Abstract
The development of assessment techniques with immediate clinical applicability is a priority for resolving the growing epidemic in metabolic disease. Many imbalances in diet-dependent metabolism are not detectable in the fasted state. Resolving the high inter-individual variability in response to diet requires the development of techniques that can detect metabolic dysfunction at the level of the individual. The intra- and inter-individual variation in lipid metabolism in response to a standardized test meal was determined. Following an overnight fast on three different days, three healthy subjects consumed a test meal containing 40% of their daily calories. Plasma samples were collected at fasting, and 1, 3, 6, and 8 h after the test meal. Plasma fatty acid (FA) concentrations within separated lipid classes and lipoprotein fractions were measured at each time point. The intra-individual variation within each subject across three days was lower than the inter-individual differences among the three subjects for over 50% of metabolites in the triacylglycerol (TG), FA, and phosphatidylcholine (PC) lipid classes at 6 h, and for 25–50% of metabolites across lipid classes at 0, 1, 3, and 8 h. The consistency of response within individuals was visualized by principal component analysis (PCA) and confirmed by ANOVA. Three representative metabolites that discriminated among the three individuals in the apolipoprotein B (ApoB) fraction, TG16:1n7, TG18:2n6, and PC18:3n3, are discussed in detail. The postprandial responses of individuals were unique within metabolites that were individual discriminators (ID) of metabolic phenotype. This study shows that the targeted metabolomic measurement of individual metabolic phenotype in response to a specially formulated lipid challenge is possible even without lead-in periods, dietary and lifestyle control, or intervention over a 3-month period in healthy free-living individuals.
Keywords: Health assessment, Metabolic phenotype, Nutritional phenotype, Lipid metabolism, Postprandial, Response to challenge
1 Introduction
Research is increasingly documenting that humans are different from each other in their responses to diet (Ordovas et al. 2002; Ordovas and Shen 2008). The high degree of variability means that dietary and lifestyle solutions must ultimately be targeted to an individual’s particular metabolic needs in order to be successful in the long term (German et al. 2003). Although the idea of personalized or individualized approaches for successful disease prevention has been widely recognized (Tegner et al. 2007; Watkins et al. 2001), the question of how to actually bring personalized health to practice is largely unanswered. New tools that can assess health with the same specificity and sensitivity as those that assess disease need to be developed (German et al. 2004; Lemay et al. 2007). In addition to analytical tools that can detect statistically smaller differences in a wider array of metabolites, and the informatic tools to interpret the data both statistically and biologically, new strategies of experimental design for nutritional trials also need to be developed.
One existing approach that accounts for high variability in metabolism in response to different diets and foods is the cross-over interventional study. The gold standard in nutrition research has long been the randomized, placebo-controlled intervention trial, in which individuals are randomized to either the treatment or control groups and the average responses of the two groups are compared. On the other hand, in cross-over studies individuals undergo both the treatment arm and the control arm in randomized order so that each individual serves as their own control, amplifying the statistical power of the study. In order to amplify the signal to noise ratio even further, researchers have combined the cross-over study design with the challenge approach, which examines the response of an individual over several hours following a meal (e.g. the postprandial state).
The importance of response to a challenge meal was first recognized in 1979 (Zilversmit 1979), when the hypothesis was proposed that triglyceride-rich lipoproteins in the postprandial state are acute mediators of atherogenesis. Since then, postprandial lipaemia—the prolonged circulation of lipids in response to a meal—has been found to be an independent predictor of risk for myocardial infarction (Stampfer et al. 1996) and has been found to be increased in patients with cardiovascular disease (Karpe 1997).
The vast majority of cross-over postprandial literature examines the effects of varying the relative fat content and FA composition of meals on postprandial lipaemia and endothelial function (Berry et al. 2007; Blackburn et al. 2003a, b; Cassader et al. 2001; Chong et al. 2007; Chung et al. 2004; Cohn et al. 1993; Fielding et al. 1996; Hennig et al. 2001; Higashi et al. 2001; Hyson et al. 2002; Nicolaiew et al. 1998; Paton et al. 2006; Potts et al. 1995; Rivellese et al. 2008; Sharrett et al. 2001; Siepi et al. 2002; Silveira et al. 1996; van Oostrom et al. 2003; Westphal et al. 2006). However, the challenge approach has been applied to investigate other aspects of responsiveness to meals including the effects of food components such as polyphenols (Papamichael et al. 2008), and lifestyle factors such as exercise (Silvestre et al. 2008; Weiss et al. 2008) and meal frequency (Murphy et al. 1996). Postprandial responsiveness has been used both in a long-term sense to investigate the effects of modulating chronic diet and lifestyle (Fuentes et al. 2008) and in an acute sense to investigate the immediate effects of varying meal composition on postprandial metabolism (Chong et al. 2007).
However, variability in response to diet remains high even with the postprandial cross-over design. For instance, Burge et al. recently found coefficients of variation for plasma TG concentrations and areas under the curve over 6 h in response to a lipid challenge as high as 98% (calculated from data provided in Burdge et al. 2003). This study included 6 similarly healthy, young, white, male subjects, indicating that even within a very narrowly defined group of individuals variation in response to a meal can be very high.
The question that remains to be addressed is whether the observed variation in response is a result of variation in the postprandial biology per se, or discrete and consistent differences among individuals. In other words, is the observed variability a function of true biological differences between individuals or is it noise attributable to the daily variations in diet and lifestyle within each individual? Is the postprandial response itself an aspect of an individual’s metabolic phenotype?
The current study was conducted to determine whether quantitative blood lipid response profiles following a standardized dietary challenge are consistent within healthy, free-living individuals when challenged at intervals of several weeks apart during which time the subjects’ diets and lifestyles were explicitly not controlled. The key question addressed is whether the individual variation in metabolic response to the defined dietary challenge within each subject is significantly lower than the variation among the three subjects. Is it possible to distinguish unique metabolic phenotypes among healthy individuals who appear similar to each other by clinical assessment at fasting? Conversely, does biological noise (e.g. weekly and monthly fluctuations of normal metabolism related to changing dietary and other environmental conditions) mask true metabolic phenotype in response to the defined challenge?
A targeted metabolomic approach was undertaken to analyze the structural and energetic lipids comprehensively, including 38 FA within each of seven lipid classes that are distributed across several lipoprotein fractions, totaling 798 lipid metabolic endpoints measured at fasting and at four time points (1, 3, 6, and 8 h) after consumption of a test meal.
