316-1. [DOI] [PubMed] [Google Scholar]
- 9.Dawson L, Eccles C, Craig T. Individualized image guided iso-NTCP based liver cancer SBRT. Acta Oncol. 2006;45:856–864. doi: 10.1080/02841860600936369. [DOI] [PubMed] [Google Scholar]
- 10.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence T, Robertson J, Anscher M, Jirtle R, Ensminger W, Fajardo L. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31(5):1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence T, Ten Haken R, Kessler M, et al. The use of 3D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23(4):781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 13.Russell A, Clyde C, Wasserman T, Turner S, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the rtog dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27(1):117–123. doi: 10.1016/0360-3016(93)90428-x. [DOI] [PubMed] [Google Scholar]
- 14.Dawson L, Normolle D, Balter J, McGinn C, Lawrence T, Ten Haken R. Analysis of radiation-induced liver disease using the lyman NTCP model. Int J Radiat Oncol Biol Phys. 2002;53(4):810–821. doi: 10.1016/s0360-3016(02)02846-8. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb M, Stratton H, Newell J, Shah D. Indocyanine green. Its use as an early indicator of hepatic dysfunction following injury in man. Arch Surg. 1984;119(3):264–268. doi: 10.1001/archsurg.1984.01390150006002. [DOI] [PubMed] [Google Scholar]
- 16.Hemming A, Scudamore C, Shackleton C, Pudek M, Erb S. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163(5):515–518. doi: 10.1016/0002-9610(92)90400-l. [DOI] [PubMed] [Google Scholar]
- 17.Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg. 2005;12(1):16–22. doi: 10.1007/s00534-004-0965-9. [DOI] [PubMed] [Google Scholar]
- 18.Oellerich M, Burdelski M, Lautz Hu, et al. Assessment of pretransplant prognosis in patients with cirrhosis. Transplantation. 1991;51(4):801–806. doi: 10.1097/00007890-199104000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Pollack D, Sufian S, Matsumoto T. Indocyanine green clearance in critically ill patients. Surg Gynecol Obstet. 1979;149:852–854. [PubMed] [Google Scholar]
- 20.Thall P, Simon R, Estey E. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14:357–379. doi: 10.1002/sim.4780140404. [DOI] [PubMed] [Google Scholar]
- 21.Casella G, George E. Explaining the Gibbs sampler. Am Stat. 1992;46:167–174. [Google Scholar]
- 22.Muler J, Mc Ginn C, Normolle D, et al. A Phase I trial using the time-to-event continual reassessment strategy to escalate cisplatin combined with gemcitabine and radiation therapy in pancreatic cancer. J Clin Oncol. 2004;22(2):238–243. doi: 10.1200/JCO.2004.03.129. [DOI] [PubMed] [Google Scholar]
- 23.Desai S, Ben-Josef E, Normolle D, et al. Phase I study of oxaliplatin, full-dose gemcitabine and concurrent radiation therapy in patients with pancreatic cancer. J Clin Oncol. 2007;25(29):4587–4592. doi: 10.1200/JCO.2007.12.0592. [DOI] [PubMed] [Google Scholar]