2 Materials and methods
2.1 Subjects
Three individuals, designated subject A, B, and C, were recruited from the University of California Davis campus. They were healthy adults aged 18–65 years with the following mean (±SEM) characteristics: age, 37 ± 16 years; weight, 65 ± 4 kg; body mass index (BMI), 21 ± 0.25 kg/m2; fasting total cholesterol, 150 ± 46 mg/dl (3.89 ± 1.19 mmol/l); fasting TG, 26 ± 18 mg/dl (0.29 ± 0.2 mmol/l); fasting insulin, 9.3 ± 0.85 μIU/ml (64.6 ± 6 pmol/l); and fasting glucose, 86.9 ± 11 mg/dl (4.82 ± 0.6 mmol/l). For individual subject characteristics, refer to Table 1. Exclusion criteria were pregnancy or nursing, any existing medical condition/disease diagnosis, prescription medications, allergy to any of the ingredients in the dietary challenge, BMI > 25, anemia and/or conditions that would influence ability to donate blood safely, and recovery from an illness, injury, or infection in the previous two weeks. The Institutional Review Board of the University of California Davis approved the study protocol, and written informed consent was obtained from all subjects.
Table 1.
Subject characteristics
| Characteristics | Subject |
Normal range | ||
|---|---|---|---|---|
| A | B | C | ||
| Age | 30 | 55 | 27 | |
| Weight (kg) | 63 | 62 | 70 | |
| Height (m) | 1.73 | 1.7 | 1.83 | |
| BMI (kg/m2) | 21 | 21 | 21 | 18.5–24.9 |
| Gender (M/F) | F | M | M | |
| Fasting TCa, mg/dl (mmol/l) | 140 (3.6) | 200 (5.2) | 109 (2.80) | ≤200 (5.2) |
| Fasting TG, mg/dl (mmol/l) | 67 (0.75) | 124 (1.4) | 34 (0.38) | ≤150 (1.7) |
| Fasting insulin, μIU/ml (pmol/l) | 8.4 (58.3) | 9.4 (65.2) | 10.1 (70.1) | 6.0–27.0 (41.7–187.5) |
| Fasting glucose, mg/dl (mmol/l) | 76.4 (4.24) | 99.1 (5.5) | 85.2 (4.73) | 60–109 (3.33–6.05) |
TC total cholesterol
2.2 Study design
The subjects were tested on three days separated by several months (once in December, once in March, and once in April). The subjects’ diets and other lifestyle factors (including exercise) were explicitly not controlled between test days in order to determine the true variability in lipid metabolism in healthy, free-living individuals maintaining their normal lifestyle. None of the subjects, however, undertook any major lifestyle changes during the course of the study, such as following a weight loss program or particular diet, starting or stopping exercise routines, and starting or stopping any prescription medications. A 24-h dietary recall was collected for the day prior to each dietary challenge. On the day of each study, the subjects arrived at the facility in the morning after an overnight (12-h) fast (no food or beverages except water after 8 pm). Subjects were weighed clothed without shoes, and a fasting blood sample was drawn by venipuncture. The subjects consumed the challenge meal within 20 min.
Postprandial blood draws were collected at 1, 3, 6, and 8 h using BD Vacutainer lavender-top EDTA tubes. Whole blood was centrifuged in a tabletop ultracentrifuge for 10 min at 4°C at 13,000 rpm within 15 min of collection. The plasma was immediately separated into 1.5-ml aliquots, transported from the clinical facility to the analytical lab on ice, and immediately frozen at −70°C until analysis. Lipid and lipoprotein composition, and glucose, insulin, and lactate were measured at each time point.
2.3 Dietary challenge
The challenge meal was calculated to provide 40% of daily calories using each subject’s BMI and activity level on the first of three study days to estimate daily caloric needs. The subjects’ weights did not change throughout the study. The challenge meal was provided in the form of a blended beverage containing 1 cup (230 g) non-fat lactose-free milk, 1 cup (227 g) low-fat vanilla-flavored yogurt (1% fat), 30 g 100% whey chocolate-flavored protein powder, 118 g banana, and 22 g flax seed oil. The subjects also consumed 3 g borage oil, 3.6 g soy lecithin, and 3 g fish oil in capsule form with their blended beverage. The entire challenge meal provided approximately 790 calories with 36 g fat (24% by weight or 41% of calories), 38 g protein (25% by weight or 19% of calories), and 79 g carbohydrate (51% by weight or 40% of calories). The lipid composition of the meal was verified by quantitative lipid analysis and is shown in Table 2. The test meal was unique in that it consisted of a high proportion of polyunsaturated fatty acids (PUFA), at 68% of fat calories, and with saturated FA and monounsaturated fatty acids (MUFA) contributing 13 and 19% of fat calories, respectively. Additionally, α-linolenic acid (18:3n3) contributed 48% of total fat calories, which is an unusually high proportion of this FA for the typical American diet. Likewise, the inclusion of lecithin, borage oil, and fish oil provided high amounts of phospholipids, γ-linolenic acid (18:3n6), and the long-chain ω3 FA eicosapentaenoic acid (20:5n3) and docosahexaenoic acid (22:6n3), relative to the typical American diet.
Table 2.
Test meal lipid composition
| FA | mg | FA | mg | FA | mg | FA | mg |
|---|---|---|---|---|---|---|---|
| 14:0 | 568.2 | 18:1n9 | 5,342.5 | 20:2n6 | 51.9 | 22:6n3 | 731.8 |
| 14:1n5 | 58.3 | 18:1n7 | 272.6 | 20:3n6 | 19.0 | 24:0 | 37.2 |
| 15:0 | 47.8 | t18:2n6 | 0.0 | 20:4n6 | 87.7 | 24:1n9 | 51.2 |
| dm16:0 | 6.5 | 18:2n6 | 5,885.0 | 20:3n3 | 0.0 | 24:6n3 | 0.0 |
| 16:0 | 3,543.3 | 18:3n6 | 681.0 | 20:4n3 | 66.1 | Total | 32,900.0 |
| t16:1n7 | 0.0 | 18:3n3 | 13,550.5 | 20:5n3 | 1,088.3 | SFA | 4,307.4 |
| 16:1n7 | 120.2 | 18:4n3 | 94.6 | 22:0 | 55.0 | MUFA | 6,135.1 |
| dm18:0 | 3.1 | 20:0 | 56.0 | 22:1n9 | 67.8 | PUFA | 22,447.2 |
| dm18:1n9 | 0.0 | 20:1n15 | 0.0 | 22:2n6 | 4.5 | n3 | 15,648.7 |
| dm18:1n7 | 0.7 | 20:1n12 | 0.0 | 22:4n6 | 53.7 | n6 | 6,797.2 |
| 18:0 | 0.0 | 20:1n9 | 222.4 | 22:5n6 | 14.4 | n7 | 392.8 |
| t18:1n9 | 0.0 | 20:3n9 | 1.4 | 22:5n3 | 117.3 | n9 | 5,685.4 |
Lipid composition is shown as mg fatty acid (FA) per test meal (including the blended beverage containing the flax seed oil, and the fish oil, borage oil, and lecithin capsules)
dm plasmalogen, t trans double bond, SFA saturated fatty acids, MUFA monounsaturated fatty acids, PUFA polyunsaturated fatty acids, n3 ω3 fatty acids, n6 ω6 fatty acids, n7 ω7 fatty acids, n9 ω9 fatty acids
2.4 Compositional lipid analysis
The plasma samples were analyzed by Lipomics Technologies, Inc. (West Sacramento, CA) as whole plasma, and as an ApoB and an apolipoprotein A (ApoA) fraction. The ApoA and ApoB fractions were separated by precipitation according to the Tung-B method (phosphotungstate/Mg2+) described by Demacker et al. (1997). Briefly, 200 μl of plasma were mixed with 500 μl of precipitation reagent (final concentrations, 1.1 μmol of phosphotungstic acid and 50 mmol of MgCl2 per ml of plasma). After incubation, the supernatant fraction was aspirated with a Pasteur pipette to collect the ApoA fraction, and the pellets were taken as the ApoB fraction.
Compositional lipid analysis was performed according to the method described by Watkins et al. (2002). Briefly, the lipids from plasma (200 μl) were extracted using a modified Folch extraction in chloroform:methanol (2:1 v/v) (Folch et al. 1957). Extracted lipids were separated by preparative HPLC into eight lipid classes—FA, diacylglycerol (DG), TG, free cholesterol, cholesterol ester (CE), lysophosphatidylcholine, phosphatidylcholine (PC), and phosphatidyl-ethanolamine (PE). FA from all lipid classes except free cholesterol were trans-esterified in methanolic HCl. The resulting FA methyl esters were extracted and analyzed by gas chromatography using an Agilent 6890 gas chromatograph (Palo Alto, CA) equipped with a 30-m HP-88 capillary column and a flame-ionization detector. The following FA were measured: 14:0, 15:0, 16:0, 18:0, 20:0, 22:0, 24:0, 14:1n5, 16:1n7, t16:1n7, 18:1n9, t18:1n9, 18:1n7, 18:2n6, t18:2n6, 18:3n6, 18:3n3, 18:4n3, 20:1n9, 20:2n6, 20:3n9, 20:3n6, 20:4n6, 20:3n3, 20:4n3, 20:5n3, 22:1n9, 22:2n6, 22:4n6, 22:5n3, 22:6n3, 24:1n9, and 24:6n3, and the plasmalogen derivatives of 16:0, 18:0, 18:1n9, and 18:1n7.
2.5 Statistical analysis
The data were analyzed as both quantitative (nmol FA/g plasma for FA, and nmol lipid class/g plasma for total lipid classes) and mol% (mol FA as a percentage of mol total FA in the lipid class), and visualization tasks were performed using JMP software (SAS Institute, Inc., Cary, NC). Separate one-way ANOVA was used to detect significant differences among subjects in FA and lipid class concentrations, as well as to detect intra-individual differences across days. All pair-wise comparisons among subjects were conducted with Tukey 95% simultaneous confidence intervals with an individual confidence level = 97.80%.
2.6 Multivariate analysis
PCA was used to overview clustering among the samples based on the multivariate data expressed as plasma mol% FA in each lipid class, and to visualize the intra- and inter-individual variation in response to the challenge meal. Information from the complete data set was reduced to a few principal components displayed as a set of scores describing maximum variation within the data. The software used for PCA was Simca-P 11.5 (Umetrics, Umeå, Sweden). Before modeling, the variables minus the 6 out of 38 lowest-abundance metabolites were mean centered, scaled to unit variance, and log transformed.
Individual Discriminators (ID) were defined as metabolites that differentiated individuals from each other by way of having an intra-individual variation that was lower than the inter-individual variation, as assessed by one-way ANOVA. The percentage of ID out of all FA measured within each lipid class was calculated and presented for each time point.
3 Results
A targeted metabolomic approach was used to quantify a total of 38 FAs present within each of seven lipid classes (TG, DG, CE, lysophosphatidylcholine, PC, PE, and FA) within whole plasma and two lipoprotein fractions (ApoB, and ApoA), for a total of 798 individual metabolites, at five time points (0, 1, 3, 6, and 8 h) on three days (once each in December, March, and April) in three individuals. The total number of discrete metabolite measurements per individual was 11,970, and the total number of measurements in the study was over 35,000. Many of the key metabolites known to be important indicators of flux through specific key lipid metabolic pathways had low variability within individuals across the three days.
3.1 Variation among individuals was greater than within individuals
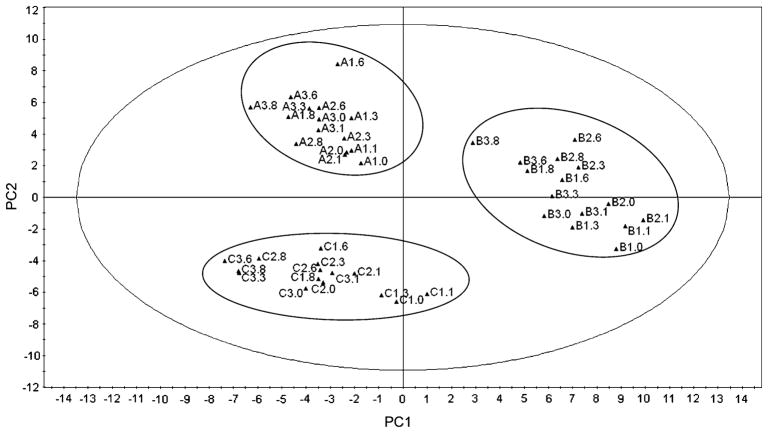
The data were first examined with PCA to determine whether the plasma lipid metabolome was visually distinguishable among the three individuals, meaning that the measurements for each individual across days and time points did not overlap with those of the other individuals. The first two components of the model (PC1 and PC2) explained 37% of the variance (R2 = 0.371, and Q2 = 0.261). When examining fasting clinical measurements only (as shown in Table 1), the individuals were indistinguishable, meaning that their fasting glucose, insulin, cholesterol, and TG concentrations were equally within the clinically accepted normal ranges. However, with multivariate analysis of the plasma lipid metabolome in response to a standardized dietary challenge, it was possible to identify distinct groups in the score plot (Fig. 1), clearly separating the individuals from each other. Inter-individual variation dominated, confirming that the inter-individual variation among the three individuals was higher than the intra-individual variation within each individual across the three days. The score plot clearly shows each individual’s postprandial time points (from all three days) grouped together in a distinct, non-overlapping cluster relative to those of the other two individuals. If the intra-individual variation had been higher than the inter-individual variation, clustering of the three days would be expected rather than of the three individuals, with significant overlap among subjects.
Fig. 1.
Score plot of principal component analysis (PCA) of plasma lipid metabolome. PC1, principal component 1; PC2, principal component 2. Each point/triangle represents a specific time point (0, 1, 3, 6, and 8 h) on a specific day (1, 2, 3) for each subject (A, B, C) such that A2.0 is the 0 h time point on day 2 for subject A, B3.6 is the 6 h time point on day 3 for subject B, C2.1 is the 1 h time point on day 2 for subject C, and so on
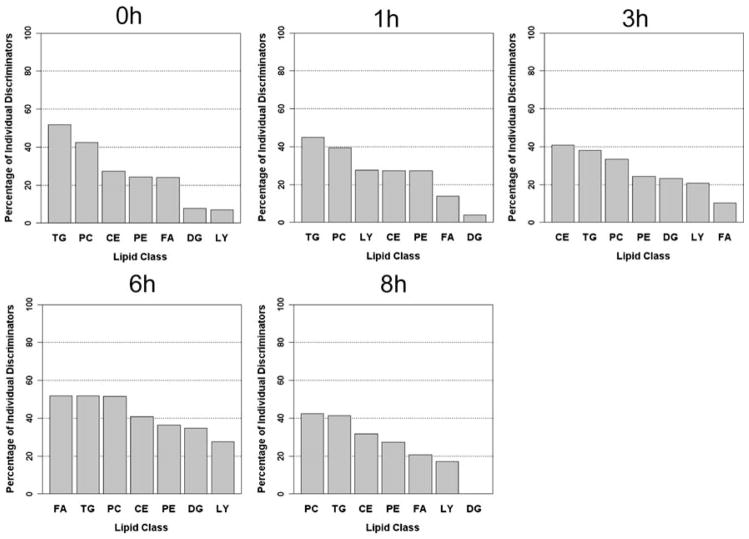
The percentage of ID was then calculated in order to determine how many of the metabolites within each lipid class behaved consistently within individuals (i.e. had a significantly lower intra- than inter-individual variation). At baseline, the TG lipid class was composed of over 50% ID, with PC at just over 40%, and CE, PE, and FA around 25% ID (Fig. 2). At 1 h the %ID across lipid classes decreased. At 3 h, CE became the lipid class with the highest proportion of ID, with PC also increasing in the percentage of ID, whereas FA became the lipid class with the lowest proportion of ID. The 6-h time point had the highest signal across lipid classes, with all lipid classes having over 25% ID, and FA, TG, and PC having over 50% ID. Finally, at 8 h PC and TG had the highest proportion of ID, whereas the DG lipid class contained a large amount of variability indicated by no metabolites that were significant ID. These data show that several lipid classes have a high abundance of metabolites that are capable of discriminating between individuals despite the explicit lack of dietary control over the months of the experiment. This suggests that although some metabolites better reflect short-term changes in dietary lipid composition and/or inconsistent metabolic response to the same challenge, a significant proportion of lipids measured are consistent markers of metabolic response and, therefore, are reflective of nutritional phenotype. The 6-h time point was particularly informative and had the highest proportion of ID across lipid classes.
Fig. 2.
Percentage of individual discriminators (ID) in each lipid class. The percentage of ID (metabolites for which the intra-individual variation across three days was lower than the inter-individual variation among subjects as assessed by one-way ANOVA) is shown for each lipid class in descending order for each time point. TG triacylglycerol, CE cholesterol ester, DG diacylglycerol, FA free fatty acids, LY lysophosphatidylcholine, PC phosphatidylcholine, PE phosphatidylethanolamine
3.2 Response curves were unique to each individual
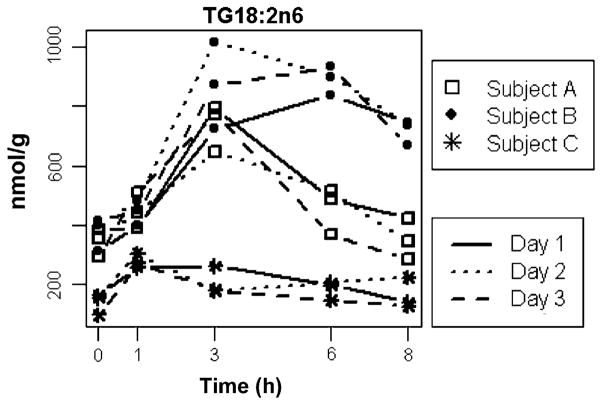
The data were then examined with statistical approaches one metabolite at a time. Three of the metabolites that were determined to be ID by ANOVA, and also by PCA to be influential in distinguishing the individuals (e.g. they were located in the periphery of the PCA loading plot), were chosen as representative metabolites that were important determinants of the inter-individual variation. Within the TG lipid class, most metabolite response curves were almost identical on each of the three days for each individual. Figure 3 shows the postprandial response curves of each of the three individuals of a key TG metabolite in the ApoB fraction—linoleic acid (18:2n6). The graphs clearly show that the intra-individual variation for each individual at each time point was low, whereas the differences among individuals were significant. The postprandial response curves were unique to each individual and were reproducible over the three days. The P-values of the ANOVA for each time point were: 0.0015, 0.0051, 0.0004, <0.0001, and <0.0001 for 0, 1, 3, 6, and 8 h, respectively, confirming that the differences among subjects were significant at each time point. At all time points except 3 h, the intra-individual variation was comparable to the variation observed for the fasted condition. Even at 3 h, the point of maximal signal (i.e. the postprandial lipid peak) and hence maximal variation, the intra-individual variability was sufficiently small to be statistically powerful in discriminating among individuals.
Fig. 3.
Postprandial response curves of 18:2n6 in TG lipid class. The concentrations of 18:2n6TG at each time point are shown in nmol FA/g. The lines connecting the time point measurements represent the 3 different days on which measurements were made
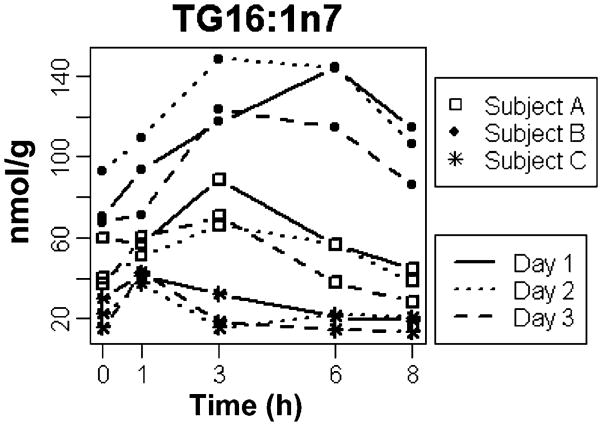
The response curves for all three individuals for another key metabolite—palmitoleic acid (16:1n7)—in the ApoB fraction of the TG lipid class are shown in Fig. 4. Again, the individual response curves for this metabolite clearly varied among individuals, yet the intra-individual variation was low. The patterns mirrored those of linoleic acid shown in Fig. 2—in subject A, the metabolite reached a peak concentration at 3 h and returned to baseline by 6 h; in subject B, the metabolite reached a similar high peak concentration at 3 h, but was only partially cleared by 8 h; and in subject C, the much lower metabolite peak occurred at 1 h and returned to baseline by 6 h. Again, ANOVA confirmed these findings and the P-values for each time point were 0.0036, 0.0043, 0.0002, <0.0001, and 0.0001 for 0, 1, 3, 6, and 8 h, respectively.
Fig. 4.
Postprandial variation in 16:1n7 in TG lipid class. The concentrations of 16:1n7TG at each time point are shown in nmol FA/g. The lines connecting the time point measurements represent the three different days on which measurements were made
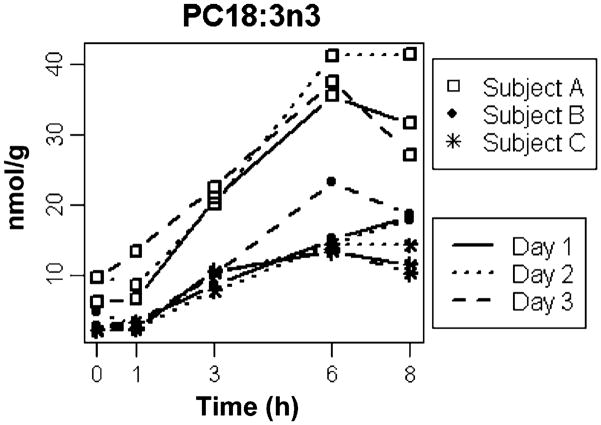
The observation of high inter-individual differences yet low intra-individual differences in metabolic response was not unique to the TG lipid class. Results for α-linolenic acid (18:3n3) in the PC lipid class in the ApoB fraction are shown in Fig. 5. Again, the pattern of response differed among the three individuals, yet was consistent within individuals. P-values from ANOVA again confirmed that the differences were significant for each time point, and were 0.0032, 0.0093, <0.0001, 0.0002, and 0.0026 at 0, 1, 3, 6, and 8 h, respectively.
Fig. 5.
Postprandial variation in 18:3n3 in phosphatidylcholine (PC) lipid class. The concentrations of 18:3n3PC at each time point are shown in nmol FA/g. The lines connecting the time point measurements represent the three different days on which measurements were made
4 Discussion
The null hypothesis of this study was that fluctuations within individuals would be sufficiently large as to preclude statistical attempts to identify individual postprandial responses as a characteristic of an individual’s metabolic phenotype. In opposition to the null hypothesis, multiple metabolic output variables related to variations in overall metabolic health were statistically different, and three representative metabolites were shown. Whereas the variation within individuals across days was low, the differences among subjects were significant. The results show that the examination of postprandial response to a standardized dietary challenge can indeed reveal aspects of diet-dependent metabolic phenotype within individuals, even without specific lead-in periods and dietary monitoring in the days and weeks prior to assessment.
It is well documented that chronic diet affects the levels of lipids and individual FA in the plasma. CE are indicative of dietary fat intake over the past 2 weeks (Katan et al. 1997). The intake of olive oil is correlated with levels of 18:1, dairy is correlated with levels of 15:0 and 17:0, and levels of long chain ω3 FA are indicative of fish intake (Fusconi et al. 2003). The FA profiles of children who are vegetarian, lacto-ovo-vegetarian, or semi-vegetarian (Krajcovicova-Kudlackova et al. 1997a), as well as adult vegetarians (Krajcovicova-Kudlackova et al. 1997b), have higher levels of 18:2n6 and 18:3n3, as well as lower levels of 16:1n7, 20:4n6, and 22:6n3. The consumption of a Mediterranean diet results in higher levels of MUFA and ω3 FA, as well as lower levels of PUFA and ω6 FA (Urquiaga et al. 2004). High pasta and low red meat consumption are correlated with lower levels of saturated FA and higher levels of MUFA (Scaglioni et al. 2004). However, in this study we examined the effects of a particular challenge on the changes in lipid metabolites rather than exploring the differences at fasting between individuals. Although we found that up to 50% of metabolites measured within lipid classes such as TG were able to distinguish among individuals and were thus considered to be discriminators of metabolic phenotype (e.g. were ID), this means that the other 50% of metabolites were, in fact, likely to be more dependent on chronic diet. However, since this study was not designed to distinguish those metabolites that are reflective of chronic diet from those that are not, we did not speculate about the nature of variation in the metabolites that were not found to be ID.
The time points in this study were chosen based on previous studies of postprandial lipid metabolism that are available in the literature (Cohn et al. 1993; Cohn et al. 1989; Li et al. 2003; Lichtenstein et al. 1993; Schaefer et al. 2001); however, the majority of such studies were designed to quantify TG and total cholesterol concentrations. The time points that best reflect the appearance and disappearance of specific FA and, indeed, specific lipid classes and lipoprotein particles may be different. Future studies examining the specific formulation of dietary challenges can now proceed in order to better detect changes in specific metabolic pathways, metabolic disorders, and nutritional phenotypes of interest.
The quantitative analysis of plasma lipid biomarkers in response to a standardized dietary pathway probe has shown to be a powerful tool for the clinical assessment of lipid metabolic status. Not all, but certainly many, of the predominant metabolic diseases associated with Western diets are lipid-related, regardless of whether dietary lipids in and of themselves are the primary cause of the disturbance in metabolism or whether it is other aspects of dietary composition that cause the lipid dysregulation. For example, in certain individuals with non-alcoholic steatohepatitis, the dietary component that causes liver lipid accumulation and its subsequent liver dysfunction is an excess of simple carbohydrates leading to increased de novo lipogenesis (Diraison et al. 2003). A surprisingly high proportion of chronic disease leads to disturbances in lipid metabolism, which ultimately cause the most deleterious health effects associated with a particular disease. For instance, in end-stage renal disease, although up to 20% of patients may need kidney transplant over 5 years, the most immediate health concern is actually the high mortality rate (up to 45% of patients) associated with cardiovascular disease and diabetes, both of which are strongly associated with dyslipidemia (Keith et al. 2004).
The results of the present study are not consistent with a model of normal humans exhibiting a common “normal” postprandial response to a lipid load. In contrast, this study found that the postprandial responses of three healthy individuals were unique and distinguishable from each other, with metabolites that were able to act as ID, or discriminators of individual metabolic phenotype. This was apparent from the unbiased and unsupervised multivariate analysis of the entire dataset through PCA, which clearly illustrated a high degree of clustering within each subject, indicating that intra-individual variation across days was low, whereas inter-individual variation among the three subjects was significant. The statistical analysis of one metabolite at a time applied to three representative metabolites confirmed these findings. Despite being equally normal by metabolic criteria as assessed clinically at fasting, the three individuals nonetheless had distinct postprandial responses to the standardized challenge.
One potential shortcoming of this study is the sample size (n = 3). If our aim had been to discover the average human response to this particular nutritional challenge then we should indeed have included a larger number of subjects that is adequately powered to detect the average response. However, in this case, our aim was not to determine the average population response. In fact, we were specifically interested in the unique response of each of the individual subjects to the defined challenge. For this reason we studied the three subjects three times. Future studies will need to determine the optimal number of times that an individual must be studied in order to be confident of their metabolic phenotype in response to a particular challenge, however, from this study we can conclude that three times was adequate in this group of individuals. The responses of individuals to different challenges must also be determined. For example, this particular challenge meal was high in PUFA relative to the typical Western diet, and was delivered in a liquid form. The influence of solid versus liquid meals, variations in FA composition and macronutrient composition, and a vast number of other dietary factors remain to be explored to determine the array of individual responses to meals and how these contribute to the assessment of individual metabolic and nutritional phenotype.
5 Conclusions
The key question addressed in this study was whether the intra-individual variation in the postprandial response to a standard challenge is lower than the inter-individual variation if administered to the same free-living individuals months apart. If so, the process of developing a standardized challenge could become part of normal human assessment as a means to define a discrete postprandial metabolic signal indicative of an individual’s nutritional and metabolic phenotype. Diet has long been thought of as a confounding variable in health assessment, with true metabolic signatures or signals being disguised by temporal fluctuations in diet and lifestyle patterns. In contrast to this long-held belief, the current study found that intra-individual variation in lipid response to a constant challenge was highly reproducible in normal individuals despite an explicit lack of dietary and lifestyle control during the course of the study. This study is a first-step application of standardizing dietary challenges based on comprehensive lipid metabolite measurements taken through the postprandial state. The significance of reproducible and measurable metabolic phenotypes in healthy people is that the data can be used to predict a pattern that may lead to disease, thus making possible early, targeted, and personalized intervention before overt disease symptoms appear.
Acknowledgments
This work was supported by University of California at Davis Graduate Group in Nutrition Block Grant, Jastro Shields Scholarship, and Superfund Training Fellowship to A. M. Zivkovic; supported in part by the National Institute of Environmental Health Sciences (NIEHS) grant R37 ES02710, the NIEHS Superfund Basic Research Program P42 ES04699, the University of California Davis CHARGE Study, Center for Children’s Environmental Health, NIEHS grant P01 ES11269, and the University of California Discovery Program.
Abbreviations
- FA
Fatty acid
- TG
Triacylglycerol
- ApoB
Apolipoprotein B
- ApoA
Apolipoprotein A
- PC
Phosphatidylcholine
- PE
Phosphatidylethanolamine
- CE
Cholesterol ester
- DG
Diacylglycerol
- PCA
Principal component analysis
- ID
Individual discriminators
- BMI
Body mass index
- MUFA
Monounsaturated fatty acid
- PUFA
Polyunsaturated fatty acid
Contributor Information
Angela M. Zivkovic, Email: amzivkovic@ucdavis.edu.
J. Bruce German, Email: jbgerman@ucdavis.edu.
References
- Berry SE, Miller GJ, Sanders TA. The solid fat content of stearic acid-rich fats determines their postprandial effects. The American Journal of Clinical Nutrition. 2007;85(6):1486–1494. doi: 10.1093/ajcn/85.6.1486. [DOI] [PubMed] [Google Scholar]
- Blackburn P, Cote M, Lamarche B, et al. Impact of postprandial variation in triglyceridemia on low-density lipoprotein particle size. Metabolism: Clinical and Experimental. 2003a;52(11):1379–1386. doi: 10.1016/S0026-0495(03)00315-9. [DOI] [PubMed] [Google Scholar]
- Blackburn P, Lamarche B, Couillard C, et al. Contribution of visceral adiposity to the exaggerated postprandial lipemia of men with impaired glucose tolerance. Diabetes Care. 2003b;26(12):3303–3309. doi: 10.2337/diacare.26.12.3303. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Jones AE, Frye SM, Goodson L, Wootton SA. Effect of meal sequence on postprandial lipid, glucose and insulin responses in young men. European Journal of Clinical Nutrition. 2003;57(12):1536–1544. doi: 10.1038/sj.ejcn.1601722. [DOI] [PubMed] [Google Scholar]
- Cassader M, Gambino R, Musso G, et al. Postprandial triglyceride-rich lipoprotein metabolism and insulin sensitivity in nonalcoholic steatohepatitis patients. Lipids. 2001;36(10):1117–1124. doi: 10.1007/s11745-001-0822-5. [DOI] [PubMed] [Google Scholar]
- Chong MF, Fielding BA, Frayn KN. Mechanisms for the acute effect of fructose on postprandial lipemia. The American Journal of Clinical Nutrition. 2007;85(6):1511–1520. doi: 10.1093/ajcn/85.6.1511. [DOI] [PubMed] [Google Scholar]
- Chung BH, Cho BH, Liang P, et al. Contribution of postprandial lipemia to the dietary fat-mediated changes in endogenous lipoprotein–cholesterol concentrations in humans. The American Journal of Clinical Nutrition. 2004;80(5):1145–1158. doi: 10.1093/ajcn/80.5.1145. [DOI] [PubMed] [Google Scholar]
- Cohn JS, Johnson EJ, Millar JS, et al. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postprandial increases in the plasma concentration of TRL triglycerides and retinyl esters. Journal of Lipid Research. 1993;34(12):2033–2040. [PubMed] [Google Scholar]
- Cohn JS, McNamara JR, Krasinski SD, Russell RM, Schaefer EJ. Role of triglyceride-rich lipoproteins from the liver and intestine in the etiology of postprandial peaks in plasma triglyceride concentration. Metabolism: Clinical and Experimental. 1989;38(5):484–490. doi: 10.1016/0026-0495(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Demacker PN, Hessels M, Toenhake-Dijkstra H, Baadenhuijsen H. Precipitation methods for high-density lipoprotein cholesterol measurement compared, and final evaluation under routine operating conditions of a method with a low sample-to-reagent ratio. Clinical Chemistry. 1997;43(4):663–668. [PubMed] [Google Scholar]
- Diraison F, Moulin P, Beylot M. Contribution of hepatic de novo lipogenesis and reesterification of plasma non esterified fatty acids to plasma triglyceride synthesis during non-alcoholic fatty liver disease. Diabetes & Metabolism. 2003;29(5):478–485. doi: 10.1016/S1262-3636(07)70061-7. [DOI] [PubMed] [Google Scholar]
- Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: The origin of an early peak studied by specific dietary fatty acid intake during sequential meals. The American Journal of Clinical Nutrition. 1996;63(1):36–41. doi: 10.1093/ajcn/63.1.36. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of Biological Chemistry. 1957;226(1):497–509. [PubMed] [Google Scholar]
- Fuentes F, Lopez-Miranda J, Perez-Martinez P, et al. Chronic effects of a high-fat diet enriched with virgin olive oil and a low-fat diet enriched with alpha-linolenic acid on postprandial endothelial function in healthy men. The British Journal of Nutrition. 2008;100(1):159–165. doi: 10.1017/S0007114508888708. [DOI] [PubMed] [Google Scholar]
- Fusconi E, Pala V, Riboli E, et al. Relationship between plasma fatty acid composition and diet over previous years in the Italian centers of the European Prospective Investigation into Cancer and Nutrition (EPIC) Tumori. 2003;89(6):624–635. doi: 10.1177/030089160308900606. [DOI] [PubMed] [Google Scholar]
- German JB, Bauman DE, Burrin DG, et al. Metabolomics in the opening decade of the 21st century: Building the roads to individualized health. The Journal of Nutrition. 2004;134(10):2729–2732. doi: 10.1093/jn/134.10.2729. [DOI] [PubMed] [Google Scholar]
- German JB, Roberts MA, Watkins SM. Personal metabolomics as a next generation nutritional assessment. The Journal of Nutrition. 2003;133(12):4260–4266. doi: 10.1093/jn/133.12.4260. [DOI] [PubMed] [Google Scholar]
- Hennig B, Toborek M, McClain CJ. High-energy diets, fatty acids and endothelial cell function: Implications for atherosclerosis. Journal of the American College of Nutrition. 2001;20(2, Suppl):97–105. doi: 10.1080/07315724.2001.10719021. [DOI] [PubMed] [Google Scholar]
- Higashi K, Shige H, Ito T, et al. Effect of a low-fat diet enriched with oleic acid on postprandial lipemia in patients with type 2 diabetes mellitus. Lipids. 2001;36(1):1–6. doi: 10.1007/s11745-001-0660-5. [DOI] [PubMed] [Google Scholar]
- Hyson DA, Paglieroni TG, Wun T, Rutledge JC. Postprandial lipemia is associated with platelet and monocyte activation and increased monocyte cytokine expression in normolipemic men. Clinical and Applied Thrombosis/Hemostasis. 2002;8(2):147–155. doi: 10.1177/107602960200800211. [DOI] [PubMed] [Google Scholar]
- Karpe F. Postprandial lipid metabolism in relation to coronary heart disease. The Proceedings of the Nutrition Society. 1997;56(2):671–678. doi: 10.1079/PNS19970067. [DOI] [PubMed] [Google Scholar]
- Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: An 18-month controlled study. Journal of Lipid Research. 1997;38(10):2012–2022. [PubMed] [Google Scholar]
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of Internal Medicine. 2004;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- Krajcovicova-Kudlackova M, Simoncic R, Bederova A, Grancicova E, Magalova T. Influence of vegetarian and mixed nutrition on selected haematological and biochemical parameters in children. Die Nahrung. 1997a;41(5):311–314. doi: 10.1002/food.19970410513. [DOI] [PubMed] [Google Scholar]
- Krajcovicova-Kudlackova M, Simoncic R, Klvanova J, Bederova A, Babinska K, Grancicova E. The plasma profile of fatty acids in vegetarians. Bratislavské Lekárske Listy (Tlacené Vydanie) 1997b;98(1):23–27. [PubMed] [Google Scholar]
- Lemay D, Zivkovic AM, German JB. Building the bridges to bioinformatics in nutrition research. The American Journal of Clinical Nutrition. 2007;86:1261–1269. doi: 10.1093/ajcn/86.5.1261. [DOI] [PubMed] [Google Scholar]
- Li Z, Otvos JD, Lamon-Fava S, et al. Men and women differ in lipoprotein response to dietary saturated fat and cholesterol restriction. The Journal of Nutrition. 2003;133(11):3428–3433. doi: 10.1093/jn/133.11.3428. [DOI] [PubMed] [Google Scholar]
- Lichtenstein AH, Ausman LM, Carrasco W, et al. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arteriosclerosis and Thrombosis. 1993;13(10):1533–1542. doi: 10.1161/01.atv.13.10.1533. [DOI] [PubMed] [Google Scholar]
- Murphy MC, Chapman C, Lovegrove JA, et al. Meal frequency; does it determine postprandial lipaemia? European Journal of Clinical Nutrition. 1996;50(8):491–497. [PubMed] [Google Scholar]
- Nicolaiew N, Lemort N, Adorni L, et al. Comparison between extra virgin olive oil and oleic acid rich sunflower oil: Effects on postprandial lipemia and LDL susceptibility to oxidation. Annals of Nutrition and Metabolism. 1998;42(5):251–260. doi: 10.1159/000012741. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Corella D, Cupples LA, et al. Polyunsaturated fatty acids modulate the effects of the APOA1 G-A polymorphism on HDL–cholesterol concentrations in a sex-specific manner: The Framingham Study. The American Journal of Clinical Nutrition. 2002;75(1):38–46. doi: 10.1093/ajcn/75.1.38. [DOI] [PubMed] [Google Scholar]
- Ordovas JM, Shen J. Gene-environment interactions and susceptibility to metabolic syndrome and other chronic diseases. Journal of Periodontology. 2008;79(8, Suppl):1508–1513. doi: 10.1902/jop.2008.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papamichael CM, Karatzi KN, Papaioannou TG, et al. Acute combined effects of olive oil and wine on pressure wave reflections: Another beneficial influence of the Mediterranean diet antioxidants? Journal of Hypertension. 2008;26(2):223–229. doi: 10.1097/HJH.0b013e3282f25b80. [DOI] [PubMed] [Google Scholar]
- Paton CM, Brandauer J, Weiss EP, et al. Hemostatic response to postprandial lipemia before and after exercise training. Journal of Applied Physiology. 2006;101(1):316–321. doi: 10.1152/japplphysiol.01363.2005. [DOI] [PubMed] [Google Scholar]
- Potts JL, Coppack SW, Fisher RM, Humphreys SM, Gibbons GF, Frayn KN. Impaired postprandial clearance of triacylglycerol-rich lipoproteins in adipose tissue in obese subjects. The American Journal of Physiology. 1995;268(4 Pt 1):E588–E594. doi: 10.1152/ajpendo.1995.268.4.E588. [DOI] [PubMed] [Google Scholar]
- Rivellese AA, Giacco R, Annuzzi G, et al. Effects of monounsaturated vs. saturated fat on postprandial lipemia and adipose tissue lipases in type 2 diabetes. Clinical Nutrition (Edinburgh, Lothian) 2008;27(1):133–141. doi: 10.1016/j.clnu.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Scaglioni S, Veduci E, Agostoni C, et al. Dietary habits and plasma fatty acids levels in a population of Italian children: Is there any relationship? Prostaglandins Leukotrienes and Essential Fatty Acids. 2004;71(2):91–95. doi: 10.1016/j.plefa.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Schaefer EJ, Audelin MC, McNamara JR, et al. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. The American Journal of Cardiology. 2001;88(10):1129–1133. doi: 10.1016/S0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- Sharrett AR, Heiss G, Chambless LE, et al. Metabolic and lifestyle determinants of postprandial lipemia differ from those of fasting triglycerides: The Atherosclerosis Risk In Communities (ARIC) study. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21(2):275–281. doi: 10.1161/01.atv.21.2.275. [DOI] [PubMed] [Google Scholar]
- Siepi D, Marchesi S, Lupattelli G, et al. Postprandial endothelial impairment and reduced glutathione levels in postmenopausal women. Annals of Nutrition and Metabolism. 2002;46(1):32–37. doi: 10.1159/000046750. [DOI] [PubMed] [Google Scholar]
- Silveira A, Karpe F, Johnsson H, Bauer KA, Hamsten A. In vivo demonstration in humans that large postprandial triglyceride-rich lipoproteins activate coagulation factor VII through the intrinsic coagulation pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(11):1333–1339. doi: 10.1161/01.atv.16.11.1333. [DOI] [PubMed] [Google Scholar]
- Silvestre R, Kraemer WJ, Quann EE, et al. Effects of exercise at different times on postprandial lipemia and endothelial function. Medicine and Science in Sports and Exercise. 2008;40(2):264–274. doi: 10.1249/mss.0b013e31815c485a. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Krauss RM, Ma J, et al. A prospective study of triglyceride level, low-density lipoprotein particle diameter, and risk of myocardial infarction. Journal of the American Medical Association. 1996;276(11):882–888. doi: 10.1001/jama.276.11.882. [DOI] [PubMed] [Google Scholar]
- Tegner J, Skogsberg J, Bjorkegren J. Thematic review series: Systems biology approaches to metabolic and cardiovascular disorders. Multi-organ whole-genome measurements and reverse engineering to uncover gene networks underlying complex traits. Journal of Lipid Research. 2007;48(2):267–277. doi: 10.1194/jlr.R600030-JLR200. [DOI] [PubMed] [Google Scholar]
- Urquiaga I, Guasch V, Marshall G, et al. Effect of Mediterranean and Occidental diets, and red wine, on plasma fatty acids in humans. An intervention study. Biological Research. 2004;37(2):253–261. doi: 10.4067/s0716-97602004000200012. [DOI] [PubMed] [Google Scholar]
- van Oostrom AJ, Sijmonsma TP, Verseyden C, et al. Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. Journal of Lipid Research. 2003;44(3):576–583. doi: 10.1194/jlr.M200419-JLR200. [DOI] [PubMed] [Google Scholar]
- Watkins SM, Hammock BD, Newman JW, German JB. Individual metabolism should guide agriculture toward foods for improved health and nutrition. The American Journal of Clinical Nutrition. 2001;74(3):283–286. doi: 10.1093/ajcn/74.3.283. [DOI] [PubMed] [Google Scholar]
- Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPAR-gamma agonist rosiglitazone. Journal of Lipid Research. 2002;43(11):1809–1817. doi: 10.1194/jlr.M200169-JLR200. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Arif H, Villareal DT, Marzetti E, Holloszy JO. Endothelial function after high-sugar-food ingestion improves with endurance exercise performed on the previous day. The American Journal of Clinical Nutrition. 2008;88(1):51–57. doi: 10.1093/ajcn/88.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal S, Taneva E, Kastner S, et al. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis. 2006;185(2):313–319. doi: 10.1016/j.atherosclerosis.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Zilversmit DB. Atherogenesis: A postprandial phenomenon. Circulation. 1979;60(3):473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]